Abstract
Translocation of bacterial-DNA in patients with cirrhosis and ascites triggers an innate immune response. Identification of characteristics to which this response is sensitive is relevant from a clinical standpoint. The aim of this study has been to determine if the proinflammatory immune response established in vivo in cirrhotic patients with ascites as a consequence of bacterial-DNA translocation is related to the identified bacterial species and their frequency of cytosine-guanosine content in serum and ascitic fluid. Patients with advanced cirrhosis and ascites were included in the study and distributed into groups I and II according to the absence or presence of bacterial-DNA translocation, respectively. Serum and ascitic fluid levels of proinflammatory cytokines after normalization of bacterial-DNA concentration and the activated form of nuclear factor-kappa B in ascitic fluid pellets were measured by enzyme-linked immunosorbent assay techniques. Translocation of bacterial-DNA with higher cytosine-guanosine content induced the highest cytokine response, which was higher than that in patients without bacterial-DNA translocation. The activated form of nuclear factor-kappa B in ascitic fluid pellets of patients with bacterial-DNA translocation was greater in patients with higher bacterial-DNA cytosine-guanosine content, whereas the amount of total nuclear factor-kappa B remained unaltered. Bacterial-DNA translocation induces a marked immune reaction in vivo in patients with advanced cirrhosis and ascites which is related, among other factors, to the bacterial-DNA cytosine-guanosine content. Therefore, the host's immune response to bacterial-DNA translocation constitutes a species-specific phenomenon.
Keywords: ascitic fluid, bacterial DNA, cirrhosis, cytokines, NF-κB
Introduction
During the last few years, evidence has been reported showing the presence of circulating bacterial DNA (bactDNA) in blood and ascitic fluid (AF) from patients with advanced cirrhosis [1,2]. Because we have also shown that bactDNA is present simultaneously in biological fluids and in mesenteric lymph nodes (MLNs) in an animal model of cirrhosis [3], we suggested that bactDNA detection in biological fluids may be considered as a surrogated marker of bacterial translocation (BT).
Circulating bactDNA in serum and AF in these patients is associated with a marked immune response. Indeed, cultured peritoneal macrophages from patients with cirrhosis and the presence of bactDNA in blood and AF show a preactivated immune status [4] and an increased intracellular expression of cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-6 [5]. Furthermore, in a recent study we showed that the response present in patients with bactDNA translocation is fully comparable with that observed in patients with spontaneous bacterial peritonitis (SBP) [6] which, in turn, is considered as the most relevant infectious complication developing in patients with advanced cirrhosis [7].
Bacterial DNA contains immunostimulatory unmethylated cytosine-guanosine (CG) dinucleotides (CpGs) that interact with Toll-like receptor-9 (TLR-9) present in several immunocompetent cells [8–10]. DNA preparations from different bacterial species produce different cytokine response patterns in vitro in a human embryonic kidney (HEK) cell line [11]. The frequency of CG content for a given genera of bacteria represents a constant and specific percentage of its genome and it has been shown that immunogenicity of bactDNA is based upon the relative abundance of these CpG sequences [11–13]. Thus, differences in the CG content between bacterial species may also be a source of variation in terms of regulating the inflammatory process.
The inflammatory status present in patients with cirrhosis may have clinical consequences. Patients with sterile ascites at admission showing increased levels of TNF-α are prone to developing nosocomial SBP episodes [14], and the exacerbated inflammatory response induced during the development of a SBP episode is related closely to the likelihood of developing renal impairment and death [14,15]. When considering animal models of cirrhosis, the blockade of TNF-α ameliorates the hyperdynamic circulation [16] and decreases BT [17]. Finally, preliminary results from our group suggest that the presence of bactDNA in patients with advanced cirrhosis is associated with an increased risk of mortality from non-infected causes [18].
The aim of this study has been to determine if the proinflammatory immune response established in vivo in cirrhotic patients with ascites as a consequence of bactDNA translocation is related to the identified bacterial species and their frequency of CG content in serum and AF. For this purpose we have studied samples from patients with bactDNA from Gram-positive microorganisms, who represent approximately 35% of bactDNA translocation episodes and in which contamination with lipolysaccharide (LPS) should be minimal. This seems relevant from a clinical point of view, as it may indicate the type of bactDNA inducing a stronger inflammatory response and is likely to be related to a worse prognosis.
