Abstract
Intravenous immunoglobulin (IVIg) preparations are reportedly effective in inhibiting the relapse of multiple sclerosis (MS), but few reports have investigated the effect of IVIg on dendritic cells (DCs), which are thought to be involved in such relapses. In the system that uses monokines to differentiate DCs from peripheral blood monocytes (Mo-DCs), we investigated the effect of immunoglobulin G (IgG) on these antigen-presenting cells. Using monocytes derived from healthy volunteers, IgG partially inhibited the expression of CD1a, a marker of immature DCs (imDCs), and CD40 and CD80, which are markers associated with T cell activation. In contrast, IgG enhanced the expression of CD83, a marker of mature DCs (mDCs). Furthermore, IgG markedly inhibited the expression of CD49d [very late activation antigen (VLA)-4 α4-integrin], the adhesion molecule required for mDCs to cross the blood–brain barrier. We obtained similar results on all the aforementioned cell surface molecules investigated in both healthy controls and MS patients. In addition, IgG treatment of cells from both healthy controls and MS patients inhibited the production of interleukin (IL)-12, a cytokine associated with mDC differentiation, but did not inhibit the production of IL-10. These results suggested the possibility that IgG treatment, apart from its known ability to regulate inflammation, may help to prevent relapses of MS by controlling DC maturation, consequently inhibiting invasion of immune cells into the central nervous system and affecting the cytokine profile.
Keywords: adhesion molecules, cell differentiation, dendritic cells, IVIg, MS
Introduction
Intravenous immunoglobulin (IVIg) has been reported to be an effective treatment for several autoimmune diseases [1–10]. In autoimmune diseases, IVIg may exert a therapeutic effect via multiple mechanisms, including the neutralization of autoantibodies with anti-idiotype antibodies [11], inhibition of complement-mediated damage [12, 13], inhibition of inflammatory cytokine production by activated lymphocytes [14, 15] and Fc receptor-mediated inhibition of inflammation [16, 17]. All these actions, however, influence effector cells; their effect on dendritic cells (DCs), which promote the development of autoreactive effector T cells involved in the onset and relapse of autoimmune disease [18, 19], have not been investigated sufficiently. Several reports on relapsing–remitting multiple sclerosis (RR-MS) assert that monocyte-derived DCs (Mo-DCs) release matrix-degrading metalloproteinases (MMP)-9 and are involved in the disruption of nerve tissues [20]. In addition, the spinal fluid of relapsing MS patients may contain some soluble factors that promote the differentiation of Mo-DCs [21], implicating Mo-DCs in MS relapses. Duddy et al. argued that Mo-DCs may be a therapeutic target cells in MS, because current effective therapies, including interferon (IFN)-β and steroids, inhibit the differentiation of monocytes into DCs [22].
To elucidate the molecular mechanism by which IVIg administration affects the course of MS, we examined Mo-DC differentiation. We investigated the effect of IgG on the differentiation of monocytes into immature DCs (imDCs) and from imDCs into mature DCs (mDCs). We analysed the effect of IgG treatment on the expression of the following cell surface molecules: CD1a, an indicator of imDC differentiation; CD83, a marker of mDC differentiation; the co-stimulatory molecules CD40, CD80 and CD86; and the adhesion molecule CD49d, which controls the migration of DCs. We used a DC differentiation system based on monocytes derived from the peripheral blood of healthy volunteers. As DCs produce the cytokines interleukin (IL)-12 and IL-10, which control the differentiation of effector T cells [23–25], we analysed the effect of IgG on the production of these cytokines. Our results demonstrated that IgG inhibited CD1a expression and CD86 down-regulation in association with impaired imDC differentiation. Also, IgG enhanced the expression of CD83, and partially inhibited the expression of the co-stimulatory molecules CD40 and CD80 associated with mDC differentiation. In addition, IgG abrogated the expression of CD49d, a marker associated with the differentiation of mDCs. These effects of IgG were confirmed using monocytes derived from the peripheral blood of RR-MS patients in remission. These findings suggest that in addition to the known actions [11–17], IVIg possesses additional anti-MS activity supporting the multi-functionality of IgG in the treatment of autoimmune disease.
