Abstract
Studies of sarcoidosis immunology have noted oligoclonal T cell populations, suggesting cell-mediated immunity that is antigen-specific. Sarcoidosis immunology and pathology are most similar to mycobacterial infections. Mycobacterium tuberculosis infection in mice and humans reflects T helper 1 (Th1) immune responses to multiple cell wall and secreted antigens. We investigated if the oligoclonal immune response in individual sarcoidosis subjects could be elicited by multiple secreted mycobacterial antigens by performing ex vivo enzyme-linked immunospot assay (ELISPOT) on peripheral blood mononuclear cells (PBMC) from 30 sarcoidosis, 26 purified protein derivative negative (PPD−) control and 10 latent tuberculosis subjects (PPD+) to assess Th1 responses to mycobacterial superoxide dismutase A (sodA), catalase-peroxidase (katG) and early secreted antigenic target protein (ESAT-6). A significant difference was noted among the sarcoidosis and PPD− control subjects to ESAT-6 [12 of 30 versus one of 26 (P = 0·0014)], katG [nine of 30 versus none of 26 (P = 0·002)] and sodA [12 of 30 versus none of 26 (P = 0·002)]. There was no significant difference between sarcoidosis and PPD+ subjects. Twelve sarcoidosis subjects recognized two or more mycobacterial proteins, as well as multiple distinct epitopes within individual proteins. One sarcoidosis subject on whom we collected bronchoalveolar lavage (BAL) fluid and PBMC had no recognition of mycobacterial antigens using PBMC, but BAL fluid demonstrated strong Th1 immune responses to ESAT-6 and katG. Individual sarcoidosis subjects recognized not only multiple mycobacterial proteins, but multiple distinct peptides within a specific protein, thus demonstrating that multiple mycobacterial epitopes elicit the Th1 immune response observed. Immune responses by sarcoidosis T cells to mycobacterial proteins may have an important role in sarcoidosis pathogenesis.
Keywords: antigens, interferon-γ, mycobacteria, sarcoidosis
Introduction
Sarcoidosis is a granulomatous disease of unknown aetiology, characterized by a T helper 1 (Th1) immunophenotype. Studies of sarcoid immunology have noted oligoclonal T cells in the sarcoid granuloma, suggesting cell-mediated immunity that is antigen-specific [1, 2]. Analysis of bronchoalveolar lavage of patients with acute sarcoidosis revealed a significant increase in lung T cells expressing the T cell antigen receptor AV2S3+ CD4+ in individuals with human leucocyte antigen D-related B3*0101 (HLA-DRB3*0101) and DRB1*0301 restrictions, suggesting restricted lung T cell expansions and a response to a specific antigen [3]. Recent reports of the detection of mycobacterial proteins in sarcoidosis granulomas, as well as humoral immune responses to mycobacterial antigens in sarcoidosis subjects, have renewed interest in the potential role of mycobacteria in sarcoidosis immunopathogenesis [4, 5]. If mycobacteria are important in sarcoidosis immunopathogenesis, one would expect immune responses to multiple mycobacterial antigens to be present in individual sarcoidosis subjects, as opposed to a dominant antigen. Studies of immunogenic antigens in subjects infected with Mycobacterium tuberculosis (MTB) or M. leprae have detected numerous immunogenic proteins [6, 7]. It has been shown in MTB-infected mice that immune recognition of secreted, cell wall or cytoplasmic mycobacterial antigens reflect growth patterns of the bacilli [8]. Demissie et al. reported recently that immune recognition of mycobacterial antigens may be stage-specific in the human host [9]. The enzyme-linked immunospot assay has been shown to identify M. tuberculosis (MTB)-specific T cells in smear-negative, tuberculosis-infected subjects [10]. In order to investigate the depth and breadth of the immune response to mycobacterial antigens in individual sarcoidosis subjects, we performed ex vivo enzyme-linked immunospot assay (ELISPOT) for Th1 immune responses to mycobacterial superoxide dismutase A (sodA), catalase-peroxidase (katG) and early secreted antigenic target protein (ESAT-6). We chose ESAT-6 and katG peptides due to a previous report of cellular immune responses to these proteins in sarcoidosis subjects [11]. SodA is a metalloenzyme present in virulent mycobacteria, which is thought to be important in inhibiting host immune responses [12, 13]. We included Trypanosome brucei whole cell wall lysate in the analysis to assess if the immune response observed was specific for mycobacterial proteins. The identification of immunogenic mycobacterial antigens in sarcoidosis subjects facilitates the eventual identification of the responsible agent(s), allows studies of genetic associations with immune recognition, as well as the role of the host immune response in clinical outcomes.
Materials and methods
Subject population
Ethical approval for the study was granted by Vanderbilt University Institutional Review Board for Human Studies. All study participants [sarcoidosis, purified protein derivative negative (PPD−) healthy volunteers and latent tuberculosis] were recruited from the same geographical area. A blood sample was drawn into tubes containing ethylenediamine tetraacetic acid (EDTA) after obtaining written consent from each study participant. Thirty sarcoidosis patients were recruited based on the following criteria: consistent clinical features including acute respiratory illness accompanied by erythema nodosum, hilar adenopathy and arthritis (Lofgren's syndrome), and indolent progressive pulmonary decomposition associated with radiographic findings, such as reticulodular infiltrates or pulmonary fibrosis. Histopathological diagnosis of sarcoidosis had to be confirmed by a pathologist (i.e. specimens from each patient had confluent non-caseating granulomas, well-circumscribed within the surrounding tissue with a variable amount of peripheral lymphocytic infiltration). Known microbial causes for granuloma formation had to be excluded by histological staining and culture for bacteria, fungi and acid-fast bacilli. Of the 30 sarcoidosis subjects, 16 had undergone tuberculin skin testing at the discretion of their physician; all 16 were skin test negative. Healthy PPD− volunteers must have undergone PPD testing by the Vanderbilt employee health services. The PPD+ subjects had written documentation of their PPD status, and had no evidence of active disease at the time of study enrolment. The PPD+ subjects were those with latent tuberculosis, as defined by the 2000 American Thoracic Society/Center for Disease Control guidelines on latent tuberculosis, using the criteria of tuberculin skin testing, chest radiographs and sputum examination by acid-fast bacilli staining and culture [14]. None of the study participants had undergone bacille Calmette–Guérin (BCG) vaccination. Approximately 60% of the sarcoidosis, PPD− and PPD+ subjects had participated in a previous investigation of immune responses to Ag85A [15].