Patients and methods
Patients and study design
We conducted a prospective study in serum and AF of patients with cirrhosis requiring a large-volume paracentesis at admission. Cirrhosis was diagnosed by histology or by clinical, laboratory and/or ultrasonographic findings. Exclusion criteria were the presence of a culture-positive blood or AF, an AF polymorphonuclear (PMN) count equal to or higher than 250/µl [19], signs or symptoms of systemic inflammatory response syndrome [20], upper gastrointestinal bleeding or intake of antibiotics in the preceding 2 weeks, hepatocellular carcinoma and/or portal thrombosis, previous liver transplantation, transjugular intrahepatic portosystemic shunt, alcoholic hepatitis, age older than 80 or younger than 18 years and refusal to participate in the study. The ethics committee of the hospital approved the study protocol and all patients gave informed consent for inclusion in the study.
Blood was obtained for routine haematological, biochemical and coagulation studies. Simultaneously, a large-volume paracentesis was performed on all patients at admission in aseptic conditions following the usual procedures [21]. All patients received intravenous albumin (8 g per litre of AF removed) as the routine protocol, if the amount evacuated was greater than 5 l. AF was centrifuged at 500 g. After washing with phosphate-buffered saline (PBS), cells were frozen and stored at −80°C in 25 × 106-cell aliquots. Samples for routine biochemical study and PMN count were obtained. Total protein, albumin, leucocyte and polymorphonuclear counts were performed in all AF specimens. Both blood and AF were inoculated at the bedside in aerobic and anaerobic blood culture bottles, 10 ml each [22]. Finally, separate blood and AF samples were inoculated, under aseptic conditions, in rubber sealed sterile Vacutainer SST II tubes (BD Diagnostics, Erembodegem, Belgium), that were never exposed to free air.
Quantification and identification of amplified bacterial DNA fragments
To detect and identify the presence of bactDNA fragments in both blood and AF, a broad-range polymerase chain reaction (PCR) and partial nucleotide sequencing analysis was performed according to the methodology described previously [1]. Briefly, DNA was isolated with QIAmp DNA Blood Mini Kit (Qiagen, Hilden, Germany) and a broad-range PCR amplification of the bacterial 16SrRNA gene conserved region was performed using the following primers: 5′-TTCCGGTTGATCCTGCCGGA-3′ forward and 5′-GGTTACCTTGTTACGACTT-3′ reverse. PCR amplicons were loaded onto DNA Laboratory-on-chips® (Agilent Technologies, Palo Alto, CA, USA) and analysed in an Agilent 2100 BioAnalyser (Agilent Technologies). BactDNA fragments were purified with QIAquick purification kit (Qiagen) and purified amplicons were used for the sequencing reactions with Big Dye Terminator version 3·1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). The same reverse oligonucleotide used for PCR amplification was used as a sequencing primer. The final product was purified by precipitation with ethanol–acetate and analysed in the ABI PRISM 310 automated sequencer (Applied Biosystems). Sequences were compared with the database from the National Center for Biotechnology Information (NCBI, http://www.ncbi.nih.gov) using the advanced blast search tool.
Quantification of serum and ascitic fluid cytokine levels
Enzyme-linked immunosorbent assays (ELISAs) for the quantitative measurement of TNF-α, interferon (IFN)-γ and IL-12 levels, as representative cytokines of the proinflammatory immune response, were carried out in basal serum and AF samples with equal concentrations of bactDNA, using Human Quantikine kits (R&D Systems, Minneapolis, USA) according to the manufacturer's instructions. All samples were tested in triplicate and read at 490 nm in a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Lower limits of detection of all cytokine assays were between 5 and 10 pg/ml. Standard curves were generated for each plate and the average zero standard optical densities were subtracted from the remaining standards, controls and samples to obtain a corrected concentration for all cytokines and endotoxin.
Measurement of lipopolysaccharide-binding protein (LBP) and LPS levels
Human LBP enzyme-linked immunosorbent assay (ELISA) test kit (HyCult Biotechnology, Uden, the Netherlands) was used, according to the manufacturer's instructions, to evaluate LBP levels in serum samples from all patients included in the study. A quantitative chromogenic limulus amoebocyte lysate (LAL) test (BioWhittaker, Nottingham, UK) was followed to evaluate LPS levels in different aliquots from the same samples. Due to LPS ubiquity, samples and reagents were handled in an airflow chamber and processed with pyrogen-free material tested by manufacturers. Escherichia coli lyophilized endotoxin (22 UE/ml) provided by the kit was used to set standard endotoxin concentrations ranging from 1·0 UE/ml to 0·1 UE/ml. To verify the lack of product inhibition by plasma protein, a dilution/heating inactivation protocol was followed prior to endotoxin measurement. Two pooled E. coli endotoxin spike solutions (0·4 UE/ml) were prepared with serum and AF samples, respectively. Dilutions ranging from 1/2 to 1/20 were performed over spiked and unspiked serum and AF samples. All test samples were then incubated at 60°C during 30 min [23]. The LAL test was performed after this period. The non-inhibitory dilution was established when the difference between spiked and unspiked endotoxin values was equal to the known concentration of the spike ± 25%, as detailed by the manufacturer. Final sample dilutions used were 1/10 in serum (spike recovery after correction of dilution: 0·36 UE/ml) and 1/4 in AF (spike recovery after correction of dilution: 0·42 UE/ml).
