Abstract
Indoleamine 2,3-dioxygenase (IDO) is a tryptophan-degrading enzyme which suppresses T lymphocyte activity. IDO activity can be determined by relating kynurenine, the main metabolite of tryptophan, to tryptophan (kyn/trp). We have demonstrated recently that systemic lupus erythematosus (SLE) is activated during the sunny season as measured by the European Consensus Lupus Activity Measurement Index (ECLAM) activity score. Our aim here was to establish whether IDO-dependent mechanisms are involved in the activation process of SLE. Kyn/trp was measured by reverse-phase high-performance liquid chromatography (HPLC) in 33 (30 female, three male) SLE patients in winter, spring and summer and in 309 healthy control subjects. At the same time-points the SLE patients were examined by a rheumatologist and a dermatologist and the activity of SLE assessed by the ECLAM score. IDO activity was higher in SLE patients than in healthy subjects. There was no seasonal variation in IDO activity in SLE patients and it did not correlate with the ECLAM activity score in winter. However, there was a significant correlation between IDO activity and the ECLAM score both in spring and in summer. High IDO activity in winter predicted subsequent activation of SLE in spring and summer. Our results indicate that IDO-dependent immunosuppressive mechanisms are activated in SLE patients. Exposure to sunlight or another factor causing seasonal variation in SLE activity leads to insufficiency of this suppression in a subgroup of patients, causing activation of SLE. High IDO activity in winter predicts activation of SLE in the sunny season.
Keywords: disease activity, ECLAM score, indoleamine 2, 3-dioxygenase, seasonal variation, systemic lupus erythematosus
Introduction
Sunlight is well known to induce skin symptoms in patients with systemic lupus erythematosus (SLE), but data on the ability of ultraviolet light also to aggravate systemic symptoms of SLE have been conflicting [1–5]. In a prospective 1-year follow-up study we have demonstrated recently that SLE is activated in the northern climate during the sunny season as measured by the European Consensus Lupus Activity Measurement (ECLAM) activity index, and this activation was due mainly to a worsening of other than cutaneous symptoms [6].
Indoleamine 2,3-dioxygenase enzyme (IDO, EC 1·13·11·52) has recently drawn much attention as a regulator of immune responses [7–10]. IDO is an enzyme catabolizing the essential amino-acid tryptophan (trp). The degradation of trp takes place via two distinct biochemical pathways: by tryptophan 5-monooxygenase (tryptophan 5-hydroxylase, EC 1·14·16·4) to the neurotransmitter serotonin and by either IDO or tryptophan 2,3-dioxygenase (EC 1·13·11·11) to N-formylkynurenine, which is then converted to kynurenine. IDO is expressed in antigen-presenting cells, macrophages and dendritic cells. The activation of IDO leads to a local decrease in tryptophan concentration, thus suppressing the activation of the surrounding T lymphocytes [7–9]. IDO is up-regulated in response to various infectious and inflammatory stimuli and increased IDO activity has been observed in several immune/inflammatory diseases [reviewed in 11], including SLE [12]. IDO activity can be estimated by determining the ratio of the serum concentration of kyn, the main metabolite of trp, to the serum concentration of trp.
To establish whether IDO-dependent regulatory mechanisms are involved in the process of activation of SLE we measured IDO activity in serial serum samples from SLE patients during different seasons. At the same time-points a detailed clinical interview and examination of the patients had been conducted and the ECLAM score of the activity of SLE measured [6].
Subjects and methods
Subjects
Of all the SLE patients treated in the Department of Internal Medicine of the Tampere University Hospital during the period 1996–98, 52 fulfilled at least four of the American College of Rheumatology (ACR) criteria for SLE [13]. Of these 52 eligible SLE patients of Finnish Caucasian origin, 33 (63%) (30 women and three men) volunteered for the study and signed informed consent.
Control subjects
Three hundred and nine healthy Finnish Red Cross Transfusion Service blood donors (139 female, 170 male) served as a control group. The mean age of these subjects was 45 ± 11 years, range 21–64 years.
