Abstract
Cysteine proteinases, termed gingipains, of Porphyromonas gingivalis are able to inactivate a broad range of host proteins involved in cellular responses and have been implicated as key virulence factors in the onset and progression of adult periodontitis. In the present study, the high molecular weight Arg-gingipain, RgpA, produced a time- and concentration-dependent hydrolysis of the tumour necrosis factor (TNF)-α receptor family member CD27 on resting T cells. As a consequence of CD27 degradation, a reduction in CD27-ligation dependent co-stimulatory CD40L expression was observed. Concomitantly, RgpA activated the protease-activated receptors (PAR)-1, PAR-2 and PAR-4 and induced CD69 and CD25 expression on T cells, thereby demonstrating T cell activation. The Lys-gingipain Kgp demonstrated a low capacity to degrade CD27 but the ability to affect CD27 expression and biological activity was increased when T cells were pretreated with blocking peptide against PAR-2. CD70, the ligand for CD27 induced on activated B cells, was significantly reduced by RgpA treatment and weakly affected by Kgp. These findings suggest that while RgpA can activate T cells through PARs, the parallel action of direct hydrolysis of membrane CD27 as well as CD70 indicates a potential down-regulatory effect through inhibition of CD27/CD70-mediated cell activation in periodontitis.
Keywords: blocking peptides, CD27, gingipains, protease activated receptors, proteolysis
Introduction
Porphyromonas gingivalis has been implicated as a major aetiological agent in periodontitis. Previous studies have shown that P. gingivalis can penetrate the epithelial barrier surrounding the gingival sulcus,and lead to increased vascular permeability [1–3]. Virulence of P. gingivalis is associated with the proteolytic enzymes expressed by this Gram-negative anaerobic bacterium due to the ability to activate and/or degrade a broad range of host proteins and cell adhesion molecules [3–5]. These cysteine proteinases referred to as Arg-gingipain (two genes code for RgpA and RgpB, respectively) and Lys-gingipain (one gene codes for Kgp) can overcome host defense mechanisms [6–8]. T cell-mediated immune responses to bacteria have emerged as playing a key role in connective tissue destruction including bone resorption in periodontitis [9]. Early periodontal lesions are characterized by T cell infiltrates, while B lymphocytes and plasma cells dominate in advanced disease [10,11]. Significantly, gingipains have been shown to reduce the surface expression of different receptors such as CD2, CD4, and membrane tumour necrosis factor (TNF)-α on lymphocytes [12,13] and to degrade major proinflammatory cytokines, including interferon (IFN)-γ [6]. These studies indicate that gingipains may reduce T cell function at periodontal lesion sites.
Activation and priming of T cells during the adaptive immune response is regulated by a complex series of signals between antigen-presenting cells (APCs) and T cells [14]. CD27 is a lymphocyte-specific member of the TNF-α receptor family [15]. Like other members of this family, CD27 is expressed as a transmembrane protein that consists of three monomeric subunits [16]. CD70, the cellular ligand for the TNF receptor family member CD27, is expressed transiently on activated T and B cells. The interaction between CD27 and its ligand, CD70, augments the induction of T cell activation [17,18] and plays a critical role in T cell-dependent B cell differentiation into plasma cells [19]. Activation of T cells by anti-CD3 monoclonal antibodies (mAb) elicits a strong up-regulation of CD27 surface expression [16]. Moreover, a defect in CD27 expression or function has been shown to contribute to the pathogenesis of certain forms of common variable immunodeficiency [20].
Protease-activated receptors (PARs) are expressed by a variety of cells and are considered to be involved in different physiological and pathological processes, including growth and development and inflammation [21]. The cellular activity of some proteases [8] including thrombin [22] is mediated mainly through the four members of the PAR family that belong to the G protein-coupled receptors [23,24]. Proteolytic cleavage of the N-terminal exo-domain of the receptor exposes ‘tethered ligands’ that appear to initiate signalling by interaction with the second extracellular loop of the receptor [21]. A proinflammatory role for PAR-2 in periodontits is supported, indirectly, by several studies. Gingipains were demonstrated to activate PAR-2 in neutrophils and in an oral epithelial cell line [25,26]. Other studies have suggested a destructive role for PAR-2 activation in inducing inflammation and bone resorption during periodontitis [27]. Further, delayed onset of inflammation has been shown in PAR-2-deficient mice [28]. However, no study has yet linked the role of PAR-1 to PAR-4 to the potential physio-pathological importance of these proteolytic effects of gingipains at the surface of immune cells. These gingipain-triggered cellular events could plausibly be associated with progression of inflammatory disease.
In this paper we show that Arg-gingipain preferentially degrades membrane CD27 and, as a consequence, affects CD27 ligation-dependent CD40L expression on CD4+ T cells. RgpA can activate PAR-1, PAR-2 and PAR-4 and induces CD69 and CD25 expression in CD4+ T cells. While Kgp does not as cleave CD27 effectively, blocking of PAR-2 activation increases total Kgp proteolytic degradation of CD27 leading to a complete loss of anti-CD27 mAb-induced CD40L expression. Our data also indicate that CD70 expressed on activated B cells can be down-regulated efficiently by RgpA and slightly affected by Kgp.
Materials and methods
Chemicals and reagents
Fetal calf serum (FCS), l-cysteine, paraformaldehyde, propidium iodide, protease-inhibitor cocktails (for mammalian tissues), RNase A, Salmonella typhimurium lipopolysaccharide (LPS), sodium azide (NaN3), sodium dodecyl sulphate (SDS), N-α-tosyl-L-lysyl chloromethyl ketone (TLCK), Triton X-100, Trizma base, Tris-hydrochloride (Tris-HCl) and Tween 20 were purchased from Sigma (St Louis, MO, USA). RPMI medium was obtained from ICN Biochemicals (Irvine, CA, USA); 3- [(3-cholamidopropyl) dimethylammonio]-1-propanesulphonate (CHAPS) was purchased from Calbiochem (La Jolla, CA, USA). Phosphate-buffered saline (PBS) and Trypticase soy broth were purchased from Oxoid (Basingstoke, UK). All reagents for electrophoresis and Western blotting were from Bio-Rad (Richmond, CA, USA.).
Antibodies
Human recombinant CD27 (rCD27) and polyclonal antibody against CD27 was obtained from R&D Systems (Minneapolis, MN, USA.). Mouse mAb specific for human CD3, CD25, CD27, CD40, CD40L, CD69, CD70, ICAM-3 and PAR-1 were purchased from Becton Dickinson Inc. (Heidelberg, Germany). Polyclonal antibodies specific for human CD27, N-terminus of PAR-2 (S-19), -3 (H-103) and -4 (S-20); and blocking peptides against the N-terminus of PAR-1 (sc-8204 P), -2 (sc-8206 P), -4 (sc-8461 P) and C-terminus of PAR-3 (sc-8208 P) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Bacterial strain and proteinase purification
P. gingivalis (ATCC 33277) was grown in enriched Trypticase soy broth under anaerobic conditions for 48 h. Arg-gingipain and Lys-gingipain proteinase–adhesin complexes were purified according to the method described previously [6].
Cell isolation and preparation
Human peripheral blood mononuclear cells (PBMC) were separated from healthy volunteers (Blood Bank, Red Cross Transfusion Service, NSW, Australia) using Ficoll-Hypaque gradients (Robbins Scientific, Sunnyvale, CA, USA). CD4+ T cells or CD19+ B cells were isolated from PBMC by positive selection using magnetic beads (Invitrogen) coated with anti-CD4+ or -CD19+ antibody (Dynal Inc., Lake Success, NY, USA), respectively, as described previously [12].
