Abstract
A successful pathogen manipulates its host for its own benefit. After ingestion, on reaching the intestine Salmonella encounters the resident tissue macrophages. Rather than being destroyed by these professional phagocytes after internalization, Salmonella survives intracellularly. Invasive Salmonella has been reported to induce apoptosis of macrophages as a part of its infection process, which may allow it to avoid detection by the innate immune system. However, the induction of apoptosis under different host environments, including the anaerobic stress encountered by the pathogen in the gut, remains to be examined. The present study is aimed at investigating the apoptotic potential of S. enterica serovar Typhi (S. typhi) grown under anaerobic conditions simulating the in vivo situation encountered by the pathogen. Apoptotic cell death was determined by assessment of nucleosomal DNA and flow cytometric analysis. Evaluation of the data revealed that anaerobically grown S. typhi could induce apoptosis in significantly more number of macrophages compared to the bacterial cells grown under aerobic conditions. A significantly enhanced generation of reactive nitrogen intermediates and caspase-3 activity during macrophage apoptosis induced by anaerobic S. typhi correlated with the increased generation of tumour necrosis factor-α, interleukin (IL)-1α and IL-6. The results indicate that reactive nitrogen intermediates and monokines induce caspase-3 mediated apoptosis of macrophages by S. typhi under anaerobic conditions. These findings may be relevant for clearer understanding of the Salmonella–macrophage interactions and may be of clinical importance in the development of preventive intervention against the infection.
Keywords: anaerobiosis, apoptosis, caspase-3, reactive nitrogen intermediates, Salmonella typhi, tumour necrosis factor
Introduction
Salmonellae are Gram-negative bacteria, which cause a variety of symptoms ranging from a mild intestinal infection to life-threatening systemic infections. Salmonellae are facultative intracellular bacteria which do not require strict conditions for their growth. These are able to proliferate and survive under different environmental conditions, including food production and processing systems as well as a diverse array of stresses encountered inside the host.
During its life cycle, Salmonella encounters low oxygen conditions while invading intestinal epithelium, as well as in other tissues of the host during systemic infection. The invasive capabilities of Salmonella have been shown to be potentiated in the presence of low oxygen, high osmolarity and alkaline pH, conditions that apparently signal the proper environment in the eukaryotic host [1]. Anaerobiosis has been reported to induce the invasiveness of Salmonella in cultured Madin-Darby canine kidney (MDCK) cells, human laryngeal tumour cells (HEp-2) and Henle cultured cell lines [2,3]. Salmonella under such conditions have also been shown to exhibit reduced LD50, greater adherence and enhanced virulence compared to aerobic bacteria [4,5]. However, the effect of enhanced virulence of anaerobically grown Salmonella upon the fate of macrophages, which form the first line of host defence, is yet to be evaluated. Salmonella exploits macrophages as a crucial tool in the initiation of the disease. Rather than being destroyed by these professional phagocytes, this pathogen is not only able to survive intracellularly, but also causes the apoptotic cell death of macrophages.
Apoptosis is an active cellular process of death triggered by certain stimuli. Apoptosis of host cells has been recognized to be involved in the regulation of immune response [6,7] and to occur during bacterial and viral infections [8]. Depending upon the pathogen, host cell apoptosis can be detrimental or beneficial to the pathogen. For example, the relevance of apoptosis by Pseudomonas aeruginosa has been demonstrated in favour of host defence [9]. Salmonella, however, has been reported to initiate apoptosis as a virulence strategy [10].
Recently, it has been observed that S. typhi grown under oxidative stress induces apoptosis in a significantly greater number of murine macrophages compared to the S. typhi grown under normal conditions [11]. However, during its infection route, anaerobic conditions are encountered by the pathogen long before the oxidative stress offered by the host within the macrophage milieu. Hence, it was worthwhile to investigate the potential of anaerobically stressed S. typhi to induce apoptosis of macrophages, which the pathogen may attempt in order to achieve immune avoidance.