Disease activity index
The study protocol included three clinical examinations by both a rheumatologist and a dermatologist and three laboratory examinations: in winter (January–February), spring (May–early June) and end of summer (August–early September) in 1999. The ECLAM score, a combination of 15 clinical and laboratory variables, was used as disease activity index [14] and was evaluated at every visit. The clinical signs in the ECLAM score include articular, mucocutaneous, pleuropulmonary, intestinal and neuropsychiatric manifestations, pericarditis, myositis, fever and fatigue. Laboratory parameters include blood cell counts, indirect Coombs test, ESR, components of complement (C3, C4 and CH50), tests of renal function (serum creatinine, creatinine clearance and 24-h urinary protein excretion, urinanalysis). Each sign or abnormal laboratory value was worth 0·5–2 points. Furthermore, evolving skin symptoms, renal manifestation or hypocomplementaemia gained an additional 1–2 points each. Thus, the theoretical maximal ECLAM score is 17·5.
Tryptophan and kynurenine determinations
Tryptophan (µmol/l) and kynurenine (µmol/l) concentrations in serum were measured by reverse-phase high-performance liquid chromatography, as described previously [15]. Samples for tryptophan and kynurenine determinations were collected on each visit and stored at −20°C. Tryptophan was separated with a Shimadzu liquid chromatograph LC-10AD VP (Shimadzu Co, Kyoto, Japan), using a 50-mm BDS Hypersil C 18 5 µm column (Thermo Electron Co., Bellefonte, PA, USA). It was monitored by fluorescence with a Shimadzu RF-10 A XL detector at 266-nm excitation and 366-nm emission wavelengths. Kynurenine was separated with a Hewlett Packard 1100 liquid chromatograph (Palo Alto, CA, USA) using a Merck LiChroCart 55–4150-mm cartridge containing a Purospher STAR RP-18 3 µm Column (Merck Co., Darmstadt, Germany). It was determined by ultraviolet absorption with a Hewlett Packard G13144 detector at 360-nm wavelength. Kyn/trp (µmol/mmol) was calculated by relating concentrations of kyn (µmol/l) to trp (mmol/l), this allowing an estimate of IDO activity.
Statistical analysis
Mann–Whitney U-test or Wilcoxon signed-rank test was applied in comparisons of continuous variables. Correlations were calculated by Spearman's rank correlation coefficient. Findings were considered statistically significant at P < 0·05. Statistical analyses were performed with spss 13·0 for Windows.
Ethical approval
The study protocol was approved by the Ethical Committee of Tampere University Hospital.
Results
The clinical characteristics of the 33 SLE patients are shown in Table 1. Two patients discontinued the study after one or two visits. Their data are included in the respective analyses when possible.
Table 1.
Demographic, clinical and immunological characteristics of the 33 patients with systemic lupus erythematosus (SLE).
| Characteristic | |
|---|---|
| Demographic | |
| Female : male | 30 : 3 |
| Age, years (mean ± s.d.) | 47 ± 12 |
| Duration of SLE, years (mean ± s.d.) | 17 ± 10 |
| Previous or present clinical characteristics, n (%) | |
| Haematological manifestations | 29 (88) |
| Musculosceletal symptoms | 28 (85) |
| Skin/mucosal manifestations | 25 (76) |
| Butterfly rash/erythema | 22 (67) |
| Photosensitivity | 21 (64) |
| Alopecia/defluvium | 14 (42) |
| Mucosal ulcers | 11 (33) |
| Extended rash | 7 (21) |
| Discoid lupus erythematosus | 3 (9) |
| Subacute cutaneous lupus erythematosus | 2 (6) |
| Peripheral vascular disease (including Raynaud's phenomenon) | 22 (67) |
| Cardiopulmonary disease | 12 (36) |
| Renal disease | 11 (33) |
| Neuropsychological manifestations | 10 (30) |
| Immunological findings at study entry | |
| Anti-nuclear antibodies | 28 (85) |
| Anti-DNA antibodies | 16 (48) |
| Anti-ENA antibodies | 21 (64) |
There were no significant changes in median IDO activity in SLE patients in different seasons (P = 0·196 for comparison of kyn/trp between spring and winter, and P = 0·946 for comparison of kyn/trp between summer and winter) (Table 2). The median tryptophan concentration was significantly lower in SLE patients in winter, spring and summer than that in healthy blood donor controls (unspecified date of sampling) (Table 2). Kynurenine concentration was significantly higher in SLE patients in winter, spring and summer than that in healthy controls (Table 2). Consequently, IDO activity (kyn/trp) was significantly higher in SLE patients in winter and spring and summer than that in healthy blood donors (Table 2).
