Abstract
Coeliac disease (CD) is an enteropathy induced in genetically susceptible individuals by gluten components, gliadin, hordein and secalin, polypeptides present in cereals such as wheat, barley and rye, respectively. Although the disease starts as intolerance to gliadins, antibodies to tissue transglutaminase (tTG) in the gut epithelium are characteristic of the disease. Whereas serum autoantibodies against tTG (tTGA) are highly specific for CD, antibodies to gliadin are less informative as they can also be detected in other enteropathies, and even in healthy individuals. However, it was shown recently that antibodies to certain gliadin peptides occur with high specificity in CD patient sera. We developed a solid phase lanthanide-based immunofluorometric assay for simultaneous detection of serum IgA and IgG antibodies to a synthetic peptide derived from gamma gliadin of wheat comprising amino acids 86–103. Three glutamine residues of this native 18-mer peptide were replaced by glutamic acids and the peptide was biotinylated. Sera from 87 individuals who had undergone duodenal biopsy and were diagnosed with CD and from 81 healthy individuals were analysed for the presence of both IgA and IgG anti-gliadin peptide antibodies. The performance of the peptide AGA assay was excellent, showing a specificity and sensitivity of 90% and 92% for IgA, and 98% and 75% for IgG, respectively. The corresponding values for conventional anti-gliadin antibody (AGA) enzyme-linked immunosorbent assay (ELISA) tests were 72% specificity and 87% sensitivity for IgA, and 64% specificity and 78% sensitivity for IgG. In a prospective study, almost all the tTGA-positive sera drawn from children who later developed CD were also positive for gliadin peptide antibodies.
Keywords: antibody, coeliac disease, dual-label assay, gliadin peptide, IFMA, lanthanide, time-resolved fluorometry
Introduction
Coeliac disease (CD) is an enteropathy caused by intake of gluten proteins present in cereals such as wheat (glutein), barley (hordein) and rye (secalin) in genetically predisposed individuals. Activation of intraepithelial lymphocytes and infiltration of DQ2 (or DQ8) restricted CD4+ T lymphocytes specific for gliadin peptides into lamina propria leads to cytokine secretion, and the immune response to these cereal proteins results ultimately in destruction of microvilli of the intestine, hyperplastic crypts and flattened appearance of the intestine, and malabsorption of nutrients [1,2]. Long-term effects include bone degeneration, osteoporosis and increased risk of malignancies in the gut. Only by following a strict gluten-free diet can the symptoms be avoided. The diagnosis is always based on small intestine biopsy before introduction of a gluten-free diet.
Serological analysis of CD patient sera has revealed the presence of IgA and IgG class antibodies to gliadin, a protein component of gluten. The reason for the insufficiency of serum anti-gliadin antibodies (AGA) in the diagnostics of CD has been the low specificity of the assay, as AGAs can also often be detected in apparently healthy individuals. In addition, specific autoantibodies for tissue transglutaminase (tTG) are present in the gut epithelium. The tTG enzyme is capable of binding and deamidating proteolytically cleaved glutamine-rich gliadin peptides to yield highly immunogenic peptides with glutamate residues [3]. It is thought thatsuch acidic peptides make up the pathogenic pool of antigens triggering CD [2].
Immune reactivity has been shown to be more prominent against partially deamidated peptides [3–5], and it has been implicated that after the primary T cell response to gliadin peptides, tTG-specific B cells may engulf tTG–gliadin peptide complexes which leads eventually to the formation of autoantibodies to tTG [2,6]. The overwhelming majority of CD patients (85–90%) carry the human leucocyte antigen (HLA)–DQ2 allele, leading to the assumption that the pathogenic peptides preferentially are presented via the DQ2 molecule. Early introduction of wheat into infant food results in an increased CD incidence among very young children [7], but postponement of it may also be detrimental. It has been proposed that gluten tolerization occurs optimally between 3 and 6 months of age [8].
Several antibody detection assays are used currently for diagnosing CD. Although induced by gliadin in wheat gluten, gliadin antibody responses are not very specific for CD. Antibodies to endomysium (EMA) have been considered the most informative and specific marker, and in fact the antigen they recognize has been shown to be the tTG protein [9]. However, with enzyme-linked immunosorbent assay (ELISA) using purified human recombinant tTG protein as the antigen, relatively high false positive rates have been obtained [10,11]. Thus indirect immunofluorescence EMA assays utilizing monkey oesophagus or human umbilical cord tissue slices provide important confirmation of the results. The gliadin protein used as the antigen in the current ELISA tests is hydrophobic, and has high proline and glutamine content and a low number of charged glutamic or aspartatic acid residues. It is poorly soluble in aqueous solutions, requiring organic solvents. In addition, differences between batches are frequent, probably due to the use of different strains of wheat with variable gliadin sequences [12], making quality control problematic.