All samples were tested in triplicate and read at 450 nm for LBP and at 405 nm in the case of LPS, in a Thermomax microplate reader (Molecular Devices).
Total versus activated nuclear factor kappa B (NF-κB) in AF pellets
Cytoplasmic NF-κB is bound typically to inhibitor κB (IκB) forming an inactive NF-κB/IκB complex. Upon stimulation, IκB is phosphorylated and NF-κB translocated into the nucleus as the activated form. Total cellular protein and nuclear extracts were obtained from 25 × 106 AF cells using the ReadyPrep total protein extraction kit and ReadyPrep cytoplasmic/nuclear protein extraction kit (Bio-Rad, Hercules, CA, USA), respectively. A Bradford protein assay was performed to determine concentration of protein; 150 µg/ml of protein were loaded onto a 96-well ELISA plate coated with an oligonucleotide containing the DNA binding NF-κB consensus sequence, according to the manufacturer's instructions (Oxford Biomedical Research, Oxford, MI, USA). All samples were tested in triplicate and read at 450 nm in a Thermomax microplate reader (Molecular Devices). Standard curves were generated for each plate and the average zero standard optical densities were subtracted from the rest of standards, controls and samples to obtain a corrected protein concentration.
Statistical analysis
Continuous variables are reported as mean ± standard deviation and categorical variables as frequency or percentages. Statistical differences of basal characteristics between groups were analysed using the Fisher test for categorical data and the Mann–Whitney U-test for quantitative data. Statistical differences between cytokines were analysed using the analysis of variance (anova) test with Bonferroni correction for multiple comparisons. All reported P-values are two-sided, and P-values lower than 0·05 were considered to indicate significance. All calculations were performed using spss version 12·0 software (Chicago, IL, USA).
Results
Characteristics of patients
Between 2003 and 2006 a consecutive series of 226 patients with cirrhosis and AF fulfilling the above-described inclusion and exclusion criteria were tested for the presence of bactDNA in blood and AF. Of those, 149 did not show the presence of bactDNA and among them, a subset of 50 patients was selected randomly for the study and constituted group I. Seventy-seven patients showed bactDNA in blood and AF and, of those, 26 (33·7%) showed bactDNA from Gram-positive bacteria and constituted group II. Clinical and analytical characteristics of patients are detailed in Table 1. No statistical differences were observed between groups for any of the studied parameters.
Table 1.
Clinical and analytical characteristics of patients included in the study. Group I: patients without bactDNA translocation; Group II: patients with bactDNA translocation; WBC: white blood cells; PMN: polymorphonuclear cells; AF: ascitic fluid.
| Group I (n = 50) | Group II (n = 26) | P value | |
|---|---|---|---|
| Age (years) | 60.05 ± 11.60 | 63.22 ± 11.53 | ns |
| Male Sex n (%) | 26 (52%) | 12 (46.7%) | ns |
| Alcohol ethiology n (%) | 33 (66%) | 18 (69.2%) | ns |
| Previous episodes of ascites n (%) | 14 (28%) | 9 (34.6%) | ns |
| Child-Pugh A/B/C (n) | 0/31/19 | 0/14/12 | ns |
| Meld mean score | 12.87 ± 4.5 | 16 ± 8 | ns |
| Mean Arterial Pressure (mmHg) | 86.5 ± 15.9 | 83.03 ± 9.44 | ns |
| Bilirubin (mg/dL) | 3.3 ± 2.1 | 3.33 ± 3.60 | ns |
| Albumin (g/dL) | 2.80 ± 0.6 | 2.75 ± 0.56 | ns |
| Quick (%) | 61.2 ± 14.6 | 59.15 ± 15.80 | ns |
| Serum creatinine (mg/dL) | 0.8 ± 0.4 | 1.02 ± 0.60 | ns |
| Serum sodium (mEq/L) | 135.0 ± 5.1 | 134.24 ± 5.44 | ns |
| Blood WBC/mm3 | 5576.4 ± 2389.3 | 6205.5 ± 3409.48 | ns |
| Total Blood PMNs/mm3 | 4920 ± 2995 | 4471.15 ± 2915.83 | ns |
| Total AF proteins (g/dL) | 1.7 ± 0.8 | 1.58 ± 0.76 | ns |
| AF WBC/mm3 | 207 ± 185.9 | 165 ± 180 | ns |
| Total AF PMNs/mm3 | 39.2 ± 42.9 | 46.4 ± 79.2 | ns |
Values shown as mean ± standard deviation; Group I: patients without bactDNA; Group II: patients with bactDNA translocation; WBC: white blood cell; PMN: polymorph nuclear cell; AF: ascitic fluid.