Preparation of peripheral blood mononuclear cells (PBMC)
PBMC were separated from blood on Ficoll-Hypaque density gradients (Amersham Biosciences, Piscataway, NJ, USA), and stored in liquid nitrogen in fetal bovine serum (FBS) with 10% dimethylsulphoxide (DMSO) until time of analysis.
Synthesis of Mycobacterium peptides
The amino acid sequences for the 17 ESAT-6 peptides, 15-mers overlapping by 10, were synthesized as described previously [16]. We tested for immune recognition of all 17 peptides in this analysis, as well as two katG peptides, based upon a previous report of cellular immune responses to katG [11]. Each peptide was synthesized by solid-phase F-moc chemistry (Genemed Synthesis, San Diego, CA, USA), to a purity of > 70%. Identity was confirmed by mass spectroscopy, and the purity was assessed by high performance liquid chromatography.
Peptide mapping to identify immunogenic sodA epitopes
Forty sodA peptides, 15-mers overlapping by 10 that, cumulatively, span the entire MTB sodA protein (GenBank no. AAD15824·1), were used individually to stimulate PBMC from a subgroup of sarcoidosis, PPD− and PPD+ subjects in order to identify immunogenic peptides. Among the sarcoidosis and PPD+ subjects, four of the 40 peptides were recognized most frequently: sodA 31, 33, 36 and 38 (Table 1). There was no recognition of any of the 40 sodA peptides among the PPD− control subjects. These four sodA peptides, the two katG peptides and the 17 ESAT-6 peptides were used for the ELISPOT analysis. The sequence of the katG and sodA peptides is listed on Table 1.
Table 1.
Mycobacterium tuberculosis (MTB) superoxide dismutase A (sodA) and catalase-peroxidase (katG) peptide sequences used in enzyme-linked immunospot assay (ELISPOT) assay.*
| Peptide | Sequence | Amino acid position |
|---|---|---|
| SodA 31 | LGIVPLLLDMWEHA | 151–165 |
| SodA 33 | MWEHAFYLQYKNVKV | 161–175 |
| SodA 36 | DFAKAFWNVVNWADV | 176–190 |
| SodA 38 | NWADVQSRYAAATSQ | 186–200 |
| KatG 31 | WTNTPTKWDNSFLEI | 321–335 |
| KatG 32 | TKWDNSFLEILYGYE | 326–340 |
Amino acid sequences were derived from MTB, GenBank Accession numbers NP 216424 and AF061030, respectively.
Trypanosome brucei cell culture and preparation of trypanosome lysate
T. brucei bloodstream cells were cultured in HMI-9 medium supplemented with 10% FCS (Atlanta Biologicals, Norcross, GA, USA) and 10% Serum Plus (JRH Biosciences, Lenexa, KS, USA). Cultures were maintained in a humidified incubator at 37°C with 5% CO2. A 100 ml culture of bloodstream T. brucei cells was grown to a density of ∼1·0 × 107 cells/ml. Cells were collected by centrifugation at 5200 g for 10 min, and washed once in cold phosphate-buffered saline (PBS). The cell pellet was then resuspended in 10 ml of fresh PBS containing protease inhibitors [1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A and 1 mM phenylmethylsulphonyl fluoride (PMSF)]. The cells were equilibrated at 1500 psi liquid nitrogen for 30 min at 4°C in a nitrogen cavitation bomb (minibomb model no. 4639; Parr Instrument Company, Moline, IL, USA). Release of the suspension from the bomb resulted in the disruption of more than 95% of the cells. The crude extract was clarified by centrifugation at 58 000 g for 10 min, aliquoted, and then stored at −80°C.
ELISPOT analysis for interferon gamma (IFN-γ) production
ELISPOT assay was performed as described previously, with modification [17]. Briefly, 96-well polyvinylidene difluoride-backed plates (MAIPS4510; Millipore, Bradford, UK) were coated with 0·5 μg/ml anti-IFN-γ monoclonal antibody (mAb) (1-DIK; Mabtech, Stockholm, Sweden) at 4°C overnight. The following day, PBMC were added directly at 105 cells/well in R10 media [RPMI-1640 supplemented with 2 mM l-glutamine, 50 IU/ml penicillin, 50 μg/ml streptomycin and 10% heat inactivated FBS (Invitrogen Corporation, Irvine, CA, USA)] and then the individual ESAT-6, katG and sodA peptides, as well as the T. brucei cell wall lysate, were added to individual wells in duplicate (20 μg/ml final concentration). Phytohaemagglutinin A (PHA) and media alone served as positive and negative controls, respectively. The plates were incubated for 18 h at 37°C in 5% CO2. After washing six times with PBS plates were incubated for 2 h with 0·5 μg/ml biotinylated anti-IFN-γ mAb (7-B6-1; Mabtech). After additional washes, a 1 : 2000 dilution of streptavidin–alkaline phosphatase conjugate (Mabtech) was added to each well for 2 h. The plates were then washed six times, and IFN-γ-producing cells detected after a 15-min colour reaction using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium substrate (BCIP/NBT Substrate Kit; Vector Laboratories, Burlingame, CA, USA). Cells were counted using an Immunospot 3 Analyser [Cellular Technology Limited (CTL), Cleveland, OH, USA]. Results were expressed as the number of spot-forming cells (SFC) per 106 PBMC. A positive response was defined as at least 50 SFC/106 PBMC, and at least three times above the mean background SFC in the negative control wells. The background number of SFC was always < 50 SFC/106 PBMC in the negative control wells. These cut-offs were defined prior to unblinding the results or the clinical diagnosis of the study participants. Assays using PBMC from PPD−, PPD+ and sarcoidosis subjects were performed simultaneously; the technicians were blinded to the clinical diagnosis of each study participant.