Recently, Schwertz et al. [13] demonstrated that short synthetic gliadin peptides bound to nitrocellulose filters were recognized by CD patient sera. Recognition of some of the epitopes showed high specificity for CD coming close to the accuracy obtained with tTG or endomysium as the antigen. We describe here the development of a sensitive solid-phase time-resolved immunofluorometric (TR-IFMA) gliadin–peptide dual-label assay which allows simultaneous determination of specific IgA and IgG in serum. We demonstrate its accuracy in the CD diagnosis and show that gliadin peptide responses parallel tTG autoantibody levels in follow-up samples of children developing CD.
Subjects and methods
Subjects and calibration material
Diagnostic serum samples from 87 subjects (mean age 51 years, range 6–79 years including five children aged 6–13 years) with coeliac disease confirmed with intestinal biopsy, and sera from 81 healthy individuals (mean age 39 years, range 21–80 years) were analysed. The samples were diluted 1 : 100 in Delfia assay buffer. AGAs and TGAs were also analysed from the samples using Biofons IgA and IgG ELISA kits (Biofile Ltd, Turku, Finland).
The calibrator series was prepared by diluting the pooled human sera with high content of AGAs in a dilution buffer containing 50 mmol/l Tris-HCl, 9 g/l NaCl, 0·5 g/l sodium azide and 75 g/l bovine serum albumin, pH 7·8. Human sera with high anti-peptide antibody IgA and IgG content were selected for assay optimization and diluted as above.
Sera collected from children participating in the Finnish Diabetes Prediction and Prevention follow-up study (DIPP) were analysed longitudinally for the presence of antibodies to the gliadin peptide (amino acids 86–103) using the lanthanide-based assay. tTGAs had been determined earlier using a commercial ELISA kit (CelikeyTM tTGA IgA, Pharmacia Diagnostics, Freiburg, Germany), and selection of transiently anti-tTG antibody (TGA)-positive children was based on this analysis [13]. The samples were analysed in accordance with the rules from the Ethics Committee of the University of Turku.
Reagents and instrumentation
Streptavidin-coated microtitre plates, DTTA (N1-(p-isothiocyanatobenzyl)-diethylenetriamine-N1,N2,N3,N3-tetraacetic acid)-Europium chelate, DTTA-Samarium chelate, Delfia assay buffer, wash solution, enhancement solution and Europium and Samarium standard solutions were obtained from PerkinElmer Life and Analytical Sciences (Wallac Oy, Turku, Finland). The Superdex 200 gel filtration column was purchased from GE Healthcare Amersham Biosciences AB (Uppsala, Sweden). The polyclonal goat anti-human IgA antibody was a product of Southern Biotechnology Associates, Inc. (Birmingham, AL, USA) and the monoclonal anti-human IgG antibody (clone HP 6043) was produced by PerkinElmer Life and Analytical Sciences.
Two partially deamidated (underlined residues, Q to E replacements) 19-mer gamma-gliadin peptides (aa 86–103), one carrying a biotin at the N-terminus (biotin-H2N-LPFPEQPEQPFPQPEQPQ-COOH), the other at the C-terminus (H2N-LPFPEQPEQPFPQPEQPQ-(biotin)K-CONH2), and a 19-mer mono-deamidated peptide from alpha-gliadin H2N-QGSFQPEQLPQFEEIRNL(biotin)K-CONH2 (aa 252–270) and its native form H2N-QGSFQPQQLPQFEEIRNL(biotin)K-CONH2 were purchased from Eurogentec SA (Seraing, Belgium), dissolved in deionized water, diluted in Delfia assay buffer and stored in aliquots at −20°C until used. An extra C-terminal lysine residue was introduced to facilitate biotinylation.
The time-resolved fluorescence assays were performed using a Delfia 1234 Plate Fluorometer, Delfia plate shake and Delfia plate wash, all products of PerkinElmer Life and Analytical Sciences.
Labelling with lanthanide chelates
Europium-labelling of the polyclonal goat anti-human IgA antibody was performed with an 80-fold molar excess of DTTA-Europium chelate in a 50-mmol/l NaHCO3–Na2CO3, 9 g/l NaCl buffer, pH 9·3 at +4°C for 16 h. The Europium-labelled antibody was purified by gel filtration on a Superdex 200 column. The molar labelling degree, the number of Europium-chelates covalently bound per one antibody molecule, was typically 5–7 when measured against a Europium standard solution.