T cell membrane molecules and gingipains
Purified CD4+ T cells at 1 × 105 cells/200 µl/well of RPMI-1640 medium in 96-well flat-bottomed plates were treated with medium alone or 5 mM l-cysteine-activated RgpA or Kgp at indicated concentrations; or TLCK (final concentration at 2 mM)-inhibited RgpA; or TLCK-inhibited Kgp under serum-free conditions for 1–2 h. To control for receptor turnover, T cells were fixed in 1% paraformaldehyde in PBS for 10 min at room temperature. After washing, fixed cells were incubated with 70 nM RgpA or Kgp for 1 h at 37°C, followed by washing and stained for surface CD27. For flow cytometric analysis, cells were washed twice with fluorescence activated cell sorter (FACS) washing buffer (0·1% BSA and 0·01% NaN3 in cold PBS), and stained with the corresponding primary monoclonal or polyclonal antibodies (1 : 50) against human: CD27, CD69, ICAM-3 and PAR-1, PAR-2, PAR-3 and PAR-4, or with matched isotype control antibodies. Cells were then labelled with 1 : 100 diluted fluorescein isothiocyanate (FITC)-conjugated anti-IgG antibody (Dako, Botany, Denmark) and quantified using a Becton Dickson FACScan analyser. Incubations were for 50 min at 4°C for each stage. Volume gates were set to include the entire T cell population. In functional studies of CD27 molecules, T cells were pretreated with or without 5 mM cysteine-activated RgpA or Kgp at 70 nM for 1 h under serum-free conditions. Cells were then washed twice to remove gingipains, and stimulated with 5 µg/ml of anti-CD27, anti-CD3 mAb; or isotype-matched mAb for 1 h. Surface expression of CD40L was detected by flow cytometry. Data were collected as histograms of relative fluorescence in a logarithmic scale on the x-axis and cell number as a linear scale on the y-axis.
Proteolytic digestion of CD27 and immunoblot analysis
RgpA or Kgp were preincubated for 15 min at 37°C with 5 mM l-cysteine. Activated RgpA or Kgp was incubated with rCD27 at a final substrate/enzyme (S/E) ratio of 1 : 1 (18 nM rCD27 with 18 nM RgpA or Kgp) in the absence of serum. Both reactions were then incubated at 37°C for a time–course study. Hydrolysis was terminated at the indicated time with TLCK (2 mM final concentration). Aliquots were then resolved by 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blot analysis [29]. Western blot staining was performed using the primary anti-human Abs (1 : 500) and the corresponding AP-conjugated secondary antibodies (1 : 1000). Membranes were washed five times in Tris-buffered saline−0·1% Tween20 between each step. Colour was developed in a solution containing nitroblue tetrazolium chloride (1·65 mg) and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (0·8 mg) in 10 ml of 100 mM Tris-HCl (pH 9·5).
CD27 release from fixed T cells following incubation with gingipains
Purified CD4+ T cells were fixed in 1% paraformaldehyde in PBS for 10 min at room temperature. After washing, fixed T cells were incubated with either activated 70 nM gingipains or TLCK-inhibited gingipains in serum-free medium for 1 h at 37°C. At termination, 20% (v/v) protease inhibitor cocktail was added. Cells were then centrifuged at 1500 g for 15 min, and the cell-free supernatants collected. The level of CD27 was measured by sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, mouse anti-CD27 mAb (1 µg/ml in PBS) was used as a capture antibody to coat 96-well high-affinity flat-bottomed ELISA plates (Sarstedt, Mawson Lakes, Australia) overnight at 4°C. Blocking was performed with 0·1% BSA in PBS for 1 h at 37°C. Subsequently, undiluted culture supernatant or rCD27 standard was added to wells overnight at 4°C, and goat anti-CD27 polyclonal antibody (1 µg/ml in PBS/0·1% Tween20) was added for 2 h at 37°C. Between each step, the plates were washed three times in PBS with 0·1% Tween20. ELISA was developed using an alkaline phosphatase-conjugated rabbit antigoat IgG (0·5 µg/ml in PBS/0·1% Tween20) and phosphatase substrate (Bio-Rad, Gladesville, USA). Plates were read at 405 nm in a Bio-Rad Benchmark microplate reader within 1 h. Optical densities of standard rCD27 were plotted against the dilution factors, and the sCD27 concentration of each sample was determined.
Cell-cycle analysis
Purified CD4+ T cells at 1 × 105 cells/200 µl/well of RPMI-1640 medium in 96-well flat-bottomed plates were treated with medium alone or 5 mM cysteine-activated RgpA or Kgp at 70 nM; or TLCK (final concentration at 2 mM) inhibited RgpA; or TLCK-inhibited Kgp under serum-free conditions for 1 h. At the end of the incubation, the cells were washed and 400 µl RPMI-1640 medium with 10% FCS added to the culture for 48 h. After incubation, cells were fixed in 70% ethanol for 1 h. After washing and suspending in PBS, cells were stained with propidium iodide (PI)/Triton X-100 staining solution with RNase A for 30 min at room temperature. DNA cell cycle analysis acquired in a monoparametric histogram (emission wavelength = 612 nm) was measured on PI solution-stained nuclei by using a FACScan. Cells were gated to exclude dead cells, doublets and triplets.
PARs blocking peptides and the cell membrane molecules
Purified CD4+ T cells were treated with medium containing 15 µg/ml of blocking peptides to PAR-1, -2, -3 and -4 for 1 h at 37°C. Cells were then washed and treated with 70 nM RgpA or Kgp for 60 min and surface expression of CD27 and CD69 was evaluated by flow cytometry. For CD40L analysis, T cells were pretreated with blocking peptide to PAR-2, washed and incubated with 70 nM gingipain for 1 h. After washing, cells were further stimulated with 5 µg/ml of anti-CD27 mAb for 1 h.
B cell membrane molecules and gingipains
Purified B cells were seeded at 1 × 105 cells/200 µl/well of RPMI-1640 medium in 96-well flat-bottomed plates and incubated in a humidified atmosphere containing 5% CO2 at 37°C. B cells were stimulated with 5 µg/ml anti-CD40 mAb and 0·25 µg/ml LPS in RPMI-1640 with 10% FCS for 48 h. After incubation, B cells were washed with serum-free RPMI-1640 medium and treated with 70 nM RgpA or Kgp, or TLCK-inhibited RgpA or TLCK-inhibited Kgp for 1 or 2 h. B cells were then analysed for surface expression of CD70 by flow cytometry. In the blocking peptide study, B cells were incubated with 15 µg/ml of blocking peptides to PAR-1, -2, -3 and -4 for 1 h after LPS and anti-CD40 mAb stimulation. After washing, B cells were treated with gingipains for 1 or 2 h and were analysed by FACScan for CD70 expression.
Statistical analysis
All data were expressed as means ± standard error of the mean (s.e.m.). Differences between groups were examined for statistical significance using Student's t-test for unpaired data and paired t-test for paired data. A P-value < 0·05 denoted the presence of a statistically significant difference.
