Abstract
We investigated whether deficiency of mannose-binding lectin (MBL), a component of innate immunity, is associated with neonatal pneumonia and sepsis during the first 72 h, i.e. early onset, and during the first month after birth. In 88 neonatal intensive care patients (71 premature), MBL2 genotype and MBL plasma levels at birth were determined prospectively by Taqman analysis and enzyme-linked immunosorbent assay, respectively. Thirty-five neonates (40%) had low, i.e. ≤ 0·7 µg/ml, MBL plasma levels at birth. Median (interquartile range) MBL plasma levels in 32 no early-onset sepsis (EOS) cases, 44 possible EOS cases and 11 EOS cases were 1·57 (0·57–2·67) µg/ml, 1·05 (0·41–1·70) µg/ml and 0·20 (0·10–0·77) µg/ml, respectively (P < 0·01). During the first month, 28 neonates (32%) had no infection, 49 (55%) had suspected infection, five (6%) had pneumonia and six (7%) had culture-proven sepsis. Low MBL levels at birth were associated both with an increased risk of developing pneumonia (OR: 12·0; 95% CI: 1·1–126·1; P = 0·04) and culture-proven sepsis (OR: 15·0; 95% CI: 1·5–151·3; P = 0·02). These results were confirmed by genetic analysis of MBL deficiency. Low MBL levels at birth are associated with an increased risk of early-onset sepsis, culture-proven sepsis and pneumonia during the first month of life.
Keywords: complement, innate immunity, mannose-binding lectin, neonate, sepsis
Introduction
Despite improved neonatal care over the past decades, infections remain common and sometimes life-threatening in neonates admitted to the neonatal intensive care unit (NICU) [1,2]. Sepsis and pneumonia have the highest morbidity [3]. Early-onset sepsis (EOS) in neonates occurs in the perinatal period, while late-onset infection, especially sepsis and pneumonia, is transmitted in the nursery. For many years, a search has been ongoing to find predictors for neonatal sepsis that identify effectively patients who are at risk of infection [4].
Mannose-binding lectin (MBL) is a plasma protein that plays an important role in the innate immune defence. MBL activates the lectin pathway of the complement system by binding to various microorganisms. This leads to opsonization and enhanced phagocytosis [5]. Circulating MBL plasma levels are determined genetically and may vary between 0 and 10 µg/ml [6,7]. Three structural mutations in exon-1 of the MBL2 gene interfere with the assembly of the protein and cause decreased functional MBL plasma levels [7]. These variant genotypes are designated O, while the normal wild-type allele is called A [8]. In addition, three polymorphisms in the promoter region affect the MBL plasma level, but only the X variant of one of these polymorphisms is associated with low plasma levels. In contrast, the Y variant is associated with high MBL plasma levels [9]. Normal MBL plasma levels are seen in individuals with the YA/YA and YA/XA wild-type genotypes, whereas the XA/XA genotype is associated with both normal and low plasma levels [6,7]. Individuals with variant structural alleles (YA/O, XA/O and O/O) have low functional MBL plasma levels; functional MBL is almost absent in the XA/O and O/O genotypes [6,10]. In clinical studies, different definitions are used to describe genetic MBL deficiency, but most MBL disease associations are found in the presence of variant structural alleles [7]. Therefore, we will compare neonates with variant MBL2 structural genotypes (YA/O, XA/O and O/O) to neonates with wild-type MBL2 structural genotypes (YA/YA, YA/XA and XA/XA).
Variant MBL2 genotypes and low MBL plasma levels can be found in approximately 40% of the European population [6,11–13], and MBL deficiency has been associated with an increased susceptibility to infections, especially in children and immunocompromised individuals [14–16]. Very recently, low MBL levels at birth were found in neonates with nosocomial sepsis, in contrast to previous observations by others [17]. Sepsis definitions varied in these studies. In neonates, low MBL levels are associated not only with variant MBL2 genotype, but also with low gestational age (GA) [10,18–20]. Therefore, detection of MBL deficiency at birth should be based on actual MBL plasma levels rather than on MBL2 genotype. However, additional genetic analyses are important because we showed that neonates with wild-type MBL2 genotypes but low MBL levels at birth were able to obtain normal levels within time, in contrast to neonates with variant MBL2 genotypes [10].
In contrast to the previously published studies on MBL deficiency and neonatal sepsis, to our knowledge we are the first to determine both MBL2 genotype and MBL plasma levels at birth in neonates admitted to the NICU. The aim of our study was to investigate whether low MBL levels or variant MBL2 genotypes were associated with the occurrence of EOS during the first 72 h after birth, and with culture-proven sepsis or pneumonia during the first month of life.