The monoclonal anti-human IgG antibody HP 6043 was labelled at +4°C for 20 h with a 60-fold molar excess of DTTA-Samarium in a 50-mmol/l NaHCO3–Na2CO3, 9 g/l NaCl buffer, pH 9·3. The Samarium-labelled anti-human IgG antibody was purified on a Superdex 200 gel filtration column as above. The labelling degree of the anti-human IgG-Samarium was typically 8–10 when a Samarium standard solution was used for the determination.
TTGA, AGA IgA and IgG ELISA assays
The ELISA assays were performed at room temperature on Biofons anti-gliadin and anti-tTG IgA and IgG ELISA kits (Biofile Ltd, Turku, Finland). Serum samples were diluted 1:100 in a dilution buffer (included in the kit). Standards and diluted samples were pipetted in duplicate in a volume of 100 µl in microtitre plate wells coated with gliadin (or recombinant tTG) protein. The plate was incubated for 30 min and washed four times with a plate washer. Alkaline phosphatase conjugated anti-human IgA or IgG tracer solution was added to the wells in a 100-µl volume, incubated for 30 min, and washed four times. One hundred µl of substrate solution was pipetted into each well, and the plate was incubated for 30 min. The stop solution (100 µl) was added to the wells, and the plate was gently shaken for 30 s. The absorbance was measured at 405 nm wave length in a microplate reader (Thermo Labsystems Ltd, Vantaa, Finland). The results were calculated against the standard curve and expressed in arbitrary units (AU). In AGA assays, a result < 30 AU was considered negative, samples > 50 AU positive, and those between 30 and 50 AU as weak positives. For children younger than 2 years of age samples below 50 AU were considered negative, those > 100 AU positive and 50–100 AU weakly positive. In tTGA IgA assays, results < 15 AU were considered negative, 15–20 AU weakly positive and > 20 AU positive. Results < 25 AU were negative in tTGA IgG assays and 25 AU or higher were positive.
Results
Establishment of the anti-gliadin peptide dual-labelled TR-IFMA assay
After preliminary peptide testing, due to higher signal-to-noise values obtained for the C-terminally biotinylated gamma-gliadin peptide (data not shown), the C-terminally biotinylated construct was chosen for further assay development. We selected to use slightly longer peptides than were the minimal (nonamer) epitopes used by Schwertz et al. [13], predicting that antibodies recognize more efficiently peptides protruding from the solid phase. The peptides were dissolved in deionized water, diluted in Delfia assay buffer and stored in aliquots at −20°C until used. In addition, deamidation of the original glutamine residues to glutamates was expected to render the peptides better binders of antibodies compared to the original one [14].
The assay was performed at room temperature on streptavidin-coated 96-well microtitre plates. The principle of the dual-labelled peptide AGA assay is presented schematically in Fig. 1. Twenty-five ng of biotinylated peptide in 100 µl of Delfia assay buffer were incubated for 45 min with continuous shaking on a Delfia plate shake. The wells were washed once with Delfia wash solution using a Delfia plate wash. One hundred µl of diluted serum sample or calibrator were added to each well and incubated for 45 min with shaking. After two washing rounds, 50 ng of Eu-labelled anti-human IgA and 100 ng of Sm-labelled anti-human IgG in 100 µl of Delfia assay buffer were added, and the incubation was continued for 30 min on a plate shake. After four washing rounds, 150 µl of Delfia enhancement solution were added to each well and incubated for 15 min on a plate shake. The Eu- and Sm-fluorescence was measured in a Delfia 1234 plate fluorometer, and the results were calculated using the Wallac Multicalc Data Management software. All samples were analysed in duplicate.
Fig. 1.
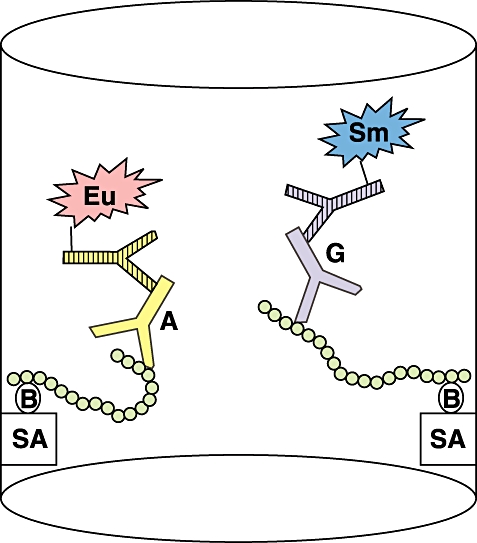
The principle of the anti-gliadin peptide dual-label immunofluorometric assay (IFMA) assay. The C-terminally biotinylated gamma gliadin peptide (residues 86–103) is attached to a streptavidin-coated microtitre plate. After incubation with serum sample and subsequent washes the plate is developed using secondary Europium-labelled anti-human IgA and Samarium-labelled anti-human IgG antibodies. The fluorescence is measured in a time-resolved fluorometer (Wallac).