Methods
Animals
Male inbred Balb/C mice, 4–6 weeks old (16–22 g in weight), used in the present study for extracting peritoneal macrophages, were procured from the Central Animal House, Panjab University, Chandigarh, India. The care and use of animals was in accordance with the guidelines of the institutional ethical committee.
Bacterial strain
The standard strain of S. typhi (Ty2), procured from Central Research Institute, Kasauli, India, was used in the present study. The strain was checked for purity and was characterized biochemically as well as serologically. The bacterial colony-forming units (CFU)/ml were calculated for infection of the macrophages.
Growth conditions
Bacteria were grown on nutrient agar plates and single colonies were used to prepare the seed culture. Inoculum 0·1% from seed culture was used to grow the bacteria under stress conditions. The bacteria were cultured by growing in nutrient broth under anaerobic conditions in accordance with the method of Schimann and Shope [12]. Anaerobic conditions were maintained by placing the culture in a Mackintosh anaerobic chamber in the presence of 85% nitrogen, 10% H2 and 5% CO2 at 37°C overnight.
Apoptotic cell death analysis
Isolation of peritoneal macrophages and their interaction with stressed bacteria.
For isolation of macrophages, Balb/C mice were injected with thioglycollate broth (2·5 ml per mouse given intraperitoneally 4 days in advance) as described earlier [13] to achieve a better yield of macrophages. Macrophages were washed with RPMI-1640 (ICN Biomedicals, CostaMesa, CA, USA) at 4000 g for 10 min. Cell viability was checked with 0·2% trypan blue staining. For interaction, 106 macrophages ml−1 were infected with 108 bacteria for 6 h in a humidified atmosphere containing 5% CO2 at 37°C. A macrophage : bacteria ratio of 106 : 108 was chosen on the basis of multiplicity of infection (MOI). This MOI (1 : 100) was selected after assessing the macrophage viability as determined by trypan blue exclusion and macrophage cytotoxicity, by measuring the release of host cytoplasmic lactate dehydrogenase (LDH). At this MOI, the macrophage viability was found to be 56% after 6 h of interaction in comparison to the viability of the uninfected macrophages (> 85%) at the initiation of the experiment. LDH release by infected macrophages was found to be increased by 38% compared to the release by uninfected macrophages.
Assessment of nucleosomal DNA.
DNA from cultured macrophages infected with the stressed Salmonella was isolated as described by Sambrook et al. [14] and nucleosomal DNA was assessed as described by Chanana et al. [11].
Fluorescein isothiocyanate (FITC)-labelled annexin V and propidium iodide staining.
The flipping of phosphatidylserine from the inner to the outer leaflet of the plasma membrane, which is a biochemical hallmark of apoptosis, is assessed by its binding with FITC-labelled annexin V (Roche Pharmaceuticals, Grenzach, Germany) for detection of apoptotic population [15]. After incubation time, cells were harvested using phosphate-buffered saline (PBS) + 0·02% ethylenediamine tetraacetic acid (EDTA), washed twice in PBS and suspended in 100 μl binding buffer (10 mM HEPES–NaOH, 140 mM NaCl, 2·5 mM calcium chloride, pH 7·4). Annexin V–FITC was added and cells were incubated for 15 min in dark conditions. After one wash, cells were resuspended in 200 μl binding buffer and counterstained with 1 μg/ml propidium iodide for determination of necrotic cells. Ten thousand cells per sample were analysed with a Becton Dickinson fluorescence activated cell sorter (FACSCalibur) equipped with a 15 mW, 488 nm air-cooled argon laser using CellQuest software.
Estimation of reactive nitrogen intermediates
Nitric oxide (NO) released from macrophages infected with stressed bacteria was measured by the determination of nitrite and citrulline levels in the culture supernatants of the cells. Nitrite levels were measured by the method of Green et al. [16]. In this assay, nitrites in the sample react with a Griess reagent to form a purple azo dye. The supernatant was collected and an equal volume of Griess reagent was added. It was incubated for 10 min at room temperature and the optical density was recorded at 546 nm. Blank and standards (sodium nitrite; SRL, Mumbai, India) (0·1–1·0 μmol) were also run in parallel. The results were expressed as μmol of nitrite-formed/106 cells.