Materials and methods
Healthy volunteers and MS patients
Anti-coagulated blood was collected from both MS patients and healthy volunteers at the Department of Neurology, Tohoku University Hospital (Sendai, Japan). Flow cytometry of surface molecules was performed using blood collected from nine MS patients (one male, eight females, aged 31–49 years, median age 42) and eight healthy volunteers (one male, seven females, aged 27–49 years, median age 32). To examine intracellular cytokine expression by flow cytometry, we used blood collected from 10 MS patients (two males, eight females, aged 31–53 years, median age 42) and 10 healthy volunteers (two males, eight females, aged 23–49 years, median age 32). All the MS patients had RR-MS that was in remission at the time of blood collection; none was receiving therapy with immunomodulatory agents, such as interferon (IFN)-β or corticosteroids. This research was approved by the Ethics Committee of the Tohoku University School of Medicine, and written informed consent was obtained from all participants.
Cytokines and antibodies
Recombinant human IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) were purchased from Biosource (Camarillo, CA, USA). Recombinant tumour necrosis factor (TNF)-α was obtained from RELIATech (Braunschweig, Germany), and IL-1β was acquired from Alexis (Lausen, Switzerland).
Fluorescein isothiocyanate (FITC)-conjugated anti-CD1a monoclonal antibodies (mAbs) were purchased from Ancell (Bayport, MN, USA). Phycoerythrin (PE)-conjugated anti-CD83, -human leucocyte antigen D-related (HLA-DR), -CD49d, -CD86, -IL-10 and -IL-12 (p40/p70) mAbs and FITC-conjugated anti-CD40 and -CD80 mAbs were purchased from BD Biosciences (San Diego, CA, USA). We purchased PE-cyanine5-conjugated anti-CD14 mAbs from Immunotech (Marseille, France). The corresponding isotype control antibodies were purchased from BD Biosciences and Immunotech.
Human IgG
Human IgG was dispensed from IVIg preparations refined by cold ethanol (Cohn) fractionation from blood plasma samples donated by more than 10 000 Japanese people. After dialysing the IgG fraction against physiological saline, we determined the IgG concentration by measuring OD280 using a spectrophotometer. Aliquots were stored at −80°C until needed. The obtained IgG contained 0·14 mg/ml IgA and < 0·05 mg/ml IgM per 100 mg/ml of the IgG, and the content of polymeric IgG was < 0·5 mg/ml. Endotoxins were not detected (< 0·05 endotoxin units/ml per 100 mg/ml). To generate F(ab′)2 fragments, we used pepsin-treated immunoglobulin for those that had been dialysed against physiological saline.
Differentiation of monocyte-derived DCs
T cells, B cells, natural killer cells and granulocytes were removed from ethylenediamine tetraacetic acid (EDTA)-treated peripheral blood using RosetteSep Human Monocyte Enrichment Cocktail (StemCell Technologies, Vancouver, Canada) to obtain monocyte fractions. After suspension at a density of 4–5 × 105 cells/well in Hank's balanced salt solution (HBSS; Sigma, St Louis, MO, USA) containing 5% heat-inactivated human AB serum, monocytes were incubated for 1 h in a 24-well culture plate at 37°C. Non-adherent cells were removed by aspiration, and the remaining cells were washed with phosphate-buffered saline (PBS). Greater than 85% of the monocytes obtained in this manner were CD14-positive. We induced DC differentiation using the method of de Jong et al. [26], with the following modifications. Briefly, monocytes were cultured in 1600 units/ml GM-CSF and 200 units/ml IL-4 in RPMI-1640 medium containing 3% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 2 mM L-glutamine, 0·05 mM 2-mercaptoethanol, 100 μg/ml streptomycin and 100 units/ml penicillin (3% complete medium) at 37°C for 7 days under humidity and 5% CO2 to differentiate the cells into imDCs. Throughout the 7 days, 30% of the medium was replaced with new medium every 2 days. After the 7-day culture, imDCs were cultured in 3% complete medium containing 50 ng/ml TNF-α and 50 ng/ml IL-1β for 2 additional days to differentiate the cells into mDCs. To examine the effects of IgG on DC differentiation, at the beginning of incubation IgG at a final concentration of 20 mg/ml or an equal volume of saline was added to the cultures. As described above, 30% of the medium was replaced with new medium every 2 days with either IgG or saline as mentioned above to maintain the incubation condition. In the experiments to clarify the involvement of FcγR, IgG, equimolar F(ab′)2 or an equal volume of saline was used in the cell culture.