During the dual assay development, we tested extensively variable concentrations of the assay components, different reaction conditions and parameters and combinations of these to obtain the best combination of sufficient signal level, optimal signal-to-noise ratio and analytical sensitivity, as well as specificity and reproducibility. As no correlation was observed in any conditions to CD using either of the alpha-gliadin peptides in IFMA, these were not analysed further (data not shown). This was contrary to the results obtained by Schwertz et al. [13].
Dual-label assay performance characteristics
Detection limit
The detection limit of the dual assay was 0·3 AU for AGA IgA and 2·5 AU for AGA IgG, when defined as the mean signal + 3 standard deviations (s.d.) detected from a negative serum.
Linearity
A serial dilution series of highly positive CD serum was measured and the IgA response was linear to approximately 3000 AU and IgG response to 6000 AU. No high-dose hook effect was observed up to 7500 AU and 15000 AU, respectively (Fig. 2a,b). Such high AGA values were observed only very rarely in the samples. It is obvious that the labelled antibody was the rate-limiting factor, as the amount of anti-human IgG-Samarium was twice that of anti-human IgA-Europium. The linear range was superior compared to AGA ELISA assays.
Fig. 2.
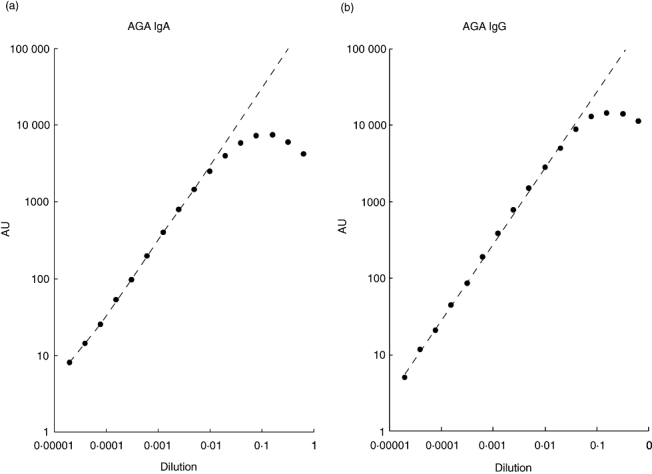
Linearity of the anti-gliadin peptide antibody response of the assay for IgA (Fig. 2a) and IgG (Fig. 2b). A strongly IgA- and IgG-positive patient serum was serially diluted in assay buffer and measured with IFMA assay as described in Subjects and methods.
Reproducibility
The variation of the dual-label peptide AGA TR-IFMA assay was determined in 10 runs (during 5 days, four replicates per run) using three serum samples that represented different, clinically relevant levels of AGA IgA (510–1169 AU) and IgG (156–1488 AU) concentrations. The mean intra-assay and interassay variations are summarized in Table 1, showing the high reproducibility of the assays. The intra-assay coefficient of variation (CV) was in the range of 1·0–2·2% and 1·2–1·7% for IgA and IgG, respectively. The interassay variation determined with three sera positive for both AGA IgA and IgG ranged from 3·1 to 3·9% and 2·3–3·6%, respectively.
Table 1.
Intra- and interassay variations of anti-gliadin antibody (AGA) peptide antibody assay.*
| Sample | IgA concentration (AU) | IgG concentration (AU) | IgA CV (%) | IgG CV (%) |
|---|---|---|---|---|
| Intra-assay variation | ||||
| 1 | 510·2 | 156·2 | 1·7 | 1·2 |
| 2 | 992·6 | 454·2 | 2·2 | 1·7 |
| 3 | 1169·4 | 1487·5 | 1·0 | 1·4 |
| Interassay variation | ||||
| 4 | 129·5 | 847·2 | 3·9 | 2·3 |
| 5 | 170·8 | 423·3 | 3·1 | 3·5 |
| 6 | 224·7 | 278·1 | 3·1 | 3·6 |
The intra-assay variation was determined on three different peptide AGA IgA- and IgG-positive sera which were applied in quadruplicate at five different locations on a microtitre plate. The interassay variations were determined with three different peptide AGA IgA- and IgG-positive sera using triplicates for each sample on a microtitre plate in 10 identical assays on eight separate days.
Recovery
Three serum samples containing a known amount of AGAs (75·5–1869 AU IgA and 194–2127 AU of IgG) were spiked with two different concentrations of an AGA-peptide antibody-containing serum. The mean recoveries obtained were 105% for AGA IgA and 101% for AGA IgG, when four replicates were analysed for each assay. The recoveries ranged from 85% to 124% for IgA and from 67% to 117% for IgG (Table 2).