Citrulline levels were measured using the method of Boyde and Rahmattulah [17]. The supernatant (50 μl) was treated with 0·1 N HCI (450 μl) and 1·5 ml of reagent [prepared by mixing two parts of 550 ml distilled water + 250 ml of H2SO4 (95–98%) + 200 ml of O-phosphoric acid (85%) + 250 mg ferric chloride and 1 part of 0·5% w/v diacetyl monoxime + 0·01% w/v thiosemicarbazide]. Tubes containing the reaction mixture were immersed in a waterbath at 100°C for 6 min followed by cooling to room temperature. The optical density was measured at 530 nm. Blank and standard (citrulline; SRL) were run simultaneously. Results were expressed as μmol of citrulline-formed/106 cells.
Measurement of caspase-3 activity
Caspase-3 activity was measured using the colorimetric CaspACE assay system (Promega, Madison, WI, USA) following the manufacturer's instructions. After treatment with stressed bacteria, cells (1 × 107) were washed twice in ice-cold PBS and resuspended in cell lysis buffer (provided by the manufacturer) at a concentration of 108 cells/ml. The cells were lysed by four cycles of freezing and thawing and incubated on ice for 15 min. The cell lysates were centrifuged at 15 000 g for 20 min at 4°C and the supernatant fraction was collected and stored at −70°C. To measure caspase activity, the concentration of protein in each sample was determined [18]. One hundred μg of total protein (in a volume of up to 20 μl) from each sample was added to each well of a 96-well plate. Then 32 μl of caspase assay buffer (provided by the manufacturer), 2 μl dimethylsulphoxide (DMSO) and 10 μl of a 100 mM dithiothreitol (DTT) solution were added to each well. The final volume was adjusted to 98 and 2 μl of the caspase-3 substrate DEVD-P-nitroanilide (DEVD-pNA) was added to the reaction. The plate was incubated at 37°C overnight. Absorbance at 405 nm was determined with a spectrophotometric microplate reader (SpectraMAX plus; Molecular Devices, Sunnyvale, CA, USA).
Monokines assay
The cell-free culture supernatants were collected at the indicated time. The cytokines [interleukin (IL)-1α, IL-6 and tumour necrosis factor (TNF)-α] activity in the culture supernatants was assessed by enzyme-linked immunosorbent assay (ELISA) using commercially available mouse cytokine ELISA kits (Chemicon, Temecula, CA, USA) according to the manufacturer's instructions. Briefly, 96-well microtitre plates precoated with monoclonal antibodies generated against mouse IL-1α, IL-6 and TNF-α were used to capture the respective monokines produced in the culture supernatants of macrophages. The assays were visualized using streptavidin alkaline phosphatase conjugate for IL-1α and TNF-α. For IL-6 the assay was visualized using a goat anti-rabbit–alkaline phosphatase conjugate and an ensuing chromagenic substrate reaction. The ELISA was sensitive to 0·2 pg/ml of the cytokines released. Linearity of the assay was assessed using different dilutions of the given standard. The precision profile was calculated by assaying replicates of standard as well as samples and then calculating the percentage coefficient of variation (%CV).
Statistical analysis
The statistical significance of results was determined by using the unpaired Student's t-test and two-way analysis of variance (anova) with multiple comparisons. Data were considered significant at P < 0·05. The linearity and CV, particularly for monokines, were assessed as per the following formula: linearity = observed value/expected value × 100; %CV = standard deviation/mean × 100. Linearity in the range of 90–94% and %CV in the range of 5–10% was considered to be satisfactory, as these ranges indicate fairly good precision of the tests.