Flow cytometry of cell surface molecules
Cells collected on days 7 and 9 were suspended for 15 min at 4°C in PBS containing 1% bovine serum albumin, 2% normal rabbit serum and 0·05% sodium azide (staining buffer). Cells were then stained with fluorescein-conjugated or isotype control antibodies for 30 min at 4°C. After washing and resuspending the cells in staining buffer, greater than 5000 cells from each sample were analysed using a fluorescence activated cell sorter (FACSCalibur) flow cytometer and CellQuest software (BD Biosciences).
Flow cytometry of intracellular cytokines
Intracellular cytokines were assessed using Cytofix/Cytoperm kits according to the manufacturer's instructions (BD Biosciences). Briefly, imDCs were collected on day 7 and resuspended in 3% complete medium containing 50 ng/ml TNF-α and 50 ng/ml IL-1β. After the addition of 1 μl/ml Golgi Stop (BD Biosciences) to prevent cytokine secretion, we incubated the cells under humidity for 12 h at 37°C in 5% CO2. We then stained the cells for accumulated IL-10 and IL-12 (p40/70) using appropriate fluorescently labelled antibodies and a FACSCalibur flow cytometer.
Statistical analysis
To calibrate the data, we used an unpaired t-test for unpaired data and a paired t-test for paired data. P-values less than 0·05 (two-tailed) were considered significant. We used sas software (SAS Institute Inc., Cary, NC, USA) for statistical analysis.
Results
Differentiation of monocyte-derived dendritic cells and the effect of IgG
Monocytes isolated from the peripheral blood of healthy volunteers were incubated with GM-CSF and IL-4 for 7 days to generate non-adherent CD14−, CD1a+ cells (74·5 ± 9·3%) as shown in Table 1, and exhibited the morphological characteristics of imDCs (data not shown). The addition of TNF-α and IL-1β to the cultures for 2 additional days did not produce any remarkable morphological changes in these cells. The expression frequency of CD1a, a characteristic DC cell surface marker, increased on day 7, but there was little additional change during the subsequent 2-day culture with TNF-α and IL-1β. During this phase, the cells matured from imDCs to mDCs. Expression of CD83, a characteristic mDC marker, increased slightly by day 7 and increased further by day 9. CD40 and CD80, co-stimulatory molecules involved in the sensitization and activation of T cells, increased with each additional day of culture from the monocytic stage. In contrast, CD86 expression, which was initially 78·0 ± 14·2% in the monocyte cultures, decreased on day 7 (51·5 ± 17·5%) and then recovered by day 9 (84·7 ± 15·5%). Expression of HLA-DR, a major histocompatibility complex (MHC) class II molecule, exhibited a similar transition, with 46·0 ± 19·5% positive in the monocyte cultures, 21·1 ± 12·2% positive on day 7 and 48·1 ± 22·3% positive on day 9 (Table 1). These results confirmed that our culture system promoted the differentiation of monocytes into imDCs (day 7) and mDCs (day 9) in a phased manner.
Table 1.
