Abstract
The adipocyte-derived hormone leptin is an important regulator of appetite and energy expenditure and is now appreciated for its ability to control innate and adaptive immune responses. We have reported previously that the leptin-deficient ob/ob mouse exhibited increased susceptibility to the Gram-negative bacterium Klebsiella pneumoniae. In this report we assessed the impact of chronic leptin deficiency, using ob/ob mice, on pneumococcal pneumonia and examined whether restoring circulating leptin to physiological levels in vivo could improve host defences against this pathogen. We observed that ob/ob mice, compared with wild-type (WT) animals, exhibited enhanced lethality and reduced pulmonary bacterial clearance following Streptococcus pneumoniae challenge. These impairments in host defence in ob/ob mice were associated with elevated levels of lung tumour necrosis factor (TNF)-α, macrophage inflammatory peptide (MIP)-2 [correction added after online publication 28 September 2007: definition of MIP corrected], prostaglandin E2 (PGE2), lung neutrophil polymorphonuclear leukocyte (PMN) counts, defective alveolar macrophage (AM) phagocytosis and PMN killing of S. pneumoniae in vitro. Exogenous leptin administration to ob/ob mice in vivo improved survival and greatly improved pulmonary bacterial clearance, reduced bacteraemia, reconstituted AM phagocytosis and PMN H2O2 production and killing of S. pneumoniae in vitro. Our results demonstrate, for the first time, that leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Further investigations are warranted to determine whether there is a potential therapeutic role for this adipokine in immunocompromised patients.
Keywords: lipid mediators, lung immunology, neutrophils, nutrition, pneumococcus
Introduction
The adipokine leptin plays an important role in the regulation of energy homeostasis by informing the satiety centre in the brain regarding peripheral lipid energy stores [1,2]. Circulating levels of this adipocyte-derived hormone are correlated with total body fat mass and decline during periods of energy deprivation [3,4]. Nearly all tissues, including cells of the immune system, express leptin receptors and blood leptin levels increase during inflammation and infection [5–8]. The function of leptin in immune responses has been demonstrated in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice [9–11]. These animals, and humans who are genetically leptin deficient, develop profound obesity and a number of hormonal and immune system abnormalities [11–13].
We have demonstrated previously that acute leptin deficiency in mice, induced by short-term starvation, was associated with reduced clearance of Streptococcus pneumoniae from the lungs compared to fed mice in a non-lethal model [14]. AMs from fasted animals also exhibited defective phagocytosis and killing of S. pneumoniae in vitro. In contrast, the provision of exogenous leptin to fasted animals restored bacterial clearance, bronchoalveolar lavage levels of PMNs and cytokines and alveolar macrophage bacterial killing [14].
With regard to chronic leptin deficiency, we have demonstrated previously that ob/ob mice displayed reduced survival and impaired bacterial clearance from the lungs following intratracheal Klebsiella challenge [6]. Associated with these impairments in ob/ob mice were decreased alveolar macrophage (AM) and neutrophil (PMN) phagocytosis of K. pneumoniae and attenuated synthesis of leukotrienes (LTs), proinflammatory lipid mediators important to pulmonary host defence. The exogenous administration of leptin in vitro restored macrophage and PMN phagocytosis of bacteria, and reconstituted LT synthesis in AMs [6,15]. Others have observed similarly that ob/ob mice are more susceptible to pulmonary Mycobacterium tuberculosis and systemic Listeria monocytogenes infections [10,16].
Despite these reports, the ability of exogenously administered leptin to reverse the observed pulmonary host defence defects in ob/ob mice has not been demonstrated in vivo. At present, it is unclear whether (a) findings from our investigations of acute starvation-associated leptin deficiency could be generalized to the situation of long-term term leptin deficiency, as is seen in states of chronic malnutrition, or (b) if leptin treatment in vivo can improve survival during pneumonia in leptin-deficient mice. Our previously reported K. pneumoniae model of infection in ob/ob mice produced a severe pneumonia that resulted in 100% lethality [6]. However, in the present report, we used an attenuated strain of S. pneumoniae serotype 3 that produces a less severe infection. This model has increased relevance to human disease, as S. pneumoniae is the most common bacterial cause of community-acquired pneumonia [17]. The reduced mortality allowed us to examine improvements in host defence end-points in vivo associated with the exogenous administration of leptin. We hypothesized that ob/ob mice would exhibit increased susceptibility to pneumococcal infection that could be corrected by the administration of leptin in vivo. The present studies confirm our hypothesis and highlight important differences in the regulation of the inflammatory response to S. pneumoniae infection in acute versus chronic states of leptin depletion.