Results
Preferential decrease in CD27 expression on CD4+ T cells following challenge with Arg-gingipain
CD27 molecules are expressed constitutively on resting CD4+ T cells [16]. The ability of gingipains to alter CD27 expression on CD4+ T cells was evaluated by flow cytometric analysis. Treatment of the T cells with 70 nM RgpA for 30 min efficiently reduced detection of CD27 from 90% to approximately 23% positive cells (Fig. 1a). In contrast, no decrease in the level of membrane CD27 expression on T cells was observed after 1 h exposure to 70 nM Kgp (Fig. 1b). A moderate decrease by 18% in CD27 levels on T cells was detected after prolonged incubation with Kgp for up to 2 h. The reduction of CD27 on T cells was due to the enzymatic activity of RgpA as the cysteine proteinase inhibitor TLCK (at 2 mM) blocked the effect completely in the absence of serum (Fig. 1b). Figure 1c demonstrates the FACs profiles of CD27 expression on CD4+ T cells after the addition of 70 nM gingipain(s) for 1 h. Treatment with RgpA under these conditions eliminated CD27 detection (Fig. 1ci), whiletreatment with Kgp did not affect CD27 detection (Fig. 1cii). We next evaluatedthe effect of gingipains on intercellular adhesion molecule (ICAM)-3 that is expressed constitutively on lymphocytes under serum-free conditions. Expression of the extracellular molecule ICAM-3, which belongs to a highly glycosylated Ig-superfamily expressed on peripheral blood lymphocytes, was not affected by treatment with 70 nM RgpA (Fig. 1d) or Kgp (data not shown) for 1 h. The results suggest that RgpA preferentially degrades CD27 on T cells.
Fig. 1.
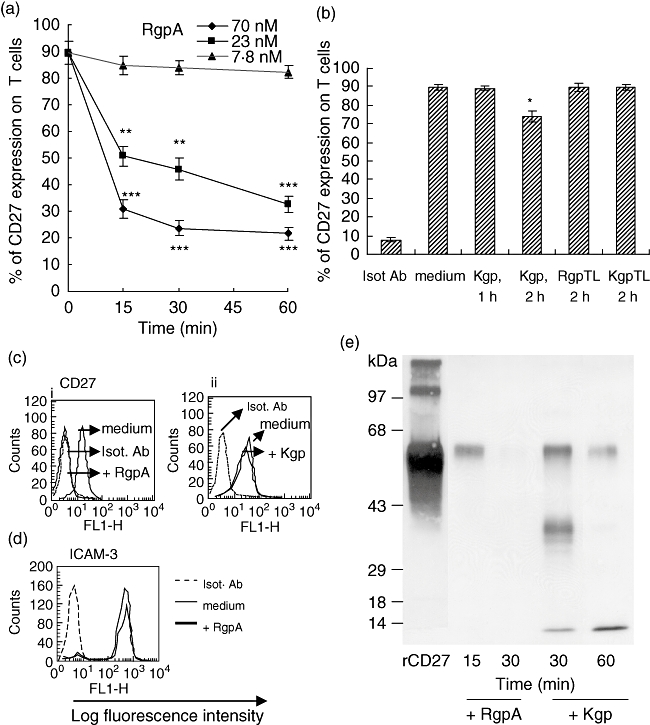
Effect of gingipains on CD27 expression on T cells. T cells were treated in the absence or presence of 5 mM cysteine activated (a) RgpA for the indicated times and concentrations or (b) 70 nM Kgp; or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited RgpA (RgpTL); or TLCK-inhibited Kgp (KgpTL) for 1 or 2 h at 37°C. After incubation, cells were labelled with anti-CD27 antibody or matched isotype (Isot) monoclonal antibody (mAb) and analysed by flow cytometry. Error bars indicate the mean ± standard error of the mean. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with untreated T cells. The percentage of CD27-expressing cells was analysed on positive CD4+ T cells over control values measured with isotype control antibody. Results are representative of three separate experiments that yielded similar results. (c, d) Fluorescence histograms obtained by fluorescence activated cell sorter of CD27 and intercellular adhesion molecule-3 expression on T cells after the addition of 70 nM gingipain for 1 h. (e) Recombinant CD27 was incubated with activated RgpA or Kgp at a final substrate to enzyme (S/E) ratio of 1 : 1 (18 nM CD27 with 18 nM RgpA or Kgp) in the absence of serum for various time periods at 37°C. Reactions were inhibited with 2 mM TLCK, and aliquots were processed for CD27 immunoblot analysis using a 12% polyacrylamide gel (sodium dodecyl sulphate-polyacrylamide gel electrophoresis).
Degradation of recombinant CD27 by gingipains
The recombinant mature CD27 protein is a disulphide-linked homodimer with subunits of 60 kDa (p60) [30]. A treatment with 70 nM RgpA for 15 min reduced p60 CD27 protein, with complete degradation after 30 min of incubation (Fig. 1e). For rCD27 following Kgp treatment, a predominant protein band of ∼39 kDa in addition to reduced p60 protein were detected after 30 min. The ∼39 kDa band disappeared as the time of exposure to Kgp increased. After 1 h of incubation with Kgp, p60 protein was reduced. These results indicate that soluble rCD27 functions as a substrate for gingipains and that Kgp degrades rCD27 slowly into small peptides. To confirm that the proteolytic activity of gingipains is responsible for the degradation of CD27, the proteinase inhibitor TLCK was incubated with gingipains before addition to T cells. Hydrolysis of the 60-kDa CD27 band was completely inhibited after 1 h treatment with either TLCK-treated RgpA or TLCK-treated Kgp (data not shown).
Effect of serum on the reduction of CD27 binding to CD4+ T cells by gingipains
As serum is a rich source of proteinase inhibitors [31,32], the effect of serum on gingipain binding to T cells was examined. As shown in Fig. 2, the hydrolysis of CD27 by 70 nM RgpA at 1 h of incubation was effective in the presence of 2·5% or 5% serum. By contrast, reduction of CD27 by Kgp was not detected at these levels of serum.
Fig. 2.
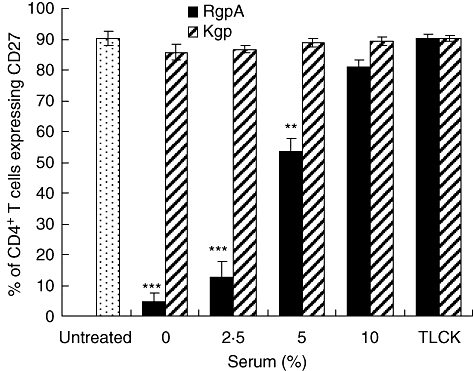
Serum inhibition of proteolysis of CD27 by gingipains. Purified T cells were treated with 70 nM cysteine-activated gingipains (RgpA or Kgp) or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited gingipains for 1 h at 37°C in the presence or absence of various levels of fetal calf serum. After incubation, cells were washed and stained with CD27 monoclonal antibody and subjected to fluorescence activated cell sorter analysis as described in Methods. Results are representative of three separate experiments on cells from different donors. Error bars indicate the mean ± standard error of the mean. **P < 0·01 and ***P < 0·001 compared with untreated T cells.
Effect of gingipains on the modulation of CD27 surface expression after paraformaldehyde treatment of CD4+ T cells
To exclude CD27 shedding resulting from an intracellular pathway [33] triggered subsequently after T cell activation by gingipains affecting another cell surface structure, T cells were fixed with 1% paraformaldehyde before any treatment. Activation of CD4+ T cells with 5 µg/ml anti-CD3 mAb resulted in slight enhancement of CD27 detection, and when fixed cells were incubated with anti-CD3 mAb for 1 h no change in binding was observed, as evaluated by flow cytometry analysis (Fig. 3). In contrast, RgpA efficiently reduced the binding of CD27 mAb to fixed as well as unfixed T cells, indicating that RgpA directly cleaves surface CD27 (Fig. 3). On the other hand, when mobilization of CD27 was inhibited by paraformaldehyde, Kgp strongly decreased surface CD27 levels, indicating that Kgp was able to cleave CD27 directly from the cell membrane. Apparent ineffectiveness of CD27 degradation by Kgp on unfixed cells could be attributed to an intracellular activation pathway induced by gingipains in T cells.
Fig. 3.