Effects of immunoglobulin G (IgG) on the expression of cell surface molecules in healthy controls.
| Day 7 | Day 9 | ||||
|---|---|---|---|---|---|
| Molecule | Monocyte | IgG | Saline | IgG | Saline |
| CD1a | 6·9 ± 4·3 | 59·8 ± 12·0** | 74·5 ± 9·3 | 64·4 ± 8·5** | 79·5 ± 8·0 |
| CD83 | 3·7 ± 2·6 | 20·9 ± 8·7** | 14·3 ± 9·0 | 50·1 ± 6·6** | 29·7 ± 2·8 |
| CD40 | 3·9 ± 2·3 | 34·0 ± 9·7 | 34·4 ± 10·3 | 53·9 ± 13·0** | 81·0 ± 8·7 |
| CD80 | 0·8 ± 0·8 | 25·4 ± 8·2* | 32·3 ± 9·5 | 48·9 ± 14·1** | 81·4 ± 7·2 |
| CD86 | 78·0 ± 14·2 | 74·3 ± 13·2** | 51·5 ± 17·5 | 96·8 ± 3·0* | 84·7 ± 15·5 |
| HLA-DR | 46·0 ± 19·5 | 21·4 ± 11·9 | 21·1 ± 12·2 | 52·6 ± 24·0 | 48·1 ± 22·3 |
| CD49d | 94·4 ± 3·4 | 39·7 ± 8·2** | 29·5 ± 8·7 | 27·5 ± 10·9** | 49·8 ± 12·3 |
Monocyte isolation and differentiation into DCs were performed as described in Materials and methods. Healthy control samples (n = 8) were each divided into a group with IgG added at the beginning of the culture period (IgG) and a group treated with vehicle alone (saline). Monocytes and cells on days 7 and 9 of treatment were analysed by flow cytometry. The results show the frequency of cells (%) positive for each cell surface molecule [CD1a, CD83, CD40, CD80, CD86, human leucocyte antigen D-related (HLA-DR), and CD49d] as the mean value ± standard deviation. To calibrate the differences between the paired mean values, we used a paired t-test
(P < 0·01
P < 0·05).
To evaluate the effect of IgG on DC differentiation, we measured the expression of these DC-specific cell surface markers after the addition of IgG to this culture system (Table 1). We utilized an IgG concentration of 20 mg/ml, which was based on the dosage administered for the medical treatment of autoimmune diseases. Expression of CD1a was significantly lower on both days 7 (P < 0·001) and 9 (P < 0·001) relative to the saline-treated group. In contrast, the expression frequency of CD83 was significantly higher on days 7 (P = 0·006) and 9 (P < 0·001) compared to the saline-treated group (Fig. 1).
Fig. 1.
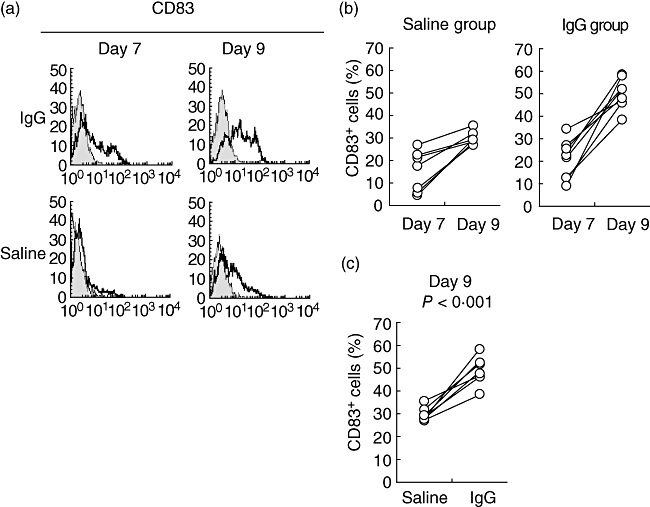
Effect of immunoglobulin G (IgG) on the expression of CD83 associated with dendritic cell (DC) differentiation. We cultured monocytes from healthy control samples (n = 8) for 7 days in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 [to produce immature DCs (imDCs)]. These cells were cultured for 2 additional days in the presence of tumour necrosis factor (TNF)-α and IL-1β to produce mature DCs (mDCs). We prepared two groups: one to which IgG was added at the beginning of culture (IgG) and a group treated with vehicle alone (saline). (a) We collected the cells on days 7 and 9 and analysed them by flow cytometry. The figure shows a representative sample stained with anti-CD83 monoclonal (open histograms) or isotype control (shaded histograms) antibodies. (b) The figure details the transition of CD83+ cells from day 7 to day 9 for the saline and IgG groups. (c) The image shows the frequency of CD83+ cells on day 9.