Table 2.
Serum tryptophan (trp) and kynurenine (kyn) concentrations (median, interquartile range) and kyn/trp ratio in systemic lupus erythematosus (SLE) patients in different seasons and in healthy blood donor controls.
| Tryptophan, µmol/l | Kynurenine, µmol/l | Kyn/trp, µmol/mmol | |
|---|---|---|---|
| SLE patients (n = 33) | |||
| Winter (n = 31) | 72·4 (61·0, 85·6) | 2·81 (2·49, 3·39) | 39·1 (33·7, 48·9) |
| Spring (n = 33) | 73·4 (61·9, 78·9) | 2·92 (2·62, 4·62) | 43·0 (32·7, 62·7) |
| Summer (n = 30) | 68·9 (62·5, 78·2) | 2·81 (2·49, 3·40) | 40·4 (34·0, 69·5) |
| Healthy controls (n = 309) | 79·4 (70·0, 88·0)a | 2·05 (1·73, 2·35)b | 25·9 (22·4, 30·5)c |
Statistics: Mann–Whitney U-test;
P = 0·025 in comparison with trp in SLE patients in winter, P = 0·009, in comparison with trp in SLE patients in spring, P = 0·001, in comparison with trp in SLE patients in summer;
P < 0·0001 in comparison with kyn in SLE patients in winter, spring and summer;
P < 0·0001 in comparison with kyn/trp in SLE patients in winter, spring and summer.
We have shown previously that the ECLAM activity scores of these SLE patients were significantly higher in spring and tended to be higher in summer than in winter [6]. IDO activity in these SLE patients did not correlate with the ECLAM activity score in winter (Fig. 1a). However, there was a significant correlation between IDO activity and the ECLAM scores both in spring and in summer (Fig. 1b,c).
Fig. 1.
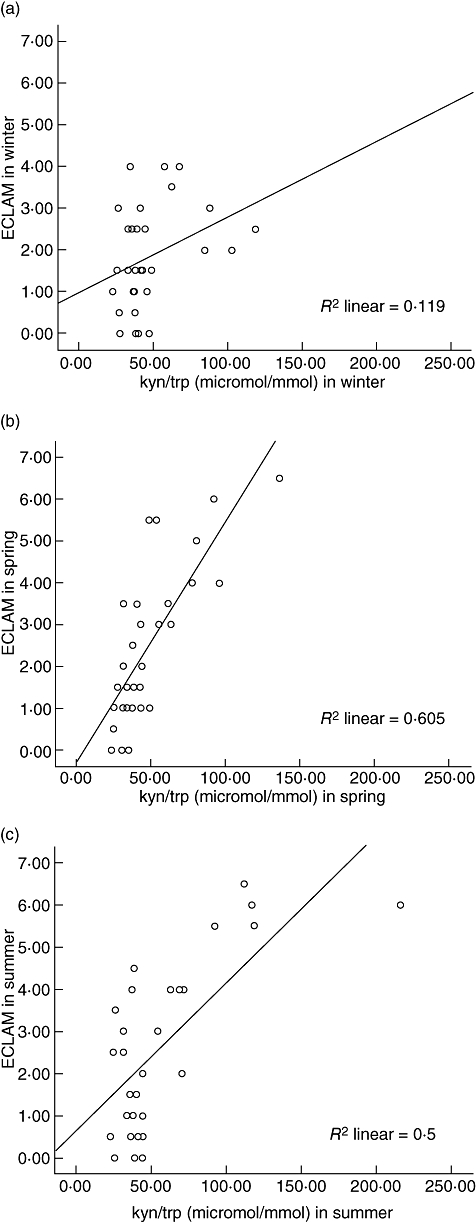
Correlation between ratio of serum kynurenine and tryptophan concentrations (kyn/trp, µmol/mmol) and European Consensus Lupus Activity Measurement Index (ECLAM) activity index in systemic lupus erythematosus (SLE) patients in (a) winter, r = 0·335, P = 0·066, (b) spring, r = 0·770, P < 0·0001 and (c) summer, r = 0·561, P = 0·002 (Spearman's correlation coefficient).
