Abstract
The aim of this study was to investigate the role of gender in histological and functional outcome, angiogenesis, neurogenesis and therapeutic effects of recombinant human erythropoietin (rhEPO) in mice after traumatic brain injury (TBI). TBI caused both tissue loss in the cortex and cell loss in the dentate gyrus (DG) in the injured hemisphere at day 35 post TBI without a significant gender difference. After TBI, sensorimotor deficits were significantly larger in male mice compared to females, while similar spatial learning deficits were present in both genders. TBI alone significantly stimulated angiogenesis and neurogenesis in the cortex and in the DG of injured hemispheres in both genders. rhEPO at a dose of 5,000 Units/kg body weight administered intraperitoneally at 6 h, and 3 and 7 days after injury significantly reduced lesion volume and DG cell loss examined at day 35 after TBI as well as dramatically improved sensorimotor and spatial learning performance without an obvious gender proclivity. rhEPO significantly enhanced neurogenesis in the cortex and the DG of the ipsilateral hemisphere in male TBI mice. rhEPO did not affect angiogenesis in the ipsilateral cortex and DG in both genders after TBI. The present data demonstrate that posttraumatic administration of rhEPO improves histological and functional outcome in both genders, which may be mediated by reducing cortical tissue damage and DG cell loss in the ipsilateral hemisphere. In addition, the major gender propensity observed in the present study with mice after TBI without treatment is limited to sensorimotor deficits and cell proliferation.
Keywords: behavior, erythropoietin, gender, mouse, neurogenesis, traumatic brain injury
1. Introduction
A growing number of reports suggest that ovarian hormones may play a role as endogenous neuroprotectants. Accumulating epidemiological data have suggested that young female patients have improved clinical prognoses after TBI (Groswasser et al., 1998; Kirkness et al., 2004; Mostafa et al., 2002; Roof and Stein, 1999). The gender difference in outcome, favoring females, has also been documented in rodents after ischemic stroke, brain hypoxia, and subarachnoid hemorrhage (Roof and Hall, 2000). However, some clinical data failed to show sexual dimorphism in posttraumatic mortality or in the incidence of acute complications after any degree of TBI (Coimbra et al., 2003; Davis et al., 2006). Gender-associated outcomes may be more apparent in experimental studies than in clinical settings. Indeed, several studies have demonstrated that female animals generally exhibit less neurodegeneration after ischemia or TBI due to the multifaceted neuroprotective effects of estrogen and/or progesterone (Garcia-Segura et al., 2001; Roof and Hall, 2000; Suzuki et al., 2006).
Gender differences in response to treatment of experimental TBI have been reported. Significant neuroprotection was noted only in injured male rodents treated with posttraumatic hypothermia (Suzuki et al., 2003), environmental enrichment (Wagner et al., 2002) or dopamine agonist (Wagner et al., 2007). Although a number of therapeutic trials for TBI have been undertaken, no efficacious treatment has been identified clinically (Narayan et al., 2002). A hematopoietic cytokine erythropoietin (EPO) has been shown to be neuroprotective in animal injury models. Administration of rhEPO 24 hours prior to or up to 6 hours after focal ischemic stroke significantly reduced the extent of infarction (Brines et al., 2000). rhEPO also attenuated concussive brain (Brines et al., 2000) and spinal cord injury (Celik et al., 2002; Grasso et al., 2006) in experimental rodent models. More recently, it has been reported that rhEPO treatment promotes spatial memory restoration and enhances neurogenesis in the dentate gyrus after TBI induced by controlled cortical impact (CCI) in the rat (Lu et al., 2005). rhEPO also improves functional outcome after stroke in rats (Chang et al., 2005; Wang et al., 2004).
The benefits of rhEPO have been demonstrated in CCI-induced TBI in the male rat (Cherian et al., 2007; Lu et al., 2005) and in weight drop-induced TBI in the male mouse (Yatsiv et al., 2005). However, the prospect of possible gender-difference in the response to rhEPO treatment after TBI has not been previously explored. In the present study, we investigated whether gender affected histological and functional outcome, angiogenesis, neurogenesis and the therapeutic effects of rhEPO after TBI in mice.
2. Results
Body weight
As compared with preinjury levels, body weight was significantly decreased at days 1 and 4 post injury in saline-treated mice (F 7, 96 = 4.25, P<0.001) and at days 1, 4, and 7 in rhEPO-treated mice (F 7, 88 = 4.87, P<0.001) (Table 1). Sham mice showed a transient but significant decrease in body weight at day 1 after surgery (F 7, 48 = 2.93, P<0.05, data not shown). However, there were no significant differences in body weight between the rhEPO- and saline-treated groups for either males or females and between males and females for the saline-treated or rhEPO treated groups (P > 0.05). At 14 days after injury, body weight almost returned to preinjury levels and continued to increase slowly afterwards.
Table 1.
Changes in body weight in both genders before and after TBI
| M-Saline | M-EPO | F-Saline | F-EPO | |
|---|---|---|---|---|
| Preinjury | 26.8 ± 0.7 | 26.7 ± 0.6 | 24.9 ± 1.1 | 24.8 ± 1.2 |
| Day 1 | 24.4 ± 0.4* | 24.2 ± 0.3* | 23.4 ± 0.6* | 23.4 ± 0.7* |
| Day 4 | 24.7 ± 0.7* | 24.5 ± 0.5* | 23.6 ± 0.5* | 23.2 ± 0.4* |
| Day 7 | 25.4 ± 0.5 | 24.7 ± 0.8* | 24.0 ± 1.3 | 23.6 ± 1.2* |
| Day 14 | 26.5 ± 1.5 | 26.0 ± 1.7 | 24.4 ± 1.1 | 24.0 ± 1.4 |
| Day 21 | 27.8 ± 1.4 | 27.2 ± 0.9 | 24.7 ± 1.0 | 24.3 ± 1.1 |
| Day 28 | 27.9 ± 1.0 | 27.5 ± 1.8 | 24.6 ± 1.0 | 24.8 ± 1.2 |
| Day 35 | 28.3 ± 1.2 | 27.6 ± 1.1 | 24.4 ± 1.5 | 24.7 ± 1.3 |
M: male; F: female. Data are mean ± SD (g).
P < 0.05 vs. corresponding Preinjury. N (mice/group) = 13 (M-Saline); 12 (M-EPO); 8 (F-Saline) and 8 (F-EPO).
Hematocrit
The baseline hematocrit (HCT) was identical for all groups before injury. TBI alone slightly reduced HCT in female mice at day 7 post injury (Table 2). As compared to saline treatment, rhEPO treatment significantly increased HCT at day 7 post TBI for both genders (P<0.05). rhEPO-induced HCT elevations remained in the male TBI mice at day 14 post injury, while HCT levels gradually returned to normal by day 14 in female mice. HCT levels returned to normal by day 21 post injury in the male mice. Although rhEPO treatment significantly increased HCT in both genders post injury, HCT remained higher in the male mice than in the female mice at day 7 post injury (P<0.001). The reason is not known.
Table 2.
Changes in hematocrit in both genders before and after TBI
| M-Saline | M-EPO | F-Saline | F-EPO | |
|---|---|---|---|---|
| Preinjury | 49 ± 2 | 48 ± 2 | 46 ± 2 | 48 ± 2 |
| Day 7 | 47 ± 2 | 56 ± 2* # | 42 ± 3 | 49 ± 3* |
| Day 14 | 46 ± 2 | 52 ± 3* | 44 ± 2 | 47 ± 2 |
| Day 21 | 46 ± 4 | 47 ± 2 | 45 ± 2 | 46 ± 1 |
| Day 28 | 47 ± 3 | 47 ± 2 | 44 ± 3 | 45 ± 3 |
| Day 35 | 46 ± 3 | 48 ± 3 | 44 ± 2 | 44 ± 3 |
M: male; F: female. Data are mean ± SD (%).
P<0.05 vs. corresponding saline-treated mice.