Intracellular cytokine staining of T cells
To identify IFN-γ- and interleukin (IL)-2-secreting T cells in response to mycobacterial peptides, staining with a combination of T cell surface markers and intracellular staining was performed as described previously [18]. Briefly, 0·5–1·0 × 106 PBMC were incubated with 10 μM peptide and the anti-CD28 and anti-CD49d mAbs (1 μg/ml each; Becton Dickinson) at 37°C under 5% CO2 for 1 h before addition of 10 μg/ml of brefeldin A (Sigma, St. Louis, MO, USA). Following a 13-h incubation at 37°C under 5% CO2, cells were washed and stained with the surface antibodies anti-CD8 and anti-CD4 (Becton Dickinson) at 4°C for 30 min. After washing, fixation and permeabilization, using a Fix & Perm Kit according to the manufacturer's instructions (Caltag, Burlingame, CA, USA), anti-IFN-γ mAb and anti-IL-2 mAb (Becton Dickinson) added at 4°C for 30 min. Cells were washed and analysed via flow cytometry.
Statistical analysis
Comparisons of the distribution of T cell frequencies were performed using the Kruskal–Wallis test. Categorical comparisons were analysed using Fisher's exact test. All P-values are two-sided, and performed using r (version 2·1.1) [19].
Results
Study participant demographics and clinical characteristics
Sixty-six subjects were recruited for study participation. Of the 30 sarcoidosis subjects, 33% were African American, 33% were male and 63% < 50 years of age. Among the 26 PPD− participants, 54% were African American, 19% were male and 63% < 50 years of age. Of 10 PPD+ subjects 50% were African American, 30% male and 70% < 50 years of age (Table 2). Among the 30 sarcoidosis subjects, 27 had pulmonary disease, and of these 27, seven also had extrapulmonary disease involving the skin or central nervous system (Table 2). The three subjects without pulmonary involvement had central nervous system (Sarcoidosis 5), cutaneous involvement (Sarcoidosis 10) and involvement of the liver and spleen only (Sarcoidosis 22). Three sarcoidosis subjects (Sarcoidosis 15, 23 and 29) presented with Lofgren's syndrome. Eleven of the 30 study participants were on immunosuppressants at the time of study enrollment. There was no disease among the PPD− healthy volunteers, and only latent disease among the PPD+ subjects.
Table 2.
Demographic and clinical characteristics of study participants.
| Subject | Age*/race†/sex‡ | Site§ | sodA¶ | katG¶ | ESAT-6¶ | T. brucei¶ | Subject | Age*/race†/sex‡ | Site§ | sodA¶ | katG¶ | ESAT-6¶ | T. brucei¶ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | 43AAF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 1 | 42CF | P, C | 90 | < 50 | < 50 | |
| Control 2 | 38CF | None | < 50 | < 50 | < 50 | Sarcoidosis 2 | 49AAF | P, C | 70 | < 50 | 50 | ||
| Control 3 | 27AAF | None | < 50 | < 50 | < 50 | Sarcoidosis 3 | 55CF | P | < 50 | 150 | 140 | ||
| Control 4 | 33AAF | None | < 50 | < 50 | < 50 | Sarcoidosis 4 | 47CF | P, CNS | 170 | 60 | 180 | ||
| Control 5 | 23CM | None | < 50 | < 50 | < 50 | Sarcoidosis 5 | 49AAF | CNS | 75 | 90 | 100 | < 50 | |
| Control 6 | 25CF | None | < 50 | < 50 | < 50 | Sarcoidosis 6 | 51CF | P | 50 | 500 | < 50 | ||
| Control 7 | 31AAM | None | < 50 | < 50 | < 50 | Sarcoidosis 7 | 49CF | P | < 50 | < 50 | < 50 | ||
| Control 8 | 52CF | None | < 50 | < 50 | < 50 | Sarcoidosis 8 | 37CM | P | 100 | < 50 | 100 | 180 | |
| Control 9 | 32CF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 9 | 31AAM | CNS, P, C | 150 | 150 | < 50 | |
| Control 10 | 34AAF | None | < 50 | < 50 | < 50 | Sarcoidosis 10 | 51AAF | C | < 50 | < 50 | < 50 | ||
| Control 11 | 29CM | None | < 50 | < 50 | < 50 | Sarcoidosis 11 | 45AAF | P | < 50 | < 50 | < 50 | < 50 | |
| Control 12 | 52CF | None | < 50 | < 50 | < 50 | Sarcoidosis 12 | 59CF | P | < 50 | < 50 | < 50 | ||
| Control 13 | 35CF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 13 | 62CM | P | < 50 | < 50 | < 50 | < 50 |
| Control 14 | 57CF | None | < 50 | < 50 | < 50 | Sarcoidosis 14 | 54AAM | P | < 50 | < 50 | < 50 | ||
| Control 15 | 54CF | None | < 50 | < 50 | < 50 | Sarcoidosis 15 | 34CF | P | 100 | 80 | 90 | < 50 | |
| Control 16 | 57CF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 16 | 50CF | P | 75 | < 50 | 80 | 80 |
| Control 17 | 55AAF | None | < 50 | < 50 | < 50 | Sarcoidosis 17 | 55AAF | P | 65 | 65 | 85 | 70 | |
| Control 18 | 37AAM | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 18 | 22AAF | P | < 50 | < 50 | 55 | < 50 |
| Control 19 | 44CF | None | < 50 | < 50 | < 50 | Sarcoidosis 19 | 43AAF | P | < 50 | < 50 | < 50 | < 50 | |
| Control 20 | 32AAF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 20 | 51CM | P | < 50 | < 50 | < 50 | < 50 |
| Control 21 | 32AAF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 21 | 61CF | P, CNS | < 50 | < 50 | < 50 | < 50 |