Expression of the co-stimulatory molecules CD40 and CD80 in the IgG-treated group on day 9 (mDCs) was significantly lower than that seen in the saline-treated group (Table 1; P < 0·001 for CD40, P < 0·001 for CD80). In contrast, IgG maintained the high expression of CD86 on day 7 (imDCs) (Fig. 2; P = 0·001). The expression of HLA-DR on both days 7 and 9 was unaffected by IgG treatment.
Fig. 2.
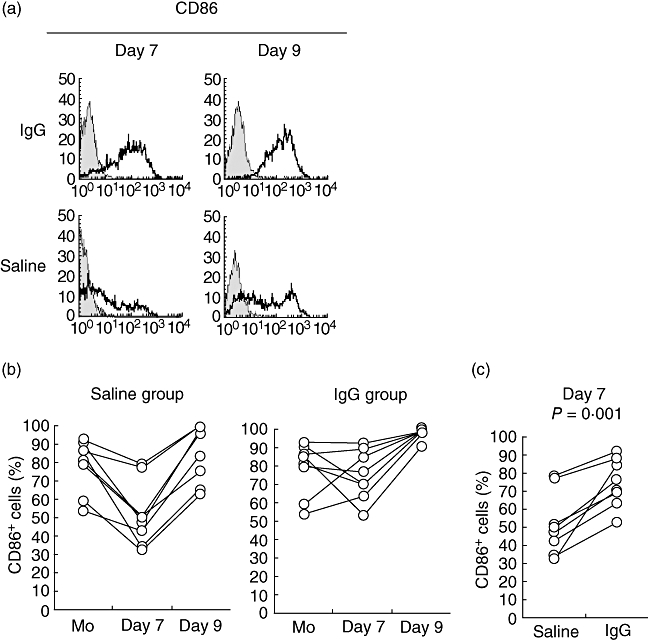
Effect of immunoglobulin G (IgG) on the expression of CD86 associated with dendritic cell (DC) differentiation. Samples from healthy controls (n = 8) were divided into groups and differentiated into DCs, as described in Fig. 1. (a) Cells collected on days 7 and 9 were analysed by flow cytometry. The figure shows a representative example of a sample stained with anti-CD86 monoclonal (open histograms) or isotype control (shaded histograms) antibodies. (b) The image details the transition of CD86+ cells [monocytes (Mo), days 7 and 9] in both the saline and IgG groups. (c) The figure details the frequency of CD86+ cells on day 7.
Effect of IgG on the expression of CD49d (α4 subunit of VLA-4) associated with DC differentiation
In MS, CD49d-mediated interactions with endocapillary cells at the brain–cerebrospinal barrier are necessary for effector cells to invade the central nervous system (CNS) [27, 28]. CD49d, which is generally expressed by all leucocytes [29], also plays an important role in the localized inflammation of the CNS during neurological diseases. No reports, however, have analysed the effect of IgG on the expression of this molecule. We evaluated the normal changes in CD49d expression throughout DC maturation and examined the effect of IgG on this process. In our system, CD49d decreased in association with the differentiation from monocytes (94·4 ± 3·4%) into imDCs (29·5 ± 8·7%), but increased again upon differentiation into mDCs (49·8 ± 12·3%) (Table 1). The expression of CD49d on day 9 (Fig. 3) decreased significantly upon the addition of IgG to the culture system (27·5 ± 10·9%, P = 0·001) compared with the saline group (49·8 ± 12·3%). IgG abrogated the recovery of CD49d expression associated with mDC differentiation. Four mg/ml IgG also significantly lowered the expression frequency of CD49d on day 9 (data not shown), although the extent of the decrease was smaller than that seen with 20 mg/ml IgG. These results suggest that IgG could influence DC passage through the cerebrospinal barrier by decreasing expression of the adhesion molecule CD49d.