P < 0.01 vs. F-EPO. N (mice/group) = 13 (M-Saline); 12 (M-EPO); 8 (F-Saline) and 8 (F-EPO).
Lesion volume
Mice were sacrificed at day 35 after injury for lesion volume measurements. TBI caused significant tissue loss in male mice (8.4 ± 2.1%) and female mice (7.7 ± 2.2%) as compared with contralateral hemispheres (P<0.05). The rhEPO-treated mice demonstrated a significantly reduced lesion volume, 4.3 ± 1.8% for males and 4.8 ± 0.7% for females, compared to the saline-treated group (P<0.001). There were no differences in lesion volume between the genders after TBI or rhEPO treatment (males vs. female, P>0.05).
Spatial learning test
In the present study the Morris water maze protocol was used to detect spatial learning deficits during the last 5 days prior to sacrifice (i.e., days 31-35 post injury). The time spent in the correct quadrant (Northeast) by sham animals of both sexes significantly increased from day 31 to day 35 post sham operation. TBI impaired spatial learning without a significant gender difference (Fig. 1). rhEPO treatment significantly improved spatial learning in the males at days 33, 34 and 35 and in the females at days 34 and 35 compared with the saline-treated group (P<0.05).
Fig. 1.
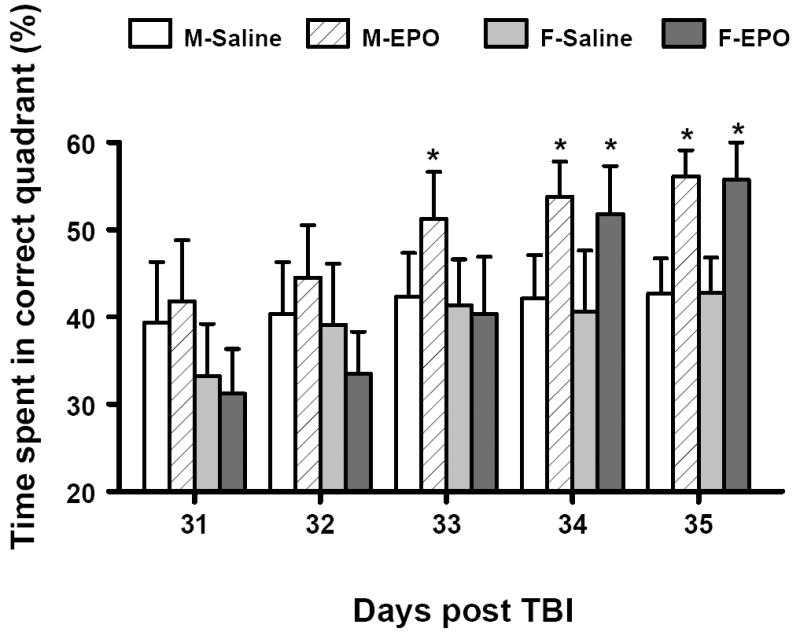
Effect of rhEPO on spatial learning function 31-35 days in both genders after TBI. Treatment with rhEPO improves spatial learning performance measured by a recent version of the Morris water maze test compared with the saline group. Data represent mean ± SD. *P < 0.05 between M-Saline vs. M-EPO or F-Saline vs. F-EPO. N (mice/group) = 13 (M-Saline); 12 (M-EPO); 8 (F-Saline) and 8 (F-EPO). M = male; F = female.
Sensorimotor function test
The incidence of forelimb footfaults during baseline (preoperatively) was approximately 4-5 % for both genders. Sham surgery alone mildly increased the frequency of footfaults at postoperative days 1 and 4. TBI significantly increased the occurrence of right forelimb footfaults contralateral to the TBI at 1 to 21 days postinjury as compared with the pre-injury baseline for both genders (Fig. 2). However, male TBI mice exhibited a significantly higher frequency of footfaults than female TBI mice at days 1, 4 and 7 post injury (P<0.05). Treatment with rhEPO significantly reduced the number of contralateral forelimb footfaults at 1 to 28 days in the males after TBI compared to treatment with saline (P<0.05). Similar effects were seen in the female TBI mice treated with rhEPO at 7 to 21 days after TBI as compared with the saline-treated group (P<0.05).
Fig. 2.
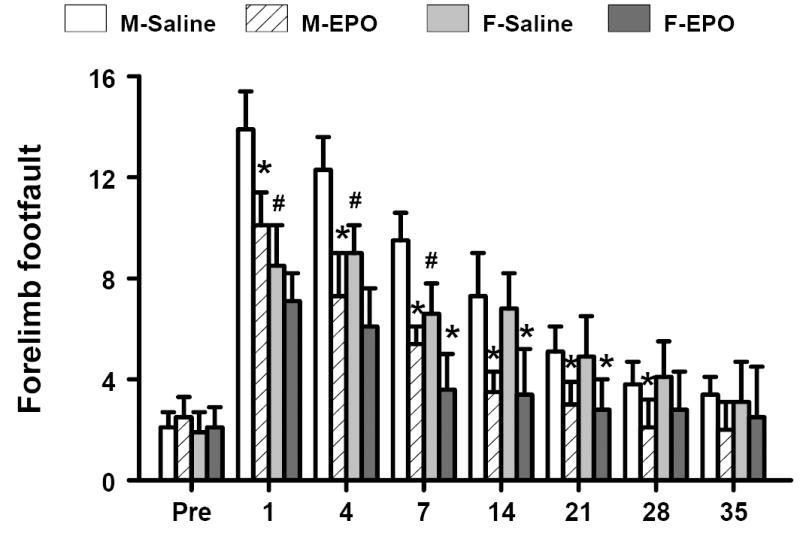
Effect of rhEPO on sensorimotor function (forelimb footfault) in both genders before and after TBI. “Pre” represents pre-injury level. Treatment with rhEPO improves recovery of sensorimotor performance in both genders as compared with the saline group. Data represent mean ± SD. *P < 0.05 between M-Saline vs. M-EPO or F-Saline vs. F-EPO; # P < 0.05 between M-Saline vs. F-Saline. N (mice/group) = 13 (M-Saline); 12 (M-EPO); 8 (F-Saline) and 8 (F-EPO). M = male; F = female.
Similar results were found for the contralateral hindlimb (Fig. 3). Sham surgery alone significantly increased the number of footfaults at postoperative day 1 relative to baseline. TBI significantly increased the incidence of contralateral hindlimb footfaults at 1 to 21 days post-injury for both genders. However, male mice displayed a significantly higher frequency of footfaults than female mice at days 7 and 14 post injury (P<0.05). Treatment with rhEPO significantly reduced the number of contralateral hindlimb footfaults 7 to 21 days post-injury compared to treatment with saline but only in male mice.
Fig. 3.
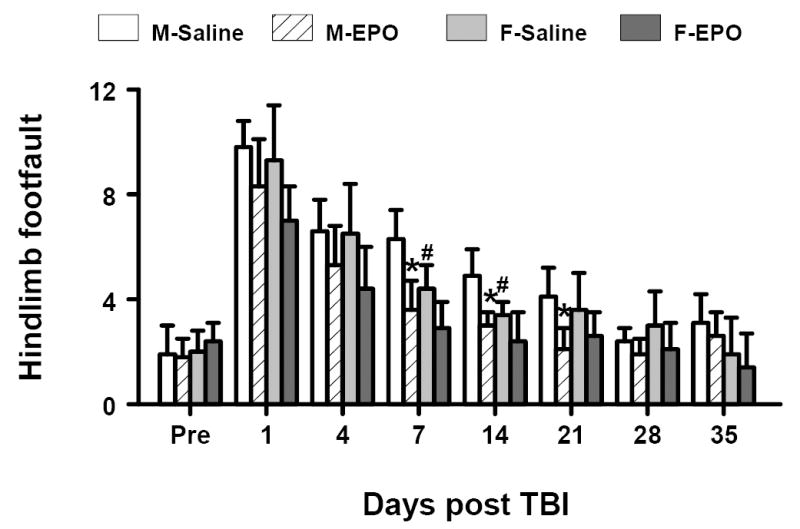
Effect of rhEPO on sensorimotor function (hindlimb footfault) in both genders before and after TBI. “Pre” represents pre-injury level. Treatment with rhEPO improves recovery of sensorimotor performance as compared with the saline group. Data represent mean ± SD. *P < 0.05 between M-Saline vs. M-EPO; # P < 0.05 between M-Saline vs. F-Saline. N (mice/group) = 13 (M-Saline); 12 (M-EPO); 8 (F-Saline) and 8 (F-EPO). M = male; F = female.