| Control 22 | 27AAF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 22 | 52CF | ABD | < 50 | < 50 | < 50 | |
| Control 23 | 42AAF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 23 | 48CF | P | < 50 | < 50 | < 50 | < 50 |
| Control 24 | 25AAF | None | < 50 | < 50 | < 50 | Sarcoidosis 24 | 47CF | P, CNS | < 50 | < 50 | < 50 | ||
| Control 25 | 50AAM | None | < 50 | < 50 | 150 | Sarcoidosis 25 | 42CM | P | < 50 | < 50 | < 50 | < 50 | |
| Control 26 | 61AAF | None | < 50 | < 50 | < 50 | < 50 | Sarcoidosis 26 | 51AAM | P | 110 | 90 | 130 | |
| PPD+ 1 | 49CF | Latent | 360 | 80 | < 50 | Sarcoidosis 27 | 45CM | P, C | < 50 | 140 | 910 | ||
| PPD+ 2 | 50AAF | Latent | 50 | < 50 | < 50 | Sarcoidosis 28 | 34CM | P | < 50 | < 50 | 250 | < 50 | |
| PPD+ 3 | 42AAF | Latent | 100 | 80 | 120 | Sarcoidosis 29 | 30CM | P | 160 | < 50 | < 50 | ||
| PPD+ 4 | 58AAM | Latent | 65 | < 50 | 500 | Sarcoidosis 30 | 34CF | P | < 50 | < 50 | < 50 | < 50 | |
| PPD+ 5 | 50CF | Latent | 50 | 60 | 100 | 70 | |||||||
| PPD+ 6 | 60AAF | Latent | < 50 | < 50 | 60 | < 50 | |||||||
| PPD+ 7 | 38CF | Latent | < 50 | < 50 | < 50 | ||||||||
| PPD+ 8 | 42AAM | Latent | 55 | 70 | 70 | < 50 | |||||||
| PPD+ 9 | 51CF | Latent | < 50 | 100 | 210 | ||||||||
| PPD+ 10 | 30CM | Latent | < 50 | < 50 | < 50 | < 50 |
Age in years
AA, African American; A, African; C, Caucasian
F, female, M, male
Site of involvement: C, cutaneous, P, pulmonary, CNS, central nervous system
inteferon-γ producing spot-forming cells per million peripheral blood mononuclear cells. ESAT6: early secreted antigenic target protein; PPD: purified protein derivative; katG: catalase-peroxidase; sodA: superoxide dismutase A.
Sarcoidosis Th1 immune responses to mycobacterial antigens are antigen-specific
Th1 immune responses to one or more of the 17 ESAT-6 peptides occurred in 12 of 30 sarcoidosis subjects, compared to one of 25 PPD− healthy volunteers (P = 0·0014) and six of 10 subjects with latent tuberculosis (P = 0·30) (Table 2). Using two katG peptides, there was recognition among nine of 30 sarcoidosis subjects, compared to none of 26 PPD− control subjects (P = 0·002) and five of 10 PPD+ subjects (P = 0·28). For sodA, we assessed for recognition of four peptides that were determined to be immunogenic by peptide mapping studies. There was recognition of one or more of these peptides among 12 of 30 sarcoidosis subjects, compared to none of 26 PPD− subjects (P = 0·002) and six of 10 PPD+ subjects (P = 0·14) (Table 2).
Due to limitations in the number of PBMC, we were not able to assess for immune recognition of T. brucei lysate in all study participants. Among the 15 sarcoidosis subjects there was recognition among three, compared to none of 10 PPD− healthy volunteers (P = 0·25) and one of four PPD+ subjects (P = 1·0). Sarcoidosis 8, 16 and 17 and PPD+ 5 had immune responses to T. brucei whole cell wall lysate, as well as two or more mycobacterial antigens, implying that some of the recognition of mycobacterial antigens may be due to common antigens among MTB and T. brucei. None of the subjects had risk factors for exposure to T. brucei. However, there were sarcoidosis subjects (sarcoidosis 5 and sarcoidosis 15) who recognized all three mycobacterial antigens, but did not recognize T. brucei (Table 1). There was no recognition of T. brucei cell wall lysate among sarcoidosis subjects who failed to recognize any of the three mycobacterial antigens.
Among the 30 sarcoidosis patients, 16 of 30 (53%) subjects recognized at least one mycobacterial peptide compared to one of 26 control volunteers (P = 0·00008) and to seven (70%) of 10 PPD+ subjects (P = 0·47). There was no distinction among sarcoidosis subjects and the negative control group for recognition of T. brucei antigens (Table 2, Fig. 1). The number of tuberculosis subjects tested for immune recognition of parasitic antigen is too small to assess critically. There was no distinction with regard to recognition of mycobacterial antigens among sarcoidosis subjects who were immunosuppressed (seven of 13), compared to those who were not (nine of 17) (P = 1·0). In addition, the distribution of the T cell frequencies among the sarcoidosis subjects was not distinct from subjects with latent tuberculosis, but was from PPD− healthy volunteers, for each of the three mycobacterial antigens (Fig. 2). There was no distinction among the three groups for immune recognition of T. brucei lysate (Fig. 2). Thus, in assessing for immune responses to mycobacterial peptides, although the sarcoidosis subjects are negative for mycobacteria by culture and histopathology, the percentage of sarcoidosis subjects responding to these antigens and the distribution of the T cell response parallels more closely subjects with latent tuberculosis than PPD− healthy volunteers (Figs 1 and 2).