Methods
Subjects and samples
From July 2002 until June 2003, we performed a prospective cohort study in the NICU of the Academic Medical Center, Amsterdam, the Netherlands. All neonates in whom blood was drawn for routine care within 24 h after birth were eligible. Patients with congenital abnormalities were excluded. Eighty-eight neonates (71 premature: gestational age < 37 weeks) were included consecutively after written informed consent was given by the parents. Recently, we described the prevalence of MBL deficiency in 85 neonates of this cohort [10]. In the remaining three patients, MBL analyses were performed recently in stored blood samples. The study protocol was approved by the local medical ethics committee.
We determined MBL2 genotype and MBL plasma levels in umbilical cord blood and neonatal blood drawn within 24 h after birth. Previously, we showed that MBL plasma levels in these samples are comparable [10]. When infection was suspected (see below), routine laboratory investigations included total leucocyte and leucocyte differentiation counts, C-reactive protein (CRP) and blood cultures. CRP levels were considered elevated above 10 mg/l [21]. The normal range for total leucocyte count was 5–30 × 109 cells/l [22]. Chest X-ray and tracheal aspirate cultures were performed when indicated clinically. Specimens were processed according to standard procedures.
Clinical data and infection classification
Along with general pre- and intrapartum clinical data, infectious signs and symptoms were recorded prospectively. They were divided into five categories: (1) temperature instability (< 37·0°C or > 38·5°C); (2) respiratory distress, e.g. dyspnoea, tachypnoea (> 60 breaths/min), apnoea, ventilation support, oxygen requirement, surfactant use; (3) cardiovascular dysfunction, e.g. tachycardia (> 160 beats/min), bradycardia (< 100 beats/min), decreased peripheral circulation, hypotension (diastolic blood pressure < 40 mm Hg), need for vasopressor support or inotropic medication; (4) neurological irregularities, e.g. hypotonia, lethargy, irritability; and (5) gastrointestinal problems, e.g. milk intolerance, vomiting, abdominal distension, suspicion of necrotizing enterocolitis [21,23]. Maternal risk factors for infection were fever (temperature > 38·0°C) and prolonged rupture of membranes > 24 h before delivery [21,24]. Diabetes gravidarum, maternal hypertension and pre-eclampsia were considered pregnancy-related diseases. Mothers who developed fever received a single shot of amoxicillin and gentamicin. When neonates were transferred to another hospital within 30 days after birth, clinical records and culture results of these hospitals were retrieved.
For our analysis, we used two outcome measures: (1) EOS within 72 h after birth and (2) infectious outcome during the first month of life. Clinical and laboratory records were reviewed and concerning EOS, three physicians (F. F., M. O. and K. D.), blind for MBL values, classified neonates independently as ‘no EOS’ cases, ‘possible EOS’ cases and ‘EOS’ cases (clinical EOS and culture-proven EOS). Discrepancies (seven of a total of 88 neonates) were solved by a consensus meeting.
For this purpose we used strictly defined criteria, which have been used previously in clinical studies on neonatal sepsis [20,21,23]: no EOS cases not had been evaluated for infection and therefore had not received any antibiotic treatment. The remaining neonates had received an EOS work-up because of maternal risk factors or neonatal clinical signs; a blood culture was drawn and antibiotics were administered. Possible EOS cases showed a combination of clinical signs, laboratory abnormalities or maternal risk factors without fulfilling the criteria of EOS. These neonates had received antibiotics (penicillin and gentamicin) for up to 7 days. Culture-proven EOS was defined as a combination of infectious signs and symptoms and a positive blood culture. Neonates were classified as having clinical EOS in the presence of maternal risk factors, increased CRP levels or pathological leucocyte counts accompanied by typical clinical signs of infection from at least three of the five categories [21,23]. EOS cases received antibiotics intravenously for at least 7 days.
The second outcome measure, the occurrence of infections during the first month of life, included the following outcome groups: (1) no infection at all; (2) suspected infection (including clinical EOS); (3) pneumonia; and (4) culture-proven sepsis. Pneumonia was defined as the presence of clinical respiratory symptoms, abnormalities on chest X-ray and positive tracheal aspirate cultures [25]. Culture-proven sepsis required the presence of typical infectious signs and symptoms in combination with a positive blood culture [26]. Severe infection was defined as the presence of culture-proven sepsis or pneumonia.
Assays
MBL measurements were performed at Sanquin Research and the Landsteiner Laboratory, Academic Medical Center, Amsterdam. MBL plasma levels were measured by an enzyme-linked immunosorbent assay as described previously [13,27]. Briefly, mannose was coated to the solid phase and after incubation with plasma, biotinylated mouse-anti-human MBL IgG (Tacx et al. [27]; 10 µg/ml, Amsterdam) was used as detection antibody. A receiver-operator characteristic curve yielded an optimal cut-off MBL plasma level of 0·7 µg/ml to discriminate between neonates with wild-type versus variant MBL2 genotypes. MBL plasma levels ≤ 0·7 µg/ml will be referred to hereafter as low MBL plasma levels.
