Abstract
Current models of adult haematopoiesis propose that haematopoietic stem cells (HSCs) differentiate into common lymphoid (CLP) and common myeloid (CMP) progenitors and establish an early separation between myeloid and lymphoid lineages. Nevertheless, the developmental potential of CMP-associated B cells suggests the existence of alternate pathways for B lymphopoesis. The aim of this study was to compare the developmental and functional properties of CMP- and CLP-derived B cells. While both populations matured through pro-B cell and transitional B cell intermediates in the bone marrow and spleen, respectively, following transfer into irradiated mice, mature CMP- and CLP-derived B cells exhibit distinct functional responses. Specifically, CMP-derived B cells did not respond to mitogenic stimulation to the same degree as their CLP-derived counterparts and secrete lower levels of IgM and the inflammatory cytokines such as interleukin (IL)-6 and IL-10. Together, these data suggest the existence of multiple pathways for generating functionally distinct B cells from bone marrow precursors.
Keywords: B cells, common lymphoid progenitor, common myeloid progenitor, cytokine
Introduction
The majority of B lymphocytes present in peripheral lymphoid tissues are produced from haematopoietic stem cells (HSCs) in the bone marrow. HSCs proceed through a series of intermediate steps during which precursors become progressively more restricted in their developmental potential [1]. Current models of adult haematopoiesis propose that HSCs differentiate into common lymphoid progenitors (CLPs) from which pro-B, pre-B and, ultimately, mature B lymphocytes develop. These newly produced B cells then migrate to the spleen wherein they undergo terminal differentiation into dominant follicular (FO) and minor marginal zone (MZ) populations. FO B cells are primarily mediators of adaptive immunity while MZ B cells, along with a minor population of B-1 B cells, participate in innate immune responses [2]. In contrast to the dominant bone marrow pathway of B cell development, B-1 B cells are the progeny of progenitors that are present at highest levels during embryogenesis. Postnatal bone marrow retains the potential to produce B-1 B cells, but with less efficiency than fetal tissues [3].
Current models of haematopoiesis propose an early dichotomy between the lymphoid and myeloid lineages. In this regard, HSC are thought to generate early lymphocyte progenitors (ELP) that possess potent B and T cell, but limited myeloid, potential, and their B cell lineage specified progeny include the CLP [4,5]. Common myeloid progenitors (CMP) are generally considered to be myeloid specified, and their immediate progeny include granulocyte–macrophage (GMP) and megakaryocyte–erythroid (MEP) progenitors [6]. While the production of most myeloid and lymphoid cells may follow this developmental progression, the separation of lymphoid and myeloid developmental pathways may not be as absolute as the model suggests. For example, bi-potential B macrophage progenitors are found in adult bone marrow of mice [7,8], indicating that a developmental relationship between the B cell and macrophage lineages is retained during postnatal haematopoiesis. In addition, residual B cell precursor activity associated with the CMP has been described [6,9]. Numerous questions exist regarding the B cells generated from CMPs. In particular, whether the characteristics and functional potential of CMP-derived B cells are distinct from CLP-derived B lymphocytes has not been addressed. Addressing this issue is relevant to understanding the potential heterogeneity of B cell developmental programmes.
In order to compare the developmental characteristics and functions between B cells generated from different progenitors, we performed adoptive transfer studies utilizing highly enriched populations of CLPs and CMPs from the bone marrow of congenic mice and examined the kinetics by which they led to mature B lymphocyte development and the functional potential of these cells. Our results indicate that, in contrast to CLP, CMP-derived B cells exhibited diminished responsiveness to LPS and CpG stimulation and produced lower levels of not only IgM but also proinflammatory cytokines such as interleukin (IL)-6 and IL-10. Taken together, these data suggest that bone marrow progenitors produce functionally distinct populations of B cells.
Materials and methods
Animals
C57BL/6 (B6 Ly5·2) and congenic C57BL/6-Ly5·1-Pep3b (B6 Ly5·1) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). B6 Ly5·1 were bred in the University of California at Davis animal facility. All mice were maintained under specific pathogen-free conditions and used at 8–12 weeks of age for these studies.