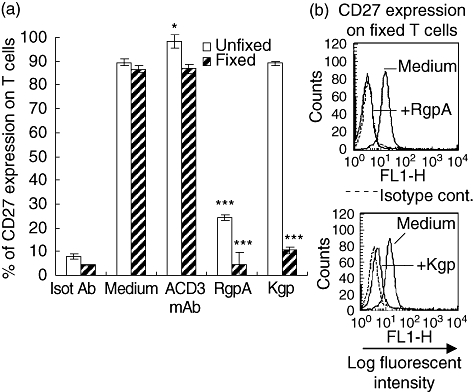
Effect of gingipains on CD27 expression after formaldehyde treatment of T cells. (a) T cells were fixed or not with 1% paraformaldehyde for 10 min at room temperature. After washing, untreated and fixed cells were incubated with medium alone or medium containing 5 µg/ml of anti-CD3 monoclonal antibody (mAb); or 70 nM RgpA or Kgp for 1 h at 37°C; and then washed with phosphate-buffered saline at 4°C. Surface expression of CD27 was then evaluated by flow cytometry. Results are mean ± standard error of the mean of three experiments. *P < 0·05 and ***P < 0·001 compared with untreated T cells in the same conditions. (b) Representative fluorescence histograms obtained by fluorescence activated cell sorter of CD27 expression on fixed T cells after the addition of gingipains for 1 h.
Measurements of soluble form of CD27 (sCD27) in the extracellular medium of gingipain-treated T cells
Using an ELISA, we next estimated the quantity of sCD27 released into the extracellular medium of T cells incubated with gingipains. T cells were fixed with 1% paraformaldehyde before any treatment. As shown in Fig. 4, basal recovery of 32 ng/ml of sCD27 was detected in supernatants of untreated T cells after 1 h of incubation. When T cells were incubated for 30 min with 70 nM gingipain, significant amounts of sCD27 in the culture medium were detected. The proteolytic activity of RgpA towards membrane CD27 of T cells was more obvious than for Kgp. Further, a decrease in the levels of sCD27 were observed after treatment of cells with gingipains for 1 h. These results indicated that the sCD27 removed from the membrane was degraded efficiently by the gingipains. Proteolysis of the sCD27 was markedly inhibited after 1 h treatment with either TLCK-treated RgpA or TLCK-inhibited Kgp.
Fig. 4.
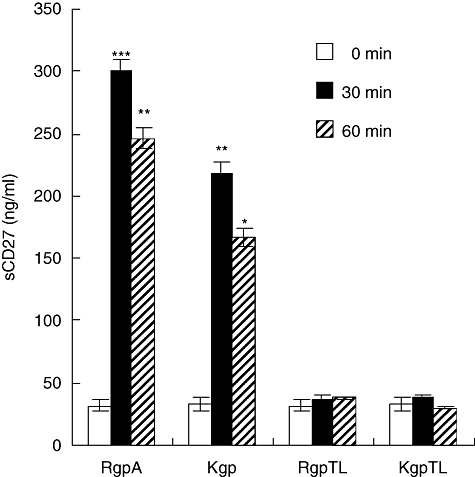
Measurements of soluble CD27 levels in the extracellular medium of gingipain-treated T cells. T cells were fixed with 1% paraformaldehyde for 10 min at room temperature. After washing, fixed T cells were treated with medium alone or medium containing 70 nM of activated RgpA or Kgp; or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited RgpA (RgpTL); or TLCK-inhibited Kgp (KgpTL) at the time indicated. Levels of CD27 in the culture media were then separated by centrifugation and analysed for soluble CD27 by enzyme-linked immunosprbent assay. Data are mean ± standard error of the mean of three experiments performed with different donors. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with untreated T cells.
Human CD4+ T cells express PAR-4 and RgpA induces PAR-1, -2 and -4 expression on the cell surface
We first evaluated the effect of RgpA on the surface expression of the PAR family in CD4+ T cells. According to flow cytometric analysis, untreated T cells stained strongly for PAR-4, but the expression of PAR-1, -2 and -3 on the cell surface was below the detectable limit (Fig. 5). T cells treated with RgpA alone increased PAR-1, -2 and -4 expression, an effect that was inhibited by TLCK. RgpA was potent in activating human T cells for expression of these receptors. Expression of PAR-1 and -2 was increased markedly after RgpA treatment for 1 h. These results indicate that RgpA induces PAR-1 and -2 receptor expression. Exposure of the cells to RgpA for up to 48 h did not further up-regulate the expression of PAR-1 and -2 (data not shown). Also, RgpA further up-regulated PAR-4 expression after 1 h incubation, but weakly induced PAR-3 on the T cell surface.
Fig. 5.
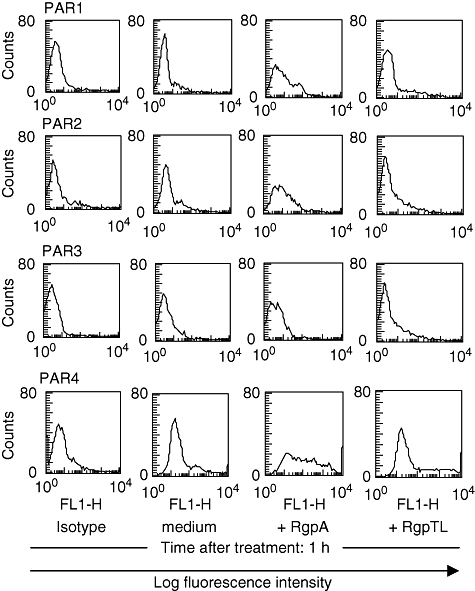
Flow cytometric analysis of the effect of RgpA on the surface expression of the protease-activated receptor (PAR) family in T cells. T cells were stimulated with medium alone or medium containing 70 nM RgpA; or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited RgpA (RgpTL) for 1 h at 37°C. After incubation, cells were stained with isotype-matched control or anti-PAR-1, -PAR-2, -PAR-3 and -PAR-4 followed by flow cytometry.
Induction of CD69 and CD25 expression on CD4+ T cells by gingipains
To determine whether gingipains could contribute to the activation of CD4+ T cells, expression of the early stage activation marker CD69 [34] on T cells was analysed by flow cytometry. Figure 6a shows a dose-dependent effect of gingipains leading to CD69 expression on T cells under serum-free conditions. CD69 was not expressed in untreated T cells during the incubation period. After 70 nM Rgp or Kgp treatment for 1 h, CD69 was detected on approximately 29% of T cells. The induction of CD69 molecules on T cells was due mainly to the enzymatic activity of gingipains as the cysteine proteinase inhibitor TLCK (at 2 mM) block nearly all the effect. The other activation marker CD25 was also increased in the gingipain- treated CD4+ T cell population (Fig. 6b). T cells expressed higher levels of CD25 after treatment with 70 nM RgpA than Kgp for 1 or 2 h, whereas the greatest difference was seen with RgpA treatment at 48 h.
Fig. 6.