Fig. 3.
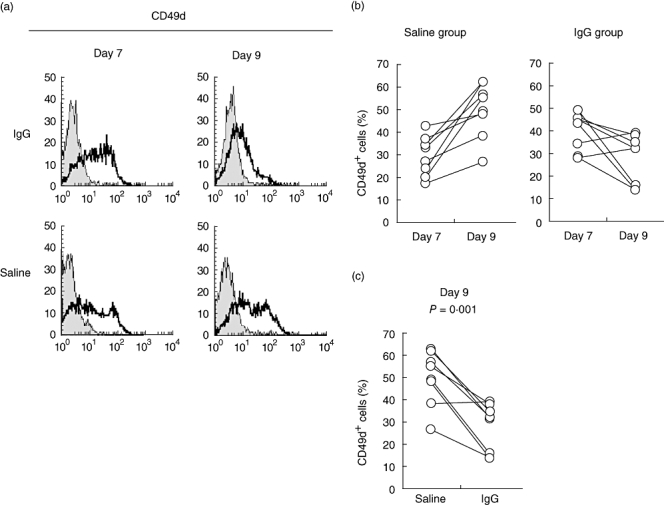
Effect of immunoglobulin G (IgG) on the expression of CD49d, the α4 subunit of very late activation antigen (VLA)-4, associated with DC differentiation. We divided samples from healthy controls (n = 8) into groups and induced differentiation into mature DCs (mDCs), as described in Fig. 1. (a) Cells collected on days 7 and 9 were analysed by flow cytometry. The figure shows a representative sample stained with anti-CD49d monoclonal (open histograms) or isotype control (closed histograms) antibodies. (b) This image shows the transition of CD49d+ cells (days 7 and 9) in both the saline and IgG groups. (c) This figure details the frequency of CD49d+ cells on day 9.
Effect of IgG on the production of IL-12 and IL-10 associated with mDC differentiation
During the differentiation of imDCs to mDCs, changes in the cytokine production profiles affect the subsequent types of T cells produced. CD1a+ CD83+ mDCs produce IL-12 and induce T helper 1 (Th1)-type immune responses, while CD1a− CD83+ mDCs produce IL-10 and induce Th2-type immune responses [23, 30]. We performed double staining for CD1a and cytokines to evaluate the effects of IgG on IL-12 and IL-10 production during imDC-to-mDC differentiation. As shown in Table 2, the percentage of IL-12-producing cells was high in the CD1a+ cells, but the expression frequency of CD1a+ IL-12+ cells was low in the IgG-treated group (P < 0·01) compared to the saline-treated group. In the CD1a− cells, the frequency of IL-10-producing cells in the IgG-treated group was significantly higher than that seen in the saline-treated group (P < 0·05). These results reveal that IgG affects both DC differentiation, as demonstrated by changes in the expression of cell surface markers associated with DC differentiation, and the subsequent cytokine production by DCs that is critical in the induction of a Th1-type immune response.
Table 2.
Effects of immunoglobulin G (IgG) on the intracellular production of interleukin (IL)-10 and IL-12 associated with mature dendritic cell (mDC) differentiation in healthy controls.
| CD1a+ | CD1a– | |||
|---|---|---|---|---|
| Cytokine | IgG | Saline | IgG | Saline |
| IL-12 | 18·1 ± 8·4** | 31·6 ± 8·3 | 5·4 ± 2·9 | 5·5 ± 4·4 |
| IL-10 | 15·1 ± 7·5 | 14·6 ± 6·5 | 11·9 ± 4·7* | 7·4 ± 3·4 |
Samples from healthy controls (n = 10) were each divided into two groups, one with IgG added at the beginning of culture (IgG) and one treated with vehicle alone (saline). Immature DCs collected from the cultures on day 7 were resuspended in medium containing tumour necrosis factor (TNF)-α, IL-1β, and Golgi Stop. Cells were collected after 12 h. We measured cell surface molecules (CD1a) and intracellular cytokines (IL-12 and IL-10) as described in Materials and methods. The results indicate the frequency of positive cells (%) in each quadrant as the mean value ± standard deviation. To calibrate the differences between paired mean values, we used a paired t-test(
P < 0·01
P < 0·05).