Fig. 1.
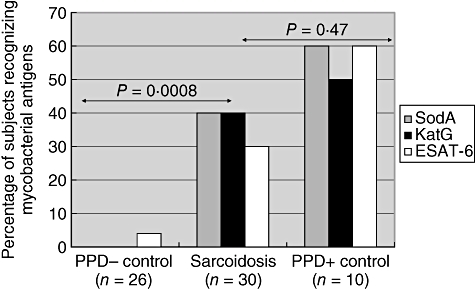
Sarcoidosis subjects recognize multiple mycobacterial antigens. Using enzyme-linked immunospot assay (ELISPOT) analysis, each of the study participants were tested for interferon-γ production after stimulation by catalase-peroxidase (katG), early secreted antigenic target protein (ESAT-6) and superoxide dismutase A (sodA) antigens. Sixteen of 30 sarcoidosis subjects had systemic responses to mycobacterial antigens which paralleled more closely the responses observed in purified protein derivative positive (PPD+) subjects, but was statistically significant from the response observed in PPD− controls.
Fig. 2.
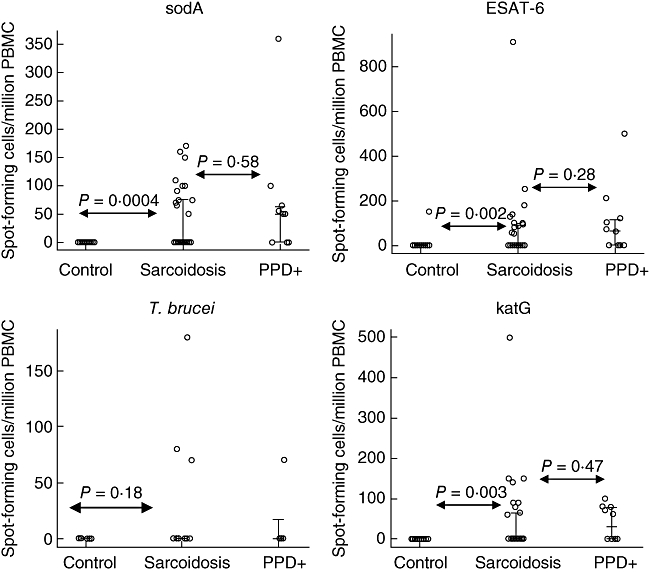
Analysis of the distribution of spot-forming cells/million peripheral blood mononuclear cells (PBMC) generated by stimulation with mycobacterial and Trypanosome brucei antigens. The bars represent the 25th, 50th and 75th percentile of each group of study participants. For the sarcoidosis subjects, the 25th and 50th percentile were the same. There was no statistically significant difference in the distribution of the T cell responses to any of the three mycobacterial antigens between the sarcoidosis and purified protein derivative positive (PPD+) controls, although there was between sarcoidosis and PPD− controls. The PPD− control that did recognize early secreted antigenic target protein (ESAT-6) antigens had a response quantitatively consistent with the sarcoidosis and PPD+ controls. Contrary to recognition of mycobacterial antigens, there was no distinction with regard to recognition of T. brucei whole cell wall lysate among sarcoidosis and PPD− controls. There were too few PPD+ controls tested for recognition of T. brucei to compare to sarcoidosis.
Individual sarcoidosis subjects recognize multiple, distinct mycobacterial peptides
Of the 16 sarcoidosis subjects whose PBMC were tested for mycobacterial proteins, 12 recognized multiple peptides within a given protein (Fig. 3). For example, sarcoidosis 5 recognized five ESAT-6 peptides (ESAT-6 peptides 12–16), both katG peptides and two sodA peptides, but did not demonstrate immune recognition to whole cell lysate from T. brucei. Sarcoidosis 15 recognized ESAT-6 peptide 9, katG peptides 31 and 32 and SodA peptides 31, 33, 36, and 38. Sarcoidosis 8 recognized ESAT-6 peptides 7–9, 15 and 16 (Fig. 3). Recognition of adjacent peptides suggests that epitope(s) are embedded within the overlapping peptide sequence. However, in some sarcoidosis subjects, immune responses to distinct peptides were observed. Although the four sodA peptides were distinct from each other, the majority of the subjects recognized two or more peptides. For example, sarcoidosis 15 recognized all four sodA peptides; sarcoidosis 16 recognized sodA peptides 31 and 33; sarcoidosis 5 recognized peptides 36 and 38 (Fig. 3).
Fig. 3.
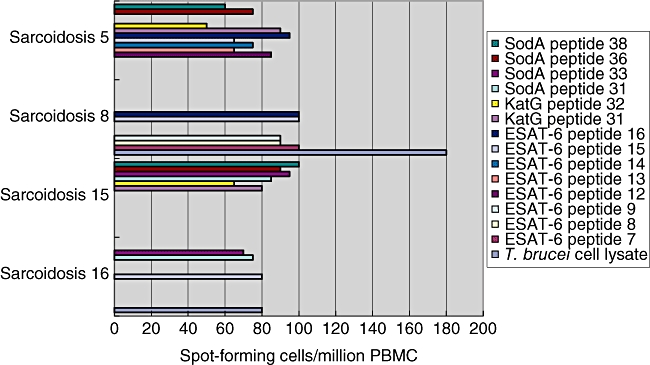
Sarcoidosis subjects recognize numerous mycobacterial epitopes. Enzyme-linked immunospot assay (ELISPOT) analysis for 17 early secreted antigenic target protein (ESAT-6), two catalase-peroxidase (katG) and four superoxide dismutase A (sodA) peptides was performed on all 66 study participants. Among the sarcoidosis subjects who recognized mycobacterial proteins, not only was there recognition of multiple mycobacterial proteins, but they frequently recognized more than one epitope within a given protein. Among some of the peptides recognized there were amino acid overlap, but recognition of peptides which were distinct from each other was also observed with similar T cell frequencies.