Materials and methods
Animals
Age-matched female C57BL/6j-ob/ob and C57BL/6j wild-type (WT) mice (8–12 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions. All experiments were conducted in compliance with the Animal Care and Use Committee of the University of Michigan.
S. pneumoniae inoculation
S. pneumoniae, serotype 3, 6303, was obtained from the American Type Culture Collection (Manassas, VA, USA), grown in Todd Hewitt broth containing 0·5% yeast extract (Difco, Detroit, MI, USA) to mid-logarithmic phage at 37°C (5% CO2). After a number of passages, we permitted the decline in virulence of this particular organism because ob/ob mice are particularly susceptible to bacterial infections [6,10]. After making a midline incision to expose the trachea, a 30-µl inoculum containing 105 or 2 × 105 colony-forming units (CFUs) of S. pneumoniae was injected through the trachea using a 30-gauge needle. The wound was closed using surgical glue (Vetbond®; 3M Animal Care Products, St Paul, MN, USA).
Leptin replacement protocol
In order to replace leptin in ob/ob mice, an intraperitoneal injection of either saline or leptin (EMD Biosciences, La Jolla, CA, USA) (1 µg/g of body weight) was administered at 9 a.m. and 6 p.m. prior to and 48 h following S. pneumoniae challenge for a total of six injections prior to blood and lung harvest. This leptin replacement protocol has been shown to achieve circulating leptin levels that were similar to that of WT mice 6–12 h post-injection [9,18]. On the day that mice were euthanized, lung and blood samples were obtained 7 h following the injection of saline or leptin.
Determination of blood and lung CFUs
S. pneumoniae CFU in blood and lung homogenate samples were determined as described previously [6]. In brief, lungs were homogenized in 1 ml sterile phosphate-buffered saline (PBS) and blood was collected from euthanized mice by orbital bleeding 48 h post-S. pneumoniae challenge. Serial dilutions of each sample were plated on soy-based blood agar plates (Difco). Bacterial CFU counts were determined after 18 h of incubation at room temperature.
Cytokine and leptin determinations
Trition X-100 was added to the lung homogenates, which sat for 30 min at 4°C, to lyse the cells and permit extraction of cytokines and leptin. Using commercially available enzyme-linked immunosorbent assay (ELISA) kits (TiterZyme EIA kits, Assay Designs, Ann Arbor, MI, USA), lung homogenates were assayed for murine leptin, interleukin (IL)-6, macrophage inflammatory peptide (MIP)-2 and tumour necrosis factor (TNF)-α [correction added after online publication 28 September 2007: definition of MIP corrected].
Cysteinyl-LT and prostaglandin E2 levels in bronchoalveolar lavage (BAL) fluid
BAL fluid was obtained from mice, as described previously [6] 48 h after S. pneumoniae challenge and centrifuged to pellet the leucocytes. The supernatants were aliquoted and stored at −70°C. BAL fluid was then assayed for cysteinyl-LTs and prostaglandin E2 (PGE2) according to the manufacturers' instructions (Cayman Chemical Co. and Assay Designs, Ann Arbor, MI, USA).
Lung leucocyte differential and total counts
A total white blood cell count was performed on cells recovered from BAL fluid as described previously [14]. Using this technique, we have found that > 95% of the cells obtained by lavage have been identified as AMs in uninfected animals [14].
AM phagocytosis of S. pneumoniae in vitro
A total of 2 × 105 AMs, obtained by lavage as described previously [6], were incubated with 2 × 106 CFUs of fluorescently labelled S. pneumoniae with 2·5% autologous serum in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) (total volume 1 ml) for 30 min. Following the incubation period, the cells were centrifuged onto glass slides, immersed in trypan blue (0·25 mg/ml) to quench the fluorescence of extracellular (adherent and not ingested) bacteria, fixed with 4% paraformaldehyde and sealed using a cover slip. The percentage of AMs containing fluorescent S. pneumoniae was determined by counting 200 macrophages in random fields using fluorescent microscopy (1000×) by an observer who was blinded to the identity of the samples.