Table 2.
Recovery of anti-gliadin peptide IgA and IgG antibodies.*
| Recovery | IgA | IgG |
|---|---|---|
| Mean | 105% | 101% |
| Max | 124% | 117% |
| Min | 85% | 67% |
Aliquots of three coeliac disease (CD) sera with different concentrations of IgA [75·5, 252·4 and 1869 arbitrary units (AU)] and IgG (193·7, 228·3 and 2127 AU) were mixed with two other CD sera positive for IgA (561·1 AU and 170·6 AU) and IgG (640·3 and 184·5 AU). The recoveries of the three former samples are given as percentages of the calculated values.
Analysis of clinical samples
In order to compare the performance of the anti-gliadin peptide IFMA assay with anti-gliadin and anti-tTG ELISAs (Biofile Ltd), sera from 87 CD patients diagnosed based on intestinal biopsy and sera from 81 healthy individuals were analysed. Two other healthy control populations, one comprising 100 young adults and the other 79 children below 5 years of age, were used for cut-off limit determination. The cut-off values in the peptide assay were set at the mean + 2 s.d. of control samples and the use of a ‘grey zone’ given in some commercial AGA kits could be avoided. It was necessary, however, to define separate cut-off values for children under 5 years of age (IgA 74 AU, IgG 160 AU), and for adults (IgA 125 AU, IgG 247 AU), as the background levels varied between these groups. This phenomenon might be explained by the different amounts of cross-reacting immunoglobulins in these age groups.
The performance characteristics of the novel IFMA method are summarized in Table 3. In the detection of AGA peptide antibodies we obtained specificities of 90% and 98% for IgA and IgG, respectively, while for the AGA ELISA (Biofile Ltd) 72% IgA and 64% IgG specificity were reached. The sensitivity of the IFMA peptide antibody method was 92% for IgA and 75% for IgG, whereas for the AGA ELISA, 87% and 78% were obtained, respectively. tTGA analysis of the same samples by the Biofons ELISA kit resulted in specificities of 90% and 86% for IgA and IgG, respectively, while the sensitivities achieved by the ELISA assay were 90% for IgA and 16% for IgG.
Table 3.
Specificity and sensitivity of anti-gliadin peptide immunofluorometric assay (IFMA) IgA and IgG assays compared with commercial anti-gliadin antibody (AGA) and anti-tissue transglutaminase antibody (tTGA) enzyme-linked immunosorbent assays (ELISA).*
| IgA | IgG | |||
|---|---|---|---|---|
| Assay | Specificity | Sensitivity | Specificity | Sensitivity |
| Peptide IFMA | 90% | 92% | 98% | 75% |
| AGA ELISA (Biofons) | 72% | 87% | 64% | 78% |
| tTGA ELISA (Biofons) | 90% | 90% | 86% | 16% |
Samples were analysed from 87 patients with biopsy-confirmed CD and 81 healthy individuals.
In order to analyse the suitability of the gliadin peptide IFMA test to reveal the emergence of anti-gliadin responses in samples collected before the gut epithelium damage, we analysed follow-up samples obtained from 20 HLA selected children at risk for developing type 1 diabetes. The sera were collected every 3 months from birth to 2 years and then at 6-month intervals, during 3–9-year periods. Ten of the children were known to be persistently tTGA-positive from the first positive serum test or were diagnosed later to have CD. Ten children tested only transiently tTGA positive, or their tTGA values fluctuated between positive and negative as measured with CelikeyTM (Pharmacia Diagnostics) tTGA IgA ELISA. Representative cases from these measurements are shown in Fig. 3. Generally, the appearance and kinetics of the gliadin peptide IgA and IgG antibodies showed high concordance with AGAs (not shown) and even higher with tTGA. In only one patient (no. 19789), the peptide AGA IgA appeared 3 months ahead of tTGA and peptide IgG (Fig. 3a). More distinct patterns were observed in the non-diseased individuals displaying fluctuating tTGA. The peptide responses raised 3–12 months prior to tTGA and peaked longer in four cases (nos 7622, 11147, 17295 and 24936). On the contrary, in child no. 14199 one single tTGA-positive peak was measured 2·5 years earlier than they became clearly positive for anti-gliadin peptide antibodies (Fig. 3b).
Fig. 3.