Results
Assessment of DNA ladder
The chromosomal DNA of macrophages infected with anaerobic stressed S. typhi was fragmented extensively into oligonucleosomes, clearly visible as a characteristic DNA ladder on agarose gels after 6 h (Fig. 1, lanes 5 and 6). However, this pattern was not detected at 4 h and 8 h post-infection of the macrophages. A single high molecular weight DNA band was observed in the control sample, in which the macrophages were not infected but the incubation time was 6 h (Fig. 1, lanes 1 and 2). Apoptosis is not in the biochemical repertoire of prokaryotes, but as a control S. typhi bacteria were subjected to phenol–chloroform extraction of DNA and no low molecular weight DNA was observed (Fig. 1, lane 3).
Fig. 1.
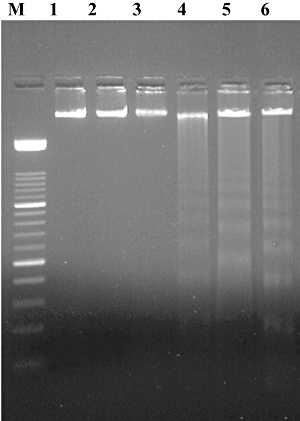
Internucleosomal cleavage of macrophage DNA following Salmonella infection (multiplicity of infection 1 : 100) after 6 h of incubation; lane M: 100 base pairs (bp) DNA ladder, lanes 1 and 2: uninfected control, lane 3: DNA of S. typhi, lane 4: DNA of macrophages infected with normal S. typhi, lanes 5 and 6: DNA of macrophages infected with anaerobic S. typhi.
FITC-labelled annexin V and propidium iodide staining
Using FITC-labelled annexin V, the macrophage population treated with normal and anaerobic stressed S. typhi for 6 h showed 34% and 50% cells, respectively, as apoptotic (Fig. 2), in contrast to 3·0% apoptotic cells in the control samples. The apoptotic cells were identified by flipping phosphatidylserine receptors on the outer leaflet of plasma membrane. Counterstaining with propidium iodide showed 6·0% and 7·11% as necrotic in macrophages treated with normal and anaerobically stressed S. typhi, respectively.
Fig. 2.
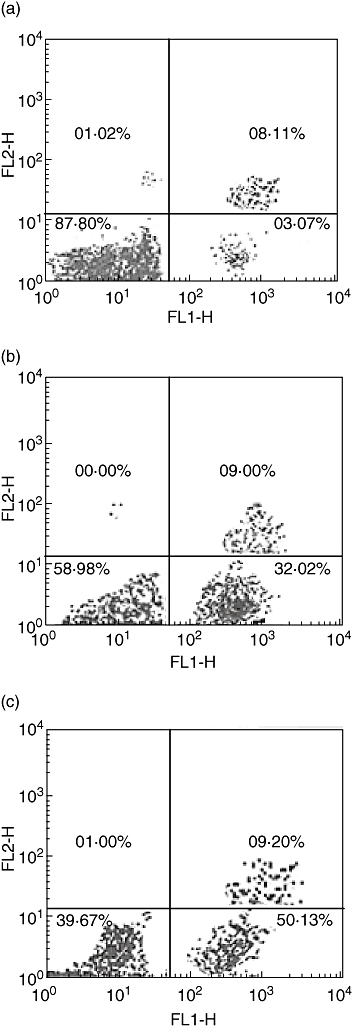
Flow cytometric analysis showing annexin V binding properties of macrophages treated with Salmonalla typhi. (a) Untreated cells shows only 3·07% of population as apoptotic (lower right chamber). (b) Normal S. typhi treated cells show 32% of population as apoptotic (lower right chamber). (c) Anaerobic S. typhi-treated cells show 50% of population as apoptotic (lower right chamber). Upper right chamber shows necrotic population which were stained with both propidum iodide and annexin-V.
Estimation of reactive nitrogen species
Infection of macrophages with stressed S. typhi induced significantly increased (P < 0·01) nitrite and citrulline levels in the mouse peritoneal macrophages compared to the macrophages infected with S. typhi grown under normal conditions (Fig. 3a,b).
Fig. 3.