The patients were grouped by median (39·1 µmol/mmol) into those with low and high IDO activity at baseline, i.e. in winter. The ECLAM index did not increase in SLE patients with low IDO activity at baseline (Fig. 2). In those with high IDO activity in winter, ECLAM activity increased significantly in spring and summer compared with winter levels (Fig. 2), although IDO activity itself did not rise significantly. There was thus a subgroup of SLE patients with high IDO activity in winter whose baseline IDO activity predicted subsequent rises in ECLAM score.
Fig. 2.
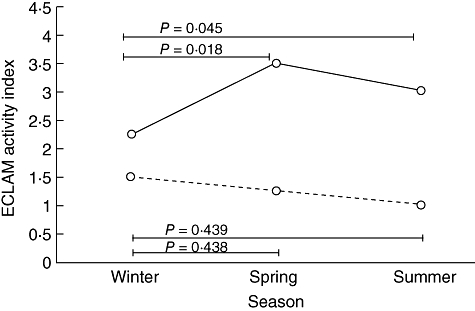
Seasonal variation of European Consensus Lupus Activity Measurement Index (ECLAM) activity index (median) in systemic lupus erythematosus (SLE) patients with high (kyn/trp ≥ median, solid line, n = 16) or low (kyn/trp < median, dashed line, n = 15) baseline IDO activity.
Discussion
SLE patients have been shown previously to have increased levels of IDO activity [12]. Our present results are consistent with this previous finding, in that we found IDO activity to be elevated in SLE patients compared with healthy control subjects. High IDO activity is probably directed to down-regulate immune responses and the activity of SLE. We sought here particularly to establish whether IDO activity changes during different seasons parallel with the activity score ECLAM. No significant changes in IDO activity were observed during different seasons.
IDO concentration did not correlate with the ECLAM score in winter. It would therefore seem that in winter elevated IDO activity functions successfully in down-regulation of the activity of SLE. In spring and in summer, on the other hand, i.e. in the sunny seasons, there was a significant correlation between IDO activity and disease activity in patients with SLE. It would appear that when an external trigger such as, for example, sunlight, activates SLE, the IDO-dependent suppressive mechanisms fail and elevated IDO levels no longer provide sufficient immunoregulation.
Our most important finding was that high IDO activity in winter predicted activation of SLE in the sunny seasons. Our results thus shed light on the functional significance of IDO activation in vivo.
IDO-mediated suppression may be operative at several stages of the immune response. Environmental factors (e.g. infections in childhood) known to drive T helper (Th) cell differentiation in the Th1 direction and protect from IgE-mediated allergic diseases have been shown to increase IDO activity [16]. The activated Th1-mediated immune response is consequently down-regulated by several negative feed-back mechanisms, one of which might be IDO enzyme activity. In this case IDO activity could merely be an indicator of the strength of an immune response or inflammation and act as a counter-regulatory response [10], i.e. to limit the intensity and extent of strong immune responses. This might be the case regarding the elevated baseline IDO levels in SLE. However, we have now demonstrated that increased IDO activity in SLE patients exposed to sunlight is not a secondary event elicited only in response to immune activation; high serum IDO concentration in fact predicts the activation of SLE in the sunny seasons, thus indicating that the effect of the ultraviolet light-induced activating signals depends on the pre-existing IDO activity. Our results suggest that higher baseline IDO activity in SLE patients in winter indicates higher probability for future SLE flare-up in the sunny seasons. To verify the significance of this finding, it would also have been enlightening to know how IDO activity and ECLAM score would develop in the following winter period.