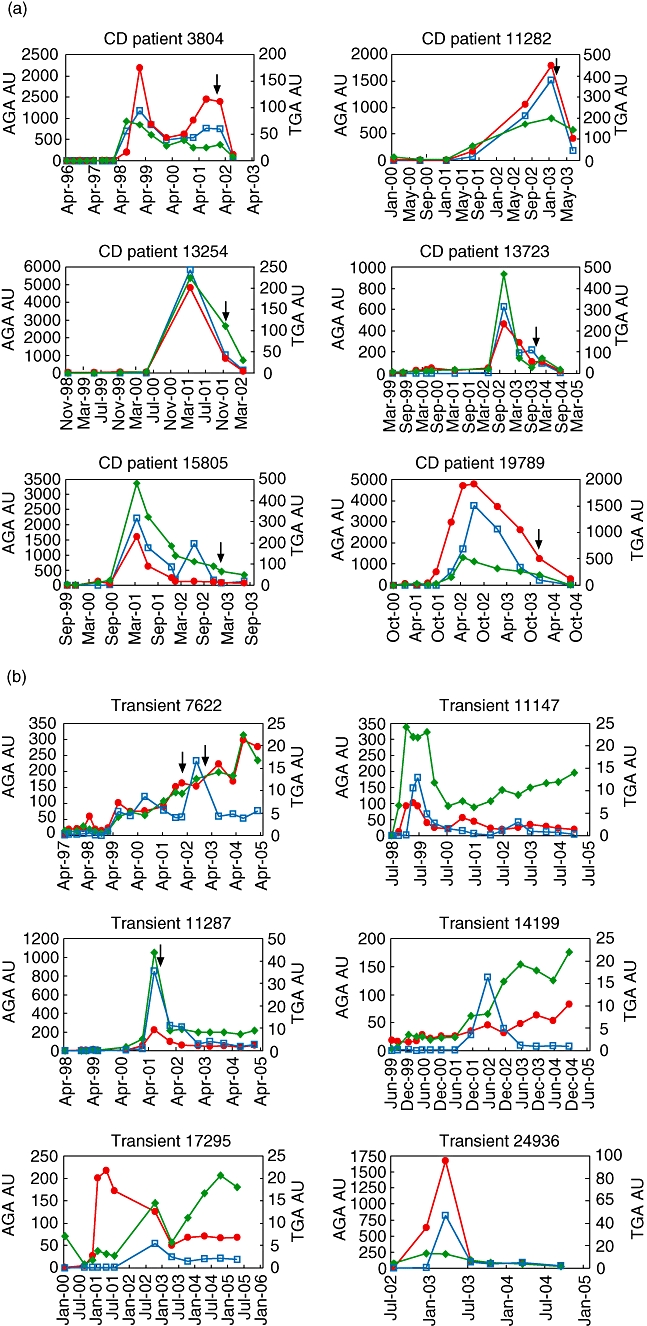
Anti-gliadin peptide IgA (red symbols) and IgG (green symbols) antibodies immunofluorometric assay (IFMA) and anti-tTG antibodies (tTGA) enzyme-linked immunosorbent assay (ELISA) (blue symbols) were analysed in follow-up sera from children at genetic risk for type 1 diabetes (Finnish Diabetes Prediction and Prevention study). Coeliac disease (CD) antibody responses were determined in individuals who eventually developed CD (a), and in healthy individuals transiently positive in tTGA IgA ELISA (b). The arrows indicate the time-point of small intestine biopsy.
Discussion
Approximately 1% of the population develop coeliac disease-associated damage of gut epithelium referring to coeliac disease at varying ages, but even the majority of the cases may remain undiagnosed despite the development of chronic disease-associated consequences. CD can be silent, and such a condition may already lead to unfavourable sequelae. One aim of the current diagnostic research is to identify with high sensitivity and specificity those individuals who most probably develop CD, as the identification of such individuals could form the basis for prophylactic intervention trials using specific preventive measures. Sensitive methods are also needed to identify changes in and response to therapy with possible preventive drugs or dietary formula. It is not sufficient to measure tTGA responses only, as antibodies to nutrients such as gliadin are important triggers and on–off regulators of emerging immunity in the gut. CD is the only autoimmune-like disease with an on–off testing possibility. Children with type 1 diabetes have an increased risk to present with CD, probably because they partly share the same HLA-alleles or due to susceptibility to non-specific activation of the immune system and subsequently altered dietary tolerance [15].
We have used a synthetic C-terminally biotinylated 19-mer peptide in a time-resolved immunofluorometric assay to recognize CD-specific antibodies in sera from CD patients. Simultaneous detection of both IgA and IgG type antibodies was achieved using Europium and Samarium labels, respectively, and combined with the excellent specificity (98%) of the peptide IgG IFMA the assay may be useful for CD diagnostics among IgA-deficient individuals [16,17]. Our observations verify the results by Schwertz et al. [13], that certain gliadin peptides are recognized preferentially by sera from individuals who develop CD. Moreover, these antibodies most often appeared simultaneously with tTG antibodies.