Antibodies
Fluorescein isothiocyanate (FITC)-conjugated Sca-1 (E13-161·7), phycoerythrin (PE)-conjugated CD19 (1D3), CD43 (Ly-48, S7), CD127 (IL-7Rα, A7R34), allophycocyanin (APC)-CD19 (1D3), purified CD4 (GK1·5) and CD11c (HL3) antibodies were purchased from BD PharMingen (San Diego, CA, USA). FITC-conjugated Ly5·2 (CD45·2, 104), PE-conjugated IgM (II/41), CD23 (B3B4), biotin-conjugated natural killer (NK)1·1 (PK136), CD21/35 (CR1/CR1, eBio8D9), IgD (11–26), Ly5·1 (CD45·1, A20), PE-Cy5·5-conjugated Ly5·1 (CD45·1, A20), Ly5·2 (CD45·2, 104), APC-conjugated c-kit (CD117, 2B8), CD11b (Mac-1, M1/70); purified CD3 (17A2), CD8α (53–6·7), CD19 (MB19-1), B220 (CD45R, RA3–6B2), CD11b (Mac-1, M1/70), Gr-1 (Ly6-G, RB6–8C5), TER119 (TER119) and CD16/32 (FcγIII/IIR, 93) antibodies were purchased from eBioscience (San Diego, CA, USA). APC-conjugated B220 (CD45R, RA3–6B2), biotin-conjugated T cell receptor (TCR)α/β (H57-597), PE-Cy5·5 and tri-colour-conjugated streptavidin were purchased from Caltag Laboratories (Burlingame, CA, USA). All isotype controls were obtained from BD PharMingen.
Transplantation of progenitor cells
CLPs and CMPs were purified by procedures described elsewhere [5,6] with some modifications. Briefly, whole bone marrow cells (BMCs) were collected from the femora and tibiae of B6 Ly5·2 mice. The low density (LD) cells (ρ = 1·077) were washed and incubated with a predetermined optimum dilution of rat monoclonal antibodies (mAbs) against lineage-specific markers CD3, CD4, CD8, CD11c, CD19, B220, CD11b, Gr-1 and TER119, followed by sheep anti-rat IgG-conjugated magnetic-beads (Dynabeads®, Dynalbiotech, Lake Success, NY, USA) to deplete the antibody-bound cells. The lineage-negative (Lin–) cells were stained with FITC-anti-CD34, PE-anti-CD16/32, PE-Cy5-anti-IL-7Rα, APC-anti-c-kit and biotin-anti-Sca-1 antibodies, followed by APC-Cy7-streptavidin, and subjected to flow cytometric assisted purification. The CLPs were cell sorter purified as IL-7Rα+Sca-1lowc-kitlow expressing cells; CMPs were cell sorter purified as IL-7Rα–Sca-1–c-kithighCD34+CD16/32lo expressing cells and HSCs were cell sorter purified as IL-7Rα–Sca-1+c-kithigh expressing cells utilizing a 10-parameter MoFlo cell sorter (Cytomation, Fort Collins, CO, USA). The purity of sorted cells was greater than 97% (data not shown).
For transplantation of progenitor cells, female B6 Ly5·1 recipient mice were irradiated with 6 Gy 7–8 h before transfer. The sorted CLPs (3 × 104) and CMPs (3 × 104) from B6 Ly5·2 mice were injected intravenously (i.v.) into the recipient mice. Animals were maintained in a pathogen-free environment and aqueous antibiotics were added to the drinking water after transfer.
At several intervals after transplantation, recipient mice were killed. Cells from spleen, BM and other organs were collected. For detection of donor-derived B cell populations, the LD cells were stained with FITC-anti-Ly5·2, PE-anti-CD19, APC-anti-CD11b and PE-Cy5·5-anti-Ly5·1 antibodies. The stained populations of cells were analysed utilizing a dual-laser fluorescence activated cell sorter (FACScalibur) using CellQuest software (BD Biosciences).
Isolation and culture of splenic B cells
Three weeks after transfer with CLPs or CMPs, spleen cells were collected from recipient mice. The LD cells were washed and incubated with a predetermined optimum dilution of rat mAbs against lineage-specific markers CD3, CD4, CD8, CD11c, CD11b, Gr-1 and TER119, followed by sheep anti-rat IgG-conjugated magnetic-beads (Dynabeads) to deplete the antibody-bound cells. The lineage-negative cells were stained with FITC-anti-Ly5·2, PE-anti-CD19, APC-anti-B220 and PE-Cy5·5-anti-Ly5·1. After washing, progenitor-derived Ly5·2+Ly5·1–CD19+B220+ cells were sorted by a 10-parameter MoFlo cell sorter (Cytomation). The purity of sorted cells was determined by reanalysing an aliquot of the collected cells and was greater than 98%.
All cell cultures were performed in RPMI-1640 culture medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS), 100 µg/ml streptomycin and 100 units/ml penicillin (Invitrogen). Aliquots of 1 × 105 B cells purified as described above were cultured in 200 µl of RPMI-1640 medium in a round-bottomed 96-well plate with or without lipopolysaccharide (LPS; 10 µg/ml, purchased from Sigma-Aldrich, St. Louis, MO, USA) or CpG-1826 (1 µM, purchased from Invitrogen, San Diego, CA, USA). After 3 days in culture, supernatants were collected and analysed by enzyme-linked immunosorbent assay (ELISA) kit for levels of IgM (purchased from ZeptoMetrix, Buffalo, NY, USA), and levels of proinflammatory cytokines utilizing the CBA kits (BD Biosciences) as we have described previously [10].
