Abstract
In vitro studies have contributed substantially to the understanding of immunopathology of respiratory syncytial virus (RSV)-mediated disease. In the present study we compared the effect of RSV-infected dendritic cells on the time–course of the primary and memory/effector T cell response in vitro. Cultures with uninfected dendritic cells known to elicit T helper 2 (Th2) responses and with polyinosinic-polycytidylic acid (poly-IC)-stimulated dendritic cells known to elicit Th1 responses served as controls. At day 1 after stimulation there was a high proportion of interleukin (IL)-2 and tumour necrosis factor (TNF)-α-producing T cells with no difference in number of producing T cells as well as concentration of secreted cytokines between RSV-infected and control cultures. However, up to day 3 generation of IFN-γ was reduced markedly. In addition, there was a reduced proliferation in RSV cultures. At day 7 the RSV-treated cultures showed a preponderance of IL-4 generation. At days 21–24, after three rounds of restimulation, memory/effector T cells matured under the influence of RSV were still not fully polarized but in contrast to the primary response displayed a predominance of Th1 cytokines. Contact with RSV-infected HEp-2 cells inhibited proliferation of T cells; memory effector T cells were less sensitive to contact inhibition than naive T cells. In addition, RSV inhibited the stimulated rearrangement of cortical actin more effectively in naive compared to memory T cells. In summary, we have shown that RSV infection of dendritic cells has a distinct modulatory effect on the primary response and a less pronounced effect on the memory response.
Keywords: cytokines, dendritic cells, proliferation, respiratory syncytial virus, T cells
Introduction
Respiratory syncytial virus (RSV) causes a significant burden of acute respiratory disease during infancy and childhood, occurring characteristically in recurrent, discrete epidemics [1]. Primary infection with RSV occurs early in life, usually before the age of 3 years [2], ranging in severity from subclinical infections to bronchiolitis and death [1]. Children with cellular immunodeficiency disease are at risk for complicated or fatal infections from RSV [3]. Therefore, T cells are the key effector cells eliminating RSV during primary infection. However, T cells not only clear virus but also cause lung pathology, as has been shown in animal models [4]. The local immune response to RSV, especially during bronchiolitis in early infancy, is characterized by low interferon (IFN)-γ generation [5]. Because intranasal gene transfer of IFN-γ protects against RSV infection [6], it is conceivable that the imbalance of cytokines may play a central role in the pathogenesis of RSV-mediated disease.
In both RSV-infected humans [7] and mice [8] dendritic cells infiltrate the bronchial epithelium and lungs. The interaction of dendritic cells and naive T cells are now recognized to play a central role in inducing immune responses. Antigen is engulfed and processed by immature dendritic cells [9]. Upon contact with antigen, dendritic cells mature and migrate from peripheral tissues into draining lymph nodes acquiring the capacity to trigger naive T cells and drive polarized T helper cell responses. Recently we [10,11] and others [12,13] were able to show that RSV interacts with myeloid dendritic cells [10] and modulates cytokine production [11,13] as well as proliferation [13] of co-cultivated naive T cells. Moreover, the individual outcome of IFN-γ generation in RSV-infected autologous co-cultures seems to be related to severity of RSV-mediated disease of the donor [14].
In contrast to IFN-γ, local production of interleukin (IL)-4 seems to be increased during severe bronchiolitis [15]; at least in the first year after severe RSV bronchiolitis there seems to be an increased risk of allergic sensitization [14,16], and even after several years an increased generation of IL-4 to RSV was found [17]. These findings support the notion that RSV might induce T helper 2 (Th2) cells. However, detailed data on development of the cytokine response to RSV-infected dendritic cells are scarce.
Not only the primary reaction but also the memory response seems to be influenced by RSV infection. Reinfections with RSV occur throughout life, but there is a reduction in severity of clinical illness upon reinfection [18]. Hall et al. report that experimental reinfection with RSV of the same strain group could occur within a few months of initial exposure [19]. In a community-based study reinfections with RSV were identified in 3·3% of the infants during a 1-year follow-up [20]. Genetic typing revealed that reinfection occurred with genetically identical viruses in nearly half of these children [21]. Thus, RSV, like other pathogenic viruses [22,23], has most probably developed mechanisms to subvert anti-viral strategies of the host. Whereas at least some, but not complete, protection is conferred by high RSV-specific serum antibody titres [24,25], the role of cell-mediated immunity in protection against RSV remains unclear [24,26]. The aim of the present study was to characterize the influence of RSV-infected dendritic cells on primary and memory/effector T cell responses in vitro.
Materials and methods
Generation of dendritic cells from cord blood
Cord blood was obtained from newborns at the Department of Obstetrics and Gynaecology (Augusta Krankenanstalt Bochum). Parents gave informed written consent before the blood was studied. The study was approved by the ethics committee of the medical faculty. Blood was drawn by puncture of the umbilical vein of the placenta after maternal blood had been wiped off. The blood for further cellular analysis was placed in a 50 ml sterile Falcon tube (Falcon, Heidelberg, Germany) containing 10 ml of Hank's balanced salt solution (HBSS) with 100 IU penicillin, 100 μg/ml streptomycin, 2·5 μg/ml amphotericin B and 7 ml citrate–phosphate–dextrose solution (Sigma-Aldrich, Taufkirchen, Germany). Only samples no older than 12 h were processed. Mononuclear cells were isolated by Biocoll (1·077 g/ml; Biochrom, Berlin, Germany) density centrifugation. Haematopoietic stem cells were isolated from mononuclear fractions through positive selection, using anti-CD34 coated microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and MiniMACS separation columns (Miltenyi Biotec). In all experiments, the isolated cells were 95–99% CD34+. Cells from the effluent of the MiniMACS columns contained all other mononuclear cells, including naive T cells. These cells were cryopreserved in freezing buffer (10% dimethylsulphoxide; Sigma-Aldrich), 45% RPMI-1640 (Biochrom), 45% fetal calf serum (FCS) (Biochrom) at −196°C. Haematopoietic progenitor cells were seeded at a density of 2 × 104 cells/ml in 24-well flat-bottomed plates (Nunc, Wiesbaden, Germany). The culture medium consisted of endotoxin free RPMI-1640 (Biochrom) containing 10% heat inactivated fetal bovine serum (FBS), 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine and 1 mM sodium pyruvate (all from Biochrom). Cultures were supplemented with granulocyte–macrophage colony-stimulating factor (GM-CSF) (100 ng/ml; Peprotech, Rocky Hill, NJ, USA), tumour necrosis factor (TNF)-α (2·5 ng/ml; Peprotech) and stem cell factor (SCF) (100 ng/ml; Peprotech). Cells were cultured at 37°C in a humidified atmosphere in the presence of 5% CO2 for 7 days and split when necessary. At day 7 cultures were supplemented with GM-CSF (100 ng/ml; Peprotech), TNF-α (2·5 ng/ml; Peprotech) and IL-4 (37·5 ng/ml, Peprotech) and cultured for another 5 days [27]. Dendritic cells were either left untreated, infected with RSV as described below or challenged with polyinosinic-polycytidylic acid (poly-IC) (10 μg/ml; Sigma-Aldrich) for 24 h.