Cell loss in the DG
At day 35 after TBI the cell number in the ipsilateral DG of injured mice was decreased to 58% (males) and 64% (females) of that seen in the ipsilateral DG of sham mice. rhEPO treatment significantly reduced cell loss in the ipsilateral DG in both genders compared to saline-treated groups (P<0.05, Fig.4). The cell number after rhEPO treatment was maintained at 77% (males) and 89% (females) of that in the ipsilateral DG of shams. The cell number in the ipsilateral DG was slightly higher in the females than in the males from the saline- or rhEPO-treated groups but did not reach a significant gender difference.
Fig. 4.
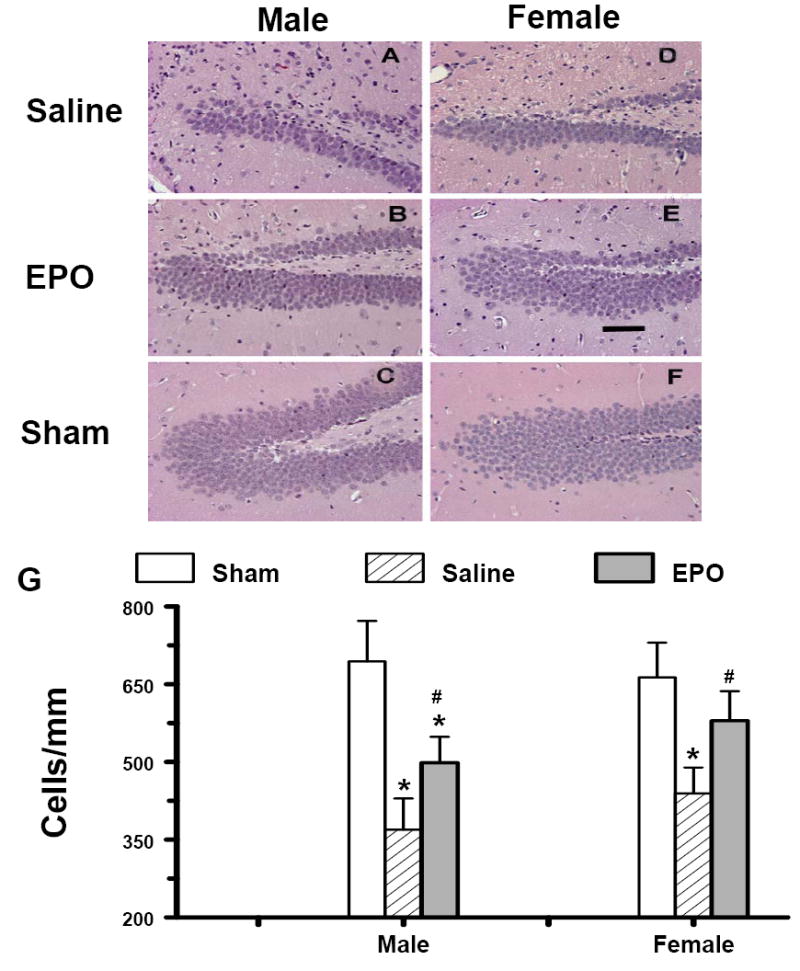
Effect of rhEPO on cell loss in the ipsilateral DG at 35 days in both genders after TBI. H & E staining: A-F. Treatment with rhEPO (B, E) reduced cell loss as compared with the saline group (A, D) in both genders. The cell number in the ipsilateral DG is shown in (G). Data represent mean ± SD. * P < 0.05 vs. corresponding Sham. # P < 0.05 between Saline vs. EPO. N (mice/group) = 13 (male-saline), 12 (male-EPO), 8 (male-sham), 8 (female-saline), 8 (female-EPO), 7 (female-sham). Scale bar = 50μm.
Angiogenesis
vWF-staining has been used to identify vascular structure in the brain after TBI (Lu et al., 2004). TBI alone significantly increased the density of vessels in the DG (Fig. 5) and the cortex (Fig. 6) of the ipsilateral hemisphere of both genders. TBI did not significantly affect the density of vessels in the CA3 and CA1 regions of the injured hemisphere compared with the ipsilateral hemisphere of sham mice (Fig. 5E). rhEPO treatment did not show significant effects on vascular density in the DG or in the cortex (Figs. 5 and 6). No gender differences in the vWF-staining were observed in mice after saline or rhEPO treatment post injury
Fig. 5.
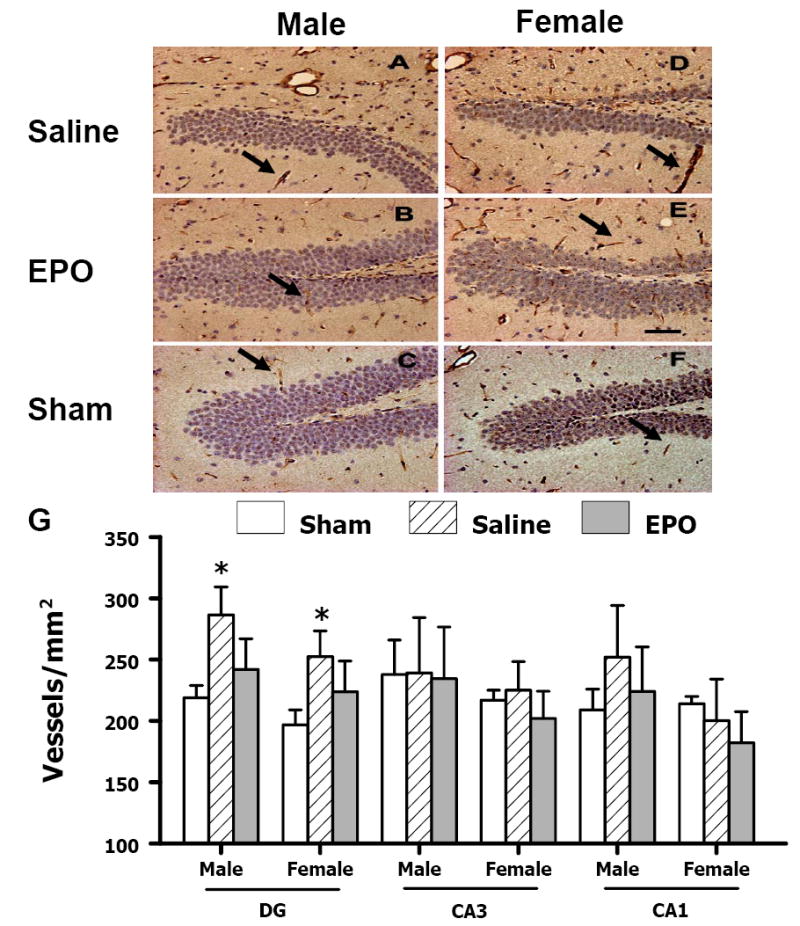
Effect of rhEPO on vWF-staining vascular structure in the ipsilateral hippocampus at 35 days after TBI. TBI (A, D) alone significantly increased the vascular density (stained brown, arrow as an example) in the DG. rhEPO (B, E) did not affect angiogenesis after TBI. The density of vWF-stained vasculature is shown in (G). Data represent mean ± SD. *P < 0.05 vs. Sham. N (mice/group) = 13 (male-saline), 12 (male-EPO), 8 (male-sham), 8 (female-saline), 8 (female-EPO), 7 (female-sham). Scale bar = 50μm.