Statistical analyses
Differences in the amount of cytokine synthesized by the B cells generated from different progenitors were analysed using the unpaired Student's t-test (Statview); P-values < 0·05 were considered to be statistically significant.
Results
Bone marrow-derived CMPs generate CD19+ cells in vivo
A previous report from our laboratory demonstrated that following direct intrasplenic transfer of Lin–IL-7Rα–Sca-1–c-kit+ enriched cells, a small number of CD19+B220+ cells were generated 10 days thereafter [11]. To determine whether this putative B cell progenitor activity was associated with a myeloid progenitor, we tested the potential of purified Lin–IL-7Rα–Sca-1–c-kit+CD34+CD16/32lo CMPs to produce B cells in vivo. As a positive control, Lin–IL-7Rα+Sca-1loc-kitlo CLPs and Lin–IL-7Rα–Sca-1+c-kithigh HSCs were injected intravenously and analysed in parallel. All donor cells were from B6 Ly5·2 mice and were transplanted intravenously into six Gy-irradiated congenic B6 Ly5·1 mice. Mice were killed at various times post-reconstitution and B cell development was assessed.
As shown in Fig. 1a, at 1 week after transfer almost 90% of the spleen cells generated from CMPs were CD11b+ myeloid cells. However, 1 week later CMP-derived cells that expressed CD19+ were observed. The production of CD19+ cells from the transfer of 3 × 104 GMP and MEP was never detected (data not shown), indicating that myeloid progenitors derived from CMP have lost the potential to generate B lineage cells. As expected, the progeny of both CLP and HSC included CD19+ cells. CLP-derived CD19+ cells were abundant as early as 1 week post-transplantation, and myeloid progeny developing from CLP were rare.
Fig. 1.
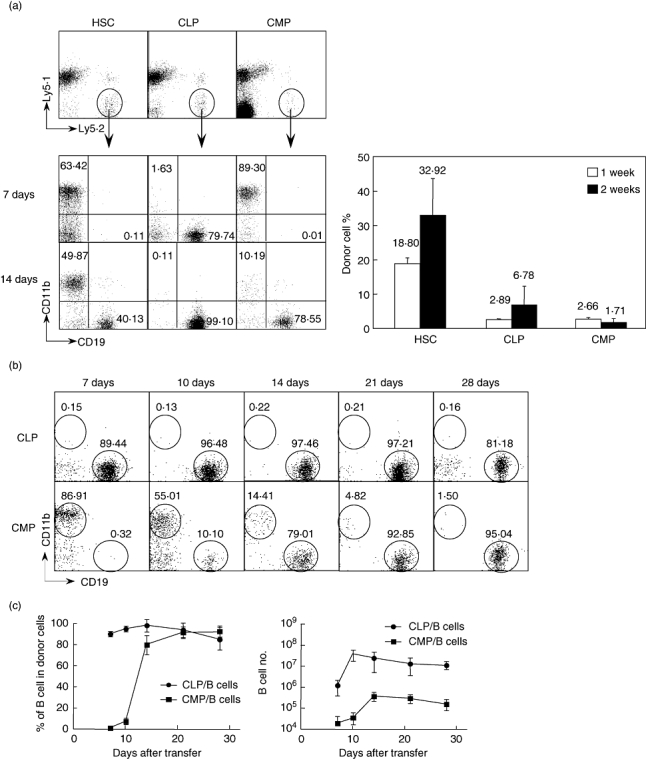
Comparison of the potential of B cell production from different progenitors. Freshly sorted haematopoietic stem cells (HSCs), common lymphoid progenitors (CLPs) and common myeloid progenitors (CMP) from B6 Ly5·2 mice were injected intravenously into B6 Ly5·1 congenic mice. At various times post-transplantation, recipient spleen cells were collected and stained with fluorescein isothiocyanate (FITC)-Ly5·2, PE-CD19, phycoerythrin (PE)-Cy5·5-Ly5·1 and allophycocyanin (APC)-CD11b. (a) Donor-type Ly5·2+Ly5·1– cells were analysed for CD19 and CD11b expression. The numbers in the figure indicate the percentage of each population in the donor-type cells (left figure). The right panel shows the percentage of donor-derived cells (Ly5·2+Ly5·1–) among total splenic low density (LD) cells. Each group included four mice. (b) CD19+ lymphoid and CD11b+ myeloid progeny from CLP and CMP at the indicated time-points. The numbers in the figure indicate the percentage of each population in the donor-type cells. (c) Percentages and cell numbers of B cells generated from CLPs and CMPs at various time-points after transfer. Each time point includes six to eight mice.