Genotyping was performed independently of the clinical data collection, as described previously [10].
Statistical analysis
The Kruskal–Wallis test was used to determine whether MBL plasma levels differed significantly between the different outcome categories, followed by Mann–Whitney U-tests for single-outcome categories comparisons with Bonferroni correction for multiple testing. As numbers in the pneumonia and sepsis categories were small, both were combined into one severe infection outcome category for statistical analysis purposes. To examine the association of low MBL levels (≤ 0·7 µg/ml) and MBL2 genotypes (variant versus wild-type) with EOS and first-month infectious outcome categories, we estimated odds ratios (ORs) and 95% confidence intervals (CIs) from multinominal logistic regression analysis. These analyses were repeated with a cut-off plasma level of 0·2 µg/ml.
Univariate associations of GA, pregnancy-related disease, fetal distress and mode of delivery with EOS (no EOS, possible EOS and EOS) were studied with multinomial logistic regression analysis. Subsequently, we performed explorative multivariate analysis to study the association between MBL deficiency and EOS, adjusted for GA and clinical characteristics associated univariately with EOS, keeping in mind that due to small numbers reliability is limited. To optimize reliability, no more than two variables were added into a model at a time [28]; spss 12·0·1 for Windows statistical software was used.
Results
Clinical characteristics and MBL
The clinical characteristics of the 88 neonates (47 males) are presented in Table 1. The median GA was 32+3 (interquartile range (IQR): 29+6−36+1) weeks. The median birth weight was 1620 (IQR: 1200–2605) g. The mothers of 42 neonates showed signs of infection: maternal fever (n = 19), prolonged rupture of membranes (n = 16), or both (n = 7).
Table 1.
Clinical characteristics of 88 neonates.
| Clinical characteristic | n (%) | Median (interquartile range) |
|---|---|---|
| Neonate characteristics | ||
| Birth weight (g) | 1620 (1200–2605) | |
| Gestational age (weeks) | 32+3 (29+6−36+1) | |
| Prematurity (< 37 weeks) | 71 (81) | |
| Duration postnatal antibiotic treatment (days) | 3 (0–5) | |
| MBL2 genotype | ||
| YA/YA | 31 (44) | |
| YA/XA | 15 (22) | |
| XA/XA | 1 (1) | |
| YA/O | 13 (19) | |
| XA/O | 5 (7) | |
| O/O | 5 (7) | |
| Delivery characteristics | ||
| Pregnancy-related disease | 23 (26) | |
| Vaginal culture | ||
| Missing | 43 (49) | |
| No bacterial growth | 29 (33) | |
| Bacterial growth | 16 (18) | |
| Maternal risk factors of infection: | 42 (48) | |
| Maternal fever | 26 (30) | |
| Prolonged rupture of membranes | 23 (26) | |
| Antenatal antibiotic administration | 26 (30) | |
| Mode of delivery | ||
| Vaginal | 35 (40) | |
| Caesarean section | 53 (60) | |
| Fetal distress | 46 (52) | |
In 87 of 88 neonates MBL plasma levels were determined at birth. The remaining neonate had the YA/YA genotype and a MBL plasma level of 2·63 µg/ml 1 week after birth. Thirty-five neonates (40%) had low MBL plasma levels (Table 2). Due to the very small size of the blood samples taken, genotypes could be determined in only 70 neonates (80%). Twenty-three neonates (33%) had variant MBL2 genotypes (Tables 1 and 2). Outcome measures, MBL plasma levels, birth weight, GA and other clinical characteristics did not differ between neonates with and without known MBL2 genotypes (P-values > 0·25). The median MBL plasma levelwas 1·63 (IQR: 0·97–2·03) µg/ml in neonates with the wild-type MBL2 genotypes and 0·17 (IQR: 0·09–0·46) µg/ml in neonates with variant MBL2 genotypes (P < 0·01).
Table 2.