Recognition of mycobacterial antigens occur in sites of active sarcoidosis involvement
We were able to obtain tissue, blood and BAL fluid on sarcoidosis 30. We performed flow cytometry on her BAL fluid to assess for Th1 immune responses to ESAT-6 and katG, as well as to identify the cells responsible for the immune responses. Flow cytometry showed increased IL-2 and IFN-γ production from CD4+ T cells (Fig. 4). Due to limitations in cell count, we assessed only for recognition of ESAT-6 peptide 14 and katG peptide 31. Th1 immune responses to both of these peptides, which possess distinct amino acid homology, were observed (Fig. 4). We performed ELISPOT analysis using PBMC from sarcoidosis 30, using each of the 17 ESAT-6 peptides, both katG peptides and all four SodA peptides. There was no recognition of any of these peptides. This suggests that assessment for immune recognition using lymphocytes for sites involved actively by sarcoidosis may improve the capability to detect immune responses to mycobacterial antigens.
Fig. 4.
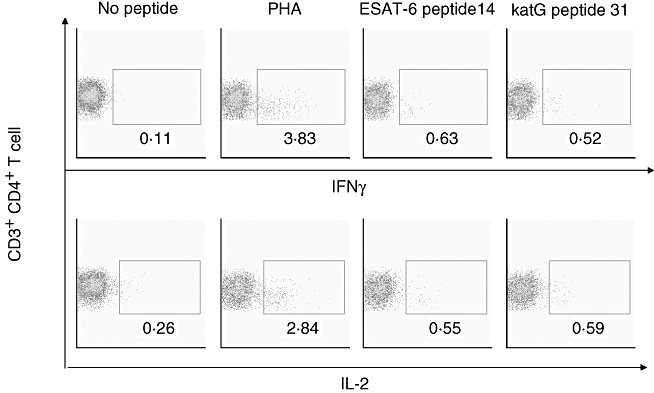
Flow cytometry of sarcoidosis bronchoalveolar fluid reveals T helper 1 (Th1) immune responses to early secreted antigenic target protein (ESAT-6) and catalase-peroxidase (katG) peptides. Intracellular cytokines staining for interleukin (IL)-2 and interferon (IFN)-γ was performed on bronchoalveolar lavage (BAL) fluid obtained from sarcoidosis 30, after stimulation with mycobacterial peptides from ESAT-6 and katG. Consistent with prior reports of sarcoidosis immunology, CD4+ T cells are increased in the sarcoidosis immune response. Both mycobacterial peptides were recognized by sarcoidosis CD4+ T cells, producing IL-2 and IFN-γ. In this subject (sarcoidosis 30), immune recognition was detected in the BAL fluid, a site of active sarcoidosis involvement but none in peripheral blood mononuclear cells.
Discussion
Analysis of the T cell antigen receptor repertoire from sarcoidosis subjects reveals selective expansion of oligoclonal populations of T cells, suggesting an antigen-driven immune response [20–22]. This study demonstrates that sarcoidosis subjects, collectively and as individuals, have a systemic, antigen-specific response to a number of mycobacterial antigens in a pattern more consistent with subjects with latent tuberculosis, as opposed to PPD−, healthy volunteers. This is consistent with immunological paradigms which report immune responses to a number of secreted, cell wall and cytoplasmic proteins in tuberculosis-infected subjects [9]. Also, consistent with previous reports, some sarcoidosis subjects recognized multiple epitopes within a given protein (Fig. 3) [16]. Furthermore, this is the first evidence that strong Th1 immune responses to mycobacterial antigens can be observed in sites of active sarcoidosis involvement such as BAL fluid. Not only was there a significant difference among the sarcoidosis and PPD− control subjects regarding the percentage of subjects recognizing the antigens, but also the T cell frequencies paralleled more closely the subjects with latent tuberculosis, despite negative histology and culture for mycobacteria in sarcoidosis specimens (Fig. 2).
At the discretion of their primary physician, approximately 60% of the sarcoidosis subjects underwent PPD skin testing. All of them were skin test negative, yet the majority recognized multiple mycobacterial antigens by ELISPOT analysis, including ESAT-6. The observation of anergy among sarcoidosis subjects has been attributed to increased T regulatory (Treg) cell activity. Treg cells accumulate at the periphery of sarcoid granulomas, in bronchoalveolar lavage fluid and in peripheral blood of patients with active disease. These cells exhibited powerful anti-proliferative activity, yet did not inhibit cytokine production completely. Sarcoidosis is therefore associated with a global Treg cell subset amplification whose activity would be insufficient to control local inflammation. At the same time, peripheral Treg cells exert powerful anti-proliferative activity that may account for the state of anergy [23]. Sensitive assays such as the ELISPOT assay can detect cytokine production. We assessed for increased Treg activity among these subjects and determined that a significant proportion of the sarcoidosis subjects possessed increased Treg activity in the peripheral blood (manuscript in preparation). Other possible explanations for the paradox of T cell anergy to PPD, yet systemic responses to mycobacterial antigens among sarcoidosis subjects, are as follows: (i) alterations in dendritic cell response in the skin as opposed to the peripheral blood; (ii) differences in the sensitivity of PPD+ skin testing compared to ELISPOT analysis; and (iii) the immune response among sarcoidosis subjects may reflect antigens of a non-tuberculous mycobacterium that is genetically similar to, yet distinct from, MTB.
Humoral immunity to mycobacterial antigens has been reported in other inflammatory diseases such as Crohn's disease, lupus and rheumatoid arthritis, which prevented us from including subjects with these diseases within the negative control group [24, 25]. In order to assess if the immune responses to mycobacterial antigens observed reflect a hyperactive immune system or were directed against secreted antigens within the sarcoidosis granulomas, we performed polymerase chain reaction (PCR) analysis for sodA on 12 archival sarcoidosis specimens from the 30 study participants. We detected mycobacterial sodA nucleic acids in eight of the 12 sarcoidosis specimens compared to two of 16 control specimens (P = 0·004, two-tailed Fisher's test) (manuscript in review). This observation suggests that the immune responses that we observed were directed against mycobacterial antigens present within the sarcoidosis granuloma.