Fig. 6.
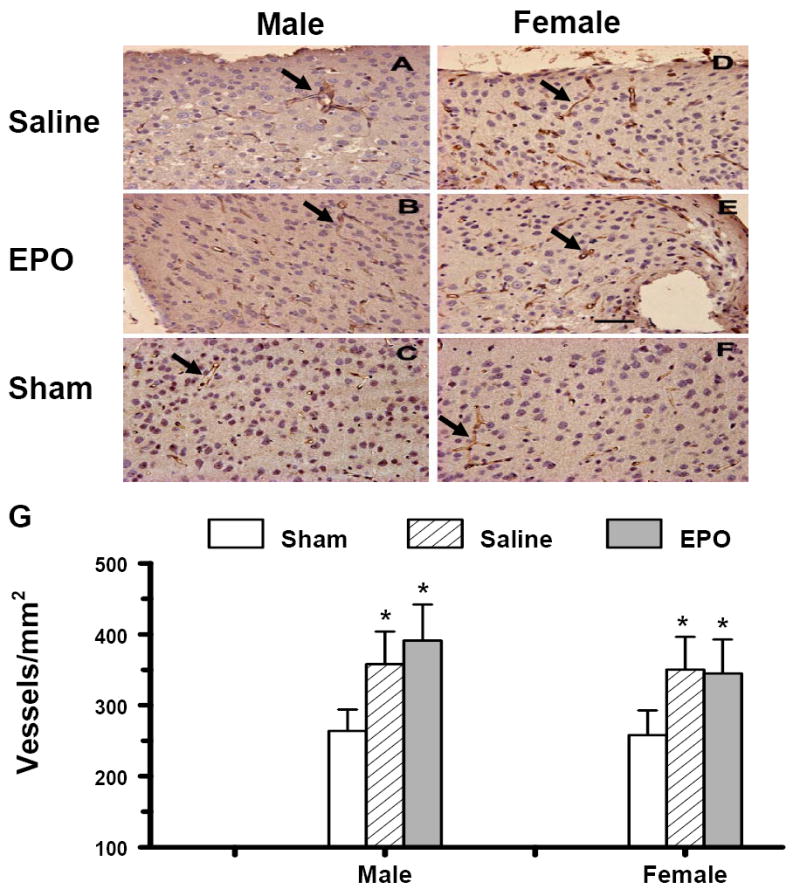
Effect of rhEPO on vWF-staining vascular structure in the ipsilateral cerebral cortex of sham or injured mice in both genders at 35 days after TBI. TBI (A, D) alone significantly increased vascular density (stained brown, arrow as an example) in the cortex. rhEPO (B, E) did not affect angiogenesis after TBI. The density of vWF-stained vasculature is shown in (G). Data represent mean ± SD. *P < 0.05 vs. Sham. N (mice/group) = 13 (male-saline), 12 (male-EPO), 8 (male-sham), 8 (female-saline), 8 (female-EPO), 7 (female-sham). Scale bar = 50μm.
Cell proliferation
An analog of thymidine, 5-bromo-2’-deoxyuridine (BrdU), is commonly used to detect proliferating cells in living tissues (Gratzner, 1982). The number of BrdU-positive cells found in the ipsilateral DG of injured mice (Fig. 7, P<0.05) was significantly increased after TBI alone, compared with the number found in the ipsilateral hemisphere of sham mice at 35 days after TBI. However, rhEPO treatment did not further influence the number of BrdU-positive cells in the DG after TBI (Fig. 7). Gender did not affect the cell proliferation in the DG after TBI or rhEPO treatment.
Fig. 7.
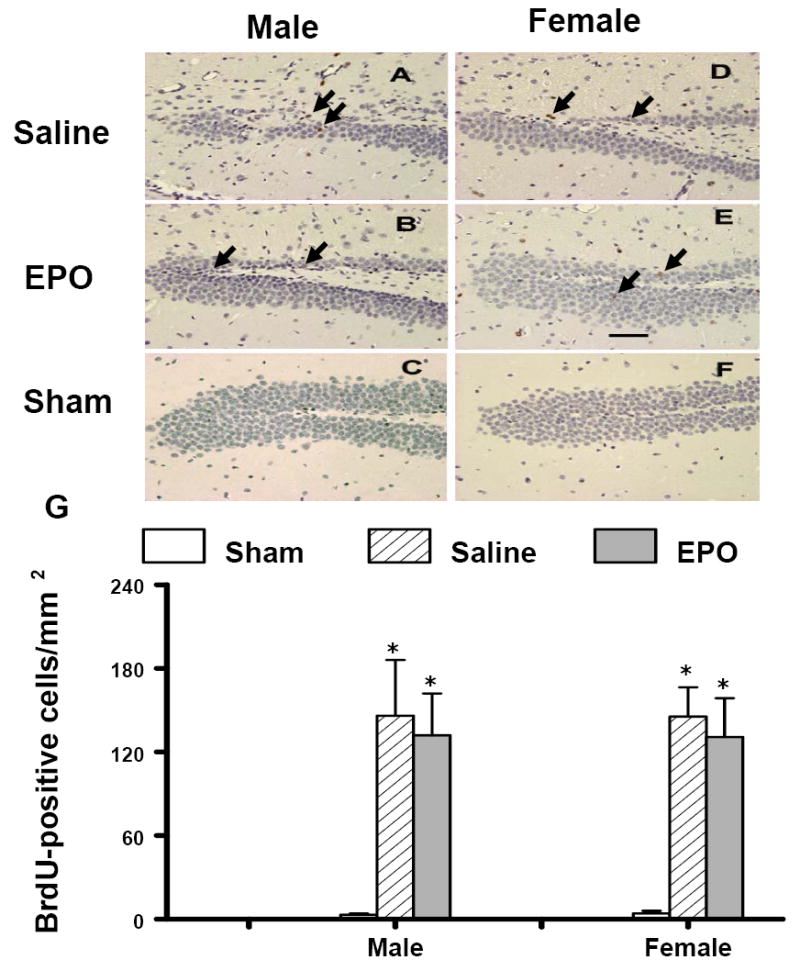
Effect of rhEPO on BrdU-positive cells in the ipsilateral DG at 35 days in both genders after TBI. TBI (A, D) alone significantly increased the number of BrdU-positive cells (brown stained, arrows) in the ipsilateral DG. However, treatment with rhEPO (B, E) did not affect cell proliferation as compared with the saline group (A, D). The number of BrdU-positive cells is shown in (G). Data represent mean ± SD. *P < 0.05 vs. Sham. N (mice/group) = 13 (male-saline), 12 (male-EPO), 8 (male-sham), 8 (female-saline), 8 (female-EPO), 7 (female-sham). Scale bar = 50μm.
BrdU-positive cells were rarely seen in the cortex of sham animals or in the contralateral cortex of mice that had undergone TBI. TBI alone significantly increased the number of BrdU-positive cells in the ipsilateral injured cortex of both genders (Fig. 8). The rhEPO-treated groups of both genders showed significantly increased BrdU-positive cells in the ipsilateral injured cortex after TBI when compared to the saline-treated group (Fig. 8, P <0.05). The number of BrdU-positive cells in the ipsilateral injured cortex of the females was significantly greater than that in the males treated with saline or rhEPO after TBI (P<0.05).
Fig. 8.