Figure 1b presents a more detailed analysis of the production of CD11b+ and CD19+ cells from CMP. As in Fig. 1a, CMPs rapidly generated myeloid cells that dominated at week 1 post-reconstitution. However, CMP-derived CD19+ cells were detected readily by 10 days post-reconstitution, and their frequency increased thereafter so that by 1 month post-transplantation nearly all the CMP-derived cells in the spleen were CD19+.
This pattern of CMP differentiation contrasted in two ways from the cells that differentiated from CLPs. First, CLP-derived B cells were detected readily by 7 days post-reconstitution. More significantly, the total number of B cells generated by CLPs was 100-fold greater than those derived from a comparable number of CMPs (Fig. 1c). Thus, CMP produce B cells far less efficiently than do CLP. In addition, while both CD4+ and CD8+ T cells were generated in the spleen 3 weeks after the transfer of CLPs and HSCs, T cells were undetectable in the CMP group (data not shown). This confirmed that the B cell generation from CMPs is not contaminated from CLPs or HSCs in our cell transfer experiments.
CMP-derived progeny populate the bone marrow
To determine if bone marrow supports the maturation of CMP and/or their progeny into B lymphocytes, we analysed that tissue for the presence of B cells and their precursors at various times after reconstitution. As shown in Fig. 2a and b, CMP-derived CD19+CD43+ pro-B cells were detected in the marrow by day 7 post-reconstitution. Surface IgM+ cells were not observed at this time-point but were present on day 14 post-reconstitution. In contrast, while the number of CLP-derived CD19+CD43+ pro-B cells was significantly lower at day 7 compared to CMPs, a higher percentage of pro-B cells generated by CLPs appeared as early as 3 days after transfer (Fig. 2b). Therefore, while the production of CD43+ pro-B cells and IgM+ mature B cells from CLPs and CMPs follow similar kinetics, the initiation and cessation of pro-B cells from CLPs occurs 4 days sooner than that of CMPs.
Fig. 2.
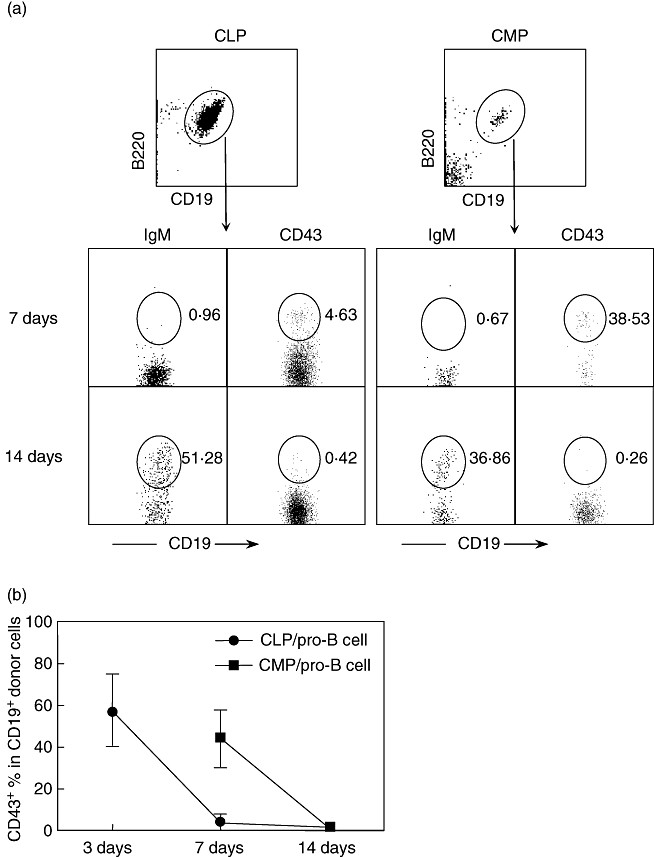
Common myeloid progenitor (CMP) progeny are present in bone marrow. At serial time-points after transfer of common lymphoid progenitors (CLPs) and CMPs, bone marrow (BM) cells from recipient mice were collected and analysed by flow cytometry. Each time-point includes four to six mice. (a) A representative pattern of IgM and CD43 expression on gated donor-type (Ly5·2+Ly5·1–) B220+CD19+ B cells after transfer. The numbers in figure indicate the percentage of each population. (b) Percentage of CD43+ pro-B cells generated from progenitors. The data presented in figure are the mean ± standard deviation.
The IL-7 receptor (CD127) is expressed on developing B lineage cells, and the generation of conventional B cells in the bone marrow of mice has been shown to be dependent upon IL-7 stimulation. However, alternative, IL-7-independent B cell developmental pathways have also been described [12]. To determine whether CD127 is expressed on CMP-derived B lineage cells, we collected BM and spleen cells from recipients reconstituted with CLPs or CMPs. As shown in Fig. 3, CMP-derived cells were CD127+, and the pattern of expression was similar to that of CLP-derived progeny.