The present IFMA peptide assay reached the specificity and sensitivity of the commercial AGA and tTGA ELISA assays but showed a much wider dynamic response range, thus enabling reliable detection of antibodies over the whole physiological range. The reproducibility of the results was excellent, confirming the applicability of the assay for clinical screenings (Table 1). The broad linear dynamic range also allowed the establishment of clear cut-off limits. We could demonstrate that several samples from patients confirmed by intestinal biopsy and positive in IFMA assay remained in the grey zone in ELISA assays. We also showed that our assay was able to detect peptide antibodies in sera that tested negative for both EMA and tTGA, originating, however, from individuals with positive biopsy specimens.
Peptide AGA IFMA assay performed similarly to our tTGA tests (IgA specificity 90% for both, sensitivity 92% for IFMA, 90% for ELISA), but clearly the peptide AGA IgG specificity (98%) and sensitivity (75%) were higher than obtained with ELISA tTGA (86% and 16%) (Table 3). The ELISA test used performed suboptimally, as usually higher IgG class responses to tTG are reported. Our peptide IFMA performance characteristics were, however, lower than those obtained with the new ELISA test by Sugai et al. [18], who reported the use of a peptide ELISA (Inova Diagnostics, San Diego, CA, USA) with sensitivity and specificity of 94·6% and 99·1% for AGA IgA and 92·4% and 100% for AGA IgG. As no sequence data were given in that paper for the peptide used, it is difficult to discuss the possible role of antigen and compare these two methods. In comparison to other reported AGA peptide tests [18], the present IFMA assay performed comparably, and given that the sensitivity and specificity are dependent on the CD prevalence in the population and on the age of the subjects tested, these parameters may vary between different studies. The unit costs for the peptide IFMA IgA–IgG dual assay were slightly lower compared to the ELISA test including both anti-tTG IgA and IgG in our routine.
Summarized in Table 4, only three of a total of 87 CD sera were negative for both peptide AGAs and ELISA TGAs. Positive samples in the control group may be true false-positives or, alternatively, they may predict the early stage of disease induction, as seen in type 1 diabetes in terms of GAD65 and IA-2 autoantibodies [19]. IgG class antibody responses to tTG and especially those for EMA are generally very low [20,21]. Four of the nine control sera testing positive in the tTG IgG ELISA were very close to the cut-off level indicating suboptimal calibration of the test kit performance. The fact that we obtained 75% IgG anti-gliadin peptide sensitivity suggests a markedly more prominent induction of IgG immunity in the gut, even in patients with a normal IgA response (Table 3).
Table 4.
Frequency of peptide anti-gliadin antibody (AGA) and tTGA antibody patterns in a total of 87 coeliac disease (CD) patients and 81 control sera.
| Frequency of pattern | |||||
|---|---|---|---|---|---|
| PEP AGA | PEP AGA | tTGA | tTGA | ||
| IgA | IgG | IgA | IgG | CD | Healthy |
| + | + | + | – | 47 | 0 |
| + | – | + | – | 15 | 1 |
| + | + | + | + | 11 | 1 |
| + | + | – | – | 4 | 0 |
| + | – | + | + | 3 | 1 |
| – | – | – | – | 3 | 59 |
| + | – | – | – | 1 | 5 |
| – | + | – | – | 1 | 1 |
| – | + | + | – | 1 | 0 |
| – | – | + | – | 1 | 5 |
| + | – | – | + | 0 | 0 |
| + | + | – | + | 0 | 0 |
| – | + | + | + | 0 | 0 |
| – | – | + | + | 0 | 0 |
| – | – | – | + | 0 | 9 |
| – | + | – | + | 0 | 0 |
It was unexpected that such a high proportion of the selected tTGA-positive sera from children recruited originally to the DIPP follow-up study also showed AGA peptide antibodies with the responses following closely the appearance and disappearance of tTGA (Fig. 3). However, in addition to being a B cell epitope, the 19-mer gliadin peptide also contains the PFPQPQ sequence included in a known T cell epitope [22], which might aid the antibody formation against this particular gliadin peptide (residues 86–103). It may also be attached preferentially to tTG via the intrinsic activity of the enzyme to form covalent complexes followed by formation hapten-carrier structures with enhanced antigenic potential.