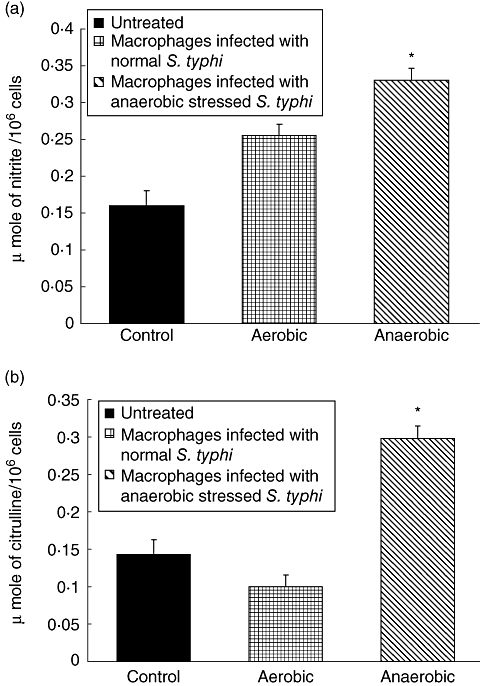
(a,b) Levels of (a) nitrite and (b) citrulline (μmol/106 cells) in the culture supernatants of macrophages infected with Salmonella typhi grown under normal and stressed conditions. Values are expressed as mean ± standard deviation of six different observations. *Significant difference (P < 0·01) from untreated control and normal S. typhi-infected macrophages.
Caspase-3 activity
Caspase-3 activity was increased during apoptosis induced by anaerobic stressed S. typhi infection of macrophages (Fig. 4). Cells treated with these stressed bacteria showed a significant increase in caspase-3 activity compared to infection with normal S. typhi as well as to untreated control macrophages after 6 h treatment.
Fig. 4.
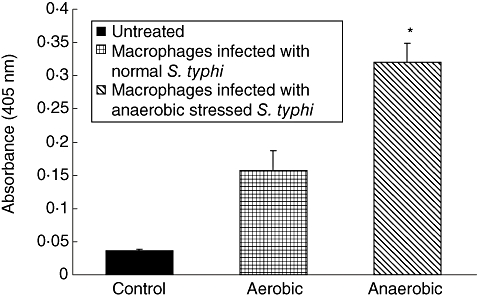
Caspase-3 activity in the culture supernatants of macrophages infected with Salmonella typhi after 6 h incubation. Values are expressed as mean ± standard deviation of six different observations. *Significant difference (P < 0·01) from untreated control and normal S. typhi-infected macrophages.
Monokine assays
Significantly enhanced levels of monokines IL-6, IL-1α and TNF-α were observed when the macrophages were infected with anaerobically stressed S. typhi compared to the untreated macrophages and the macrophages infected with S. typhi grown under normal conditions for 6 h (Fig. 5). Of the cytokines assayed, generation of TNF-α was found to be highest (P < 0·001).
Fig. 5.
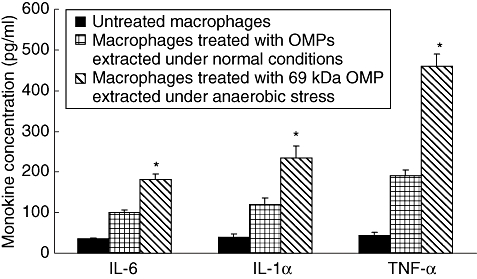
Estimation of monokines levels (picogram/ml) in the culture supernants of macrophages interacted with Salmonella typhi after 6 h of incubation. Values are expressed as mean ± standard deviation of six different observations. *Significant difference (P < 0·01) from untreated control and normal S. typhi-infected macrophages.