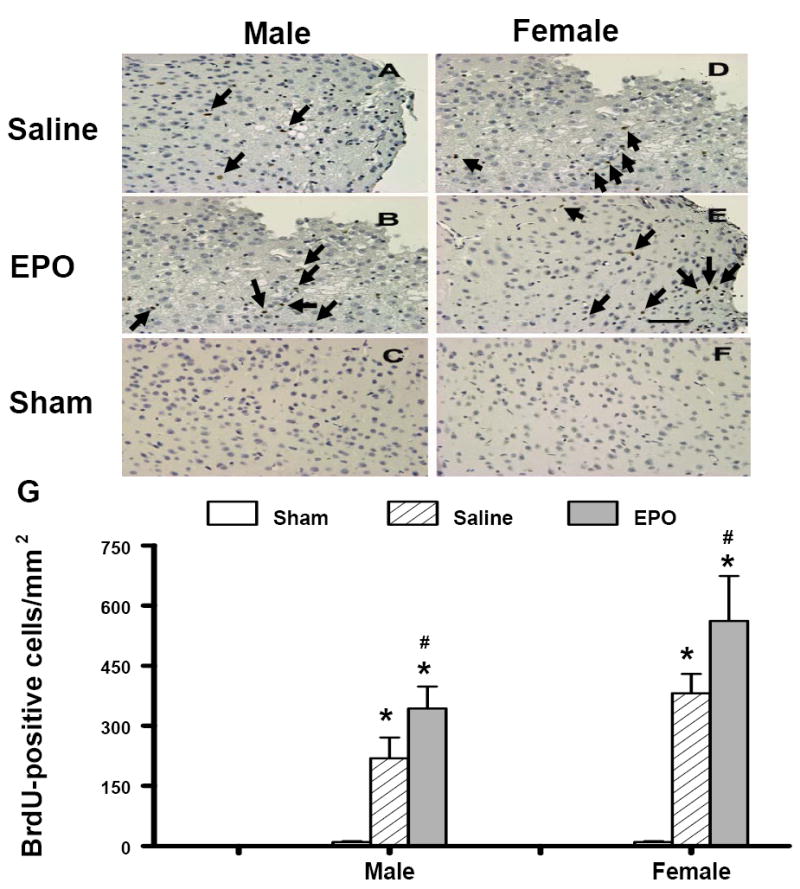
Effect of rhEPO on BrdU-positive cells in the ipsilateral cerebral cortex of sham or injured mice at 35 days in both genders after TBI. TBI (A, D) alone significantly increased the number of BrdU-positive cells (brown stained, arrows) in the ipsilateral cortex. Treatment with rhEPO (B, E) significantly increased BrdU-positive cells in the cortex as compared with the saline group (A, D). The number of BrdU-positive cells is shown in (G). Data represent mean ± SD. *P < 0.05 vs. Sham; #P < 0.05 vs. corresponding saline treatment. N (mice/group) = 13 (male-saline), 12 (male-EPO), 8 (male-sham), 8 (female-saline), 8 (female-EPO), 7 (female-sham). Scale bar = 50μm.
Neurogenesis
Newly generated neurons were identified by double labeling for BrdU (proliferating marker) and NeuN (mature neuronal marker). There was no gender difference in the percentage of NeuN/BrdU-colabeled cells in both ipsilateral DG (Fig. 9a-A vs. 9a-C) and cortex (Fig. 9b-A vs. 9b-C) of injured mice after TBI. Approximately 10% of BrdU-positive cells were NeuN/BrdU-colabeled cells in the ipsilateral DG and cortex of injured mice after TBI in both male and female mice (Fig. 9d). The percentage of NeuN/BrdU-colabeled cells was significantly higher in the rhEPO-treated group than in the saline-treated group in the DG (Fig. 9a-A vs.9a-B) and cortex (Fig. 9b-A vs. 9b-B) in male mice. The percentage of NeuN/BrdU-colabeled cells was about 47% in the ipsilateral DG and 36% in the ipsilateral cortex in the male mice treated with rhEPO. Only 10 % of BrdU-positive cells in the ipsilateral DG (Fig. 9a-C vs.9a-D) and cortex (9b-C vs. 9b-D) were NeuN/BrdU-colabeled cells in the rhEPO-treated female mice (Fig. 9d). After treatment with rhEPO, male mice exhibited a significantly higher percentage of NeuN/BrdU-colabeled cells in the DG (Fig. 9a-B, 9b-B, and 9d) and cortex (Fig. 9a-D, 9b-D, and 9d) than did the female mice.
Fig. 9.
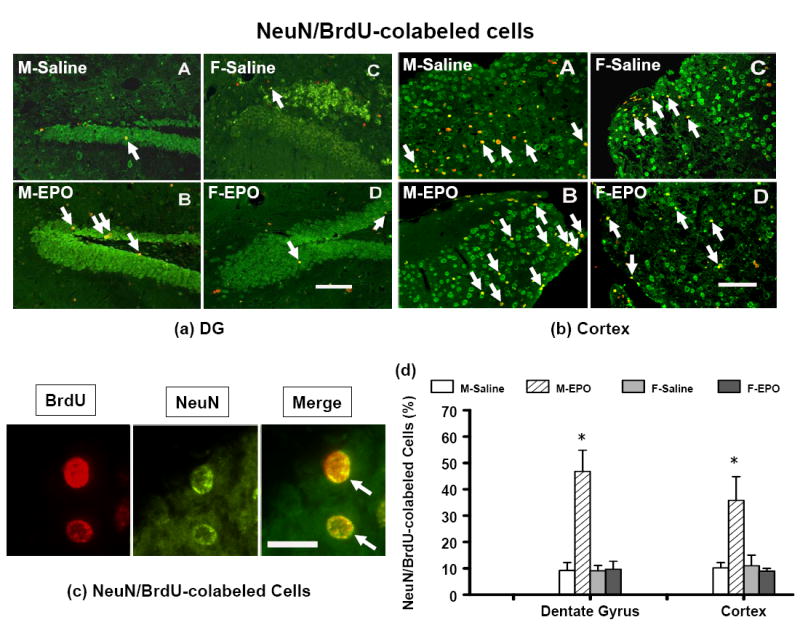
Photographs show the double fluorescent staining for BrdU (red) and NeuN (green) to identify the newly generated cells (red) and neurogenesis (yellow) in the dentate gyrus (a) and the cortex (b) of the ipsilateral, injured hemisphere of the saline-treated and the rhEPO-treated groups at 35 days in both genders after TBI. (c) NeuN/BrdU-double staining (merge in yellow): Newborn BrdU-positive cells (red) can differentiate into neurons expressing NeuN (green). (d) The percentage of NeuN/BrdU-colabeled cells. TBI alone significantly increased the percentage of NeuN/BrdU-colabeled cells (merge in yellow, indicated by arrow) in the DG (a-A, a-C) and the cortex (b-A, b-C). Treatment with rhEPO significantly increased the percentage of NeuN/BrdU-colabeled cells in the DG (a-B vs. a-A) and the cortex (b-B vs. b-A) in male TBI mice as compared with the saline group. Data represent mean ± SD. *P < 0.05 vs. male; # P < 0.05 vs. corresponding saline treatment. N (mice/group) = 13 (M-saline); 12 (M-EPO); 8 (F-saline) and 8 (F-EPO). M = male; F = female. Scale bar = 50μm (a, b); 25 μm (c).
3. Discussion
In the present study, we investigated effects of postinjury administration of rhEPO on histological and functional outcome, angiogenesis, and neurogenesis in both female and male mice after TBI. To our knowledge, our data are the first observations on gender effects of rhEPO treatment and demonstrate that rhEPO is neuroprotective in both genders after TBI. The main findings of the present study are: (1) TBI caused cortical tissue loss and dentate gyrus cell loss in the ipsilateral hemisphere without an obvious gender difference; rhEPO treatment significantly reduced cortical injury and dentate gyrus cell loss similarly in both genders. (2) TBI caused more sensorimotor deficits in male mice than in females; rhEPO treatment significantly reduced sensorimotor deficits. (3) The spatial learning function was similarly impaired in both genders after TBI and significantly improved by rhEPO treatment. (4) Cell proliferation and neurogenesis did not show a gender difference in the DG after TBI, while cell proliferation in the ipsilateral cortex was significantly higher in the female mice than that in the males; treatment with rhEPO significantly enhanced neurogenesis in the DG and cortex in male TBI mice as compared to females. (5) TBI significantly stimulated angiogenesis without a gender propensity; rhEPO treatment did not affect angiogenesis. (6) The improvements in spatial learning and sensorimotor function appear to result substantially from early rhEPO treatment’s neuroprotective effect in reducing tissue damage and cell loss. (7) The footfault test and this moving-platform version of the water maze test are sensitive to sensorimotor and spatial performance deficits in both female and male mice even after a month post TBI. Data in this present study indicate that the gender propensity observed after TBI without treatment is mainly limited to the sensorimotor deficits and cell proliferation.