Fig. 3.
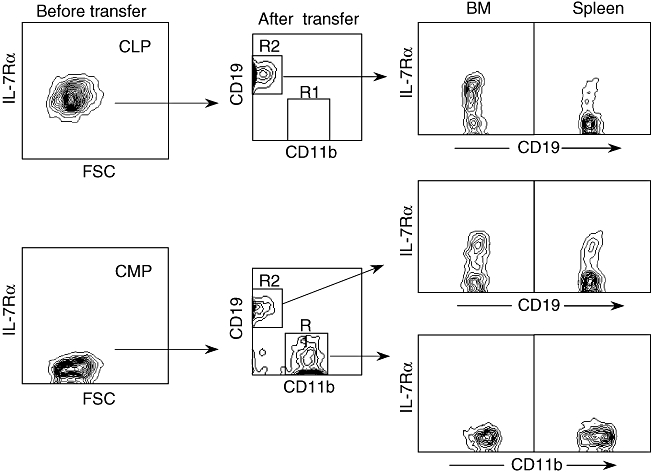
Expression of interleukin (IL)-7Rα on common myeloid progenitor (CMP)-derived B lineage cells. At 10 days after transfer of common lymphoid progenitors (CLPs) and CMPs, bone marrow (BM) and spleen cells from recipient mice were collected and analysed expression of IL-7Rα on donor-derived (Ly5·2+Ly5·1–) CD19+ B lymphoid cells and CD11b+ myeloid cells by flow cytometry. The left panel demonstrates expression of IL-7Rα on freshly sorted CLP and CMP. Each group includes three mice; representative data are shown.
CMP-derived CD19+ B cells are present in lymph node but no B-1 B cells are present in the peritoneal cavity
The above data demonstrate that CMP-derived B lineage cells are present in both the bone marrow and spleen. As shown in Fig. 4a, a very low number of CMP-derived CD19+ cells could also be detected in the liver and the peritoneal cavity and to a lesser extent in lymph nodes. Because the bone marrow retains the potential to repopulate B-1 B cells [13], we also examined the peritoneal cavity to determine whether CMP could generate cells along that lineage. As shown in Fig. 4b, no donor-derived CD19+ cells that expressed CD11b or CD5 were generated from either CMP or CLP.
Fig. 4.
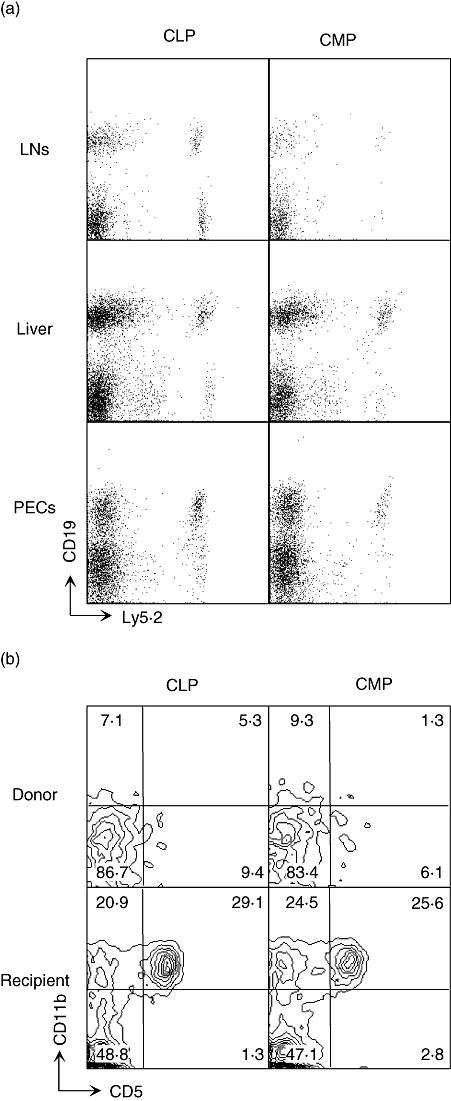
Common myeloid progenitor (CMP)-derived B lineage cells are present in multiple lymphoid tissues. Three weeks after transplantation of common lymphoid progenitors (CLPs) or CMPs, donor-derived CD19+ B lymphoid cells in various organs were analysed by flow cytometry. (a) CD19 expression on donor-type Ly5·2+Ly5·1– cells in lymph nodes, liver and peritoneal cavity cells (PECs) of recipient mice. (b) Expression of CD11b and CD5 on CD19+ cells in Ly5·2+Ly5·1– donor type lymphocytes was compared to recipient type cells (Ly5·2–Ly5·1+). The lymphocytes were collected from the peritoneal cavity of recipient mice. Numbers in each panel represent the percentages of cells in the indicated region.