Preparation of and infection with RSV
A viral stock (RSV long strain) was prepared by infection of HEp-2 cells with a low input multiplicity of infection (MOI), as described previously [28]. When infection was advanced, cell supernatants were harvested, disrupted by ultrasonication and debris pelleted by low-speed centrifugation. RSV was purified further by sucrose gradient separation and virus preparations were frozen at −80°C. Infectivity titres of stock viruses were determined by inoculation of serial dilutions into HEp-2 cells. Virus growth was detected by observation of typical cytopathic effects followed by immunocytochemical staining of infected cell monolayers. Cell lines and virus preparations were tested for mycoplasma by polymerase chain reaction (PCR) with a mycoplasma detection kit (Venor GeM®), as described in the manufacturer's manual (Minerva Biolabs, Berlin, Germany).
To infect dendritic cells with RSV, cell suspension (200 μl) was incubated for 2 h with 1 ml purified virus at an MOI of 20. The inoculum was removed and replaced by culture medium containing 10% FCS. Co-culture experiments were set up 24 h after infection. Preliminary experiments showed that 85% of the dendritic cells were still viable.
Isolation and stimulation of T cells
Cells from the effluent of the stem cell separations were cryopreserved in freezing buffer (10% dimethylsulphoxide; Sigma-Aldrich), 45% RPMI-1640 (Biochrom) and 45% FCS (Biochrom) at −196°C. To set up a co-culture the effluent was thawed and CD4+CD45RA+ T cells were obtained by negative sorting with anti-human leucocyte antigen D-related (HLA-DR), anti-glycophorin, anti-CD8, anti-CD56 and anti-CD45RO magnetic beads (Milteyi Biotech) using an AutoMACS separation device (Milteyi Biotech). T cells were cultured in RPMI-1640 (Biochrom) with 10% FCS (Biochrom). For cultures analysed by fluorescent staining, 106 CD4+CD45RA+ T cells/ml were co-cultured with 105 dendritic cells/ml and 0·1 ng/ml toxic shock syndrome toxin-1 (TSST-1) (Toxin Technology, Sarasota, Florida, USA) in a 24-well tissue culture plate (Nunc). For measurement of [3H]-thymidine incorporation, 2 × 105 T cells/well and 2 × 104 dendritic cells/well and 0·1 ng/ml TSST-1 were co-incubated in a 96-well tissue culture plate (Nunc). TSST-1 was chosen because the TSST-1 binding T cell receptor (TCR)-Vβ2 T cells can be detected readily by flow cytometry. In cultures with purified T cells alone TSST-1 does not induce T cell proliferation or cytokine generation. In most experiments, dendritic cells were either untreated, poly-IC (10 μg/ml)-stimulated or RSV-infected (MOI 20) for 24 h.
Fluorescence staining
Cultured cells were washed twice, the concentration adjusted to 2 × 105 cells/ml in HBSS and incubated with the appropriately diluted antibodies against surface proteins for 20 min at 4°C. Expression of co-stimulatory molecules by dendritic cells was detected by appropriate phycoerythrin (PE)-labelled antibodies specific for HLA-DR, CD86 (BD Biosciences, Heidelberg, Germany) and CD83 (Beckman Coulter, Krefeld, Germany), respectively.
Expression of T cells surface markers was detected by staining with anti-CD45RA-fluorescein isothiocyanate (FITC), anti-CD45RO-phycoerythrin (PE) or anti-CD49a-PE (all from BD Biosciences), in parallel with anti-CD3-Tricolor (Caltag, Hamburg, Germany) or TCR-Vβ2-PE (Beckman Coulter).
To determine the rate of dendritic cells infected by RSV, permeabilization of the cell membrane was achieved by resuspension in 100 μl HBSS containing 0·1% saponin and 0·01 M HEPES buffer (saponin buffer). The permeabilized cells were incubated with biotinylated goat-anti-RSV (Biodesign, Saco, ME, USA) for 20 min at 4°C, washed with saponin buffer and subsequently incubated with FITC-labelled streptavidin (Caltag) for 20 min at 4°C in the dark. After washing with saponin buffer the cells were resuspended in 200 μl HBSS for flow cytometric analysis.
Intracellular cytokine staining
At different time-points after stimulation the T cells were activated with 2·4 μg/ml phytohaemagglutinin (PHA) (Biochrom) plus 1 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich) for 6 h. Monensin (1·3 μM/ml, Sigma-Aldrich) was added for the last 4 h [29], and cells were collected. The cytokines were measured as described previously [30]. Cells were washed in HBSS (Biochrom) and then fixed in ice-cold HBSS containing 4% paraformaldehyde (Riedel-de Haën, Seelze, Germany) for 10 min. Cells were washed twice. Permeabilization of the cell membrane was achieved by resuspension in 100 μl HBSS containing 0·1% saponin (Sigma-Aldrich) and 0·01 mM HEPES buffer (saponin buffer). The permeabilized cells were incubated with either anti-IL-2 FITC and anti-TNF-α-PE, anti-IFN-γ-FITC and anti-IL-10-PE or anti-IL-4-Alexa 488 and anti-IL-13-PE (all from BD Biosciences). To identify TCR-Vβ2-positive T cells the cells were counterstained with biotin-labelled antibodies against TCR-Vβ2 chain (Beckman Coulter) for 20 min at 4°C, washed and then incubated with streptavidin-Tricolor (Caltag) for 20 min at 4°C. After washing with saponin buffer the cells were resuspended in 200 μl HBSS for flow cytometric analysis.