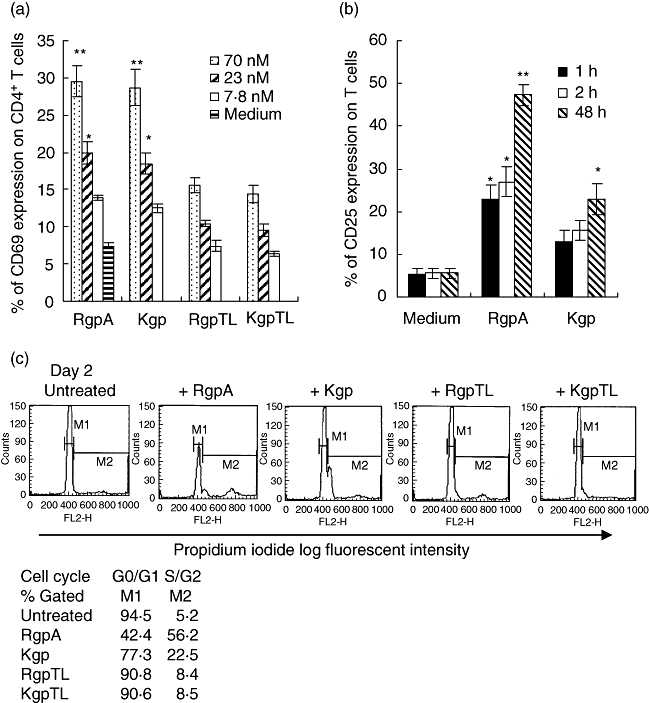
Effect of gingipains on activation markers expression and proliferation of T cells. (a) T cells were stimulated with medium alone or medium containing various concentrations of activated RgpA or Kgp; or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited RgpA (RgpTL); or TLCK-inhibited Kgp (KgpTL) for 1 h at 37°C. (b) T cells were stimulated with medium alone or medium containing 70 nM of activated RgpA or Kgp for 1 h, 2 h and 48 h at 37°C. After incubation, cells were washed and the expression of (a) CD69 and (b) CD25 of the cells were analysed by flow cytometry. Results are means ± standard error of the mean of three experiments. *P < 0·05 and **P < 0·01 compared with untreated T cells in the same conditions. (c) T cells were exposed to cysteine-activated RgpA or Kgp; or TLCK-inhibited RgpA (RgpTL); or TLCK-inhibited Kgp (KgpTL) for 1 h, washed and incubated at 37°C for 48 h before DNA cell cycle analysis.
Cell cycle status following gingipain treatment
In the absence of stimulation, 94% of untreated CD4+ T cells were in the G0/G1 phase of the cell cycle (Fig. 6c). The remaining 5% of cells were either in the S or G2/M phases of the cell cycle. Following treatment of T cells with RgpA followed by 48 h of incubation, the percentage of cells in G0/G1 was decreased to 42%, with a corresponding increase in the numbers of cells in S and G2/M phase, indicating the stimulatory effect of RgpA. Kgp induced a weak activation after 48 h of culture. TLCK-treatment of RgpA significantly inhibited this mitogenic action on T cells, as under these conditions the percentage of cells in G0/G1 phase was maintained at 90%, while the percentage of cells in S and G2/M phase was reduced from 56% to 8%, indicating that proteolytic activity is required for the cell cycle progression observed.
Arg-gingipain reduced anti-CD27 mAb induced CD40L expression on CD4+ T cells
To evaluate whether the proteolytic cleavage of CD27 correlated with reduced functional activity of the molecule, membrane-bound CD40L expressed on activated CD4+ T cells was determined. Analytical flow cytometry revealed that T cells treated with increasing concentrations of anti-CD27 mAb for 1 h showed a dose-dependent increase of CD40L expression in comparison to treatment with control antibody, with a plateau in the level of CD40L expression reached at 5 µg/ml (Fig. 7a). Preliminary treatment of CD4+ T cells by RgpA down-regulated the expression of CD40L induced by anti-CD27 mAb in a dose-dependent manner (Fig. 7b). Increasing concentrations of RgpA attenuated the induction of CD40L by anti-CD27 mAb with inhibition greatest at 70 nM (P < 0·001). Inhibition of CD27 induced CD40L surface expression on T cells did not occur in the presence of TLCK-treated RgpA, indicating that proteolysis of CD27 was a requirement for this effect. By contrast, T cells pretreated with 70 nM Kgp for up to 2 h did not reduce anti-CD27 mAb-induced CD40L surface expression (Fig. 7c). Further, T cells pretreated with 70 nM RgpA or Kgp followed by anti-CD3 mAb treatment did not show reduced CD40L surface expression (Fig. 7c), indicating that Arg-gingipain preferentially reduced functional CD27.
Fig. 7.
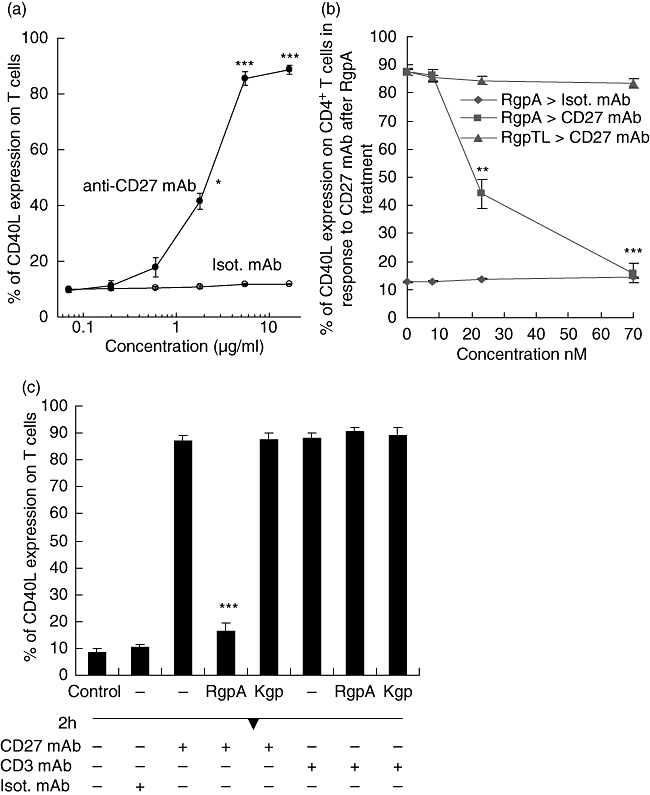
Modulation of T cell CD40L expression by CD27 monoclonal antibody (mAb) following gingipain treatment. (A) Purified T cells were stimulated with the indicated concentrations of anti-CD27 mAb alone or an isotype-matched (Isot.) mAb for 1 h. Data represent the mean ± standard error of the mean (s.e.m.) from three independent experiments. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with untreated T cells. (b) T cells were preincubated with different concentrations of RgpA or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited RgpA (RgpTL) for 1 h. Cells were then washed and treated with 5 µg/ml of anti-CD27 mAb or isotype-matched (Isot.) mAb for 1 h. (c) T cells were preincubated with or without 70 nM concentrations of activated RgpA or Kgp for 1 h. Cells were then washed and stimulated with 5 µg/ml of anti-CD27 mAb; or anti-CD3 mAb; or isotype-matched (Isot.) mAb for 1 h. Surface expression of CD40L was then evaluated by flow cytometry. Data show differences against the control CD40L expression generated by the same conditions but without gingipain treatment.
Blockade of the N-terminus of PAR-1, -2 and -4 increase gingipain-mediated proteolysis of CD27
To determine whether blockade of PARs affected CD27 expression mediated by gingipains, T cells were pretreated with a blocking peptide directed against the N-termini of PAR-1, -2 and -4 and C-terminus of PAR-3. As shown in Fig. 8a, preincubation of T cells with one of the PAR-1, -2 or -4 blocking peptides at 15 µg/ml increased RgpA-mediated CD27 hydrolysis by approximately 20%. Significantly, pretreatment of T cells with one of the PAR-1, -2 or -4 blocking peptides for 1 h increased Kgp proteolytic activities for CD27. Figure 8b demonstrates the FACs profile of enhanced proteolytic activity of Kgp on CD27 after treatment with PAR-2 blocking peptide for 1 h. Further, blocking peptide against the C-terminus of PAR-3 did not enhance RgpA or Kgp proteolytic activity. Significantly, blocked PAR-2 activation increases total Kgp proteolytic degradation of CD27, leading to a complete loss of anti-CD27 mAb-induced CD40L surface expression (Fig. 8c). In agreement with these data, pretreatment of the cells with PAR-2 blocking peptide also inhibited CD69 surface expression following gingipain challenge (Fig. 8d), whereas PAR-2 blocking peptide alone did not induce CD69 surface expression (data not shown). These results provide evidence that the blockade of PARs on T cells can affect CD27 and its interaction with target cells. Together with data presented in Fig. 3, these results provide evidence that the gingipains can hydrolyse CD27 on the cell surface of T cells and diminish CD27-mediated activity as well as T cell activation, possibly through gingipain interaction with PAR-1, -2 or -4, as demonstrated for Kgp.