Bacterial species identified by PCR and automated sequencing analysis were the same in serum and AF for a given patient in all cases. Three species were identified among patients from group II: Staphylococcus aureus (46·2%, n = 12), Enterococcus faecalis (34·6%, n = 9) and Streptococcus pneumoniae (19·2%, n = 5). Patients from group II were studied further according to the CG dinucleotide content of the identified bacteria. Neither clinical nor analytical differences were observed among this group after patients' distribution according to bacterial species.
T helper 1 (Th1)-biased cytokine cascade in patients with ascites and presence of bactDNA
The first stage of innate immune activation by bactDNA leads to activation of cell signalling pathways driven to Th1-promoting cytokine secretion [24]. The proinflammatory immune response was evaluated by quantitative analysis of TNF-α, IFN-γ and IL-12 levels in serum and AF. Results were analysed according to the frequency of CG content present in each identified bacterial species. CG dinucleotide content of bacteria isolated in our series of patients was obtained from published whole-genome data: Staph. aureus 2·54%, S. pneumoniae 2·73% and E. faecalis. 3·50% [11]. Figure 1 represents serum and AF levels of TNF-α, IFN-γ and IL-12 in patients with cirrhosis and ascites, after amplified bactDNA concentration in all samples was normalized to 30 ng/µl. All cytokines studied in serum and AF showed higher levels of expression as the CG content was incremented. Levels of all serum and AF cytokines in group I (bactDNA–) were significantly lower than values observed in patients with bactDNA translocation (group II), independently of the identified bacterial species (Fig. 1). Serum from a series of 15 healthy donors was included in the analysis. In all cases, levels of proinflammatory markers stayed below each cytokine lower standard (TNF-α and IFN-γ < 15·6 pg/ml; IL-12 < 5·6 pg/ml).
Fig. 1.
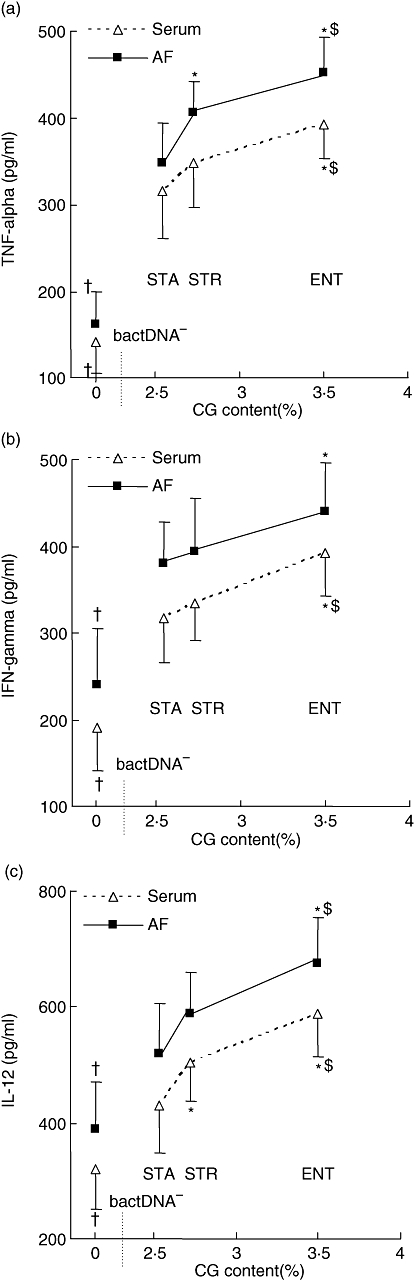
Serum and ascitic fluid (AF) levels of tumour necrosis factor (TNF)-α (a), interferon (IFN)-γ (b) and interleukin (IL)-12 (c) in cirrhotic patients with ascites and bacterial DNA translocation, according to CG dinucleotide content of each identified species. CG content frequency for the four species was: Staphylococcus aureus 2·54%; Streptococcus pneumoniae 2·73%; and Enterococcus faecalis. 3·50%. STA: Stap. aureus; STR: S. pneumoniae; ENT: Enterococcus faecalis. *P < 0·05 when compared with STR; †P < 0·05 when compared with the remaining groups.