T cell proliferation assays
Plates were tested for [3H]-thymidine incorporation at different time-points as indicated for each experiment. For the final 16 h cultures were pulsed with 1 μCi/well [3H]-thymidine (GE Healthcare, Freiburg, Germany), and [3H]-thymidine uptake was measured using a scintillation beta counter.
At days 5 and 24, cell divisions were visualized by carboxyfluorescein diacetate succinimyl esther (CFSE) dilution. At days 0 or 21, respectively, naive or memory/effector T cells were washed twice with serum-free medium, labelled with 1 μM CFSE (Invitrogen, Karlsruhe, Germany) in serum-free medium for 5 min at room temperature. The cells were then washed twice with culture medium. The labelled cells were co-cultured with dendritic cells and 0·1 ng/ml TSST-1 and T cell proliferation was analysed at days 5 or 23, respectively, using a fluorescence activated cell sorter (FACScan®) cytometer. To be able to detect selectively the TCR-Vβ2-bearing T cells, cells were stained with biotinylated anti-TCR-Vβ2 (Beckman-Coulter) and streptavidin-Tricolor (Caltag) directly before analysis.
Assays in which inhibition of proliferation of naive or memory/effector T cells was analysed were by co-cultivation of 5 × 104 T cells, 5 × 103 dendritic cells and 0·1 ng/ml TSST-1 with RSV-infected and ultraviolet (UV)-inactivated HEp-2 cells in ratios of 1 : 1–1 : 1024. Incorporation of [3H]-thymidine was determined after 72 h of co-cultivation.
Data acquisition and analysis
A FACScan® flow cytometer (Becton Dickinson, Mountain View, USA) equipped with filter settings for FITC (530 nm) (FL-1), for PE (585 nm) (FL-2) and for Tricolor emitting in deep red (<650 nm) (FL-3) was used. Ten to 50 000 events were acquired in list mode and analysed using CellQuest® software.
Enzyme-linked immunosorbent assay (ELISA)
Cytokines were measured with ELISA kits (R&D, Wiesbaden, Germany) on cell-free supernatants. Data are expressed as nanograms per ml ± standard deviation of six cultures.
T cell spreading
Naive (CD4+CD45RA+) and memory (CD4+CD45RO+) T cells were isolated freshly from the peripheral blood of healthy adults using the appropriate magnetic bead isolation kit (Miltenyi Biotech).
T cells were transferred for 30 min at 37°C on chamber slides coated with either poly L-lysine (0·01% in water; Sigma-Aldrich) or anti-CD3 [OKT-3; American Type Culture Collection (ATCC Promochem, Wesel, Germany)] and anti-CD28 (CD28.2, Becton Dickinson) antibodies (1 μg/ml each). Slides were additionally coated with RSV at an MOI of 1 or, for control, to mock preparations (prepared from uninfected Hep-2 cells) for 2 h at 4°C.
For staining T cells were fixed with 4% paraformaldehyde and permeabilized with 0·1% saponin. F-actin was stained with Alexa555-conjugated phalloidin (Molecular Probes, Eugene, OR, USA). Optical sections were acquired by fluorescence microscopy using a Zeiss Axioimager (Zeiss, Oberkochen, Germany) equipped with an ApoTome and an Axiocam MRm. T cells were analysed with the Axiovision 4·5 software. The proportion of T cells showing significant actin reorganization was calculated by choosing randomly 15 different fields for each condition (×40 objective). We counted a minimum of 200 T cells for each condition.
Statistical analysis
Data were analysed by two-way analysis of variance (anova) and Bonferroni test for post-testing of selected data sets, one-way anova or Student's t-test as appropriate using GraphPad Prism version 4·0 software package (GraphPad Software, San Diego, CA, USA).
Results
Experimental approach
For each experiment naive T cells, memory/effector T cell and dendritic cells were generated from cord blood of the same blood donor. Immature dendritic cells were generated from haematopoietic stem cells by culture for 12 days under the influence of SCF, GM-CSF, TNF-α and IL-4. In most experiments either uninfected, RSV-infected (MOI 20) or poly-IC (10 μg/ml)-treated dendritic cells were used as stimulators. A proportion of 25% ± 4·4% dendritic cells were infected with RSV, as revealed by flow cytometric analysis. Phenotypic differences between these populations of dendritic cells were found essentially as described before [11]. Whereas poly-IC up-regulated the expression of HLA-DR, CD86 and CD83, RSV infection induced only a slight increase in CD86 and CD83 (Fig. 1).
Fig. 1.
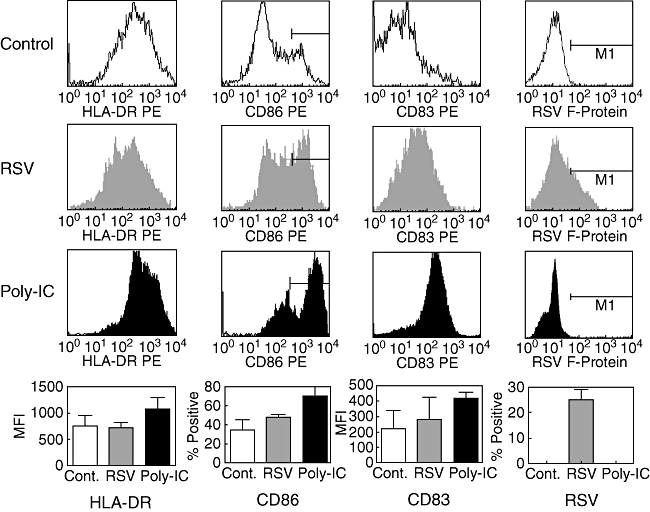
Surface phenotype of dendritic cells. Immature dendritic cells were left untreated, infected with respiratory syncitial virus (RSV) [multiplicity of infection (MOI) 20] or stimulated for 24 h with polyinosinic-polycytidylic acid (poly-IC) (10 μg/ml). The histograms show fluorescence values of gated large cells. The percentage ± standard deviation of positive cells (CD86, RSV F-protein) or mean fluorescence intensity ± SD (human leucocyte antigen D-related, CD83) from all six experiments are shown in the bottom panel.
Primary immune response was studied by co-incubation of naive T cells, dendritic cells and TSST-1 (0·1 ng/ml), a superantigen that activates preferentially T cells bearing the TCR-Vβ2 chain. Analyses were performed on days 0–10 as indicated. To study the memory/effector phase naive T cells were restimulated at days 7, 14 and 21 with one of the different types of autologous dendritic cells and were analysed at days 21–24.