CMP-derived B cells mature through transitional stages
Following their production in the bone marrow, newly produced B cells migrate to the spleen where they undergo further maturation through various transitional stages. To determine if CMP-derived B cells mature through similar intermediates, we measured the expression of surface markers that define transitional stages of splenic B lymphopoesis. As shown in Fig. 5a, CMP-derived IgM+IgDlo transitional type 1 (T1) and IgM+IgD+ T2 B cells were detected as early as 10 days after transfer. Transitional stages of splenic B cell maturation can be distinguished further by the differential expression of CD21 and CD23. Follicular and marginal zone B cells are CD21intCD23int and CD21hiCD23lo, respectively [14,15]. As shown in Fig. 5b, both FO and MZ B cells were produced from CMPs.
Fig. 5.
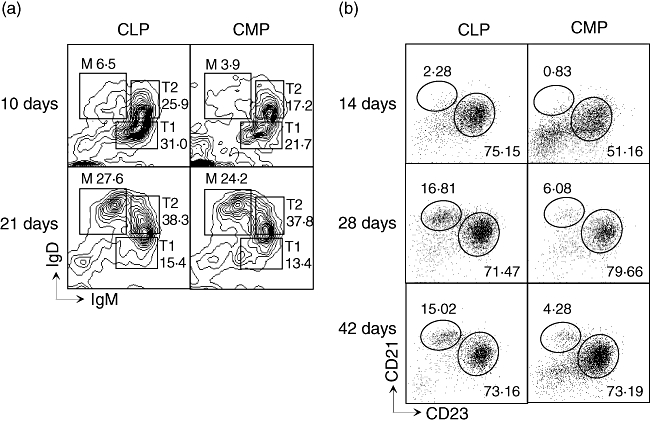
Distribution and subsets of common myeloid progenitor (CMP)- and common lymphoid progenitor (CLP)-derived B cells. (a) IgM/IgD expression on gated donor (Ly5·2+ Ly5·1–) CD19+ B cells in spleen 10 days and 21 days after transfer of progenitors. Transitional type 1 (T1), (T2 and mature B cells (M) are gated. (b) CD21/CD23 expression on donor-derived (Ly5·2+ Ly5·1–) splenic CD19+ B cells at indicted time-point after transfer. Follicular (FO) and marginal zone (MZ) B cells are gated as CD21intCD23int and CD21hiCD23lo, respectively. The numbers in figures indicate the percentage of each population.
CMP- and CLP-derived B cells are functionally distinct
Both bacterial LPS and CpG motif-containing oligonucleotides (CpG ODN) are potent B cell mitogens [16–18]. Activation of B cells by LPS triggers immunoglobulin secretion [18,19]. In efforts to determine if the functional properties of the B cells derived from CMPs behave similarly to those derived from CLPs, we measured the ability of B cells generated from different progenitors to secrete antibodies and cytokines in vitro after stimulation with LPS and CpG1826. As shown in Fig. 6a, following LPS or CpG stimulation, CLP-derived B cells produced relatively higher levels of IgM than CMP-derived B cells. These cells also produced higher relative amounts of IL-6 and IL-10, indicating that CMP-derived B cells may be less able to respond to in vitro stimulation than B cells generated from CLPs.
Fig. 6.
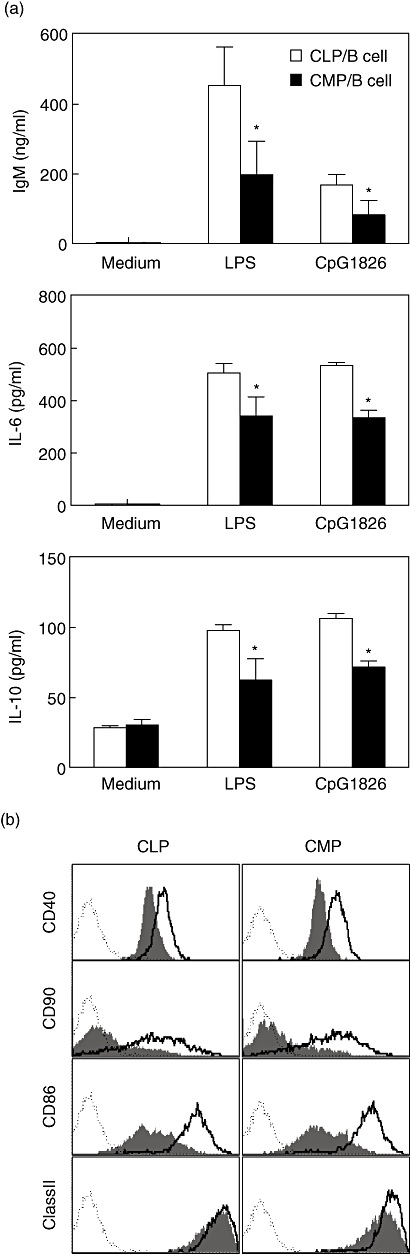
Common myeloid progenitor (CMP)- and common lymphoid progenitor (CLP)-derived B cells are functionally distinct. (a) CLP/CMP-derived CD19+B220+ B cells were sorted from the recipients as described in Materials and methods, and aliquots of 1 × 105 cells cultured with or without lipopolysaccharide (LPS) or CpG-1826 at 37°C for 3 days. The concentrations of IgM and cytokines were measured by enzyme-linked immunosorbent assay (ELISA) or CBA kit. Data represent the average values of triplicate samples ± standard deviation and were consistent in two repetitions. (*P < 0·05, CMP-derived B cells versus CLP-derived B cells). (b) Expression of CD40, CD80, CD86 and ClassII on original different B cells. CLP- or CMP-derived B cells were sorted and cultured with or without LPS at 37°C overnight. The expressions of CD40, CD80, CD86 and ClassII were analysed by flow cytometry before (grey-filled histogram) and after (unfilled histogram) culture. The dashed histogram represents isotype.