The powerful immunostimulatory capacity of LPS was of concern in this study as a possible source of contamination. Both LPS and LBP levels were measured in serum and AF samples and no statistically significant differences between bactDNA– patients (group I) and any of the subgroups of bactDNA+ patients were found (Fig. 2).
Fig. 2.
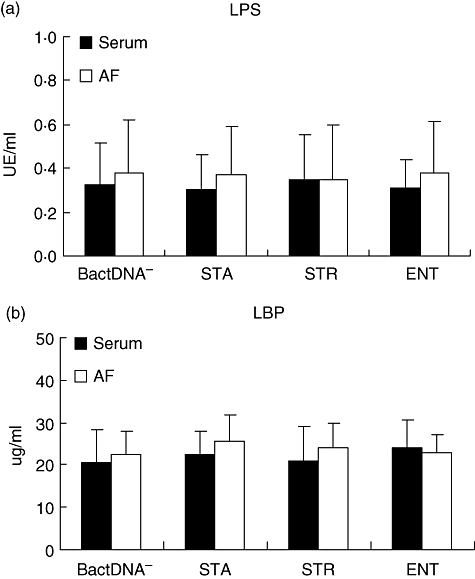
Serum and ascitic fluid (AF) levels of lipopolysaccharide (LPS) (a) and lipopolysaccharide-binding protein (b) levels in patients included in the study. STA: Staphylococcus aureus; STR: Streptococcus pneumoniae; ENT: Enterococcus faecalis.
NF-kB signalling pathway is activated in patients with bactDNA translocation
The signalling pathway triggered by CpGs involves TLR-9 interaction and translocation of NF-κB [24,25]. Thus, differences in CG content may also be crucial in terms of NF-κB activation. AF cells from patients in group I (absence of DNAbact) showed reduced levels of the NF-κB activated form compared with patients with bactDNA translocation (group II) (Fig. 3a). Patients from this group were studied further according to the CG dinucleotide content of the identified bacteria. Higher levels of the NF-κB activated form were present in AF cells from patients with bacterial DNA showing a higher content of CG dinucleotides, whereas levels of total NF-κB remained stable (Fig. 3b). This solid tendency was significant when comparing values in patients with bactDNA from E. faecalis versus Staph. aureus (P = 0·002), S. pneumoniae versus Staph. aureus (P = 0·01) and E. faecalis versus S. pneumoniae (P = 0·02).
Fig. 3.
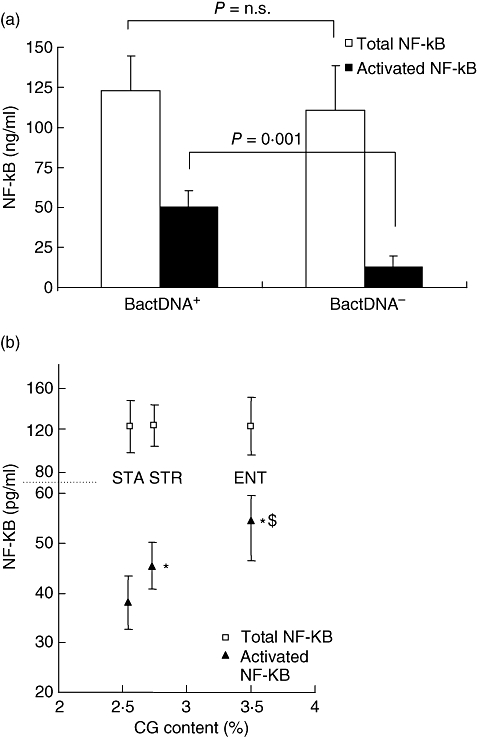
(a) Ascitic fluid (AF) cells from patients with bacterial DNA (bactDNA) translocation show higher levels of activated nuclear factor-kappa B (NF-κB) than patients without bactDNA translocation. (b) NF-κB activation is increased in patients with translocation of bacterial DNA with higher CG content. STA: Staphylococcus aureus; STR: Streptococcus pneumoniae; ENT: Enterococcus faecalis. *P < 0·05 when compared with STA; †P < 0·05 when compared with STR.