While the percentage of TSST-1 binding TCR-Vβ2-bearing cells was approximately 5% at day 1, almost all T cells were TCR-Vβ2-positive at day 22. In addition, T cells lost the naive (CD45RA) and acquired a memory/effector phenotype (CD45RO, CD49a). There were no differences between T cells cultured with either RSV infected, poly-IC-treated or uninfected dendritic cells (Fig. 2).
Fig. 2.
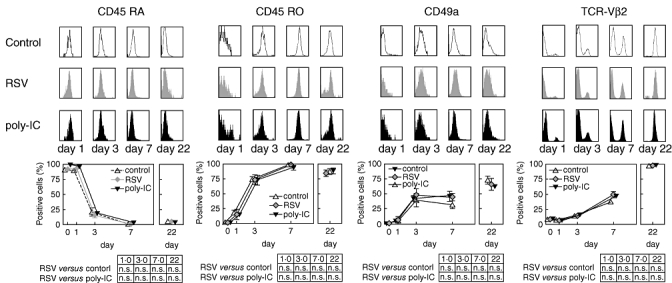
Loss of the naive T-cell marker CD45RA and acquisition of the memory T cell markers CD45RO, CD49a and T cell receptor-VCβ2 upon co-culture with non-infected, respiratory syncitial virus (RSV)-infected and polyinosinic-polycytidylic acid-treated dendritic cells. T cells and dendritic cells were cultured as delineated. The top panels show representative histograms of surface marker distributions on T cells co-cultured with RSV-infected and non-infected dendritic cells. The bottom panel shows the results from at least three independent experiments. The results are expressed as mean ± standard deviation.
Generation of cytokines during the primary and memory response
In previous clinical and experimental studies RSV has been shown to suppress cytokine generation, in particular the generation of IFN-γ [11]. In this study we followed the cytokine response during the primary and the memory response. From days 1 to 10 cytokines were produced in a sequential manner. At day 1 high frequencies of IL-2 and TNF-α-producing T cells were found (Fig. 3). A maximal peak of IFN-γ generation was detected at day 3, paralleled only by a marginal rate of IL-10-positive T cells (Fig. 3). At about day 7 there was a corresponding peak of IL-4 and IL-13 production, with a significant preponderance of IL-4 but not IL-13 in RSV-infected cultures. Maximal IL-13 generation was seen in cultures with untreated control dendritic cells (Fig. 3). IL-2, TNF-α and IFN-γ showed a second peak at day 7, although to a lesser extent than the first peak (Fig. 3). In contrast to the primary response, all cytokines peaked simultaneously at days 22–23 during the memory response (Fig. 3).
Fig. 3.
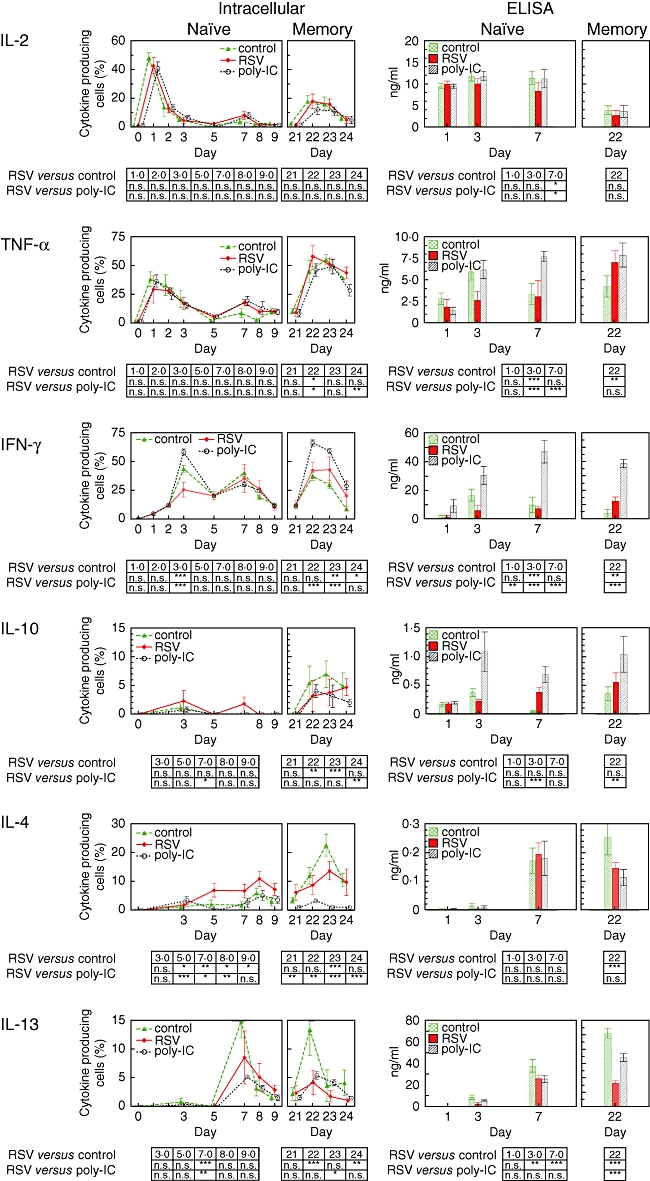
Cytokine production during primary and memory immune response in cultures with untreated, polyinosinic-polycytidylic acid-stimulated and respiratory syncitial virus (RSV)-infected dendritic cells. At the time-points indicated co-cultures were restimulated with phytohaemagglutinin (2·4 μg/ml) and phorbol myristate acetate (1 ng/ml) in the presence of monensin (1·3 μM/ml) for the last 6 h before cells were harvested. Rates of cytokine producing cells (left panel) and concentrations in the supernatant (right panel) were determined for interleukin (IL)-2 and tumour necrosis factor-α, interferon-γ and IL-10 as well as IL-4 and IL-13. The results are expressed as mean ± standard deviation. Data were analysed by two-way analysis of variance and data sets were compared against RSV by Bonferroni post-test.