Analysis of peripheral blood samples from MS patients
To suggest a possible therapeutic benefit of IVIg in MS, we needed to confirm that the effects of IgG on the differentiation of DCs (with regard to the expression of surface markers, co-stimulatory molecules and the adhesion molecule) were the same in both MS patients and healthy controls. Using blood obtained from volunteer RR-MS patients in remission who had not been treated with immune-modulating drugs, we examined surface molecule expression and cytokine production by DCs, as described above. Monocytes separated from the peripheral blood of RR-MS patients differentiated in the same morphological manner as those from the healthy controls. The expression of DC cell surface markers displayed the same temporal pattern as that seen in healthy controls. The addition of IgG to the cultures exerted similar effects on DCs as seen on the monocytes of healthy controls (Fig. 4). The increased expression of CD49d associated with mDC differentiation was abrogated by IgG treatment (Fig. 4c). In addition, a decrease in the frequency of IL-12-producing cells was seen in the IgG-treated group, similar to that exhibited by the samples from the healthy controls (data not shown).
Fig. 4.
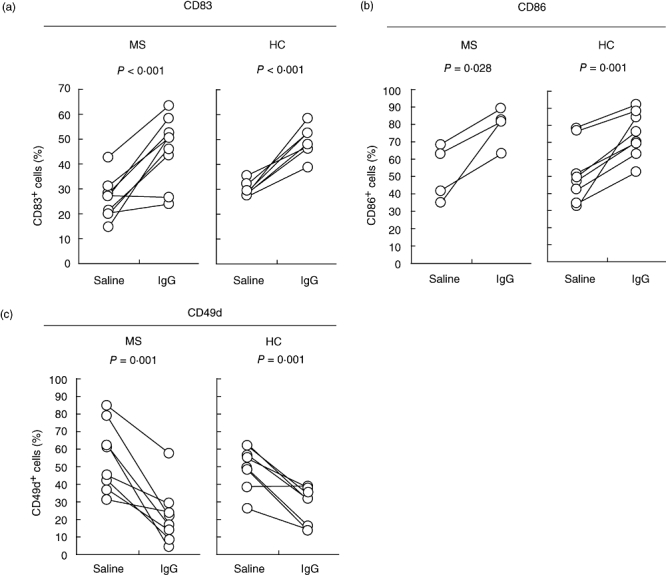
Effects of immunoglobulin G (IgG) on the expression of CD83, CD86 and CD49d in multiple sclerosis (MS) patients. Samples from MS patients (MS: CD83, n = 9; CD86, n = 4; CD49d, n = 8) and healthy controls (HC, n = 8) were analysed by flow cytometry for (a) CD83 on day 9, (b) CD86 on day 7 and (c) CD49d on day 9.
Discussion
In RR-MS patients, relapse is inhibited by IVIg administration during remission [7–10]. The mechanism of action by which IVIg exerts this effect has not been elucidated, although several theories have been put forth. Few analyses of the effects of IVIg on MS relapse and the associated differentiation of Mo-DCs have been attempted, so we analysed the effects of IgG on the differentiation of Mo-DCs.
mDCs can be obtained by initially culturing monocytes isolated from peripheral blood in the presence of IL-4 and GM-CSF to obtain imDCs, then culturing these cells successively with lipopolysaccharide (LPS) or other monokines to produce mDCs [26, 31, 32]. In the LPS-mediated differentiation of mDCs at least two factors in our system may influence our results: the individual differences on LPS response and the presence of anti-LPS antibodies in IVIg preparations [33, 34]. When monocytes are isolated from a buffy coat, the cells may be activated in the process. Given the importance of Mo-DCs in the control of effector cell differentiation in the CNS of MS patients at the time of relapse [25, 35, 36], we adopted a system that induces mDC differentiation from peripheral blood-derived monocytes using purified monokines (TNF-α and IL-1β) rather than LPS.