PMN killing of S. pneumoniae in vitro
A total of 1 × 106 glycogen elicited-peritoneal PMNs, obtained from mice 5 h following an intraperitoneal injection of a 1% glycogen solution as described previously [15], were incubated with 1 × 106 CFUs of S. pneumoniae in RPMI-1640 and 10% autologous serum in 5 ml polypropylene tubes at 37°C with 5% CO2 in air. Prior to incubation (time zero) and 60 min after incubation, two samples of each tube were serially diluted and plated on soy-based blood agar plates (Difco). Following 16 h of incubation at 37°C, S. pneumoniae CFU were enumerated.
H2O2 determinations
PMNs (2 × 105) were adhered to 96-well plates for 1 h and incubated overnight in RPMI-1640 and 10% fetal calf serum (Invitrogen). On the following day, the PMNs were rinsed with Hank's buffered salt solution, pretreated with and without leptin (1 ng/ml) for 30 min, and stimulated with 5 × 106 heat-killed S. pneumoniae. H2O2 production was determined using the hydrogen peroxide/peroxidase assay kit (Invitrogen) 30 min following the addition of bacteria.
Statistical analysis
Survival was evaluated for differences using a log-rank test. Where appropriate, mean values were compared using a paired t-test or a one-way analysis of variance (anova). The Student–Newman–Keuls test was used for mean separation. In all cases, a P-value of < 0·05 was considered significant.
Results
Leptin treatment improves survival in ob/ob mice challenged with S. pneumoniae
To determine if leptin plays a role in host defence against Gram-positive pathogens, we compared survival in WT, ob/ob mice treated with saline and ob/ob mice treated with leptin in response to intratracheal S. pneumoniae inoculation (105 CFU). As shown in Fig. 1, we observed an 83% mortality rate in ob/ob mice 10 days following S. pneumoniae challenge. In contrast, only 10% of the WT animals expired in response to this organism. Interestingly, we also found that leptin treatment increased the survival of ob/ob mice significantly following challenge with 105 CFU S. pneumoniae. We did not, however, observe a statistically significant improvement in survival with leptin in ob/ob mice challenged with 2 × 105 CFU S. pneumoniae (data not shown). This result indicates that ob/ob mice are exquisitely susceptible to pneumococcal pneumonia and that leptin can enhance host defence in vivo.
Fig. 1.
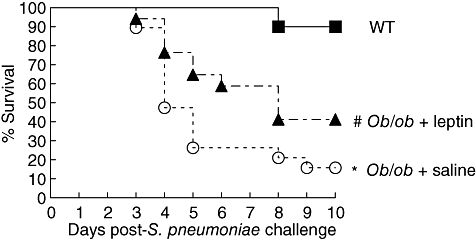
Effect of leptin deficiency on survival following intratracheal Streptococcus pneumoniae challenge. Wild-type (WT) (▪) and ob/ob mice given an intraperitoneal injection of either saline (○) or leptin (▴) twice daily 24 h prior to and 48 h following S. pneumoniae challenge (105 colony-forming units) and monitored over a 10-day period (n = 10–18 mice per group). *P < 0·05 using log-rank test versus WT and ob/ob + leptin. #P < 0·05 using log-rank test versus WT and ob/ob + saline.
Leptin administration to ob/ob mice improves defective bacterial clearance and reduces bacterial dissemination following S. pneumoniae challenge
The pulmonary bacterial burden of the ob/ob mice was 5-log-fold greater (7·8 ± 0·6 log-CFUs) than that of their WT counterparts (2·2 ± 0·9 log-CFUs). In addition, 70% of the ob/ob mice developed bacteraemia compared with none of the WT animals 48 h after S. pneumoniae challenge (Fig. 2). The provision of exogenous leptin to ob/ob mice improved pulmonary bacterial clearance and reduced the dissemination of S. pneumoniae to the peripheral circulation 48 h post-infection. This result suggests that leptin contributes to host defence by regulating pulmonary bacterial clearance.