Risks of possible early-onset sepsis (EOS) and EOS compared to no EOS according to mannose-binding lectin (MBL) plasma levels, MBL2 genotype, gestational age and other selected clinical characteristics.
| Possible EOS | EOS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | OR | 95% CI | P-value | n | OR | 95% CI | P-value |
| Pregnancy-related disease | ||||||||
| No | 37 | Ref | 10 | Ref | ||||
| Yes | 8 | 0·3 | 0·1–0·8 | 0·02 | 1 | 0·1 | 0·0–1·1 | n.s. |
| Fetal distress | ||||||||
| No | 25 | Ref | 4 | Ref | ||||
| Yes | 20 | 0·5 | 0·2–1·4 | n.s. | 7 | 1·2 | 0·3–4·9 | n.s. |
| Gestational age (1 week↑) | 45 | 1·0 | 0·9–1·1 | n.s. | 11 | 1·0 | 0·8–1·2 | n.s. |
| Mode of delivery | ||||||||
| Vaginal | 22 | Ref | 7 | Ref | ||||
| Caesarean section | 23 | 0·2 | 0·1–0·7 | < 0·01 | 4 | 0·1 | 0·0–0·6 | < 0·01 |
| MBL plasma level (1 µg/ml↑) | 44 | 0·7 | 0·4–1·1 | n.s. | 11 | 0·2 | 0·1–0·7 | 0·01 |
| MBL plasma level | ||||||||
| Normal (> 0·7 µg/ml) | 27 | Ref | 3 | Ref | ||||
| Low (≤ 0·7 µg/ml) | 18 | 1·7 | 0·6–4·5 | n.s. | 8 | 6·8 | 1·5–31·6 | 0·01 |
| MBL plasma level | ||||||||
| Plasma level > 0·2 µg/ml | 38 | Ref | 5 | Ref | ||||
| Plasma level (≤ 0·2 µg/ml | 7 | 2·8 | 0·5–14·3 | n.s. | 6 | 18·0 | 2·8–115·6 | < 0·01 |
| MBL2 genotype | ||||||||
| Wild-type (YA/YA, YA/XA, XA/XA) | 25 | Ref | 3 | Ref | ||||
| Variant (YA/O, XA/O, O/O) | 13 | 3·3 | 0·8–13·2 | n.s. | 7 | 14·8 | 2·4–91·2 | < 0·01 |
OR, odds ratio; CI, confidence interval; Ref, reference; n.s., not significant. Multinominal logistic regression was used.
Early-onset sepsis
Thirty-two (36%) neonates were not suspected of EOS (no EOS cases; median GA: 32+6 weeks). Forty-five (48%) neonates were classified as possible EOS cases (median GA: 30+3 weeks). Eleven (16%) neonates were classified as EOS cases (median GA: 32+5 weeks). Of these, one had a positive blood culture (Haemophilus parainfluenzae); the remaining EOS cases were clinical EOS cases. Listeria monocytogenes was cultured in the amniotic fluid of three clinical EOS cases. The CRP level was elevated in one no EOS case, in eight possible EOS cases and in five EOS cases (P = 0·01). The median duration of antibiotic treatment started within 72 h after birth was 5 (IQR: 2–7) days in neonates with low MBL plasma levels compared to 3 (IQR: 0–4) days in neonates with normal MBL plasma levels (P = 0·01).
Neonates with low MBL plasma levels had an increased risk of EOS compared to neonates with normal levels (OR: 6·8; 95% CI: 1·5–31·6; P = 0·01; Table 2). A cut-off MBL plasma level of 0·20 µg/ml yielded similar results (Table 2). Median MBL plasma levels were 1·57 (IQR: 0·57–2·67) µg/ml in no EOS, 1·05 (IQR: 0·41–1·70) µg/ml in possible EOS and 0·20 (IQR: 0·10–0·77) µg/ml in EOS cases (Kruskal–Wallis test, P < 0·01; Fig. 1). Only median MBL plasma levels of EOS cases were decreased significantly compared to no EOS cases (Bonferroni-corrected Mann–Whitney U-test, P < 0·01). The MBL plasma level was 0·20 µg/ml in the culture-proven EOS case.
Fig. 1.
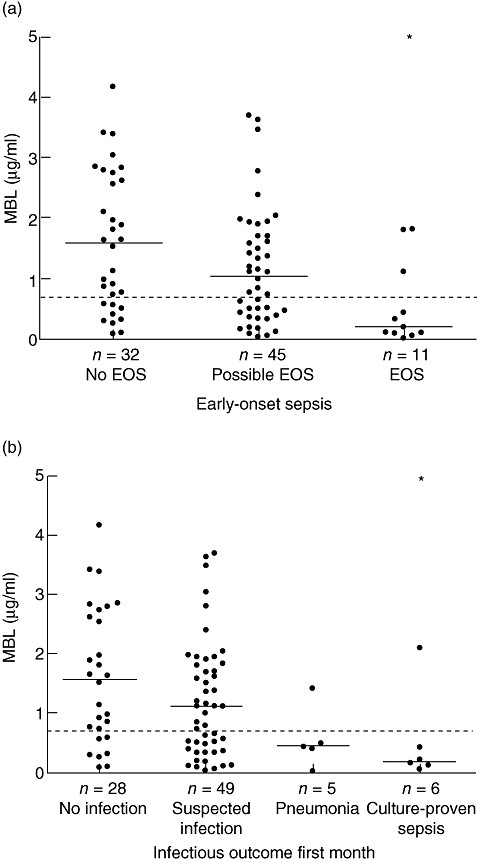
(a) Early-onset sepsis (EOS). Mannose-binding lectin (MBL) plasma levels in 32 no EOS cases, 45 possible EOS cases and 11 EOS cases. P-value (Kruskal–Wallis test) between groups < 0·01. (b) First month infectious outcome. MBL plasma levels in 28 no infection cases, 49 suspected infection cases, five pneumonia cases and six culture-proven sepsis cases. P-value (Kruskal–Wallis test) between groups is 0·03. MBL plasma level of one neonate missing. Dotted lines represent MBL plasma level of 0·7 µg/ml *P-value < 0·01 compared to reference (no EOS or no infection) group; Mann–Whitney U-test, adjusted for multiple comparisons.