Fig. 8.
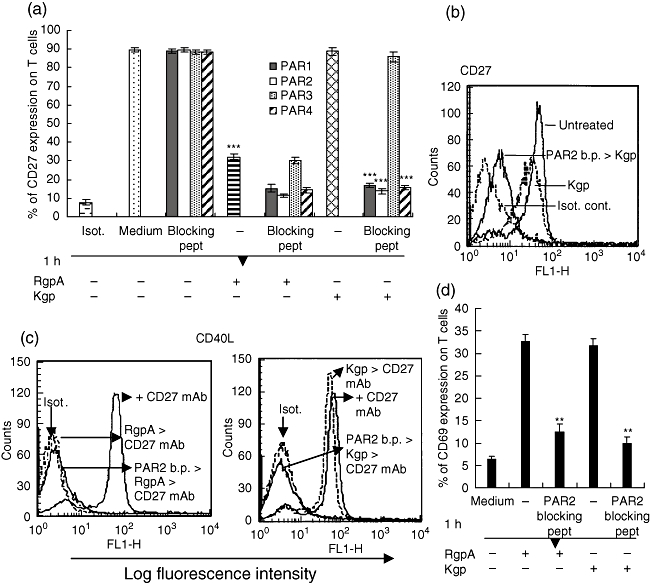
Effect of PARs blocking peptides on surface CD27, CD40L, and CD69 expression on T cells. (a) T cells were incubated with medium alone or medium containing 15 µg/ml of blocking peptides to PAR-1,- 2, -3 and monoclonal antibody (mAb) 4 for 1 h at 37°C. Cells were washed and treated with 70 nM RgpA or Kgp for 1 h and surface expression of CD27 was then evaluated by flow cytometry. Data are presented as mean ± standard error of the mean of three experiments performed with cells from different donors. Substantial reduction of CD27 expression was produced by incubation with RgpA without PARs blocking peptide treatment (***P < 0·001). In contrast, for Kgp, preincubation with blocking peptides to protease-activated receptor (PAR)-1, -2 or -4 was required to achieve reduction of CD27 levels (***P < 0·001). Preincubation with blocking peptide to PAR-3 was not effective. (b) Representative fluorescence histograms obtained by fluorescence activated cell sorter analysis of CD27 expression on T cells after treatment with blocking peptide (b.p.) to PAR-2 as indicated. (c) After treatment with 15 µg/ml of blocking peptide (b.p.) to PAR-2, T cells were washed and incubated with 70 nM of RgpA or Kgp for 1 h. After washing, cells were stimulated with 5 µg/ml of anti-CD27 mAb for 1 h. Surface expression of CD40L was then evaluated by flow cytometry. (d) Flow cytometric analysis of CD69 expression on T cells in the presence or absence of PAR-2 blocking peptide, followed by 70 nM of gingipain treatment for 1 h. For reduction of CD69 expression, preincubation with blocking peptide to PAR-2 was essential for both RgpA and Kgp, in contrast to the findings for CD27 (**P < 0·01).
Analysis of CD70 surface expression on B cells activated in vitro
We next examined the effect of gingipains on the surface expression of CD27 ligand (CD70) on lymphocytes by flow cytometry. CD70 is not expressed on resting B cells, but can be induced readily upon activation in vitro [35]. Purified CD19+ B cells were stimulated with anti-CD40 mAb and LPS for 2 days. On such activated B cells, ∼80% were positive for CD70 (Fig. 9A). After 1 h RgpA treatment ∼75% of the CD70 antigen on B cells was lost, while Kgp induced a weak reduction of the CD70 expression after 2 h incubation. The reduction of CD70 on B cells was due apparently to the proteolytic activity of gingipains as TLCK (at 2 mM) blocked the effect completely.
Fig. 9.
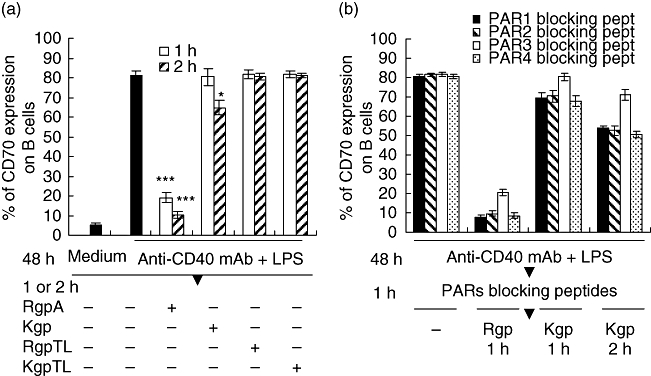
Effects of blocking the PARs/gingipain interactions on induced CD70 expression in B cells. (a) Purified B cells were cultured in medium alone or stimulated with 5 µg/ml anti-CD40 monoclonal antibody (mAb) and 0·25 µg/ml lipopolysaccharide (LPS). After 48 h culture, B cells were washed and treated with medium alone, or 70 nM RgpA or Kgp, or or N-α-tosyl-L-lysyl chloromethyl ketone (TLCK)-inhibited RgpA (RgpTL) or TLCK-inhibited Kgp (KgpTL) for 1 h. B cells were then analysed by flow cytometry for surface expression of CD70. Showing significant difference against CD70 expression on stimulated B cells generated by the same conditions but without gingipain treatment. (b) Purified B cells were stimulated with anti-CD40 mAb and LPS for 48 h. After the incubation, B cells were washed and incubated with 15 µg/ml of blocking peptides to PAR-1, -2, -3 and -4 for 1 h. Cells were then washed and treated with medium alone, or 70 nM RgpA or Kgp for 1 or 2 h. Surface expression of CD70 was then evaluated by flow cytometry. Data represent the mean ± standard error of the mean from three independent experiments. Significant difference against CD70 expression on stimulated B cells generated by the same conditions without protease-activated receptors (PARs) blocking peptide treatments (a).
Blockade of PAR-1, -2 and -4 enhancement of proteolysis of CD70 on B cells
To determine the effect of PARs blockade on CD70 expression mediated by gingipains, B cells activated by anti-CD40 mAb and LPS were pretreated with the blocking peptides directed against PAR-1, -2, -3 and -4. As shown in Fig. 9b, preincubation of B cells with 15 µg/ml PAR-1, -2 or -4 blocking peptides increased RgpA-mediated proteolytic activity for CD70. However, blocked PAR-1, -2 and -4 activation revealed only a weak reduction of CD70 expression by Kgp within 1–2 h (Fig. 9b), unlike the proteolytic activity of Kgp on CD27 expressed by T cells (Fig. 8a). Further, blocking peptide against the C-terminus of PAR-3 did not enhance Kgp proteolytic activity against this target.