Fig. 2.
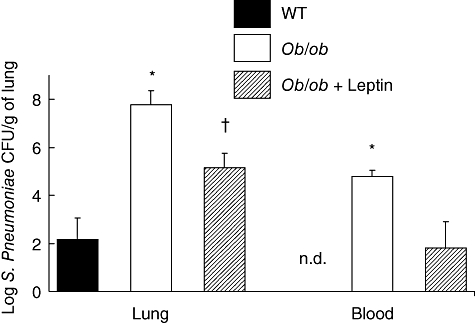
Partial restoration of defective pulmonary Streptococcus pneumoniae clearance in ob/ob mice with exogenous leptin. Wild-type (WT) and ob/ob mice were given an intraperitoneal injection of either saline or leptin twice daily 24 h prior to and 48 h following S. pneumoniae challenge [105 colony-forming units (CFUs)]. Lung homogenate and blood were assessed for bacterial CFUs 48 h post-S. pneumoniae challenge. Bars represent the mean ± standard error of the mean. n = 4–5 mice per group; *P < 0·05 versus WT and ob/ob + leptin. †P < 0·05 versus WT and ob/ob; n.d., none detected.
Pulmonary leucocyte recruitment following S. pneumoniae challenge
In order to determine if the differences between the treatment groups in bacterial clearance could be explained by variations in pulmonary cellular recruitment following S. pneumoniae challenge, we assessed the total number and differential counts of lung leucocytes in BAL fluid 48 h post-infection. Compared with WT and ob/ob mice given leptin, we found a greater number of total leucocytes/ml of BAL fluid in ob/ob mice. This increase was due largely to a greater number of PMNs/ml of BAL fluid (WT: 4·3 × 104 ± 9·5 × 103, ob/ob: 6·4 × 105 ± 2·0 × 105, and ob/ob + leptin: 9·0 × 104 ± 6·6 × 104) (P < 0·05, ob/ob versus WT and ob/ob + leptin). We did not observe differences between our treatment groups in the number of mononuclear/macrophages or lymphocytes (data not shown).
Increased pulmonary cytokine and lipid mediator levels in ob/ob mice in response to S. pneumoniae
The levels of leptin, IL-6, TNF-α and MIP-2 in lung homogenate and cysteinyl-LTs and PGE2 in BAL fluid were assessed 48 h post-S. pneumoniae challenge, as these mediators have been identified to play an important role in the induction of an inflammatory response during the early phase of pneumococcal pneumonia [14,19–22]. The administration of leptin restored blood leptin to levels similar to that observed in WT mice 48 h post-S. pneumoniae challenge. Compared with the WT animals, lung homogenate leptin levels were approximately 40% lower in the ob/ob mice given leptin (Fig. 3a). Compared with the WT and ob/ob mice treated with leptin, we found higher levels of TNF-α, MIP-2 and PGE2 in the ob/ob mice (Fig. 3b,c). Interestingly, the increased levels of lung homogenate MIP-2 were associated with elevated PMNs in the BAL fluid of ob/ob mice. Although there was a trend for greater levels of IL-6 and cysteinyl-LTs (Fig. 3c) in the lung homogenates and BAL fluid of ob/ob mice, these differences were not found to be significant.
Fig. 3.
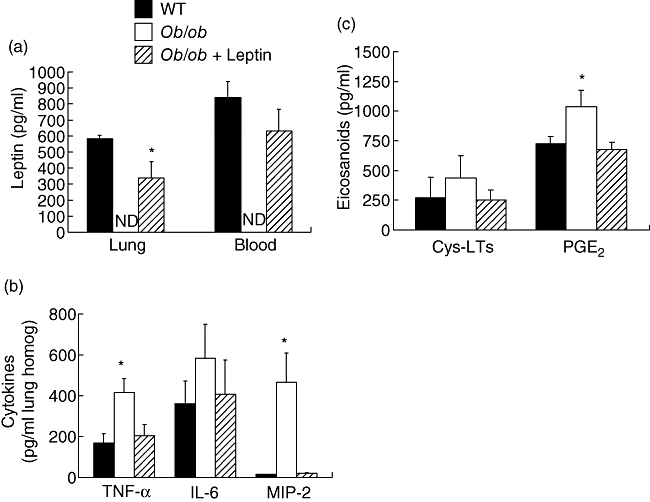
Lung and blood leptin (a), lung homogenate cytokine (b) and bronchoalveolar lavage fluid lipid mediator (c) levels 48 h post-Streptococcus pneumoniae challenge in wild-type (WT) and ob/ob mice given saline or leptin. WT and ob/ob mice were given an intraperitoneal injection of either saline or leptin twice daily 24 h prior to and 48 h following S. pneumoniae challenge. Bars represent the mean ± standard error of the mean. n = 4–5 mice per group; n.d., not detected. *P < 0·05 versus WT and ob/ob + leptin.