AF neutrophilic response is not affected by bactDNA translocation
No significant changes in the AF total count of polymorphonuclear cells were observed between patients with bactDNA translocation and those without bactDNA translocation (Table 1). Among the first, distribution of patients according to the CG dinucleotide content of the identified bacteria showed that AF total count of PMNs was increased in patients with bacterial DNA with a higher content of CG dinucleotides (Staph. aureus: 22·23 ± 23·54; S. pneumoniae: 33·72 ± 74·35; E. faecalis: 39·10 ± 54·42), although statistical significance was not achieved due to the high variability of the data.
Discussion
In this study we report evidence that the cytokine response in patients with translocation of bactDNA maintains a direct relationship with the bactDNA CG content, representing a species-specific response. This means that not only should the translocation phenomenon be considered in the study of patients with cirrhosis, but also the bacteria spp. origin of the detected bactDNA. Quantitative differences in levels of the activated form of NF-κB in these patients support further that the host's immune response to bactDNA is a species-specific dependent event, among others.
BactDNA is a potent modulator that constitutes a sufficient danger signal to establish a Th1-biased cytokine network of response. Immunostimulatory unmethylated CG dinucleotides present in bactDNA induce a cell-mediated innate immune response by triggering dendritic cells, macrophages and NK cells to express proinflammatory and effector cytokines [26–28]. They also increase the expression of classes I and II major histocompatibility complex (MHC) molecules and surface co-stimulatory molecules, such as CD40, CD80 and CD86 [24,26,29–31]. All these effects are canalized through the CpG–TLR-9 interaction in the host. However, the percentage of CG content in the sequence of bacterial genome is species-specific [11] and, based on this, genera of bacteria may be a source of variation in terms of regulation of the inflammatory process. This may be relevant, as we have shown previously that the amount of bactDNA present in blood in patients varies greatly with time [2], and the absolute amount of CGs present in these patients is related directly to the total bactDNA levels. Because the inflammatory status in patients with cirrhosis has been shown to induce relevant clinical consequences, obtaining detailed information regarding the intimate causes of immune response may be of clinical interest.
Different studies carried out so far in cirrhotic patients have shown a constant proportion of samples with presence of bactDNA from Gram-positive microorganisms [1,2]. Further, it has been reported that an increasingly relevant percentage of bacterial episodes challenging hospitalized patients undergoing invasive procedures involving Gram-positive microorganisms [32]. The powerful immunostimulatory capacity of LPS was a concern in this study as a possible source of contamination and, together with the increasing relevance of Gram-positive population, constituted the main reasons to evaluate the effect of bactDNA molecular composition over the host's inflammatory response in this subgroup of cirrhotic patients. The existence of high levels of endotoxin has been described in a proportion of patients with advanced cirrhosis and ascitic fluid [33]. In our series, neither LPS nor LBP were different among patients with bactDNA from different Gram-positive bacterial species and compared with bactDNA– patients (Fig. 2), suggesting that an LPS effect, if any, is not responsible for statistical differences in cytokine levels. However, translocation of other bacterial products, such as flagellin or peptidoglycan, was not assessed. Therefore, their effects cannot be ruled out.
In the present study, from a series of 26 patients with bactDNA translocation from Gram-positive microorganisms, soluble immune response showed differences according to the species origin of bactDNA, both in serum and AF (Fig. 1). We looked for molecular-based explanations for these results and, as can be observed in Fig. 1, relative abundance of CG dinucleotides can partially explain those differences.
Immunomodulation properties of unmethylated CpG sequences have been studied extensively during the last years. CG-containing synthetic oligodeoxynucleotides (CpG ODNs) have been shown to mimic bactDNA effects in the immunological system. In terms of an early innate response, they are able to interact with TLR-9 and initiate intracellular signalling through translocation of the NF-κB and the expression of co-stimulatory molecules, to induce resistance to apoptosis and to favour migration of PMNs [24,34]. Regarding the adaptive immune response, CpG ODNs are also effective to enhance the Th1 humoral and cellular responses, activating plasmacytoid dendritic cells to secrete IFN-α [35–37]. However, differences have also been described between synthetic ODNs and bactDNA preparations regarding their immunogenic capabilities. As an example, in macrophages CpG ODNs are not as potent activators as E. coli bactDNA [38]. To explain these differences, ideas related to E. coli bactDNA double-strandedness, length, extra uncharacterized sequences or epigenetic factors have been proposed. More recently, the length of DNA has been reported to be critically important for efficient uptake of DNA and enhanced immunostimulatory activity [39]. None of these studies, however, provide anything other than in vitro evidence of immunostimulatory properties of bactDNA.