Role of TNF-α in mediating bacteria-induced apoptosis
To evaluate the contribution of TNF-α in apoptosis, we determined whether TNF-α, produced as a part of the macrophage response to Salmonella infection, played a role in activating apoptosis after bacterial infection. Therefore, the apoptotic cells were again enumerated by acridine orange ethidum bromide co-staining after blocking the TNF-α activity using 20 μg/ml of anti-TNF-α antibodies (Sigma, St. Louis, MO, USA) at the time when macrophages were infected with S. typhi, as described previously [11]. A significant reduction in the apoptotic cell population was observed (Fig. 6). As a control, normal goat IgG antibodies were added instead of anti-TNF-α antibodies.
Fig. 6.
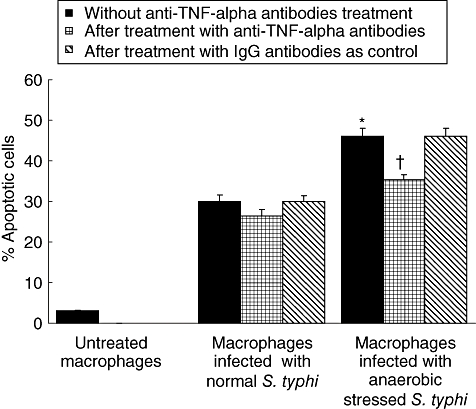
Assessment of macrophage apoptosis (by acridine orange ethidium bromide co-staining) following infection with Salmonella typhi after 6 h of incubation after blocking tumour necrosis factor (TNF)-α activity. Values are expressed as mean ± standard deviation of six different observations. *Significant difference (P < 0·01) from untreated control and normal S. typhi-infected macrophages. †Significant difference from anaerobic S. typhi-infected macrophages without anti-TNF-α antibody treatment.
Discussion
Macrophages serve essential functions as regulators of immunity and homeostasis [19,20]. As participants in native immunity, macrophages phagocytose, kill invading microorganisms and elaborate signalling molecules that amplify acute inflammatory responses. Macrophages also contribute to acquired immune responses by means of specialized functions that include antigen presentation and regulation of T cell responses. However, some bacterial pathogens, including Salmonella, have the capacity to induce macrophage apoptosis in order to elude innate immune responses and colonize the host successfully [21–24]. When a microbe such as Salmonella reaches the distal ileum, it must adapt to an anaerobic environment in order to carry out essential metabolic activities [2,25]. However, very little is known about the outcome of the microbe–macrophage interactions occurring under these situations.
In this study, it was observed that anaerobically stressed S. typhi cells were able to induce apoptosis of peritoneal macrophages after 6 h of interaction, as demonstrated by the results of assessment of nucleosomal DNA as well as flow cytometric analysis. The significantly increased number of macrophage apoptotic cells resulting from interaction with bacterial cells grown under anaerobic conditions may be due to the up-regulation or modification of certain genes and subsequently their corresponding proteins during the infection of macrophages with the pathogen under anaerobic conditions. It has been well documented that a number of genes including orgA (induced by anaerobiosis), prgH (a member of PhoPQ regulon) and invasion locus hil are expressed in Salmonella under anaerobic stress conditions [26]. It has been observed that bacteria growing under anaerobic conditions exhibit greater adherence as well as invasiveness and it has been proposed that increased invasiveness of anaerobically grown Salmonella was due to synthesis of at least two additional proteins [2]. It has also been reported recently that anaerobically grown S. typhi expresses 69 kDa OMP with enhanced intensity [27]. Expression of this protein under the anaerobic condition might be playing a role in induction of macrophage apoptosis.
NO has also been implicated in the induction of apoptosis of macrophages and monocytes. Reactive nitrogen intermediates (RNI) such as nitrites and citrulline are known to be the end-products of oxidative metabolism of labile nitric oxide and their quantification is regarded as an indicator of NO generation [28]. Significantly increased levels of nitrite and citrulline observed in the present study may contribute to S. typhi pathogenesis by inducing NO generation by the macrophages. Increased levels of NO might have resulted in the increased activity of caspase-3 observed in this study, as NO has been reported to increase cellular ceramide level through the enhancement of caspase-3 activity [29]. The ceramide formation can induce several apoptotic signal pathways, including the release of mitochondrial cytochrome c into cytosol, the activation of caspase-9 and -3, the inhibition of protein kinase B/Akt and the suppression of Bcl-2 expression. Activation of caspase-3 has been reported to cleave the inhibitor for caspase activated DNase (ICAD), allowing CAD to enter the nucleus and degrade chromosomal DNA [30]. Increased activity of caspase-3 might be responsible for the nucleosomal fragmentation observed in the present study.