MBL2 genotypes were detected in 22 no EOS cases, 38 possible EOS cases and 10 EOS cases. Neonates with variant MBL2 genotypes had a 15-fold higher risk of EOS compared to neonates with wild-type MBL2 genotypes (OR: 14·8; 95% CI: 2·4–91·2; P < 0·01; Table 2).
Explorative multivariate analysis EOS
The risk of EOS was decreased in neonates born by caesarean section (OR: 0·1; 95% CI: 0·0–0·6; P < 0·01, Table 2). GA and the remaining selected clinical characteristics were not associated with EOS. Explorative multivariate analyses suggested that the association between low MBL levels and EOS was maintained when adjusted for GA (adjusted OR of low MBL: 7·0; 95% CI: 1·5–32·9, P = 0·01) or mode of delivery (adjusted OR: 7·3, 95% CI: 1·5–36·0, P = 0·02, Table 3). The association between variant MBL2 genotypes and EOS was also maintained after adjustment for GA and mode of delivery, respectively (Table 3).
Table 3.
Multivariate analysis of the association between early-onset sepsis (EOS) and low mannose-binding lectin (MBL) levels and variant structural MBL2 genotypes, respectively.
| Possible EOS | EOS | |||||
|---|---|---|---|---|---|---|
| Model | Adjusted OR | 95% CI | P-value | Adjusted OR | P-value | 95% CI |
| Model 1 | ||||||
| Gestational age (1 week↑) | 1·0 | 0·9–1·1 | n.s. | 1·0 | 0·8–1·2 | n.s. |
| MBL plasma level | ||||||
| Normal (> 0·7 µg/ml) | Ref | Ref | ||||
| Low (≤ 0·7 µg/ml) | 1·7 | 0·6–4·6 | n.s. | 7·0 | 1·5–32·9 | 0·01 |
| Model 2 | ||||||
| Mode of delivery | ||||||
| Vaginal | Ref | Ref | ||||
| Caesarean section | 0·2 | 0·1–0·7 | < 0·01 | 0·1 | 0·0–0·6 | 0·01 |
| MBL plasma level | ||||||
| Normal (> 0·7 µg/ml) | Ref | Ref | ||||
| Low (≤ 0·7 µg/ml) | 1·8 | 0·6–4·9 | n.s. | 7·3 | 1·5–36·0 | 0·02 |
| Model 3 | ||||||
| Gestational age (1 week↑) | 1·0 | 0·9–1·2 | n.s. | 1·0 | 0·8–1·2 | n.s. |
| MBL2 genotype | ||||||
| Wild-type (YA/YA, YA/XA, XA/XA) | Ref | Ref | ||||
| Variant (YA/O, XA/O, O/O) | 3·3 | 0·8–13·2 | n.s. | 14·9 | 2·4–92·3 | < 0·01 |
| Model 4 | ||||||
| Mode of delivery | ||||||
| Vaginal | Ref | Ref | ||||
| Caesarean section | 0·2 | 0·1–0·8 | 0·03 | 0·1 | 0·0–0·8 | 0·02 |
| MBL2 genotype | ||||||
| Wild-type (YA/YA, YA/XA, XA/XA) | Ref | Ref | ||||
| Variant (YA/O, XA/O, O/O) | 3·1 | 0·7–13·0 | n.s. | 13·5 | 2·1–89·2 | < 0·01 |
OR, odds ratio; CI, confidence interval; NS, not significant. Multinominal logistic regression was used.