Here we report that the immunogenic activity of circulating bactDNA in serum and AF in patients with cirrhosis is related to the content of CG dinucleotides in their sequences which, in turn, suggests a species-specific quantitative proinflammatory immune response in vivo. Although data presented here relate to translocation of bactDNA from Gram-positive microorganisms, this phenomenon and others described previously, such as the effect of bactDNA concentration in the serum of these patients or the synergy shown between bactDNA and LPS in in vitro systems [40], may interact to induce a species-based differential immune response in cirrhotic patients with ascites and bactDNA translocation. To our knowledge, this is the first time that the inflammatory response that has been observed in asymptomatic patients with advanced cirrhosis has been related to the bacterial species origin of the detected bactDNA.
In our series the number and proportion of AF PMNs is not different in patients with or without bactDNA translocation, confirming data reported previously in a smaller series of patients [1]. However, although a trend was noted towards a higher number of PMNs in patients showing bactDNA with higher proportion of CGs, numbers were not statistically significant, due probably to dispersion of data. Regarding factors that play a role in the attraction of PMNs to a specific location, we have already shown that AF levels of C5a, one of the PMN attracting factors, are similar in patients with or without bactDNA translocation [41].
In summary, bactDNA translocation induces a marked immune reaction in vivo in patients with advanced cirrhosis and AF which is related, among other factors, to the bactDNA CG content. This is supported further by the absence of LPS and the increased levels of activated NF-κB in patients with higher bactDNA CG content. Therefore, the host's immune response to bactDNA translocation constitutes a species-specific phenomenon. The clinically relevant consequences of these events require future specifically designed investigations.
Acknowledgments
This work was supported with grants (PI05/0005, PI05/1784 and PI06/1453) from the Plan Nacional de I +D, Instituto de Salud Carlos III, Madrid, Spain. Rocío Caño is the recipient of a grant from Fundación de Investigación del Hospital General Universitario de Alicante.
References
- 1.Such J, Frances R, Munoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135–41. doi: 10.1053/jhep.2002.33715. [DOI] [PubMed] [Google Scholar]
- 2.Frances R, Benlloch S, Zapater P, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484–91. doi: 10.1002/hep.20055. [DOI] [PubMed] [Google Scholar]
- 3.Guarner C, Gonzalez-Navajas JM, Sanchez E, et al. The detection of bacterial DNA in blood of rats with CCl(4)-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006;44:633–9. doi: 10.1002/hep.21286. [DOI] [PubMed] [Google Scholar]
- 4.Frances R, Munoz C, Zapater P, et al. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860–4. doi: 10.1136/gut.2003.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frances R, Rodriguez E, Munoz C, et al. Intracellular cytokine expression in peritoneal monocyte/macrophages obtained from patients with cirrhosis and presence of bacterial DNA. Eur J Gastroenterol Hepatol. 2005;17:45–51. doi: 10.1097/00042737-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Frances R, Gonzalez-Navajas JM, Zapater P, et al. Soluble mediators engagement by bacterial DNA in cirrhotic patients with culture-negative ascitic fluid. Hepatology. 2006;44(Suppl. 1):461A. [Google Scholar]
- 7.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669–74. doi: 10.1086/514940. [DOI] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H. Toll meets bacterial CpG-DNA. Immunity. 2001;14:499–502. doi: 10.1016/s1074-7613(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 10.Chuang TH, Lee J, Kline L, Mathison JC, Ulevitch RJ. Toll-like receptor 9 mediates CpG-DNA signaling. J Leukoc Biol. 2002;71:538–44. [PubMed] [Google Scholar]
- 11.Dalpke A, Frank J, Peter M, Heeg K. Activation of Toll-like receptor 9 by DNA from different bacterial species. Infect Immun. 2006;74:940–6. doi: 10.1128/IAI.74.2.940-946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Such J, Hillebrand DJ, Guarner C, et al. Tumor necrosis factor-alpha, interleukin-6, and nitric oxide in sterile ascitic fluid and serum from patients with cirrhosis who subsequently develop ascitic fluid infection. Dig Dis Sci. 2001;46:2360–6. doi: 10.1023/a:1012342929326. [DOI] [PubMed] [Google Scholar]