During the primary response there were no differences in IL-2 and TNF-α. IFN-γ was diminished significantly in RSV-infected cultures at day 3 and, conversely, a significant increase of IL-4-producing cells was seen at day 7. Concurrently, the percentage of IL-13-producing cells in RSV-treated cultures was higher compared to poly-IC but lower compared to untreated cultures. Throughout the memory response there were no significant differences in the rates of IL-2 and TNF-α-producing T cells; the rates of IFN-γ and IL-10 as well as IL-4-producing T cells were found to be between untreated and poly-IC-treated cultures. In contrast, frequencies of IL-13-producing T cells were low in RSV-treated cultures (Fig. 3).
In order to detect not only frequencies of cytokine-producing T cells but also the amount of secreted cytokines, supernatants were analysed by ELISA. There was no difference in IL-2 and TNF-α secretion between RSV-infected cultures and controls at day 1. At day 3, however, considerably less IL-2, TNF-α and IFN-γ was found in the supernatants of RSV-infected cultures, whereas there was no reduction of IL-4 and IL-13 generation at day 7 (Fig. 3). At day 22 T cells restimulated with RSV-infected dendritic cells secreted considerable amounts of IFN-γ, but only intermediate concentrations of IL-4 and low IL-13 concentrations were found (Fig. 3).
Proliferation
RSV has been reported to influence not only cytokine generation but also proliferation of T cells [13,31]. T cells were labelled with CSFE and proliferation determined by flow cytometry as a function of the reduction of fluorescence signal on the cells. For untreated and poly-IC-treated cultures, histograms showed a maximum at four cell cycles, whereas for RSV-treated cultures the maximum was found at three cell cycles. In contrast, in the memory response peaks of all three CSFE histograms correspond to three cell divisions (Fig. 4a).
Fig. 4.
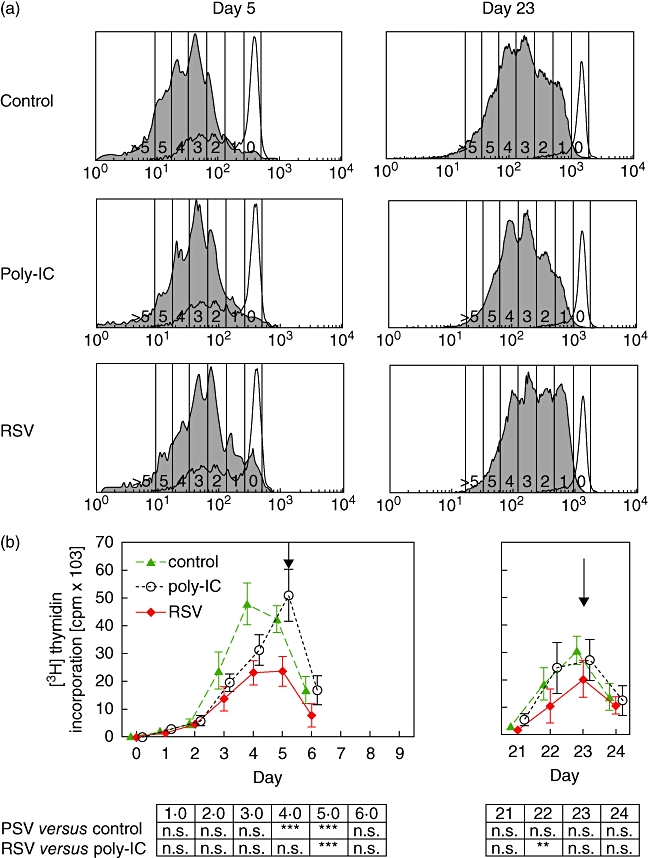
Induction of T cell proliferation by dendritic cells. Carboxyfluorescein diacetate succinimyl esther (CFSE)-labelled naive CD4+CD45RA+ T cells or memory/effector cells were co-cultured with uninfected, polyinosinic-polycytidylic acid-treated or respiratory syncitial virus (RSV)-infected dendritic cells and toxic shock syndrome toxin-1 (TSST-1) (0·1 ng/ml) as an activation stimulus. Upper panels: after 3 days, cell divisions of T cell receptor-Vβ2 bearing cells were visualized by CFSE dilution. Grey histograms denote results obtained with TSST-1-stimulated T cell receptor-Vβ2 bearing cells. Open histograms denote results obtained with unstimulated control cultures. Results shown are from one representative experiment of six. Lower panel: proliferation was determined by [3H]-thymidine incorporation. Proliferation of T cells when stimulated with RSV-dendritic cells was significantly decreased (**P < 0·01). Arrows denote the time of CFSE testing.
In addition, reduced [3H]-thymidine incorporation in RSV-treated cultures [23 520 ± 2189 counts per minute (cpm)] compared to untreated (42 385 ± 1952 cpm) and poly-IC-treated cultures (50 880 ± 3842 cpm) was apparent at day 5, whereas memory/effector T cells generated by restimulation with RSV-infected dendritic cells displayed only a slight reduction of proliferation (20 129 ± 2744 cpm) compared to untreated (30 724 ± 2153 cpm) and poly-IC-treated (27 201 ± 3021 cpm) cultures (Fig. 4b).
Contact-mediated inhibition
A mechanism described for RSV-mediated inhibition of proliferation is contact with RSV F-protein presented on cell surfaces [31]. UV-irradiated HEp-2 cells presenting RSV antigens on their surface, or as control UV-irradiated uninfected HEp-2 cells were co-cultivated with non-infected dendritic cells and T cells. HEp-2 cells were mixed with T cells at ratios in the range of 1 : 16–1 : 1024, and the mixed cells were co-incubated for 72 h. Proliferation of was monitored by [3H]-thymidine incorporation for a further 16 h. A 80–90% reduction of proliferation was observed with naive T cells. A significant lower rate of inhibition of lymphocyte proliferation was determined with memory T cells (Fig. 5).
Fig. 5.
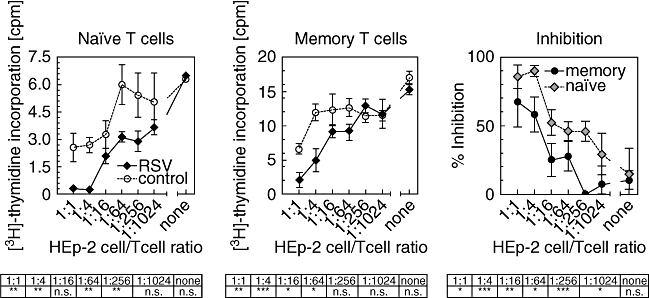
Cells presenting respiratory syncitial virus (RSV) antigens inhibit proliferation of toxic shock syndrome toxin-1 (TSST-1) stimulated naive and memory cells. RSV-infected (solid squares) or mock-infected (open circles) inactivated HEp-2 cells were co-cultivated with naive or memory/effector T cells, dendritic cells and TSST-1 0·1 ng/ml in the ratios indicated. Proliferation was determined by [3H]-thymidine incorporation. Values represent the result of six experiments. The right panel shows the calculated inhibition of proliferation of naïve and memory/effector cells in based on the appropriate mock-infected control.