First-month infectious outcome
During the first month of life, 28 neonates (32%) did not show any signs of infection at all, 49 (55%) had a suspected infection at least once, five (6%) had pneumonia and six (7%) had culture-proven sepsis (including the culture-proven EOS case). The microorganisms cultured in the tracheal aspirates of the neonates with pneumonia were Citrobacter, coagulase-negative Staphylococcus epidermidis (CNS), Ureaplasma urealyticum, Klebsiella pneumoniae and Stenothrophomonas maltophilia. CNS was cultured in the blood of the five neonates with late-onset culture-proven sepsis. Only one neonate died, but this was not due to infectious complications.
During the first month, neonates with low MBL levels had an increased risk of severe infections (pneumonia or culture-proven sepsis) (OR: 13·5; 95% CI: 2·3–78·1; P < 0·01) compared to neonates with normal levels (Table 4). Four of five neonates with pneumonia and five of six neonates with culture-proven sepsis had low MBL levels at birth (Fig. 1). A cut-off MBL plasma level of 0·2 µg/ml yielded similar results (data not shown). Median MBL plasma levels were 1·57 (IQR: 0·62–2·70) µg/ml in the no infection group, 1·11 (IQR: 0·37–1·81) µg/ml in the suspected infection group and 0·41 (IQR: 0·14–0·46) µg/ml in the severe infection group (Kruskal–Wallis, P < 0·01). After Bonferroni correction, only the median MBL plasma level of the severe infection compared to the no infection group was decreased significantly (P < 0·01). Median plasma levels in the pneumonia and culture-proven sepsis group separately were 0·43 (IQR: 0·21–0·95) µg/ml and 0·18 (IQR: 0·10–0·85 µg/ml), respectively (Fig. 1).
Table 4.
Risks of infectious outcome during the first month of life.
| MBL plasma level | |||||
|---|---|---|---|---|---|
| Outcome measure | Normal (> 0·7 µg/ml) n | Low (≤ 0·7 µg/ml) n | OR | 95% CI | P-value |
| (a) According to mannose-binding lectin (MBL) plasma level (n = 88) | |||||
| Severe infection | 2 | 9 | 13·5 | 2·3–78·1 | < 0·01 |
| Culture-proven sepsis | 1 | 5 | 15·0 | 1·5–151·3 | 0·02 |
| Pneumonia | 1 | 4 | 12·0 | 1·1–126·1 | 0·04 |
| Suspected infection | 30 | 19 | 1·9 | 0·7–5·3 | n.s. |
| No infection | 21 | 7 | Ref | – | |
| MBL2 genotype | |||||
| Wild-type | Variant | ||||
| (b) According to MBL2 genotype (n = 70) | |||||
| Severe infection | 3 | 5 | 10·0 | 1·5–65·7 | 0·02 |
| Culture-proven sepsis | 1 | 2 | 12·0 | 0·8–177·4 | n.s. |
| Pneumonia | 2 | 3 | 9·0 | 1·0–78·6 | 0·05 |
| Suspected infection | 26 | 15 | 3·5 | 0·9–13·7 | n.s. |
| No infection | 18 | 3 | Ref | – | |
Multinominal logistic regression was used. The odds ratio (OR) can be interpreted as an estimation of the relative risk for the infectious outcome category compared to the ‘no infection’ category. CI, confidence interval; n.s., not significant.
Neonates with variant MBL2 genotypes had a statistically significant increased risk of developing any severe infection during the first month of life (OR: 10·0; 95% CI: 1·5–65·7; P = 0·02), compared to neonates with wild-type MBL2 genotypes (Table 4). Of the 11 neonates with a severe infection, five had variant, three had wild-type and three had unknown MBL2 genotypes. The separate risks of pneumonia (OR: 9·0; 95% CI: 1·0–78·6, P = 0·05) and culture-proven sepsis (OR: 12·0; 95% CI: 0·8–177·4, P = 0·07) also tended to be increased (Table 4).
Discussion
Our findings suggest that MBL deficiency increases the susceptibility to infections in premature and term NICU-patients. Both the presence of low (≤ 0·7 µg/ml) MBL plasma levels at birth and variant MBL2 genotypes appeared to be associated with EOS. In addition, neonates with low MBL plasma levels appeared to have an increased risk of severe infections during the first month of life compared to neonates with normal plasma levels. Although our study was hampered by small numbers, these results are in agreement with a recently reported increased risk of nosocomial sepsis with low MBL levels in a cohort of 206 neonates [17]. Furthermore, variant MBL2 genotypes have been associated with an increased susceptibility to develop sepsis and septic shock in critically ill children and adults previously [11,29,30]. Low MBL levels might increase the susceptibility for sepsis by a decreased capacity of phagocytosis or opsonization of microorganisms.