Effects of exogenous leptin administration to ob/ob mice in vivo on AM phagocytosis
Because we had identified previously a phagocytic defect in AMs from ob/ob mice, we next asked if the observed attenuation in S. pneumoniae clearance from the lung was associated with a phagocytic defect in AMs and if the administration of exogenous leptin to ob/ob mice in vivo could restore this response. Compared with AMs from WT mice (61 ± 6·5%), a significantly greater percentage of cells had phagocytosed S. pneumoniae than ob/ob mice (21 ± 3·4%) and this impairment could be reconstituted with the provision of exogenous leptin to ob/ob mice (48 ± 6·4%) in vivo.
Leptin enhances PMN killing of S. pneumoniae and H2O2 synthesis in vitro
We next sought to determine if bactericidal capacity was defective in these cells. In comparison with cells from WT mice, killing of S. pneumoniae by PMNs form ob/ob mice was reduced and treatment of ob/ob mice with leptin reconstituted this defect (Fig. 4a). Next, we assessed the production of H2O2 in PMNs as S. pneumoniae are killed by oxidative mechanisms [23]. As shown in Fig. 4b, we found that, compared with cells from WT animals, PMNs recovered from ob/ob mice produced approximately 30% less H2O2 after stimulation with heat-killed S. pneumoniae and the addition of a physiological dose of leptin (1 ng/ml) to PMNs from ob/ob mice reconstituted H2O2 synthesis.
Fig. 4.
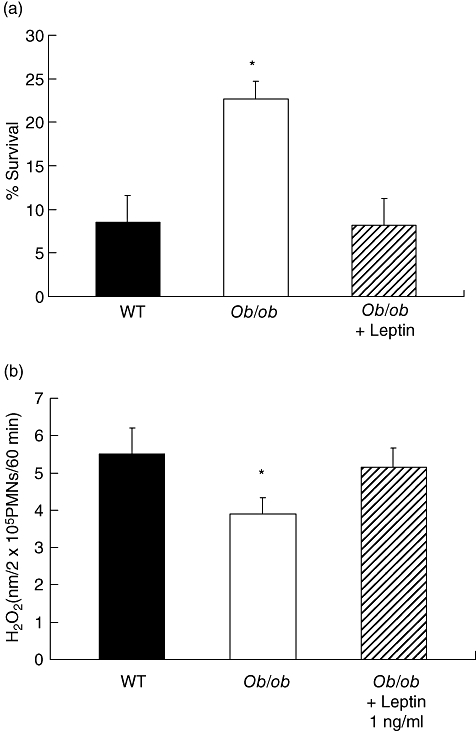
Leptin enhances polymorphonuclear leukocyte killing of Streptococcus pneumoniae (reduces survival) (a) and heat-killed S. pneumoniae stimulated H2O2 production in vitro (b). Bars represent the mean ± standard error of the mean of three to four separate experiments performed in duplicate. *P < 0·05 versus WT and ob/ob + leptin.
Discussion
The consequence of chronic leptin deficiency in ob/ob mice is associated with marked immune suppression against bacterial pathogens [6,10,16]. It is notable that the mortality of infected ob/ob mice was dramatic (83%) compared to the usually low degree of death observed in WT animals (10%). This result confirms our previous study of acute leptin deficiency modelled under conditions of short-term starvation, where the bacterial clearance of starved mice was significantly less than that of the fed control mice [14]. These new data, and another study comparing host defence against K. pneumoniae where survival was 50% in WT and 0% in ob/ob mice, suggest a more potent immunosuppressive effect associated with long-term leptin deficiency than short-term depletion, although further studies are needed to clarify differences between these two models [6].