TNF-α, IL-1α, IL-6 and IFN-γ have been reported to induce apoptosis in various cell types [31–33]. Evidence that TNF-α induces apoptosis via NO production is provided by the studies of Song et al. [34], in which it is demonstrated that TNF-α generation along with parallel increases in inducible type nitric oxide synthase (iNOS) mRNA expression and NO production induced apoptosis in wild-type mice cardiomyocytes. Schuerwegh et al. [31] have also suggested a significant role of NO in cytokine (TNF-α- and IL-1α)-induced bovine chondrocytes apoptosis. Relationship of TNF-α with caspase-3 as well as NO [35] in activation of apoptosis has also been established. Accordingly, Gupta and Gollapudi [36] have also shown that increased apoptosis of T cells in aged humans is associated with increased activation of caspase-3 and TNF-α. In immune system-responsive cells, activation of caspase-3 has been shown to be required for apoptosis induced by Fas–FasL or TNF-α–TNF receptor interactions [37]. In the present study TNF-α-mediated apoptosis is also indicated with S. typhi grown under anaerobic conditions. These results are in concurrence with the earlier study wherein cellular sensitivity or resistance to TNF-α is correlated with decreased or increased levels of nitric oxide, respectively [34]. In our study, blocking of TNF-α activity in infected macrophage cultures using anti-TNF-α antibodies blocked, in part, the induction of apoptosis. These results are in accordance with an earlier study [38], wherein 20% reduction in epithelial cell apoptosis by S. dublin was observed.
Our results are suggestive of a central role of RNIs, caspase-3 and TNF-α in conjunction with IL-1α and IL-6 in inducing the apoptotic cell death of macrophages following infection with S. typhi grown under anaerobic stress conditions. These findings may be relevant for the better understanding of the disease pathophysiology and for the future developments of diagnostic and preventive strategies.
Acknowledgments
Grants from the India Council of Medical Research (ICMR) for conducting the study are gratefully acknowledged.
References
- 1.Johnston C, Pegues DA, Hueck CJ, Lee A, Miller SI. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–27. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee CA, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–8. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst RK, Dombroski DM, Merrick JM. Anaerobiosis, type-I fimriae, and growth phase are factors that affect invasion of Hep-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–16. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh RD, Khullar M, Ganguly NK. Role of anaerobiosis in virulence of Salmonella typhimurium. Mol Cell Biochem. 2000;215:39–46. doi: 10.1023/a:1026545630773. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor S, Singh RD, Sharma PC, Khullar M. Anaerobiosis induced virulence of Salmonella typhi. Ind J Med Res. 2002;115:184–8. [PubMed] [Google Scholar]
- 6.Williams P, Wiede F, Meissner A, Donhauser N, Bogdan C, Korner H. TNF but not Fas ligand provides protective anti-L. major immunity in C57BL/6 mice. Microbes Infect. 1995;7:1461–8. doi: 10.1016/j.micinf.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Osborne BA. Apoptosis and the maintenance of homoeostasis in the immune system. Curr Opin Immunol. 1996;8:245–54. doi: 10.1016/s0952-7915(96)80063-x. [DOI] [PubMed] [Google Scholar]
- 8.Zychlinsky A, Thirumalai K, Arondel J, Cantey JR, Aliprantis AO, Sansonetti PJ. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–65. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buommino E, Morelli F, Metafora S, et al. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect Immun. 1999;67:4794–800. doi: 10.1128/iai.67.9.4794-4800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boise LH, Collins CM. Salmonella induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–7. doi: 10.1016/s0966-842x(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 11.Chanana V, Majumdar S, Rishi P. TNF-α mediated apoptosis in murine macrophages by Salmonella enterica serovar Typhi under oxidative stress. FEMS Immunol Med Microbiol. 2006;47:278–86. doi: 10.1111/j.1574-695X.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 12.Schimann DA, Shope SR. Anaerobic growth of Salmonella typhimurium results in increased uptake by Henle 407 epithelial and mouse peritoneal cells in vitro and repression of a major outer member protein. Infect Immun. 