T cell spreading
To study whether RSV surface contact affects the ability of human T cells to reorganize their actin cytoskeleton, these were exposed to purified RSV or to mock preparations. When seeded onto slides coated with CD3- and CD28-specific antibodies (co-stimulatory slides), about 81% of mock-treated naive T cells and 89% of mock-treated memory T cells spreaded in reorganizing their cortical actin as indicated by the formation of prominent filamentous actin-containing membrane protrusions within 30 min (Fig. 6a,d). In contrast, only about 42% naive T cells but 75% of memory T cells spreaded after CD3/CD28 ligation in RSV-exposed cultures (Fig. 6c). As shown in Fig. 6d, RSV-mediated suppression is significantly higher in naive compared to memory T cells.
Fig. 6.
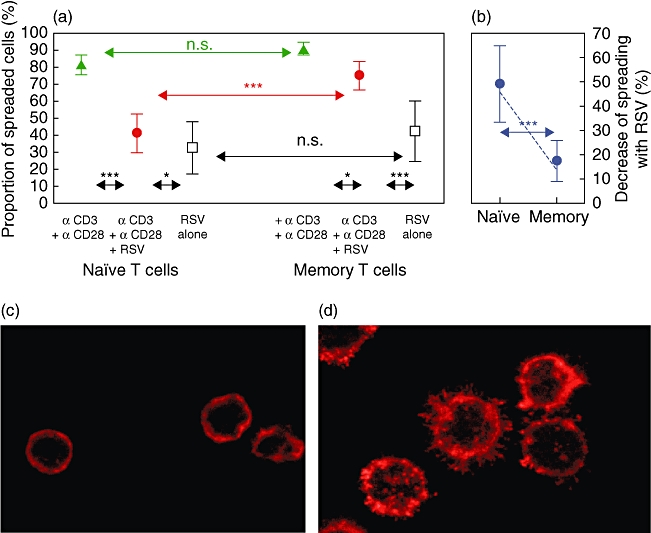
Respiratory syncitial virus (RSV) contact impairs spreading (stimulated rearrangement of cortical actin) of naive but not memory T cells. Poly L-lysine-treated chamber slides were coated with anti-CD3 and anti-CD28, anti-CD3 and anti-CD28 plus RSV or RSV alone. Microscopic evaluation after phalloidin staining revealed cells showing significant actin reorganization (spreading) (d) and non-spreaded cells (c). Addition of RSV inhibited spreading of naive but not of memory cells (a). Decrease of spreading is significantly higher with naive cells (b). The results are from 10 independent experiments. Given are mean ± standard deviation. ***P < 0·001,n *P < 0·05.
Discussion
There is ample evidence that RSV interacts with dendritic cells and that the primary T cell response to RSV is influenced significantly by this interaction [7,8,10,11,13,14,32]. Most of the in-vitro studies used isolated cells which were analysed after a single predefined time interval after stimulation [10,11,13,14,32]. However, it is well known in both humans [33] and mice [34,35] that during primary T cell response activation of effector functions follows a distinct sequence. In addition, the memory/effector response to RSV is of particular interest as symptomatic reinfection by RSV occurs throughout life [18,19,21], and an effective vaccine is still pending [36]. Therefore, in the present study we compared the effect of RSV-infected dendritic cells on the course of the primary and memory/effector T cell response in vitro. As a source of cells we chose cord blood, which contains a high number of naive T cells and in addition haematopoietic stem cells from which large numbers of dendritic cells can be grown. The possibility of storing cells in liquid nitrogen enabled us to restimulate co-cultures under controlled conditions with thawed autologous cells. The superantigen TSST-1 reacting with the TCR-Vβ2 chain was used to mimic antigenic stimulation. Therefore, reactions observed throughout the study were superantigen- rather than antigen-specific. Proliferation and cytokine generation were assessed in a sequential manner. Cultures with uninfected dendritic cells known to elicit Th2 responses [37] and with poly-IC-stimulated dendritic cells known to elicit Th1 responses [38] served as controls.
RSV did not alter the temporal order, but rather the magnitude of the response at definite intervals after stimulation. At day 1 after stimulation there was a high proportion of IL-2 and TNF-α-producing T cells (Fig. 3), with no difference in the numbers of producing T cells as well as concentration of secreted cytokines between the RSV-infected and control cultures. However, up to day 3, secretion of both cytokines measured by ELISA was diminished in RSV-infected cultures. TNF-α has a clear-cut anti-viral activity [39], and animal studies indicate that IL-2 may also contribute to viral clearance [40]. Thus, subversion of the immune response by RSV might be due in part to reduced secretion of these cytokines. Furthermore, while loss of the marker for naive T cells CD45RA and acquisition of the effector T cell markers CD45RO and CD49a was not affected by RSV, generation of IFN-γ was reduced markedly in RSV-infected cultures in comparison to both uninfected and poly-IC-treated cultures.
This early phase of the primary response characterized by an RSV-induced decrease of IFN-γ production corresponds to low IFN-γ levels in clinical samples from infants with bronchiolitis [5,15,41]. While reduced rates of IFN-γ-producing T cells correspond to reduced IFN concentrations in supernatants, there were discrepant results for IL-10. Despite low numbers of IL-10-producing lymphocytes, considerable concentrations of IL-10 were measured in the supernatants, especially in poly-IC-stimulated cultures. IL-10 found in the supernatants was produced most probably by antigen-presenting cells, which are known producers of IL-10 and which are stimulated by poly-IC.
Proliferation reached its peak at days 4–5 and was also diminished significantly in RSV-treated cultures (Fig. 4). This finding is consistent with most [13,31] but not all [11] previous reports. Discrepancies might be due to a lower viral load of the dendritic cells used previously [11].