We also examined the expression of various co-stimulatory molecules before and after LPS stimulation. As shown in Fig. 6b, both CMP- and CLP-derived B cells express CD40, CD80, CD86 and major histocompatibility complex (MHC) class II cell surface antigens. Following LPS stimulation, the expression of CD40, CD80 and CD86 was up-regulated on both CMP- and CLP-derived B cells, suggesting that differences in the ability of CMP- compared with CLP-derived B cells to synthesize IgM and the cytokines were not secondary to differential expression of the common co-stimulatory molecules.
Discussion
According to the present models of haematopoiesis, haematopoietic stem cells in the BM differentiate into CLPs. CLP progeny include pro-B and pre-B cells which, following functional rearrangement and expression of immunoglobulin genes, mature into B cells expressing surface IgM [20–22]. While the majority of B cells are, arguably, produced through a CLP intermediate stage of development, alternative pathways for the production of B cells are emerging [7,8,13]. For example, the bone marrow contains bi-potential B cell/macrophage progenitors, and the data in this report provide evidence that CMPs indeed retain B cell potential. The aim of the present study was to compare the developmental and functional properties of B cells derived from CMP and CLP populations.
Our data indicate that the developmental characteristics of CMP- and CLP-derived B cells are similar. For example, the bone marrow provides a supportive microenvironment for the generation of B cells from both progenitors, and development occurs through a phenotypically similar pro-B cell stage. The cytokine response of CMP- and CLP-derived B cell progenitors may also be similar, as they both express the IL-7 receptor. Following maturation in the bone marrow, newly produced B cells are functionally immature and migrate to the spleen wherein they respond to antigenic and microenvironmental signals that potentiate their development into follicular or marginal zone subpopulations. Similar to what has been described previously, CMP-derived B lineage cells progress through well-characterized T1 and T2 stages of development and their progeny can enter both the MZ and FO compartments [23–25].
At least some B-1 B cells are generated from progenitors that are produced preferentially during embryogenesis. However, adult bone marrow retains the potential to generate B-1b and, to a lesser extent, B-1a B cells [3]. This activity has been attributed recently to a rare population of lineage-negative, CD45Rlow/neg CD19+ cells. In order to determine if CMP might also be a source of B-1 B cells, we examined the peritoneal cavity of recipient mice for such cells. The data indicated clearly that no detectable donor-derived B-1 B cells were generated from CMP (Fig. 4). Thus, the B lymphocytes produced from CMP are conventional, B-2 B cells.
Taken together, our data indicate both CMP and CLP can generate B cells. However, their properties are not identical. For example, the data clearly indicate that the efficiency by which CMPs produced B cells is significantly lower (Fig. 1) than that of CLPs on a cell-for-cell basis. This is perhaps not surprising, given that CLP are lymphoid-specified progenitors while the primary fate of CMP is to generate myeloid cells. It is unclear what proportion of B cells in the organism are CMP-derived. That CMPs can be demonstrated to produce B cells following injection into irradiated mice may simply reflect selective pressures in an artificial environment, and not a normal event under homeostatic conditions. Nevertheless, the fact that CMPs are present at a 10-fold greater frequency in BM than CLPs [5,6] raises the possibility that a significant population of CMP-derived B cells could contribute to the peripheral B cell pool. However, in our transfer model, the differences between B cells derived from lymphoid and myeloid progenitors may be based on the fact that B cell development from CLP or CMP transplanted animals were exposed to different milieus in vivo; this is reflected by in vitro analysis of IgM and cytokine production. Thus, to exclude environmental influences, it will be interesting to reconstitute Ly5·1+/Ly5·2+ recipient mice with the same number of Ly5·1+ CMPs and Ly5·2+ CLPs.