Discussion
The interaction of certain members of the tumour necrosis factor (TNF) superfamily with their receptors is important in the development an efficient adaptive immune response [36]. The present study shows a down-regulatory effect of RgpA on T cell membrane expression of CD27, a type I transmembrane phospho-glycoprotein which belongs to the TNF receptor family. Our investigation demonstrated a direct and extensive proteolytic effect of RgpA on membrane CD27 that is independent of PARs activation. RgpA was more efficient than Kgp in degrading both membrane and soluble forms of CD27, with the latter confirmed by demonstrating the degradation of human rCD27 by these proteases. It is noteworthy that human CD27 contains 20 Arg-X and 8 Lys-X bonds, that are possible cleavage sites by RgpA and Kgp, respectively.
In this study, hydrolysis of membrane CD27 by gingipains was linked with increase of soluble CD27 in the culture medium. It is possible that gingipains may hydrolyse several susceptible bonds present in soluble and membrane CD27 molecules. Hence, release of CD27 is followed apparently by rapid degradation of the soluble molecule, reducing accumulation of CD27 in the culture medium. There is increasing evidence for a functional role of soluble receptors in vivo. Many truncated soluble receptors can retain their ligand binding capacity [37]. It is possible that the soluble receptors may prevent ligands approaching their cell surface receptors, or bring ligands to their site of action. The level of gingipain in the gingival crevicular fluid at inflamed sites of adult periodontitis patients was estimated at 40–90 nM [38], comparable to the amount utilized in this study. Hence, the proteolytic cleavage of CD27 on T cells by RgpA is likely to take place in vivo.
Antigen-induced T cell activation in vitro is known to result in the surface expression of activation markers in an orderly sequence: CD69 and CD25. In our study, the activation markers CD69 and CD25 were both up-regulated after gingipain treatment, suggesting that these changes were the result of recent activation. To elucidate further whether the expression of activation markers was associated with T cell proliferation, we assessed DNA content by propidium iodide staining of T cells. The majority of the T cells were in S and G2/M phases after RgpA treatment, and fewer cells were in G0/G1 phase. T cell proliferation was greater after RgpA treatment than for Kgp. These findings suggest that T cells express activation markers in association with a gingipain-induced proliferation.
Four members of the PAR family have been identified and they can be activated by different proteases [23,39]. In vitro study has shown that Arg-gingipain can activate PAR-1 and PAR-4 expressed on the surface of platelets [40]. In this study, we reported that PAR-4 is strongly expressed on resting T cells; therefore, cleavage and activation of PAR-4 by RgpA could be an important early event. Findings from the present study support this contention: (1) PAR-1, -2 and -4 expression on the cell surface was up-regulated by RgpA; (2) up-regulation of CD69 on T cells by gingipain alone was inhibited by blocking peptide against PAR-2. These results indicating the capacity of RgpA to activate PAR-2 on T cells are in agreement with previous reports [25,26] that PAR-2 plays a crucial role in the local inflammatory reaction within the pathological periodontal pocket. Disruption of cellular immune processes by blocking PAR activity and concomitantly diminishing T cell activation did not prevent gingipain-mediated hydrolysis of CD27 and reduction of CD40L expression through CD27. Taken together, these data suggest that gingipains can disrupt cellular immune cascades by multiple and independent pathways.
Activation and priming of T cells during the adaptive immune response is regulated by a series of complex signals between APCs and T cells [14]. Naive T cells generally cannot expand efficiently upon T cell receptor (TCR) stimulation alone. A second signal is required, which is provided by membrane receptors on T cells and their ligands on APCs. Studies have implicated CD27 in co-stimulation of peripheral CD4+ T cells and CD27 ligation has been shown to increase T cell proliferation induced after TCR/CD3 triggering [41]. Several TNF superfamily members, including CD40L and TNF, are involved in regulating T cell activation events. CD40L is expressed by activated CD4+ T cells. CD40 engagement by CD40L plays a central role in promoting the survival and activation of APCs, which subsequently deliver costimulation signals and provide a cytokine environment for the appropriate activation of T cells [42]. In line with these studies, the present data confirm that CD27 ligation can promote CD40L expression in T cell activation. We have shown previously that gingipains do not directly affect CD3 and CD40L expression on T cells [12]. It is significant that in the present study degradation of functional CD27 by Arg-gingipain inhibited the surface expression of CD40L in response to CD27 ligation. These observations suggest that Arg-gingipain can significantly reduce CD40L-mediated T cell activation via proteolysis of CD27. As CD40L plays a central role in promoting the survival and activation of APCs, the findings demonstrate that a major consequence of the proteolysis of CD27 by gingipains could be a significant inhibition of T cell co-stimulation of B cells.
CD70 is a TNF-related type II transmembrane glycoprotein induced upon activation on T and B cells [17,18]. Expression of CD70 by B cells activated in vitro has been shown to be dependent on the continuous presence of LPS and CD40 ligation. Our study shows that CD70 was detected readily at the cell surface of activated B cells and could be significantly depleted by RgpA and weakly affected by Kgp. It is notable that human CD70 contains 16 Arg-X and only one Lys-X bond that are possible cleavage sites by RgpA and Kgp, respectively. For Kgp, limited proteolysis of CD70 could be due to restricted access to the only Lys93-Glu94 peptide bond available. These results indicate that gingipains interfere with the CD27 ligand on B cells and play a key role in the modulation of CD27/CD70 interaction in T cell-dependent B cell responses.
Kgp has been shown to degrade both membrane TNF-α [13] and proinflammatory cytokine TNF-α [43], but exhibiting much weaker proteolytic activity than RgpA. The present study demonstrated that Kgp only weakly suppressed the level of membrane CD27. This could be attributed to activation of other pathways in T cells, as Kgp was shown to cleave the CD27 on paraformaldehyde-fixed T cells. In agreement with these data, the present study established that blocking peptides to PAR-1, -2 and -4 on T cells also augmented the proteolysis of CD27 by gingipains, suggesting that PAR activation is not required for Kgp hydrolysis of CD27. Further, the presence of PAR-2 blocking peptide increased significantly the inhibitory effect of Kgp on CD27 ligation-induced activities. This is consistent with our current findings and supports the ability of gingipains to cleave surface CD27 directly rather than a consequence of gingipain-induced cell activation.
The ability of PARs blocking peptides to inhibit T cell activation by gingipains suggests that the N-termini of PARs receptors were required for cell activation. The PARs are a family of G-protein coupled receptors that are activated by proteolytic cleavage [28]. We speculate that the blockade of any one of the three PARs can possibly block signal transference to G protein. After this site becomes saturated, gingipains may begin to degrade the CD27 molecule on T cells more efficiently. While PAR-2 activation appears to be involved in some inflammatory activities [25,26], our data suggest that the use of PAR-2 antagonists might promote some of the high proteolytic activities that have been found in gingival crevicular fluid [38]. Hence, the use of specific proteinase inhibitors with low toxicity, rather than PARs antagonists, may inhibit both the degradation of host cell membrane molecules and activation of PARs by RgpA. Taken together, our results provide evidence that RgpA elicits PARs activation on T cells that could contribute to the host inflammatory response resulting in an increased severity of the disease. At the same time, it is clear that gingipain degradation of CD27 on T cells and CD70 on activated B cells could disrupt this critical co-activation loop. Thus, the combination of PARs activation and CD27/CD70 hydrolysis by gingipains could influence the outcome of the T cell-mediated immune response.
Acknowledgments
This work was supported by a grant (ID352479) from the National Health and Medical Research Council of Australia.