During a second wave of cytokine production at day 7, all cytokines were generated including IL-4 and IL-13 (Fig. 3). The RSV-treated cultures showed a preponderance in the rate of IL-4-producing T cells with equal concentrations of IL-4 in the supernatants compared to untreated controls and poly-IC-treated cultures. As RSV impedes proliferation and thus absolute lymphocyte numbers, the higher rate of IL-4 generation most probably compensates for lower lymphocyte numbers.
In contrast to IL-4, the rate of IL-13-producing T cells and IL-13 concentrations in the supernatant were significantly higher in untreated cultures known to be confined to a Th2 response. Thus, with diminished IFN-γ and relatively increased IL-4, we found a partial Th1/Th2 polarization with a prevalence of Th2 cytokines during the RSV primary reaction. The clinical association of allergic sensitization with RSV bronchiolitis is consistent with this finding [16,42].
After three rounds of restimulation, T cells not only exhibited the typical markers of memory cells CD45RO and CD49a, but were almost exclusively positive for TCR-Vβ2 as a consequence of clonal expansion after stimulation with the superantigen TSST-1. In addition, restimulated T cells produced all cytokines shortly after stimulation and thus displayed the typical features of memory/effector cells [34].
According to the classical paradigm, a decrease of IFN-γ and an increase of IL-4 during the primary reaction would favour the Th2 phenotype in the memory reaction by cross-regulatory properties of the two cytokines [43,44]. However, RSV-restimulated T cells produced IL-4 as well as IFN-γ at rates between clear-cut Th1 and Th2 responses, with a predominance of IFN-γ. Furthermore, T cells expressed the Th1-associated surface marker CD49a [45]. In addition, Th2 cells are known to display a typical concomitant expression of IL-4 and IL-13 [46]. Almost no IL-4/IL-13 co-expressing T cells were found in RSV-infected cultures, whereas a considerable number was found in uninfected Th2-prone control cultures. Overall, despite several rounds of restimulation T cells matured under the influence of RSV were not fully polarized and in contrast to the primary response displayed a prevalence of Th1 cytokines. This is in line with clinical observations in adults that the immune response to RSV compared to other common upper respiratory tract infections such as adenovirus, rhinovirus or influenza virus induces only a weak but distinct IFN-γ generation [47,48]. Because RSV replication is controlled by IFN-γ [6], its relatively low but distinct formation might be responsible for reduced severity of clinical illness upon reinfection with RSV [18].
Taken together, RSV restrains the magnitude and cytokine patterns of both primary and memory responses but seems to exert a stronger effect on primary than on memory responses. As activation of memory T cells is less dependent on strong stimulatory signals [49,50], a conceivable explanation for the differential response of memory and naive T cells to RSV might be the weak activation of dendritic cells by RSV, as found in this and our previous study, with cord blood-derived dendritic cells [11]. However, RSV seems to activate monocyte-derived dendritic cells fully [13], which also inhibit T cell functions. De Graaff et al. [13] proposed that inhibiting activities might be conferred by soluble factors in the supernatant of RSV-infected dendritic cells. This is in line with our previous finding of increased prostaglandin E2 (PGE2) and IL-11 concentration in supernatants of RSV-infected dendritic cells [10]. Both PGE2 and IL-11 are inflammatory mediators with well-known immunmodulatory activities.
Another possible mechanism of inhibition exerted by RSV products was described by Schlender et al. [31]. Contact with RSV-infected cells inhibits proliferation of T cells [31]. We were able to reproduce this result and, remarkably, memory effector T cells were less sensitive to contact inhibition by infected HEp-2 cells than naive T cells.
Interestingly, microscopic examination of activated lymphocytes revealed a marked RSV-mediated inhibition of spreading. Furthermore, this inhibition was more prominent in naive compared to freshly isolated memory T cells. Inhibition of TCR-induced spreading was reported for measles virus [51] which shares genetic and functional similarities with RSV [52,53]. Measles virus contacted T cells failed to support the formation of a stable immunological synapse [51] and the majority of T cells pre-exposed to measles virus failed to acquire a detectable polar front–rear organization [51]. In addition it has been shown that contact with measles virus interferes with phosphatidyl-inositol-3-kinase [54]. Interestingly, greater inositol triphosphate generation occurs in naive than in memory T cells upon stimulation of the TCR [55]. Thus, it is conceivable that comparable mechanisms play a role in RSV-mediated immunomodulation.
In summary, we have shown that RSV infection of dendritic cells has a distinct modulatory effect on the primary response and a less pronounced effect on the memory response. In addition, we have identified contact with RSV proteins as a potential mechanism for RSV-mediated immunodulation.
Acknowledgments
This work was supported a grant from the Deutsche Forschungsgemeinschaft (DFG Scha 385/4-1) and from FoRUM. The expert technical assistance of Veronika Baumeister and Angelika Michel is acknowledged.