- 15.Navasa M, Follo A, Filella X, et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227–32. doi: 10.1002/hep.510270507. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Talavera JC, Merrill WW, Groszmann RJ. Tumor necrosis factor alpha: a major contributor to the hyperdynamic circulation in prehepatic portal-hypertensive rats. Gastroenterology. 1995;108:761–7. doi: 10.1016/0016-5085(95)90449-2. [DOI] [PubMed] [Google Scholar]
- 17.Frances R, Chiva M, Sanchez E, et al. Bacterial translocation is downregulated by anti-TNF-alpha monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol. 2007;46:797–803. doi: 10.1016/j.jhep.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Zapater P, Frances R, Gonzalez-Navajas JM, et al. Ascitic and serum bacterial DNA is a short-term survival indicator in patients with cirrhosis and culture-negative, non-neutrocytic ascites. Gastroenterol Hepatol. 2006;29(Suppl. 1):117. [Google Scholar]
- 19.Albillos A, Cuervas-Mons V, Millan I, et al. Ascitic fluid polymorphonuclear cell count and serum to ascites albumin gradient in the diagnosis of bacterial peritonitis. Gastroenterology. 1990;98:134–40. doi: 10.1016/0016-5085(90)91301-l. [DOI] [PubMed] [Google Scholar]
- 20.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–23. [PubMed] [Google Scholar]
- 21.Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med. 1986;146:2259–61. [PubMed] [Google Scholar]
- 22.Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351–5. doi: 10.1016/0016-5085(88)90372-1. [DOI] [PubMed] [Google Scholar]
- 23.Roth RI, Levin FC, Levin J. Optimization of detection of bacterial endotoxin in plasma with the Limulus test. J Lab Clin Med. 1990;116:153–61. [PubMed] [Google Scholar]
- 24.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 25.Hacker H. Signal transduction pathways activated by CpG-DNA. Curr Top Microbiol Immunol. 2000;247:77–92. doi: 10.1007/978-3-642-59672-8_5. [DOI] [PubMed] [Google Scholar]
- 26.Iho S, Yamamoto T, Takahashi T, Yamamoto S. Oligodeoxynucleotides containing palindrome sequences with internal 5′-CpG-3′ act directly on human NK and activated T cells to induce IFN-gamma production in vitro. J Immunol. 1999;163:3642–52. [PubMed] [Google Scholar]
- 27.Lipford GB, Sparwasser T, Bauer M, et al. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;27:3420–6. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 28.Sparwasser T, Miethke T, Lipford G, et al. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur J Immunol. 1997;27:1671–9. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 29.Cowdery JS, Chace JH, Yi AK, Krieg AM. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–5. [PubMed] [Google Scholar]
- 30.Martin-Orozco E, Kobayashi H, Van Uden J, Nguyen MD, Kornbluth RS, Raz E. Enhancement of antigen-presenting cell surface molecules involved in cognate interactions by immunostimulatory DNA sequences. Int Immunol. 1999;11:1111–18. doi: 10.1093/intimm/11.7.1111. [DOI] [PubMed] [Google Scholar]
- 31.Sparwasser T, Koch ES, Vabulas RM, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–54. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–8. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 33.Guarner C, Soriano G, Tomas A, et al. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139–43. [PubMed] [Google Scholar]
- 34.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–5. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 35.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 37.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–4. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 38.Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J Immunol. 2000;165:4165–73. doi: 10.4049/jimmunol.165.8.4165. [DOI] [PubMed] [Google Scholar]
- 39.Roberts TL, Dunn JA, Terry TD, et al. Differences in macrophage activation by bacterial DNA and CpG-containing oligonucleotides. J Immunol. 2005;175:3569–76. doi: 10.4049/jimmunol.175.6.3569. [DOI] [PubMed] [Google Scholar]
- 40.Gao JJ, Zuvanich EG, Xue Q, Horn DL, Silverstein R, Morrison DC. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264·7 macrophages. J Immunol. 1999;163:4095–9. [PubMed] [Google Scholar]
- 41.Frances R, Gonzalez-Navajas JM, Zapater P, et al. Bacterial DNA induces the complement system activation in serum and ascitic fluid from patients with advanced cirrhosis. J Clin Immunol. 2007;27:438–44. doi: 10.1007/s10875-007-9090-2. [DOI] [PubMed] [Google Scholar]