T. brucei antigen was included to assess if the recognition observed was secondary to non-specific reactivity from a hyperactive immune response. While Th1 immune response to T. brucei has been described in infected mice and humans [26], there was no significant difference in its recognition among the three study groups. This finding is somewhat limited by our inability to test all 66 study participants, due to limitations in PBMC. Of the three sarcoidosis and one PPD+ subject who did recognize T. brucei, there was also recognition of two or more mycobacterial antigens. This detection of immune responses to T. brucei in subjects with no risk factors suggests two possibilities: (i) common microbial epitopes may exist between mycobacteria and T. brucei; and (ii) sarcoidosis subjects with robust immune responses may be prone to non-specific responses to foreign antigen. The former seems possible, as there was recognition of T. brucei among one of the PPD+ subjects (PPD+ 5). Also, the detection of mycobacterial sodA nucleic acids in sarcoidosis granulomas also supports that the immune responses are driven by proteins present within the granuloma (manuscript in review). We performed analysis for immune recognition of Escherichia coli sodA among the sarcoidosis, PPD− and PPD+ subjects, according to PBMC availability; there was no significant difference in immune recognition to E. coli sodA among the three groups (personal observation).
The observation of a strong immune response in sarcoidosis BAL fluid to mycobacterial antigens is a compelling argument that mycobacteria may have a role in sarcoidosis pathogenesis (Fig. 4). Flow cytometry revealed that sarcoidosis 30 had a Th1 immune response composed primarily of CD4+ T cells, which secreted IFN-γ and IL-2, after stimulation by ESAT-6 peptide 14 and katG peptide 31. We chose these two peptides because they had been shown recently to be immunogenic in systemic sarcoidosis [11]. The detection of Th1 immune responses in a site involved actively by sarcoidosis adds further support to the hypothesis that, in a subset of sarcoidosis subjects, mycobacterial antigens are important. We were also able to detect mycobacterial nucleic acids in archival sarcoidosis specimens of subjects whose PBMC did not recognize any of the three mycobacterial antigens, such as sarcoidosis 30 and sarcoidosis 20 (manuscript in review). The detection of immune responses in sites involved actively by sarcoidosis, as well as the presence of mycobacterial nucleic acids in the sarcoidosis granulomas, suggests that the current study could be an underestimation of the prevalence of mycobacterial involvement.
There were sarcoidosis subjects who did not respond to any of the mycobacterial antigens. This lack of reactivity in some sarcoidosis subjects may reflect that sarcoidosis subjects do not, in fact, have sarcoidosis but a disease with common clinicopathological phenotypes, such as chronic beryllium disease (CBD). Two recent reports suggest that CBD can be inappropriately classified as sarcoidosis, without proper testing [27, 28]. It would be interesting to perform mutual assessment for recognition of mycobacterial antigens and beryllium in the same sarcoidosis population. An alternative possibility is that during active disease immune responses are present, but as their disease resolves the memory cells present are below the level of detection, which has been described in some tuberculosis-infected subjects [29]. Assessment for site-specific responses during the initial diagnosis will help to delineate this more clearly. Unfortunately, we did not have diagnostic BAL fluid on the other sarcoidosis subjects. It is also possible that mycobacteria do not have a role in all sarcoidosis cases; the lack of recognition reflects an alternative aetiology.
It is unclear whether the immune responses to mycobacterial antigens are secondary to persistent, poorly degraded antigens or immune responses to slowly replicating mycobacteria. It is beyond the capability of this study to delineate the actual Mycobacterium species. Immune recognition of mycobacterial ESAT-6 has been described in MTB complex, non-tuberculous mycobacteria such as M. gordonae and M. kansasii, as well as Streptomyces and Nocardia[9, 30]. Cellular immune responses to ESAT-6 have been shown to discriminate between pulmonary infection with M. tuberculosis and M. avium[31]. Molecular analyses of sarcoidosis specimens have noted the presence of 16S rRNA and rpoB sequences consistent with M. tuberculosis complex, non-tuberculosis species, and species genetically similar, yet distinct, to MTB [32]. The observation that sarcoidosis subjects improve clinically with steroid therapy without the development of tuberculosis, as well as reports of subjects developing sarcoidosis in the midst of anti-tuberculosis therapy [33], suggests that the reactivity that we are observing reflects immune recognition to a member of MTB complex or a genetically similar mycobacterial species.
These findings contribute to recent reports suggesting that mycobacteria have a role in sarcoidosis immunopathogenesis [4, 5]. They reveal that a significant subset of sarcoidosis subjects demonstrates a Th1 systemic response to multiple mycobacterial antigens, and that assessment from sites of active sarcoidosis involvement reveals more vigorous responses, again to multiple antigens. Future studies will involve identification of genetic associations with immune recognition, and a delineation of whether the immune response detected reflects responses to persistent antigen or a slowly replicating Mycobacterium.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HL83839-01; AI65744-01).