The dose of rhEPO was selected based on our previous studies (Lu et al., 2005; Mahmood et al., 2007; Wang et al., 2004; Wang et al., 2007). Our previous study found that rhEPO was detected in cerebrospinal fluid and brain parenchyma 30 minutes after an intravenous bolus dose (from 1,000 to 10,000 units/kg) given 6 hr after stroke in male rats (Wang et al., 2007). Interestingly, there was no statistically significant difference in rhEPO levels in the ipsilateral ischemic hemisphere versus the contralateral non-ischemic hemisphere. A recent study showed that rhEPO (a single intraperitoneal injection of 5,000 units/kg) crossed the blood-brain barrier, peaked in brain at 10 h (3.3 ± 1.5 mU/mg protein), and was increased after stroke in neonatal male rats (Statler et al., 2007). Whether there is a gender difference in the pharmacokinetics of rhEPO in the brain is unknown. The pharmacokinetics of rhEPO after TBI may differ after stroke. Further studies are therefore warranted to provide useful information on pharmacokinetics of rhEPO after TBI in both genders. Dose response and therapeutic time window studies of rhEPO treatment for TBI in both genders are also warranted.
Based on our previous studies (Lu et al., 2003; Mahmood et al., 2007), the neurorestorative processes of neurogenesis, angiogenesis, and synaptogenesis are translated into improvement of spatial learning measured using the Morris water maze test at approximately 31-35 days post-injury. Time points for the footfault test were selected based on a report that sensorimotor deficits (footfaults) peak at early stage after TBI and spontaneously recover over time (Baskin et al., 2003). Accordingly, lesion volume, cell loss, neurogenesis and angiogenesis were examined at the end of the study (Day 35). Although other studies have demonstrated beneficial effects of post-injury administration of rhEPO in male mice at much earlier time points (Yatsiv et al., 2005), a different mouse TBI model (weight-drop) and a different paradigm of rhEPO treatment (1 h and 24 h post injury) were employed. Cognitive function was evaluated based on the differential exploration of familiar and new objects. A difference in this cognitive function was highly significant between vehicle-treated and rhEPO-treated mice at Day 3 but diminished at Day 8 post injury (Yatsiv et al., 2005). This functional test may not be suitable to evaluate long-term cognitive functional recovery. Their study also demonstrated that rhEPO treatment improved recovery of motor function as early as 3 days and up to 14 days (the longest time points examined) after TBI. The early beneficial effects on sensorimotor functions (footfault) were also observed in our present study with rhEPO treatment in both genders after TBI. The Morris water maze test has been widely used to detect spatial learning deficits in rats and mice after TBI (Clausen et al., 2005; Dixon et al., 1999; Kline et al., 2002; Lu et al., 2007; Mahmood et al., 2007; Smith et al., 1995). Our present data from the Morris water maze test showed that males exhibited a marked improvement after rhEPO treatment by as early as day 33 while females did not until day 34, suggesting that there is a gender-related one-day delay in spatial learning in females. However, rhEPO significantly improved spatial learning performance at days 34 and 35 without a gender difference. Our study further shows that the spatial learning deficits may be related to cell loss in the DG because rhEPO treatment significantly reduced cell loss in the ipsilateral injured DG in both genders.
Gender difference after TBI may be outcome-specific. Female rats were partially protected from bilateral frontal contusion-induced edema, while no gender difference in the lesion volume was observed (Roof et al., 1993). A recent study reports that CCI-induced TBI in mice does not demonstrate any gender differences in lesion volume and neurodegeneration (Hall et al., 2005). Gender and estrogen manipulation (ovariectomy) do not affect CCI-induced cortical and hippocampal injury, as well as injury-related inflammation in mice (Bruce-Keller et al., 2007). In contrast to the “focal” CCI-induced TBI (Hall et al., 2005), mice subjected to a “diffuse” weight-drop induced TBI show substantial gender-different time courses and magnitudes of calpain-mediated cytoskeletal degradation and neurodegeneration (Kupina et al., 2003). In our present study with mice, there was no significant gender difference in lesion volume and spatial learning deficits observed after TBI. However, female mice displayed less sensorimotor deficits than males after TBI. These findings are in agreement with a recent study, in which female rats perform better than males on motor tasks after CCI-induced TBI (Wagner et al., 2004).
No gender difference was observed in cell proliferation and neurogenesis in the DG and cortex in sham mice in the present study. This is in agreement with a report that gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice (Lagace et al., 2007). In our present study, more extensive cell proliferation was detected in the injured cortex of saline- or rhEPO-treated female mice after TBI compared to male mice. However, 90% of these proliferating cells were not NeuN/BrdU-colabeled, indicating that very limited neurogenesis occurs in the injured cortex after TBI. Furthermore, our present study showed that rhEPO treatment significantly enhanced the percentage of NeuN/BrdU-colabeled cells in the DG and cortex of male mice post injury, suggesting that rhEPO treatment enhanced neurogenesis that favored male mice. This is in agreement with our previous study that rhEPO treatment increases neurogenesis in the DG in male rats after TBI (female rats not studied) (Lu et al., 2005). Further investigations as to why rhEPO treatment dramatically enhanced neurogenesis in males are warranted. Neurogenesis normally only occurs in the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampus in the rodent adult brain (Arlotta et al., 2003; Itoh et al., 2005; Magavi et al., 2000). The source of the newborn neurons in the cortex after injury is unclear. However, degeneration (Magavi et al., 2000) or injury (Itoh et al., 2005) induces cortical neurogenesis. Neural precursors were also isolated from damaged rat cerebral cortex after TBI (Itoh et al., 2005). A second source is the SVZ. The neural precursor cells migrate toward cortical lesions from the SVZ after TBI (Goings et al., 2004; Ramaswamy et al., 2005; Sundholm-Peters et al., 2005). Our recent work also demonstrates that newly generated neurons in the SVZ migrate toward the ischemic boundary in rats after stroke (Zhang et al., 2007). Whether there is a gender difference in migration of SVZ cells into injured cortex after TBI is not known.
In the present study, TBI significantly stimulated angiogenesis in the ipsilateral cortex and DG without a significant sex difference. In our previous studies with male rats, we have shown that angiogenesis is stimulated by injury induced by stroke (Wang et al., 2004) or TBI (Lu et al., 2004). Treatment with rhEPO promotes angiogenesis (Wang et al., 2004), neurogenesis and improves functional outcome in male rats after brain injury (Lu et al., 2005; Wang et al., 2004). However, rhEPO did not affect angiogenesis in mice after TBI. The disparate effects of rhEPO on angiogenesis shown in our previous and present studies may be attributed to the following: 1) different species used (rats vs. mice), and 2) rhEPO administration time (initiated at day 1 after stroke or TBI for rats vs. at 6 h after TBI for mice). Thus, further studies are warranted to clarify the effects of rhEPO on angiogenesis in rodent models of brain injuries.
A growing body of literature suggests that neuroprotective agents or treatments proven in males may not work effectively in females after TBI (Suzuki et al., 2003; Wagner et al., 2002; Wagner et al., 2007). rhEPO provides neuroprotection in males in a number of brain injury models such as stroke, TBI, and subarachnoid hemorrhage (Brines et al., 2000; Chang et al., 2005; Lu et al., 2005; Mahmood et al., 2007; Siren et al., 2001; Springborg et al., 2002; Verdonck et al., 2007; Villa et al., 2003; Wang et al., 2004; Wang et al., 2007; Wen et al., 2002; Yatsiv et al., 2005). In addition, rhEPO has been demonstrated to reduce equally excitotoxic damage in newborn female and male mice (Keller et al., 2006). In light of the positive outcome in using EPO to treat stroke in a recent small clinical trial (Ehrenreich et al., 2002) and to increase hippocampal response during memory retrieval in humans (Miskowiak et al., 2007), and our present findings that rhEPO significantly improves histological outcome, sensorimotor function and spatial learning recovery in both genders, rhEPO is a promising neuroprotective agent for TBI and warrants further investigation.