References
- 1.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368:312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 4.Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18:541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168:633–9. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 6.Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. Intranasal IFN-gamma gene transfer protects BALB/c mice against respiratory syncytial virus infection. Vaccine. 1999;18:558–67. doi: 10.1016/s0264-410x(99)00185-1. [DOI] [PubMed] [Google Scholar]
- 7.Gill MA, Palucka AK, Barton T, et al. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis. 2005;191:1105–15. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- 8.Beyer M, Bartz H, Horner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol. 2004;113:127–33. doi: 10.1016/j.jaci.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 10.Bartz H, Buning-Pfaue F, Turkel O, Schauer U. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129:438–45. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones A, Morton I, Hobson L, Evans GS, Everard ML. Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus. Clin Exp Immunol. 2006;143:513–22. doi: 10.1111/j.1365-2249.2005.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Graaff PM, de Jong EC, van Capel TM, et al. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol. 2005;175:5904–11. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- 14.Schauer U, Hoffjan S, Rothoeft T, et al. Severe respiratory syncytial virus infections and reduced interferon-gamma generation in vitro. Clin Exp Immunol. 2004;138:102–9. doi: 10.1111/j.1365-2249.2004.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristjansson S, Bjarnarson SP, Wennergren G, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local Th2-like response. J Allergy Clin Immunol. 2005;116:805–11. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–5. [PubMed] [Google Scholar]
- 17.Pala P, Bjarnason R, Sigurbergsson F, Metcalfe C, Sigurs N, Openshaw PJ. Enhanced IL-4 responses in children with a history of respiratory syncytial virus bronchiolitis in infancy. Eur Respir J. 2002;20:376–82. doi: 10.1183/09031936.02.00249902. [DOI] [PubMed] [Google Scholar]
- 18.Henderson FW, Collier AM, Clyde WA, Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–4. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–8. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 20.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. J Infect Dis. 2004;190:1828–32. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 21.Scott PD, Ochola R, Ngama M, et al. Molecular analysis of respiratory syncytial virus reinfections in infants from coastal Kenya. J Infect Dis. 2006;193:59–67. doi: 10.1086/498246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klagge I, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–33. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 23.Wiertz EJ, Mukherjee S, Ploegh HL. Viruses use stealth technology to escape from the host immune system. Mol Med Today. 1997;3:116–23. doi: 10.1016/S1357-4310(96)10059-9. [DOI] [PubMed] [Google Scholar]
- 24.Fernald GW, Almond JR, Henderson FW. Cellular and humoral immunity in recurrent respiratory syncytial virus infections. Pediatr Res. 1983;17:753–8. doi: 10.1203/00006450-198309000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–8. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 26.Bont L, Versteegh J, Swelsen WT, et al. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res. 2002;52:363–7. doi: 10.1203/00006450-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 28.Thurau AM, Streckert HJ, Rieger CH, Schauer U. Increased number of T cells committed to IL-5 production after respiratory syncytial virus (RSV) infection of human mononuclear cells in vitro. Clin Exp Immunol. 1998;113:450–5. doi: 10.1046/j.1365-2249.1998.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Meth. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 31.Schlender J, Walliser G, Fricke J, Conzelmann KK. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J Virol. 2002;76:1163–70. doi: 10.1128/JVI.76.3.1163-1170.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan S, Halonen M, Welliver RC. Innate immune responses in respiratory syncytial virus infections. Viral Immunol. 2004;17:220–33. doi: 10.1089/0882824041310612. [DOI] [PubMed] [Google Scholar]
- 33.Rothoeft T, Gonschorek A, Bartz H, Anhenn O, Schauer U. Antigen dose, type of antigen-presenting cell and time of differentiation contribute to the T helper 1/T helper 2 polarization of naive T cells. Immunology. 2003;110:430–9. doi: 10.1111/j.1365-2567.2003.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohning M, Richter A, Radbruch A. Cytokine memory of T helper lymphocytes. Adv Immunol. 2002;80:115–81. doi: 10.1016/s0065-2776(02)80014-1. [DOI] [PubMed] [Google Scholar]
- 35.Cardell S, Sander B. Interleukin 2, 4 and 5 are sequentially produced in mitogen-stimulated murine spleen cell cultures. Eur J Immunol. 1990;20:389–95. doi: 10.1002/eji.1830200223. [DOI] [PubMed] [Google Scholar]
- 36.Piedra PA. Clinical experience with respiratory syncytial virus vaccines. Pediatr Infect Dis J. 2003;22:S94–9. doi: 10.1097/01.inf.0000053893.15894.ff. [DOI] [PubMed] [Google Scholar]
- 37.Mandron M, Aries MF, Brehm RD, et al. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol. 2006;117:1141–7. doi: 10.1016/j.jaci.2005.12.1360. [DOI] [PubMed] [Google Scholar]
- 38.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong GH, Tartaglia LA, Lee MS, Goeddel DV. Antiviral activity of tumor necrosis factor is signaled through the 55-kDa type I TNF receptor. J Immunol. 1992;149:3350–3. [PubMed] [Google Scholar]
- 40.Bukreyev A, Whitehead SS, Prussin C, Murphy BR, Collins PL. Effect of coexpression of interleukin-2 by recombinant respiratory syncytial virus on virus replication, immunogenicity, and production of other cytokines. J Virol. 2000;74:7151–7. doi: 10.1128/jvi.74.15.7151-7157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aberle JH, Aberle SW, Rebhandl W, Pracher E, Kundi M, Popow-Kraupp T. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin Exp Immunol. 2004;137:146–50. doi: 10.1111/j.1365-2249.2004.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schauer U, Hoffjan S, Bittscheidt J, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20:1277–83. doi: 10.1183/09031936.02.00019902. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 44.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 45.Ray SJ, Franki SN, Pierce RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–79. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 46.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 47.Chonmaitree T, Roberts NJ, Jr, Douglas RG, Jr, Hall CB, Simons RL. Interferon production by human mononuclear leukocytes: differences between respiratory syncytial virus and influenza viruses. Infect Immun. 1981;32:300–3. doi: 10.1128/iai.32.1.300-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douville RN, Bastien N, Li Y, Pochard P, Simons FE, HayGlass KT. Human metapneumovirus elicits weak IFN-gamma memory responses compared with respiratory syncytial virus. J Immunol. 2006;176:5848–55. doi: 10.4049/jimmunol.176.10.5848. [DOI] [PubMed] [Google Scholar]
- 49.Fischer H, Gjorloff A, Hedlund G, et al. Stimulation of human naive and memory T helper cells with bacterial superantigen. Naive CD4+45RA+ T cells require a costimulatory signal mediated through the LFA-1/ICAM-1 pathway. J Immunol. 1992;148:1993–8. [PubMed] [Google Scholar]
- 50.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 51.Muller N, Avota E, Schneider-Schaulies J, Harms H, Krohne G, Schneider-Schaulies S. Measles virus contact with T cells impedes cytoskeletal remodeling associated with spreading, polarization, and CD3 clustering. Traffic. 2006;7:849–58. doi: 10.1111/j.1600-0854.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 52.Klagge IM, Abt M, Fries B, Schneider-Schaulies S. Impact of measles virus dendritic-cell infection on Th-cell polarization in vitro. J Gen Virol. 2004;85:3239–47. doi: 10.1099/vir.0.80125-0. [DOI] [PubMed] [Google Scholar]
- 53.Schlender J, Schnorr JJ, Spielhoffer P, et al. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–9. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avota E, Harms H, Schneider-Schaulies S. Measles virus induces expression of SIP110, a constitutively membrane clustered lipid phosphatase, which inhibits T cell proliferation. Cell Microbiol. 2006;8:1826–39. doi: 10.1111/j.1462-5822.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- 55.Hall SR, Heffernan BM, Thompson NT, Rowan WC. CD4+ CD45RA+ and CD4+ CD45RO+ T cells differ in their TCR-associated signaling responses. Eur J Immunol. 1999;29:2098–106. doi: 10.1002/(SICI)1521-4141(199907)29:07<2098::AID-IMMU2098>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]