References
- 1.Andrian E, Grenier D, Rouabhia M. In vitro models of tissue penetration and destruction by P. gingivalis. Infect Immun. 2004;72:4689–98. doi: 10.1128/IAI.72.8.4689-4698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–43. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun LWP, DeCarlo AA, Chapple CC, Hunter N. Functional implication of the hydrolysis of platelet endothelial cell adhesion molecule 1 (CD31) by gingipains of Porphyromonas gingivalis for the pathology of periodontal disease. Infect Immun. 2005;73:1386–98. doi: 10.1128/IAI.73.3.1386-1398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadowaki T, Nakayama K, Yoshimura F, Okamoto K, Abe N, Yamamoto K. Arg-gingipain acts as a major processing enzyme for various cell surface proteins in Porphyromonas gingivalis. J Biol Chem. 1998;273:29072–6. doi: 10.1074/jbc.273.44.29072. [DOI] [PubMed] [Google Scholar]
- 5.Potempa J, Travis J. Porphyromonas gingivalis proteinases in periodontitis, a review. Acta Biochim Pol. 1996;43:455–65. [PubMed] [Google Scholar]
- 6.Yun LWP, DeCarlo AA, Hunter N. Modulation of major histocompatibility complex protein expression by human gamma interferon mediated by cysteine proteinase-adhesin polyproteins of Porphyromonas gingivalis. Infect Immun. 1999;67:2986–95. doi: 10.1128/iai.67.6.2986-2995.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavloff N, Potempa J, Pike RN, Prochazka V, Kiefer MC, Travis J, Barr PJ. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis: Biosynthesis as a proteinase-adhesin polyprotein. J Biol Chem. 1995;270:1007–10. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 8.Pavloff N, Pemberton PA, Potempa J, et al. Molecular cloning and characterization of Porphyromonas gingivalis Lys-gingipain. A new member of an emerging family of pathogenic bacterial cysteine proteinases. J Biol Chem. 1997;272:1595–600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 9.Baker PJ, Garneau J, Howe L, Roopenian DC. T-cell contributions to alveolar bone loss in response to oral infection with Porphyromonas gingivalis. Acta Odontol Scand. 2001;59:222–5. doi: 10.1080/00016350152509247. [DOI] [PubMed] [Google Scholar]
- 10.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–74. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodontal Res. 1993;28:478–86. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 12.Yun LWP, DeCarlo AA, Chapple CC, Collyer CA, Hunter N. Binding of Porphyromonas gingivalis gingipains to human CD4+ T cells preferentially down-regulates surface CD2 and CD4 with little affect on co-stimulatory molecule expression. Microb Pathog. 2005;38:85–96. doi: 10.1016/j.micpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Mezyk-Kopec R, Bzowska M, Potempa J, et al. Inactivation of membrane tumor necrosis factor alpha by gingipains from Porphyromonas gingivalis. Infect Immun. 2005;73:1506–14. doi: 10.1128/IAI.73.3.1506-1514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison JP, Lanier LL. Structure, function and serology of the T-cell antigen receptor complex. Annu Rev Immunol. 1987;5:503–40. doi: 10.1146/annurev.iy.05.040187.002443. [DOI] [PubMed] [Google Scholar]
- 15.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 16.Van Lier RA, Borst J, Vroom TM, et al. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987;139:1589–96. [PubMed] [Google Scholar]
- 17.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154:2612–23. [PubMed] [Google Scholar]
- 18.Hendriks J, Gravestein LA, Tesselaar K, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–40. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 19.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27–CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci USA. 1995;92:11249–53. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquot S, Lemaitre LM, Paris E, et al. B cell co-receptors regulating T cell-dependent antibody production in common variable immunodeficiency: CD27 pathway defects identify subsets of severely immuno-compromised patients. Int Immunol. 2001;13:871–6. doi: 10.1093/intimm/13.7.871. [DOI] [PubMed] [Google Scholar]
- 21.Ossovskaya VS, Bunnet NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 22.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 23.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 24.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. 1994;91:9208–12. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lourbakos A, Chinni C, Thompson P, et al. Cleavage and activation of proteinase-activated receptor-2 on human neutrophils by gingipain-R from Porphyromonas gingivalis. FEBS Lett. 1998;435:45–8. doi: 10.1016/s0014-5793(98)01036-9. [DOI] [PubMed] [Google Scholar]
- 26.Lourbakos A, Potempa J, Travis J, et al. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect Immun. 2001;69:5121–30. doi: 10.1128/IAI.69.8.5121-5130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzhausen M, Spolidorio LC, Ellen RP Jobin, et al. Protease-activated receptor-2 activation. A major role in the pathogenesis of Porphyromonas gingivalis infection. Immunopath Infect Dis. 2006;168:1189–99. doi: 10.2353/ajpath.2006.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner JR, Kahn ML, Coughlin SR, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–10. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- 29.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loenen WAM, De Vries E, Gravestein LA, Hintzen RQ, Van Lier RA, Borst J. The CD27 membrane receptor, a lymphocyte-specific member of the nerve growth factor receptor family, give rise to a soluble form by protein processing that does not involve receptor endocytosis. Eur J Immunol. 1992;22:447–55. doi: 10.1002/eji.1830220224. [DOI] [PubMed] [Google Scholar]
- 31.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 32.Pizzo SV, Grøn H, Pike R, et al. The potential role of α2-macroglobulin in the control of cysteine proteinases (gingipains) from Porphyromonas gingivalis. J Periodont Res. 1997;32:61–8. doi: 10.1111/j.1600-0765.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 33.Hintzen RQ, De Jong RD, Hack CE, et al. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 34.Santis AG, Lopez-Cabrera M, Sanchez-Madrid F, Proudfoot N. Expression of the early lymphocyte activation antigen CD69, a C-type lectin, is regulated by mRNA degradation associated with AU-rich sequence motifs. Eur J Immunol. 1995;25:2142–6. doi: 10.1002/eji.1830250804. [DOI] [PubMed] [Google Scholar]
- 35.Arens R, Nolte MA, Tesselaar K, et al. Signaling through CD70 regulates B cell activation and IgG production. J Immunol. 2004;173:3901–8. doi: 10.4049/jimmunol.173.6.3901. [DOI] [PubMed] [Google Scholar]
- 36.Hehlgans T, Mannel DN. The TNF–TNF receptor system. Biol Chem. 2002;383:1581–5. doi: 10.1515/BC.2002.178. [DOI] [PubMed] [Google Scholar]
- 37.Gatanaga T, Hwang C, Kohr W, et al. Purification and characterization of an inhibitor (soluble tumor necrosis factor) for tumor necrosis factor and lymphotoxin obtained from the serum ultrafiltrates of human cancer patients. Proc Natl Acad Sci USA. 1990;87:8781–4. doi: 10.1073/pnas.87.22.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eley BM, Cox SW. Correlation between gingivain/gingipain and bacterial dipeptidyl peptidase activity in gingival crevicular fluid and periodontal attachment loss in chronic periodontitis patients. J Periodontol. 1996;67:703–16. doi: 10.1902/jop.1996.67.7.703. [DOI] [PubMed] [Google Scholar]
- 39.Storck J, Kusters B, Vahland M, Morys-Wortmann C, Zimmermann ER. Trypsin induced von Willebrand factor release from human endothelial cells is mediated by PAR-2 activation. Thromb Res. 1996;84:463–73. doi: 10.1016/s0049-3848(96)00214-9. [DOI] [PubMed] [Google Scholar]
- 40.Lourbakos A, Yuan YP, Jenkins AL, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood. 2001;97:3790–7. doi: 10.1182/blood.v97.12.3790. [DOI] [PubMed] [Google Scholar]
- 41.Borst J, Sluyser C, Vries ED, Klein H, Melief CJ, van Lier RA. Alternate molecular form of human T cell specific antigen CD27 expressed upon T cell activation. Eur J Immunol. 1989;19:357–64. doi: 10.1002/eji.1830190221. [DOI] [PubMed] [Google Scholar]
- 42.Grewal IS, Flavell RA. The CD40 ligand. Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 43.Calkins CC, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-α by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. J Biol Chem. 1998;273:6611–4. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]