The transiently tTGA-positive children, sometimes with fluctuating antibody levels [23], represent an interesting group. If they do not develop CD, it may imply that there is a subclinical form of gluten intolerance which can be kept under control. It is known that both the gene dose (HLA-DQ2) and high exposure to gluten are associated with the risk of developing CD [24]. In contrast to CD patients, in some cases in transiently positive children, the AGA peptide IgG responses in particular were relatively independent of IgA responses and also of tTGA responses (Fig. 3). It remains to be seen whether these children with fluctuating antibody responses present eventually with CD and whether the gliadin peptide dual-assay can contribute to identifying individuals eventually progressing to disease.
Acknowledgments
This study was supported in part by the National Technology Agency of Finland (Tekes) and PerkinElmer Life and Analytical Sciences, Wallac Oy, Turku Finland.
References
- 1.Koning F, Schuppan D, Cerf-Bensussan N, Sollid LM. Pathomechanisms in celiac disease. Best Pract Res Clin Gastroenterol. 2005;19:373–87. doi: 10.1016/j.bpg.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Aleanzi M, Demonte AM, Esper C, Garcilazo S, Waggener M. Celiac disease: antibody recognition against native and selectively deamidated gliadin peptides. Clin Chem. 2001;47:2023–8. [PubMed] [Google Scholar]
- 4.Sjostrom H, Lundin KE, Molberg O, et al. Identification of a gliadin T-cell epitope in coeliac disease: general importance of gliadin deamidation for intestinal T-cell recognition. Scand J Immunol. 1998;48:111–5. doi: 10.1046/j.1365-3083.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 5.Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 6.Qiao SW, Bergseng E, Molberg O, Jung G, Fleckenstein B, Sollid LM. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: importance of proline spacing and glutamine deamidation. J Immunol. 2005;175:254–61. doi: 10.4049/jimmunol.175.1.254. [DOI] [PubMed] [Google Scholar]
- 7.Ivarsson A, Hernell O, Stenlund H, Persson LA. Breast-feeding protects against celiac disease. Am J Clin Nutr. 2002;75:914–21. doi: 10.1093/ajcn/75.5.914. [DOI] [PubMed] [Google Scholar]
- 8.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343–51. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 10.Feighery L, Collins C, Feighery C, et al. Anti-transglutaminase antibodies and the serological diagnosis of coeliac disease. Br J Biomed Sci. 2003;60:14–8. doi: 10.1080/09674845.2003.11783671. [DOI] [PubMed] [Google Scholar]
- 11.Lock RJ, Stevens S, Pitcher MC, Unsworth DJ. Is immunoglobulin A anti-tissue transglutaminase antibody a reliable serological marker of coeliac disease? Eur J Gastroenterol Hepatol. 2004;16:467–70. doi: 10.1097/00042737-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 12.van Herpen TW, Goryunova SV, van der Schoot J, et al. Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genomics. 2006;7:1. doi: 10.1186/1471-2164-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwertz E, Kahlenberg F, Sack U, et al. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem. 2004;50:2370–5. doi: 10.1373/clinchem.2004.036111. [DOI] [PubMed] [Google Scholar]
- 14.Osman AA, Gunnel T, Dietl A, et al. B cell epitopes of gliadin. Clin Exp Immunol. 2000;121:248–54. doi: 10.1046/j.1365-2249.2000.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson MA, Bowman MA, Kao KJ, et al. Lack of immune responsiveness to bovine serum albumin in insulin-dependent diabetes. N Engl J Medical. 1993;329:1853–8. doi: 10.1056/NEJM199312163292505. [DOI] [PubMed] [Google Scholar]
- 16.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Gut. 1998;42:362–5. doi: 10.1136/gut.42.3.362. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and ‘Club del Tenue’ Working Groups on Coeliac Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prince HE, Norman GL, Binder WL. Immunoglobulin A (IgA) deficiency and alternative celiac disease-associated antibodies in sera, submitted to a reference laboratory for endomysial IgA testing. Clin Diagn Lab Immunol. 2000;7:192–6. doi: 10.1128/cdli.7.2.192-196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugai E, Vazquez H, Nachman F, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4:1112–7. doi: 10.1016/j.cgh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Wasserfall CH, Atkinson MA. Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmun Rev. 2006;5:424–8. doi: 10.1016/j.autrev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Korponay-Szabo IR, Dahlbom I, Laurila K, et al. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut. 2003;52:1567–71. doi: 10.1136/gut.52.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin a deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol. 2002;9:1295–300. doi: 10.1128/CDLI.9.6.1295-1300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan L, Molberg O, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 23.Simell S, Kupila A, Hoppu S, et al. Natural history of transglutaminase autoantibodies and mucosal changes in children carrying HLA-conferred celiac disease susceptibility. Scand J Gastroenterol. 2005;40:1182–91. doi: 10.1080/00365520510024034. [DOI] [PubMed] [Google Scholar]
- 24.Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–8. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]