Increased IDO activity is by no means specific for SLE or any other immunoinflammatory disease; we have noted elevated levels, e.g. in patients with primary Sjögren's syndrome, in particular in those with immunologically active disease [17]. However, the present results confirm that IDO-dependent immunosuppressive mechanisms are activated in SLE patients. Moreover, they demonstrate that exposure to sunlight or another factor causing seasonal variation in SLE activity leads to insufficiency of this suppression in a subgroup of SLE patients, causing activation of systemic SLE symptoms. Most importantly, a novel finding was that high IDO activity in winter predicted activation of SLE in the sunny season. One can only speculate that IDO-dependent mechanisms could also be operative in the activation of SLE in general, not only in the case of ultraviolet exposure. If data from independent and larger materials confirm our findings, IDO levels could perhaps also be used in the follow-up of patients with SLE, elevated levels warranting careful monitoring of the patient or perhaps even modification of treatment, similarly to anti-DNA antibodies.
Acknowledgments
This study was supported by the Medical Research Fund of Tampere University Hospital, Tampere, Finland and The Academy of Finland. We thank Ms Raija Repo, Ms Sinikka Repo-Koskinen and Ms Eija Spåre for skilful technical assistance.
References
- 1.Sturfelt G, Nived O. Clinical inconsistency, benign course and normal employment rates in unselected systemic lupus erythematosus. Clin Exp Rheumatol. 1985;3:303–10. [PubMed] [Google Scholar]
- 2.Wysenbeek AJ, Block DA, Eries LE. Prevalence and expression of photosensitivity in systemic lupus erythematosus. Ann Rheum Dis. 1989;48:461–3. doi: 10.1136/ard.48.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amit M, Molad Y, Kiss S, Wysenbeek AJ. Seasonal variations in manifestations and activity of systemic lupus erythematosus. Br J Rheumatol. 1997;36:449–52. doi: 10.1093/rheumatology/36.4.449. [DOI] [PubMed] [Google Scholar]
- 4.Krause I, Shraga I, Molad Y, Guedj D, Weinberger A. Seasons of the year and activity of SLE and Behçet's disease. Scand J Rheumatol. 1997;26:435–9. doi: 10.3109/03009749709065715. [DOI] [PubMed] [Google Scholar]
- 5.Haga HJ, Brun JG, Rekvig OP, Wetterberg L. Seasonal variations in activity of systemic lupus erythematosus in a subarctic region. Lupus. 1999;8:269–73. doi: 10.1191/096120399678847858. [DOI] [PubMed] [Google Scholar]
- 6.Hasan T, Pertovaara M, Yli-Kerttula U, Luukkaala T, Korpela M. Seasonal variation of disease activity of systemic lupus erythematosus in Finland − a one-year follow-up study. Ann Rheum Dis. 2004;63:1498–500. doi: 10.1136/ard.2003.012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 8.Mellor AL, Munn DH. Tryptophan catabolism and regulation of adaptive immunity. J Immunol. 2003;170:5809–13. doi: 10.4049/jimmunol.170.12.5809. [DOI] [PubMed] [Google Scholar]
- 9.Mellor AL, Munn DH. Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nature Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 10.Mellor AL. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–5. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Widner B, Sepp N, Kowald E, et al. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology. 2000;201:621–30. doi: 10.1016/S0171-2985(00)80079-0. [DOI] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;118:412–6. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bencivelli W, Isenberg DA, et al. Disease activity in systemic lupus erythematosus: report of the consensus Study Group of the European workshop for rheumatology research. II. Identification of the variables indicative of disease activity and their use in the development of an activity score. Clin Exp Rheumatol. 1992;10:541–7. [PubMed] [Google Scholar]
- 15.Laich A, Neurauter G, Widner B, Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. 2002;48:579–81. [PubMed] [Google Scholar]
- 16.Raitala A, Karjalainen J, Oja SS, Kosunen TU, Hurme M. Indoleamine 2,3-dioxygenase (IDO) activity is lower in atopic than in non-atopic individuals and is enhanced by environmental factors protecting from atopy. Mol Immunol. 2006;43:1054–6. doi: 10.1016/j.molimm.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Pertovaara M, Raitala A, Uusitalo H, et al. Mechanisms dependent on tryptophan catabolism regulate immune responses in primary Sjögren's syndrome. Clin Exp Immunol. 2005;142:155–61. doi: 10.1111/j.1365-2249.2005.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