1991;56:437–40. doi: 10.1128/iai.59.1.437-440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chander H, Majumdar S, Sapru S, Rishi P. Macrophage cell death due to Salmonella enterica serovar Typhi and its acid stress protein has features of apoptotsis. Microbiol Immunol. 2005;49:323–30. doi: 10.1111/j.1348-0421.2005.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 15.Vermes I, Haanen C, Steffens-Nakken H, Reutelinsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidlyserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Method. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 16.Green LC, Wagner DA, Glogowski J, Shipper PL, Wishnok J, Rannerbaum SR. Analysis of nitrate, nitrite and 15N nitrate in biological fluids. Anal Biochem. 1982;126:121–58. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Boyde TRC, Rahmatulah M. Colorimetric determination of citrulline. Anal Biochem. 1980;107:424–31. doi: 10.1016/0003-2697(80)90404-2. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin's phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Celada A, Nathan C. Macrophage activation revisited. Immunol Today. 1994;15:100–2. doi: 10.1016/0167-5699(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 20.Gordon S. The role of the macrophage in immune regulation. Res Immunol. 1998;149:658–88. doi: 10.1016/s0923-2494(99)80039-x. [DOI] [PubMed] [Google Scholar]
- 21.Zychlinsky A, Prevosst MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;58:167–9. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 22.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasion Sip B induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valledor AF, Li-Chung H, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA. 2004;101:17813–18. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takaya A, Suzuki A, Kikuchi Y, et al. Derepression of Salmonella pathogenicity island 1 genes within macrophages leads to rapid apoptosis via caspase-1- and caspase-3-dependent pathways. Cell Microbiol. 2005;7:79–90. doi: 10.1111/j.1462-5822.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith MW, Neidhardt FC. Proteins induced under anaerobiosis in Escherichia coli. J Bacteriol. 1983;154:336–43. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones BD, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–52. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chanana V, Majumdar S, Sharma M, Ray P, Rishi P. Coordinated expression and immunogenicity of an outermembrane protein in Salmonella enterica serovar Typhi under iron limitation, oxidative stress and anaerobic conditions. J Biomed Sci. 2006;13:303–12. doi: 10.1007/s11373-005-9047-5. [DOI] [PubMed] [Google Scholar]
- 28.Steuher DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to E. coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–42. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schubert KM, Scheid MP, Duronio V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J Biol Chem. 2000;275:13330–5. doi: 10.1074/jbc.275.18.13330. [DOI] [PubMed] [Google Scholar]
- 30.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 31.Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck S. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr Cartilage. 2003;11:681–7. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 32.Yamane D, Nagai M, Ogava Y, Tohya Y, Akashi H. Enhancement of apoptosis via an extrinsic factor, TNF-α, in cells infected with cytopathic bovine viral diarrhea virus. Microbes Infect. 2005;7:1482–91. doi: 10.1016/j.micinf.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:583–90. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 34.Song K, Chen Y, Göke R, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binder C, Schulz M, Hiddemann W, Oellerich M. Induction of inducible nitric oxide synthase is an essential part of tumor necrosis factor-alpha-induced apoptosis in MCF-7 and other epithelial tumor cells. Lab Invest. 1999;79:1703–12. [PubMed] [Google Scholar]
- 36.Gupta S, Gollapudi S. TNF-α-induced apoptosis in human naive and memory CD8+ T cells in aged humans. Exp Gerontol. 2006;41:69–77. doi: 10.1016/j.exger.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim JM, Eckmann L, Savidge TC, Lowe TW, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–23. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]