We and others have observed that leptin increases in the blood and lungs of mice during bacterial pneumonia [6,14,24]. At 48 h after S. pneumoniae challenge, we observed that the provision of exogenous leptin to ob/ob mice almost restored pulmonary bacterial clearance and reduced pneumococcal dissemination to the blood. Lung and blood leptin levels were approximately 75 and 60%, respectively, of that seen in WT animals at this particular time-point. This result indicates that exogenous leptin reaches the site of infection, where it can affect the host response to S. pneumoniae.
An effective host response against pneumococcal pneumonia requires the elaboration of proinflammatory cytokines and lipid mediators that activate resident AMs and adjacent cells in the alveolar milieu [25]. In particular, IL-6, TNF-α and the LTs have been shown to play protective roles in murine pneumococcal pneumonia [19,20,22]. However, in our experiments, the levels of these mediators were elevated in ob/ob mice compared with either the WT or ob/ob mice given exogenous leptin. This difference was due probably to the greater bacterial counts in the lungs of the ob/ob mice because exogenous leptin treatment was associated with reduced lung bacterial counts and reduced levels of these proinflammatory mediators. In contrast to the responses in ob/ob mice in this report, we have observed previously that pulmonary IL-6 and MIP-2 levels were reduced in mice that had been fasted to lower circulating leptin levels [14]. Therefore, the impairments in host defence observed in genetically induced leptin deficiency (ob/ob mice) did not result in the suppression of pulmonary cytokine or LT production during pneumococcal pneumonia in vivo.
We were intrigued by the increased production of PGE2 in the lungs of infected ob/ob mice relative to the WT animals. While the fivefold greater bacterial burden in the lungs of ob/ob mice probably provided a greater stimulus for PGE2 synthesis, these animals may possess an enhanced capacity for stimulus-induced prostanoid synthesis [26]. We speculate that the increased levels of PGE2 in the BAL fluid of ob/ob mice following infection may have contributed to the greater pulmonary bacterial counts by inhibiting bacterial clearance mechanisms in vivo. This lipid mediator has been shown to attenuate potently both phagocytosis and killing of bacteria in AMs and PMNs in vitro [27,28], and inhibiting PGE2 production in vivo has been shown to improve pulmonary host defence during bacterial pneumonia [29]. Furthermore, the overproduction of PGE2 was reported recently to contribute to the enhanced susceptibility of mice to bacterial pneumonia following bone marrow stem cell transplantation [30]. Lastly, PGE2 facilitates the ingress of PMNs to pulmonary areas of inflammation via vascular endothelial cell-mediated vasodilation and this may have contributed to the greater dissemination of S. pneumoniae from the alveolar space to the peripheral circulation in ob/ob mice.
PMNs, recruited to the lungs during pneumococcal pneumonia, contribute to host defence by phagocytosing and killing bacteria and the observed defect in PMNs from ob/ob mice to kill S. pneumoniae (a previously unrecognized response) may have contributed to the increased pulmonary bacterial burden in these animals [31,32]. The mechanism by which leptin deficiency impairs PMN bactericidal capacity may be due to an impairment in H2O2 synthesis. Similarly, others have reported that leptin enhances PMN reactive oxygen intermediate production and phagocytosis [15,33]. These results suggest that leptin promoted pulmonary anti-bacterial host defence by restoring AM and PMN clearance of bacteria without inducing an exaggerated inflammatory response. A similar result was observed by Ikejima and coworkers [10], who demonstrated that exogenous leptin administered in vivo could partially restore the impairment in hepatic L. monocytogenes clearance in ob/ob mice.