The present study on the association between MBL deficiency and neonatal infections is, to our knowledge, the first to involve both MBL plasma level and MBL2 genotype. This is very important in studies on MBL in neonates, as premature neonates without MBL2 gene mutations can have only temporary low MBL levels at birth [10]. De Benedetti et al. [17] might have overestimated the infection risk because they determined MBL serum levels only once on admission to the NICU. Premature neonates with low MBL levels at birth that had wild-type MBL-2 genotypes might have developed normal MBL levels before the onset of sepsis [10,19]. However, our genetic analyses support the findings of de Benedetti et al. [17]. Therefore, persistently low MBL levels indeed appear to play a role in susceptibility to neonatal infections during the first weeks of life. Ahrens et al. [4] did not find an increased infection risk in premature neonates with exon-1 mutations, but they probably underestimated the infection risk because the effect of low MBL plasma levels due to prematurity and the X promoter polymorphism were not investigated [4]. Hilgendorff et al. [20] did not find an increased EOS risk in neonates with low MBL levels, but they had a smaller cohort.
Comparison of studies on MBL and neonatal sepsis is also hampered by different sepsis definitions used in different studies. For instance, Ahrens et al. [4] and De Benedetti et al. [17] used culture-proven sepsis at any time during hospital stay, while Hilgendorff et al. [20] studied congenital sepsis diagnosed clinically within 72 h of life. Although culture-proven infections are preferred in clinical research, it is now accepted widely that early-onset neonatal sepsis is often a clinical diagnosis because blood cultures can be false-negative after antenatal administration of antibiotics [20–23]. By using generally accepted criteria for clinical EOS, similar to previous reports on the subject, exclusion of other explanations of the clinical symptoms is pursued [20–23]. An advantage of our study is that in contrast to the three previously published studies in neonates, we investigated the presence of early-onset sepsis and infectious outcome during the first month separately. In this way we overcome sepsis definition difficulties.
Our cohort consisted of primarily premature neonates. Given the fact that low gestational age is associated with low MBL levels and increased risk of infection [10,24], the selection of younger children may lead to an overestimation of the observed association between MBL and infection risk. As a consequence, these results may not be applicable to term neonates. However, as the association between low MBL plasma levels and risk of EOS was maintained after adjustment for GA in multivariate analysis, we do not expect that maturational MBL plasma level differences affected our results. The association between low MBL plasma levels and increased risk of EOS is also independent of mode of delivery. However, because our study number was small, a larger prospective study is needed to confirm these findings. The most likely explanation for the univariate association between mode of delivery and EOS is that children born by uncomplicated vaginal delivery were admitted to the NICU only in case of (suspected) infection.
In conclusion, neonates with low MBL plasma levels appear to be at increased risk of severe neonatal infections, both of early- and late-onset. Therefore, MBL measurements might be used to identify which neonates are prone for infections. Furthermore, in the near future substitution of plasma-purified or recombinant MBL may protect neonates with low MBL plasma levels from sepsis or pneumonia [31]. However, a large, well-designed prospective multi-centre study should confirm the association between low MBL plasma levels and both early-onset and late-onset sepsis, taking into account the complicated developmental profile of MBL in neonates. This study must include serial MBL level measurements during the first weeks after birth and at the onset of and during infection. In addition, MBL2 genetic polymorphisms are required to validate these measurements.
Acknowledgments
We would like to thank Charlotte Dorrepaal MD for collecting clinical data and plasma samples and Joris van der Post MD, PhD for helpful discussion. This study was supported financially by the Landsteiner Foundation for Blood Transfusion Research (grant LSBR 0207).