4. Experimental Procedure
4.1 Animal models
All experimental procedures have been approved by the IACUC of Henry Ford Health System. Young adult male and female C57BL/6 mice (24-29 g, Charles River Laboratories, Inc., Wilmington, MA) were anesthetized intraperitoneally with 400 mg chloral hydrate/kg body weight. Body temperature was maintained with a circulating water-heating pad (37 °C). CCI was delivered as previously described (Lu et al., 2005; Smith et al., 1995; Xiong et al., 2005) with minor modifications. Each animal was placed in a stereotactic frame. One 4-mm diameter craniotomy was performed over the left parietal cortex adjacent to the central suture, midway between lambda and bregma. The dura was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 2.5-mm diameter tip at a rate of 4 m/s and 0.8 mm of compression. Velocity was measured with a linear velocity displacement transducer. Sham groups of mice underwent the same craniotomy but were not injured (n = 8 for male; n = 7 for female). The TBI animals were divided into four groups: 1) M-Saline group (TBI+saline, male, n = 13), 2) M-EPO group (TBI+rhEPO, male, n = 12), 3) F-Saline group (TBI+saline, female, n = 8), and 4) F-EPO group (TBI+rhEPO, female, n = 8).
4.2 rhEPO and BrdU administration
The dose of rhEPO was selected based on previous studies (Lu et al., 2005; Wang et al., 2004). rhEPO at a dose of 5,000 units/kg body weight (Epoetin alpha, AMGEN, Thousand Oaks, CA) was administered i.p. at 6 h and at 3 and 7 days (total dosage = 15,000) after TBI or sham. Mice in the saline-treated group received an equal volume of saline at 6 h, and at 3 and 7 days after TBI. To label proliferating cells, BrdU (100 mg/kg; Sigma, St. Louis, MO) was injected i.p. into mice daily for 10 days, starting 1 day after TBI. All animals were sacrificed at 35 days after TBI or surgery.
4.3 HCT and body weight
To determine the effects of rhEPO on HCT, a blood sample (50 μl) was collected via tail vein before injury and weekly after TBI or sham up to 5 weeks. HCT was measured in micro-HCT capillary tubes (Fisher Scientific, Pittsburgh, PA) using standard procedures (Readacrit Centrifuge, Clay Adams, Parsippany, NJ). Body weight was recorded before TBI and at 1, 4, 7, 14, 21, 28 and 35 days after TBI or surgery.
4.4 Behavioral tests
Morris water maze test
To detect spatial learning impairments, a recent version of the Morris water maze test was used (Choi et al., 2006). The procedure was modified from previous versions (Day et al., 1999; Morris, 1984; Morris et al., 1982; Sutherland et al., 1982) and has been found to be useful for chronic spatial memory assessment in rats and mice with brain injury (Choi et al., 2006; Lu et al., 2005). All animals were tested during the last five days (i.e., from 31-35 days after TBI or surgery) before sacrifice. Data collection was automated by the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA.). To collect data, a white pool (1.2 m in diameter) was subdivided into four equal quadrants formed by imaging lines. At the start of a trial, the mouse was placed randomly at one of four fixed starting points, facing toward the wall (designated North, South, East and West) and allowed to swim for 90 seconds or until they found the platform within 90 seconds. If the animal found the platform, it was allowed to remain on it for 10 seconds. If the animal failed to find the platform within 90 seconds, it was placed on the platform for 10 seconds. Throughout the test period, the platform was located in the NE quadrant 1 cm below water in a randomly changing position, including locations against the wall, toward the middle of the pool or off-center but always within the target quadrant. If the animal was unable to find the platform within 90 s, the trial was terminated and a maximum score of 90 s was assigned. If the animal reached the platform within 90 s, the percentage of time traveled within the NE (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform and employed for statistical analysis. The advantage of this version of the water maze is that each trial takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant (Schallert, 2006).
Footfault test
To evaluate sensorimotor function, the footfault test was carried out before TBI and at 1, 4, 7, 14, 21, 28 and 35 days after TBI or surgery by an investigator blind to the treatment groups. The mice were allowed to walk on a grid (12 cm × 57 cm with 1.3 cm × 1.3 cm diameter openings). With each weight-bearing step, a paw might fall or slip between the wires (Barth et al., 1990; Baskin et al., 2003) and if this occurred it was recorded as a footfault. A total of 50 steps were recorded for each right forelimb and hindlimb.
4.5 Tissue preparation and measurement of lesion volume
At day 35 after TBI, animals were anesthetized i.p. with chloral hydrate, and perfused transcardially first with saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4. Their brains were removed and post-fixed in 4 % paraformaldehyde at room temperature for 48 hours. The brain tissue was cut into 7 equally spaced (1 mm) coronal blocks, and processed for paraffin sectioning. A series of adjacent 6-μm thick sections were cut from each block in the coronal plane and stained with hematoxylin and eosin (H&E). For lesion volume measurement, the brain sections were traced by a microcomputer imaging device (MCID) (Imaging Research, St. Catharine’s, Ontario, Canada), as previously described (Chen et al., 2005). The indirect lesion area was calculated (i.e., the intact area of the ipsilateral hemisphere is subtracted from the area of the contralateral hemisphere) (Swanson et al., 1990), and the lesion volume presented as a volume percentage of the lesion compared with the contralateral hemisphere. H&E sections from Block E and F containing hippocampus were used to acquire images of the DG at 20 × magnification. To evaluate the cell loss after TBI, we counted the cell number per millimeter in the DG.
4.6 Immunohistochemistry
Angiogenesis
Brain sections were deparaffinized, rehydrated, and then incubated with 0.4% Pepsin solution at 37 °C for 1 h. After washing with PBS, the sections were blocked with 1% BSA at room temperature for 1 h. To identify vascular structure, sections were incubated with rabbit anti-human von Willebrand factor (vWF, 1:200; DakoCytomation, Carpinteria, CA) at 4 °C overnight (Lu et al., 2004). Sections were incubated with biotinylated anti-rabbit antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 min. Sections were incubated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc., Burlingame, CA). Each of the steps was followed by three 5-min rinses in PBS. Diaminobenzidine (Sigma, St. Louis, MO) was then used as a sensitive chromogen for light microscopy. Sections were counterstained with hematoxylin. The vWF-positive vessels were examined at 20 × magnification and counted in the boundary zone of the lesion and the DG, CA3 and CA1 regions of the hippocampus, using the MCID system (MCID, St. Catherine’s, Ontario, Canada). The vascular density in these regions was determined by dividing the immunoreactive vessels by the corresponding area and was used as a parameter of angiogenesis.
Cell proliferation
To examine cell proliferation, coronal sections were histochemically stained with mouse anti-BrdU antibody. Paraffin-embedded coronal sections were deparaffinized and rehydrated. Antigen retrieval was performed by boiling sections in 10 mM citrate buffer (pH 6.0) for 10 min. After washing with PBS, sections were incubated with 0.3 % H2O2 in PBS for 10 min, blocked with 1 % BSA containing 0.3 % Triton-X 100 at room temperature for 1 h, and incubated with mouse anti-BrdU (1:200; Dako, Carpinteria, CA) at 4 °C overnight. Sections were incubated with biotinylated anti-mouse antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 min. Sections were incubated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc., Burlingame, CA). Each of the steps was followed by three 5-min rinses in PBS. Diaminobenzidine (Sigma, St. Louis, MO) was then used as a sensitive chromogen for light microscopy. Sections were counterstained with hematoxylin. BrdU-positive cells in the DG and the cortex of the ipsilateral, injured hemispheres were examined at 20 × magnification and counted.