Although the observed attenuation of bacterial clearance in ob/ob mice was not due to deficient recruitment of PMNs, these cells can damage lung tissue by elaborating proteases that degrade the extracellular matrix of the lung [34] and possibly contribute to overall lung injury and enhanced dissemination of S. pneumoniae to the peripheral circulation. Surprisingly, we had observed reduced PMN accumulation and less inflammatory mediator generation in the lungs of fasted mice in response to S. pneumonia and found that exogenous leptin restored PMN recruitment during pneumococcal pneumonia in fasted mice [14]. These conflicting results observed in ob/ob and fasted WT mice following S. pneumoniae challenge might be explained partially by differences in peripheral PMN counts at baseline. Faggioni and coworkers have demonstrated previously that peripheral blood PMN counts in ob/ob mice were twice that of WT animals [35].
It is unlikely that the host defence impairments observed in ob/ob mice in this study were due to phenotypic abnormalities in ob/ob mice, such as obesity, hyperglycaemia and glucocorticoid excess, for a number of reasons. First, bacterial clearance improved dramatically in ob/ob mice given exogenous leptin despite the fact that these animals were still obese. Secondly, Murray and coworkers did not observe impairments in host defence against S. pneumoniae-induced peritonitis in mice manipulated genetically to produce elevated blood glucocorticoids levels [36]. However, it is important to note that diet-induced obesity and obesity that arises from leptin deficiency may have different effects on the host's immune system, as a recent publication by Smith and colleagues demonstrated that diet-induced obese mice are more susceptible to influenza virus infection [37].
These results extend our previous findings regarding defective host defence against Klebsiella pneumonia and similar reports from others regarding increased susceptibility to bacterial infections in female ob/ob mice [6,10,16,38]. However, it is relevant that a recent study failed to detect differences between male WT and ob/ob mice in pulmonary bacterial outgrowth following intranasal challenge with either Gram-negative or Gram-positive bacteria [24]. It is uncertain whether their negative results were due to differences in the route of infection or mouse gender. It is important to note that we administered S. pneumoniae via the intratracheal route in our experiments and were unable to deliver equal amounts of bacterial suspensions to WT and ob/ob mice via the intranasal route (data not shown). Interestingly, a report by Yagasaki et al. demonstrated that, compared with their WT counterparts, the length of the skull and nasal bone are shorter (suggesting smaller nasal volume) in ob/ob male mice and this might explain why these mice were more prone to expelling the inoculum [39]. In addition, male mice are inherently more susceptible to infectious agents than females and both of these differences might have masked the effects of leptin deficiency on host defence [40,41].
In summary, we have demonstrated that ob/ob mice are very susceptible to Gram-positive pneumonia induced by S. pneumoniae and that exogenous leptin administration in vivo improves pulmonary bacterial clearance and survival in ob/ob mice. These novel and clinically relevant results suggest that exogenously administered leptin should be investigated further as an adjunctive therapeutic agent in the treatment of bacterial pneumonia, particularly in patients who are immunocompromised as a result of energy-malnutrition, a common secondary consequence of chronic disease.
Acknowledgments
Support for this research was provided by grants RG056N (P. M.) and RG8909N (D. M. A.) from the American Lung Association and HL077417, GM69438 (P. M.) and HL078727 (D. M. A.) from the National Institutes of Health.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Frederich RC, Lollmann B, Hamann A, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–3. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;12:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–23. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 5.Hoggarda N, Mercerb JG, Raynera DV, Moarb K, Trayhurna P, Williams LM. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT–PCR and in situ hybridization. Biochem Biophys Res Commun. 1997;232:383–7. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- 6.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 7.Moshyedi AK, Josephs MD, Abdalla EK, et al. Increased leptin expression in mice with bacterial peritonitis is partially regulated by tumor necrosis factor alpha. Infect Immun. 1998;66:1800–2. doi: 10.1128/iai.66.4.1800-1802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–6. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 10.Ikejima S, Sasaki S, Sashinami H, et al. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005;54:182–9. doi: 10.2337/diabetes.54.1.182. [DOI] [PubMed] [Google Scholar]