References
- 1.Semenzato G, Bortoli M, Agostini C. Applied clinical immunology in sarcoidosis. Curr Opin Pulm Med. 2002;8:441–4. doi: 10.1097/00063198-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Moller DR. Genetic basis of remitting sarcoidosis. Triumph of the trimolecular complex? Am J Respir Cell Mol Biol. 2002;27:391–5. doi: 10.1165/rcmb.2002-0164PS. [DOI] [PubMed] [Google Scholar]
- 3.Vourlekis JS, Sawyer RT, Newman LS. Sarcoidosis: developments in etiology, immunology, and therapeutics. Adv Intern Med. 2000;45:209–57. [PubMed] [Google Scholar]
- 4.Dubaniewicz A, Dubaniewicz-Wybieralska M, Sternau A, et al. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J Clin Microbiol. 2006;44:3448–51. doi: 10.1128/JCM.01433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201:755–67. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill PC, Fox A, Jeffires DJ, et al. Quantitative T cell assay reflects infectious load of M. tuberculosis in an endemic case contact model. Clin Infect Dis. 2005;40:273–8. doi: 10.1086/427030. [DOI] [PubMed] [Google Scholar]
- 7.Thole JE, Wieles B, Clark-Curtiss JE, Ottenhoff TH, Rinke de Wit TF. Immunological and functional characterization of M. leprae protein antigens: an overview. Mol Microbiol. 1995;18:791–800. doi: 10.1111/j.1365-2958.1995.18050791.x. [DOI] [PubMed] [Google Scholar]
- 8.Andersen P. The T cell response to secreted antigens of Mycobacterium tuberculosis antigens. Immunobiology. 1994;191:537–47. doi: 10.1016/S0171-2985(11)80460-2. [DOI] [PubMed] [Google Scholar]
- 9.Demissie A, Leyten EMS, Abebe M, et al. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2006;13:179–86. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafari C, Ernst M, Kalsdorf B, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med. 2006;174:1048–54. doi: 10.1164/rccm.200604-465OC. [DOI] [PubMed] [Google Scholar]
- 11.Drake WP, Dhason MS, Nadaf M, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75:527–30. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harth G, Horwitz MA. Export of recombinant Mycobacterium tuberculosis superoxide dismutase is dependent upon both information in the protein and mycobacterial export machinery. A model for studying export of leaderless proteins by pathogenic mycobacteria. J Biol Chem. 1999;274:4281–92. doi: 10.1074/jbc.274.7.4281. [DOI] [PubMed] [Google Scholar]
- 13.Edwards KM, Cynamon MH, Voladri RK, et al. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164:2213–19. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR. 2000;49:18–19. [Google Scholar]
- 15.Hajizadeh R, Sato H, Carlisle J, et al. Mycobacterium tuberculosis antigen 85A induces Th-1 immune responses in systemic sarcoidosis. J Clin Immunol. 2007;27:445–54. doi: 10.1007/s10875-007-9080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pathan AA, Wilkinson KA, Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–25. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 17.Altfeld MA, Livingston B, Reshamwala N. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J Virol. 2001;75:1301–11. doi: 10.1128/JVI.75.3.1301-1311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulder PJ, Tang Y, Brander C, et al. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J Exp Med. 2000;192:1819–32. doi: 10.1084/jem.192.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.r Development Core Team. T: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 20.Moller DR. T-cell receptor genes in sarcoidosis. Sarc Vasc Diff Lung Dis. 1998;15:158–64. [PubMed] [Google Scholar]
- 21.Forrester JM, Wang Y, Ricalton N, et al. TCR expression of activated T cel clones in the lungs of patients with pulmonary sarcoidosis. J Immunol. 1994;153:4291–302. [PubMed] [Google Scholar]
- 22.Jones CM, Lake RA, Wijeyekoon JB, Mitchell DM, du Bois RM, O'Hehir RE. Oligoclonal V gene usage by T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients. Am J Resp Cell Mol Biol. 1996;14:470–7. doi: 10.1165/ajrcmb.14.5.8624252. [DOI] [PubMed] [Google Scholar]
- 23.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen I, Wiker HG, Johnson E, Langeggen H, Reitan LJ. Elevated antibody responses in patients with Crohn's disease against a 14-kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Scand J Immunol. 2001;53:198–203. doi: 10.1046/j.1365-3083.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsoulfa G, Rook GA, Van-Embden JD, et al. Raised serum IgG and IgA antibodies to mycobacterial antigens in rheumatoid arthritis. Ann Rheum Dis. 1989;48:118–23. doi: 10.1136/ard.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi M, Wei G, Pan W, Tabel H. Experimental African trypanosomiasis: a subset of pathogenic, IFN-gamma-producing, MHC class II-restricted CD4+ T cells mediates early mortality in highly susceptible mice. J Immunol. 2006;176:1724–32. doi: 10.4049/jimmunol.176.3.1724. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Quernheim J, Gaede KI, Fireman E, Zissel G. Diagnoses of chronic beryllium disease within cohorts of sarcoidosis patients. Eur Resp J. 2006;27:1190–5. doi: 10.1183/09031936.06.00112205. [DOI] [PubMed] [Google Scholar]
- 28.Contini S, Mattioli G, Berretta F, et al. Berylliosis in Italy: a case of ‘sarcoidosis’ under the threshold limit value. Med Del Lavoro. 2006;97:592–6. [PubMed] [Google Scholar]
- 29.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;174:831–9. doi: 10.1164/rccm.200511-1783OC. [DOI] [PubMed] [Google Scholar]
- 30.Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797–807. doi: 10.1086/345760. [DOI] [PubMed] [Google Scholar]
- 31.Lein AD, von Reyn CF, Ravn P, Horsburgh CR, Jr, Alexander LN, Andersen P. Cellular immune responses to ESAT-6 discriminate between patients with pulmonary disease due to Mycobacterium avium complex and those with pulmonary disease due to Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 1999;6:606–9. doi: 10.1128/cdli.6.4.606-609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake WP, Pei Z, Pride DP, Collins RD, Cover TL, Blaser MJ. Molecular analysis of sarcoidosis and control tissues for Mycobacteria DNA. Emerg Infect Dis. 2002;8:1328–35. doi: 10.3201/eid0811.020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CF, Yew WW, Wong PC, Joseph L. A case of concomitant tuberculosis and sarcoidosis with mycobacterial DNA present in the sarcoidosis lesion. Chest. 1998;114:626–9. [PubMed] [Google Scholar]