References
- 1.Klein JO. Bacterial sepsis and meningitis. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infants. Philadelphia: WB Saunders; 2001. pp. 943–98. [Google Scholar]
- 2.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–03. Pediatr Infect Dis J. 2005;24:635–9. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 3.Sinha A, Yokoe D, Platt R. Epidemiology of neonatal infections: experience during and after hospitalization. Pediatr Infect Dis J. 2003;22:244–51. doi: 10.1097/01.inf.0000055060.32226.8a. [DOI] [PubMed] [Google Scholar]
- 4.Ahrens P, Kattner E, Kohler B, et al. Mutations of genes involved in the innate immune system as predictors of sepsis in very low birth weight infants. Pediatr Res. 2004;55:652–6. doi: 10.1203/01.PDR.0000112100.61253.85. [DOI] [PubMed] [Google Scholar]
- 5.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer N, Dolman KM, van Zwieten R, et al. Mannan-binding lectin (MBL)-mediated opsonization is enhanced by the alternative pathway amplification loop. Mol Immunol. 2006;43:2051–60. doi: 10.1016/j.molimm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency − revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 8.Madsen HO, Garred P, Kurtzhals JA, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 9.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 10.Frakking FN, Brouwer N, Zweers D, et al. High prevalence of mannose-binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol. 2006;145:5–12. doi: 10.1111/j.1365-2249.2006.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidler KJ, Wilson P, Davies JC, Turner MW, Peters MJ, Klein NJ. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intens Care Med. 2004;30:1438–45. doi: 10.1007/s00134-004-2303-8. [DOI] [PubMed] [Google Scholar]
- 12.Crosdale DJ, Ollier WE, Thomson W, et al. Mannose binding lectin (MBL) genotype distributions with relation to serum levels in UK Caucasoids. Eur J Immunogenet. 2000;27:111–7. doi: 10.1046/j.1365-2370.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 13.Frakking FN, van de Wetering MD, Brouwer N, et al. The role of mannose-binding lectin (MBL) in paediatric oncology patients with febrile neutropenia. Eur J Cancer. 2006;42:909–16. doi: 10.1016/j.ejca.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 15.Cedzynski M, Szemraj J, Swierzko AS, et al. Mannan-binding lectin insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol. 2004;136:304–11. doi: 10.1111/j.1365-2249.2004.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–8. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 17.De Benedetti F, Auriti C, D'urbano LE, et al. Low serum levels of mannose binding lectin are a risk factor for neonatal sepsis. Pediatr Res. 2007;61:325–8. doi: 10.1203/pdr.0b013e318030d12f. [DOI] [PubMed] [Google Scholar]
- 18.Lau YL, Chan SY, Turner MW, Fong J, Karlberg J. Mannose-binding protein in preterm infants: developmental profile and clinical significance. Clin Exp Immunol. 1995;102:649–54. doi: 10.1111/j.1365-2249.1995.tb03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terai I, Kobayashi K. Perinatal changes in serum mannose-binding protein (MBP) levels. Immunol Lett. 1993;38:185–7. doi: 10.1016/0165-2478(93)90004-l. [DOI] [PubMed] [Google Scholar]
- 20.Hilgendorff A, Schmidt R, Bohnert A, Merz C, Bein G, Gortner L. Host defence lectins in preterm neonates. Acta Paediatr. 2005;94:794–9. doi: 10.1111/j.1651-2227.2005.tb01987.x. [DOI] [PubMed] [Google Scholar]
- 21.Nupponen I, Andersson S, Jarvenpaa AL, Kautiainen H, Repo H. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. 2001;108:E12. doi: 10.1542/peds.108.1.e12. [DOI] [PubMed] [Google Scholar]
- 22.Ottolini MC, Lundgren K, Mirkinson LJ, Cason S, Ottolini MG. Utility of complete blood count and blood culture screening to diagnose neonatal sepsis in the asymptomatic at risk newborn. Pediatr Infect Dis J. 2003;22:430–4. doi: 10.1097/01.inf.0000068206.11303.dd. [DOI] [PubMed] [Google Scholar]
- 23.Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54:1251–7. doi: 10.1016/s0895-4356(01)00400-0. [DOI] [PubMed] [Google Scholar]
- 24.Baltimore RS. Neonatal sepsis: epidemiology and management. Paediatr Drugs. 2003;5:723–40. doi: 10.2165/00148581-200305110-00002. [DOI] [PubMed] [Google Scholar]
- 25.van der Zwet WC, Kaiser AM, van Elburg RM, et al. Nosocomial infections in a Dutch neonatal intensive care unit: surveillance study with definitions for infection specifically adapted for neonates. J Hosp Infect. 2005;61:300–11. doi: 10.1016/j.jhin.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 27.Tacx AN, Groeneveld AB, Hart MH, Aarden LA, Hack CE. Mannan binding lectin in febrile adults: no correlation with microbial infection and complement activation. J Clin Pathol. 2003;56:956–9. doi: 10.1136/jcp.56.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz MH. Multivariable analysis: a primer for readers of medical research. Ann Intern Med. 2003;138:644–50. doi: 10.7326/0003-4819-138-8-200304150-00012. [DOI] [PubMed] [Google Scholar]
- 29.Garred P, Strom J, Quist L, Taaning E, Madsen HO. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J Infect Dis. 2003;188:1394–403. doi: 10.1086/379044. [DOI] [PubMed] [Google Scholar]
- 30.Gordon AC, Waheed U, Hansen TK, et al. Mannose-binding lectin polymorphisms in severe sepsis: relationship to levels, incidence, and outcome. Shock. 2006;25:88–93. doi: 10.1097/01.shk.0000186928.57109.8d. [DOI] [PubMed] [Google Scholar]
- 31.Valdimarsson H, Vikingsdottir T, Bang P, et al. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol. 2004;59:97–102. doi: 10.1111/j.0300-9475.2004.01357.x. [DOI] [PubMed] [Google Scholar]