4.7 Immunofluorescent staining
Newly generated neurons were identified by double labeling for BrdU and NeuN. After dehydration, tissue sections were boiled in 10 mM citric acid buffer (pH 6) for 10 min. After washing with PBS, sections were incubated in 2.4 N HCl at 37 °C for 20 min. Sections were incubated with 1% BSA containing 0.3% Triton-X-100 in PBS. Sections were then incubated with mouse anti-NeuN antibody (1:200; Chemicon, Temecula, CA) at 4 °C overnight. FITC-conjugated anti-mouse antibody (1:400; Jackson ImmunoResearch, West Grove, PA) was added to sections at room temperature for 2 h. Sections were then incubated with mouse anti-BrdU antibody (1:200; Dako, Glostrup, Denmark) at 4°C overnight. Sections were then incubated with Cy3-conjugated anti-mouse antibody (1:400; Jackson ImmunoResearch, West Grove, PA) at room temperature for 2 hours. Each of the steps was followed by three 5-min rinses in PBS. Tissue sections were mounted with Vectashield mounting medium (Vector laboratories, Burlingame, CA). Images were collected with fluorescent microscopy. NeuN/BrdU-colabeled cells in the DG and the cortex were counted at a magnification of 20.
4.8 Cell counting
Cell counts were performed by observers blinded to the individual treatment status of the animals. Five sections with 100-μm intervals from the dorsal DG were analyzed with a microscope at 400× magnification. For cell proliferation, the total number of BrdU-positive cells was counted in the boundary zone of the lesion and the DG, CA3 and CA1 regions of the hippocampus, using the MCID system (Imaging Research, Inc., St. Catharine’s, Canada). The cells with BrdU (brown stained) that clearly localized to the nucleus (hematoxylin stained) were counted as BrdU-positive cells. For immunofluorescent staining, the number of BrdU-positive cells (red stained) and NeuN/BrdU-colabeled cells (yellow after merge) were counted in the DG and the lesion boundary zone. The percentage of NeuN/BrdU-colabeled cells over the total number of BrdU-positive cells in the corresponding regions was estimated and used as a parameter to evaluate neurogenesis.
4.9 Statistical analyses
All data are presented as mean ± standard deviation. For lesion volume, cell counting, and vWF-stained vascular density, a one-way analysis of variance (ANOVA) followed by post hoc Student-Newman-Keuls (SNK) tests were used to compare the difference between the rhEPO-treated, saline-treated and sham groups. Data were analyzed by ANOVA for repeated measurements of body weight, hematocrit and functional tests (spatial performance and sensorimotor function). Differences between genders were analyzed by two-way ANOVA followed by SNK analysis. Statistical significance was set at P<0.05.
Acknowledgments
This work was supported by NINDS grants RO1 NS52280 and PO1 NS42345.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arlotta P, Magavi SS, Macklis JD. Molecular manipulation of neural precursors in situ: induction of adult cortical neurogenesis. Exp Gerontol. 2003;38:173–182. doi: 10.1016/s0531-5565(02)00156-0. [DOI] [PubMed] [Google Scholar]
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Dimayuga FO, Reed JL, Wang C, Angers R, Wilson ME, Dimayuga VM, Scheff SW. Gender and estrogen manipulation do not affect traumatic brain injury in mice. J Neurotrauma. 2007;24:203–215. doi: 10.1089/neu.2006.0163. [DOI] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Mu D, Wendland M, Sheldon RA, Vexler ZS, McQuillen PS, Ferriero DM. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58:106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L, Goodman CJ, Robertson CS. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Clausen F, Lewen A, Marklund N, Olsson Y, McArthur DL, Hillered L. Correlation of hippocampal morphological changes and morris water maze performance after cortical contusion injury in rats. Neurosurgery. 2005;57:154–163. doi: 10.1227/01.neu.0000163412.07546.57. discussion 154-163. [DOI] [PubMed] [Google Scholar]
- Coimbra R, Hoyt DB, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma. 2003;54:689–700. doi: 10.1097/01.TA.0000058314.31655.5F. [DOI] [PubMed] [Google Scholar]
- Davis DP, Douglas DJ, Smith W, Sise MJ, Vilke GM, Holbrook TL, Kennedy F, Eastman AB, Velky T, Hoyt DB. Traumatic brain injury outcomes in pre- and post- menopausal females versus age-matched males. J Neurotrauma. 2006;23:140–148. doi: 10.1089/neu.2006.23.140. [DOI] [PubMed] [Google Scholar]
- Day LB, Weisand M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Erbayraktar S, Passalacqua M, Meli F, Gokmen N, Yilmaz O, La Torre D, Buemi M, Iacopino DG, Coleman T, Cerami A, Brines M, Tomasello F. Amelioration of spinal cord compressive injury by pharmacological preconditioning with erythropoietin and a nonerythropoietic erythropoietin derivative. J Neurosurg Spine. 2006;4:310–318. doi: 10.3171/spi.2006.4.4.310. [DOI] [PubMed] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–808. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- Hall ED, Gibson TR, Pavel KM. Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J Neurotrauma. 2005;22:669–679. doi: 10.1089/neu.2005.22.669. [DOI] [PubMed] [Google Scholar]
- Itoh T, Satou T, Hashimoto S, Ito H. Isolation of neural stem cells from damaged rat cerebral cortex after traumatic brain injury. Neuroreport. 2005;16:1687–1691. doi: 10.1097/01.wnr.0000183330.44112.ab. [DOI] [PubMed] [Google Scholar]
- Keller M, Yang J, Griesmaier E, Gorna A, Sarkozy G, Urbanek M, Gressens P, Simbruner G. Erythropoietin is neuroprotective against NMDA-receptor-mediated excitotoxic brain injury in newborn mice. Neurobiol Dis. 2006;24:357–366. doi: 10.1016/j.nbd.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Kirkness CJ, Burr RL, Mitchell PH, Newell DW. Is there a sex difference in the course following traumatic brain injury? Biol Res Nurs. 2004;5:299–310. doi: 10.1177/1099800404263050. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kupina NC, Detloff MR, Bobrowski WF, Snyder BJ, Hall ED. Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp Neurol. 2003;180:55–73. doi: 10.1016/s0014-4886(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Chopp M. Biologic transplantation and neurotrophin-induced neuroplasticity after traumatic brain injury. J Head Trauma Rehabil. 2003;18:357–376. doi: 10.1097/00001199-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- Miskowiak K, O’Sullivan U, Harmer CJ. Erythropoietin enhances hippocampal response during memory retrieval in humans. J Neurosci. 2007;27:2788–2792. doi: 10.1523/JNEUROSCI.5013-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mostafa G, Huynh T, Sing RF, Miles WS, Norton HJ, Thomason MH. Gender-related outcomes in trauma. J Trauma. 2002;53:430–4. doi: 10.1097/00005373-200209000-00006. discussion 434-435. [DOI] [PubMed] [Google Scholar]
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Roof RL, Zhang Q, Glasier MM, Stein DG. Gender-specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res. 1993;57:47–51. doi: 10.1016/0166-4328(93)90060-4. [DOI] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol Behav. 1999;68:81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000;17:1155–69. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Springborg JB, Ma X, Rochat P, Knudsen GM, Amtorp O, Paulson OB, Juhler M, Olsen NV. A single subcutaneous bolus of erythropoietin normalizes cerebral blood flow autoregulation after subarachnoid haemorrhage in rats. Br J Pharmacol. 2002;135:823–829. doi: 10.1038/sj.bjp.0704521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler PA, McPherson RJ, Bauer LA, Kellert BA, Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de Looij Y, Remy C, Segebarth C, Payen JF. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav Brain Res. 2007;181:200–209. doi: 10.1016/j.bbr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, Zafonte RD, Dixon CE. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS. Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J Neurochem. 2005;95:732–744. doi: 10.1111/j.1471-4159.2005.03412.x. [DOI] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. Faseb J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