The most striking difference between CMP- and CLP-derived B cells is functional. Both B cell populations can respond to LPS and CpG in vitro to secrete IgM and cytokines such as IL-6 and IL-10 (Fig. 6). However, the magnitude of the response to these mitogens and the concentration of cytokines produced by CMP-derived B cells was significantly lower, suggesting that CMP-derived B cells are functional B cells with distinct developmental characteristics. These differences were not secondary to differences in kinetics in the synthesis of IgM and the cytokines (data not shown). The molecular basis for these differences remains to be elucidated. However, the fact that the CMP-derived B cells are the progeny of a precursor that is clearly of the myeloid lineage that is basically programmed for distinct functional activity could contribute to the differences observed. It is also important to consider the fact that the differences in the in vitro potential of the CMP compared with the CLP-derived B cells could be limited by the environment in which they are being analysed. Thus, this lineage may function differently if they are provided with cytokines/growth factors that are potentially uniquely required by this cell lineage. Further studies are required to address this issue.
The CMP-derived B cells in our transfer model were not generated from CLPs or HSCs that contaminated our CMP fraction for a number of reasons. First, we detected a significant CD3+ T cell population in the spleen of recipients 3 weeks after transfer with CLPs and HSCs but not with CMPs (data not shown). Secondly, while our CMP fraction generated CD11b+ myeloid cells only transiently, the HSC gave rise to a stable population of CD11b+ myeloid cells (Fig. 1). Finally, CLP-derived B cells produced more IgM and the proinflammatory cytokines in response to stimulation with LPS or CpG, indicating that functional differences exist within the CLP- and CMP-derived B cells in vitro (Fig. 6). Confirmatory studies could be performed using allotype congenic mice wherein the transferred CLP- and CMP-derived donor progenitors could originate from allotype congenic mice and the IgM synthesized by the B cells from these two progenitors could be analysed for allotype-specific IgM. There are multiple implications for these data and we suggest that further study of precursor populations in both thymus and bone marrow will be critical for understanding not only differentiation, but also implications for human autoimmune disease [26–28].
Acknowledgments
We thank Carol Oxford for FACS sorting, Loreli Coleman for mouse irradiation and Nikki Phipps for manuscript preparation. Financial support was provided by National Institutes of Health Grant DK39588.
References
- 1.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a ‘natural immune memory’. Immunol Rev. 2000;175:70–9. [PubMed] [Google Scholar]
- 3.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–9. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–30. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 5.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 6.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–7. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 7.Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;2:83–8. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- 8.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19- hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019–30. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang YH, Lian ZX, Tsuneyama K, et al. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–40. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Yang GX, Lian ZX, Kikuchi K, et al. Plasmacytoid dendritic cells of different origins have distinct characteristics and function: studies of lymphoid progenitors versus myeloid progenitors. J Immunol. 2005;175:7281–7. doi: 10.4049/jimmunol.175.11.7281. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(–/–) mice. J Exp Med. 2001;194:1141–50. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 14.Loder F, Mutschler B, Ray RJ, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–40. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 16.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho A, Forni L, Melchers F, Watanabe T. Genetic defect in responsiveness to the B cell mitogen lipopolysaccharide. Eur J Immunol. 1977;7:325–8. doi: 10.1002/eji.1830070517. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 19.Yuan D, Vitetta ES. Structural studies of cell surface and secreted IgG in LPS-stimulated murine B cells. Mol Immunol. 1983;20:367–75. doi: 10.1016/0161-5890(83)90018-4. [DOI] [PubMed] [Google Scholar]
- 20.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–35. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 21.Tudor KS, Payne KJ, Yamashita Y, Kincade PW. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity. 2000;12:335–45. doi: 10.1016/s1074-7613(00)80186-7. [DOI] [PubMed] [Google Scholar]
- 22.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–25. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy RR, Li YS, Allman D, Asano M, Gui M, Hayakawa K. B-cell commitment, development and selection. Immunol Rev. 2000;175:23–32. [PubMed] [Google Scholar]
- 24.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Inaba M, Naiki M, Lian ZX, Gershwin ME, Ikehara S. Comparative immunobiology of thymic DC mRNA in autoimmune-prone mice. J Autoimmun. 2007;28:41–5. doi: 10.1016/j.jaut.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Krebs P, Kurrer MO, Kremer M, et al. Molecular mapping of autoimmune B cell responses in experimental myocarditis. J Autoimmun. 2007;28:224–33. doi: 10.1016/j.jaut.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Blank M, Shoenfeld Y. B cell targeted therapy in autoimmunity. J Autoimmun. 2007;28:62–8. doi: 10.1016/j.jaut.2007.02.001. [DOI] [PubMed] [Google Scholar]