- 11.Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–82. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 12.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–42. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 13.Faggioni R, Fantuzzi G, Gabay C, et al. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–42. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 14.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects following acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2006;173:212–8. doi: 10.1164/rccm.200506-909OC. [DOI] [PubMed] [Google Scholar]
- 15.Moore SI, Huffnagle GB, Chen G-H, White E, Mancuso P. Leptin modulates neutrophil phagocytosis of K. pneumoniae. Infect Immun. 2003;71:4182–5. doi: 10.1128/IAI.71.7.4182-4185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieland CW, Florquin S, Chan ED, et al. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol. 2005;17:1399–408. doi: 10.1093/intimm/dxh317. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control (CDC) Defining the public health impact of drug-resistant Streptococcus pneumoniae: report of a working group. MMWR. 1996;45:1–14. [PubMed] [Google Scholar]
- 18.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 19.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–44. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 20.van der Poll T, Keogh C, Buurman W, Lowry S. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997;155:603–8. doi: 10.1164/ajrccm.155.2.9032201. [DOI] [PubMed] [Google Scholar]
- 21.Greenberger MJ, Strieter RM, Kunkel SL, et al. Neutralization of MIP-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–65. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 22.Schultz MJ, Wijnholds J, Peppelenbosch MP, et al. Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J Immunol. 2001;166:4059–64. doi: 10.4049/jimmunol.166.6.4059. [DOI] [PubMed] [Google Scholar]
- 23.Letiembre M, Echchannaoui H, Bachmann P, et al. Toll-like receptor 2 deficiency delays pneumococcal phagocytosis and impairs oxidative killing by granulocytes. Infect Immun. 2005;73:8397–401. doi: 10.1128/IAI.73.12.8397-8401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieland CW, Stegenga ME, Florquin S, Fantuzzi G, van der Poll T. Leptin and host defense against Gram-positive and Gram-negative pneumonia in mice. Shock. 2006;25:414–9. doi: 10.1097/01.shk.0000209524.12873.da. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee F-YJ, Li Y, Yang EK, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Physiol Cell Physiol. 1999;276:C386–94. doi: 10.1152/ajpcell.1999.276.2.C386. [DOI] [PubMed] [Google Scholar]
- 27.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–65. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 28.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–9. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 29.Sadikot RT, Zeng H, Azim AC, et al. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol. 2007;37:1001–9. doi: 10.1002/eji.200636636. [DOI] [PubMed] [Google Scholar]
- 30.Ballinger MN, Aronoff DM, McMillan TR, et al. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol. 2006;177:5499–508. doi: 10.4049/jimmunol.177.8.5499. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron Y, Ouellet N, Deslauriers A-M, Simard M, Olivier M, Bergeron MG. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–22. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dallaire F, Ouellet N, Bergeron Y, et al. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J Infect Dis. 2001;184:292–300. doi: 10.1086/322021. [DOI] [PubMed] [Google Scholar]
- 33.Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson M-P. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414–8. [PubMed] [Google Scholar]
- 34.Sibille Y, Marchandise FX. Pulmonary immune cells in health and disease: polymorphonuclear neutrophils. Eur Respir J. 1993;6:1529–43. [PubMed] [Google Scholar]
- 35.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–72. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray SE, Lallman HR, Heard AD, Rittenberg MB, Stenzel-Poore MP. A genetic model of stress displays decreased lymphocytes and impaired antibody responses without altered susceptibility to Streptococcus pneumoniae. J Immunol. 2001;167:691–8. doi: 10.4049/jimmunol.167.2.691. [DOI] [PubMed] [Google Scholar]
- 37.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–43. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 38.Conge GA, Gouache P, Joyeux Y, Goichot J, Fournier JM. Influence of different types of experimental obesity on resistance of the mouse to infection by Salmonella typhimurium and Klebsiella pneumoniae. Ann Nutr Metab. 1988;32:113–20. doi: 10.1159/000177423. [DOI] [PubMed] [Google Scholar]
- 39.Yagasaki Y, Yamaguchi T, Watahiki J, Konishi M, Katoh H, Maki K. The role of craniofacial growth in leptin deficient (ob/ob) mice. Orthod Craniofacial Res. 2003;6:233–41. doi: 10.1034/j.1600-0544.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 40.Mock BA, Nacy CA. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect Immun. 1988;56:3316–9. doi: 10.1128/iai.56.12.3316-3319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yancey AL, Watson HL, Cartner SC, Simecka JW. Gender is a major factor in determining the severity of Mycoplasma respiratory disease in mice. Infect Immun. 2001;69:2865–71. doi: 10.1128/IAI.69.5.2865-2871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