The effects of IgG on the differentiation of Mo-DCs were reported by Bayry et al. using peripheral blood samples from healthy volunteers. Using an LPS-mediated mDC differentiation system, the addition of IgG inhibited significantly the expression of CD1a, CD83, HLA-DR, CD40, CD80 and CD86 [37]. In this study, the expression of CD1a, CD40 and CD80 was inhibited in a similar fashion by the addition of IgG to monocytes of both healthy controls and MS patients in remission. In contrast, the expression of CD83 was enhanced by IgG treatment. Several previous reports have observed this enhancement of CD83 expression by IgG. Reddy et al. reported that the differentiation of mDCs from imDCs, as assessed by CD83 expression, was promoted by conditioned media from cultures of human monocytes on IgG solid-phase plates [38]. Nuclear factor (NF)-κB is reportedly activated by the cross-linking of FcγRII (CD32), promoting the differentiation of Mo-DCs [39]. These reports suggest a mechanism by which IgG could enhance the expression of CD83.
In the present study, as seen by Bayry et al., the expression of CD40 and CD80 was inhibited by IgG treatment. In contrast, IgG treatment abrogated CD86 down-regulation, and levels remained high on day 7 (Fig. 2). We could not find any other reports of similar phenomena. CD86, however, exhibits strong binding to cytotoxic T lymphocyte-associated antigen (CTLA)-4 [40]. As the levels of CD86 expression were significantly higher than those of CD80, signalling through CD86-CTLA-4 may inhibit T cell activation [41]. To verify this, future studies must confirm that CD86 interacts with CTLA-4 with a higher avidity than that of the binding to CD28, and that an inhibitory signal is transmitted in the presence of IgG.
Anti-α4-integrin antibodies inhibit the binding of CD49d to its ligand, vascular cell adhesion molecule (VCAM)-1. Such treatment diminishes lesions in the brains of MS patients, inhibits relapse and alleviates symptoms [42, 43]. Lapointe et al. reported that IVIg inhibits the interaction of CD49d-expressing activated leucocytes with endocapillary cells expressing VCAM-1 in MS patients [44]. In the present study, we have determined that IgG significantly inhibits the expression of CD49d associated with mDC differentiation. These results suggest that IVIg, unlike preparation of anti-α4-integrin antibody, inhibits the invasion of immune cells into the CNS by inflammatory cells by inhibiting CD49d expression by mDCs.
We also performed experiments to clarify the involvement of FcγR in the therapeutic effect of IgG treatment. We performed a comparative experiment using pepsin-treated globulin (F(ab′)2) at concentrations equimolar to those of IgG examined in this study. The F(ab′)2-treated group exhibited an enhancement of CD83 expression on day 9 similar to that seen in the IgG-treated group (Fig. 5a), suggesting that the effect of IgG was not mediated by interactions with the FcγR. The inhibitory effect on CD86 down-regulation seen on day 7 after treatment with IgG was reduced significantly in the F(ab′)2-treated group (61·7 ± 17·4%) compared to the IgG-treated group (80·1 ± 9·8%), although both were still higher frequencies compared to the saline-treated group (39·9 ± 11·4%) (Fig. 5b). While the F(ab′)2 group exhibited inhibitory effects on day 9 CD49d expression similar to those seen with the IgG group, there were differences between individuals (Fig. 5c). For both CD86 and CD49d, however, the differences in expression levels between the F(ab′)2 group and the saline group were statistically significant. These results suggest that it is difficult to explain all of the above-mentioned effects of IgG only by the FcγR-mediated mechanism.
Fig. 5.
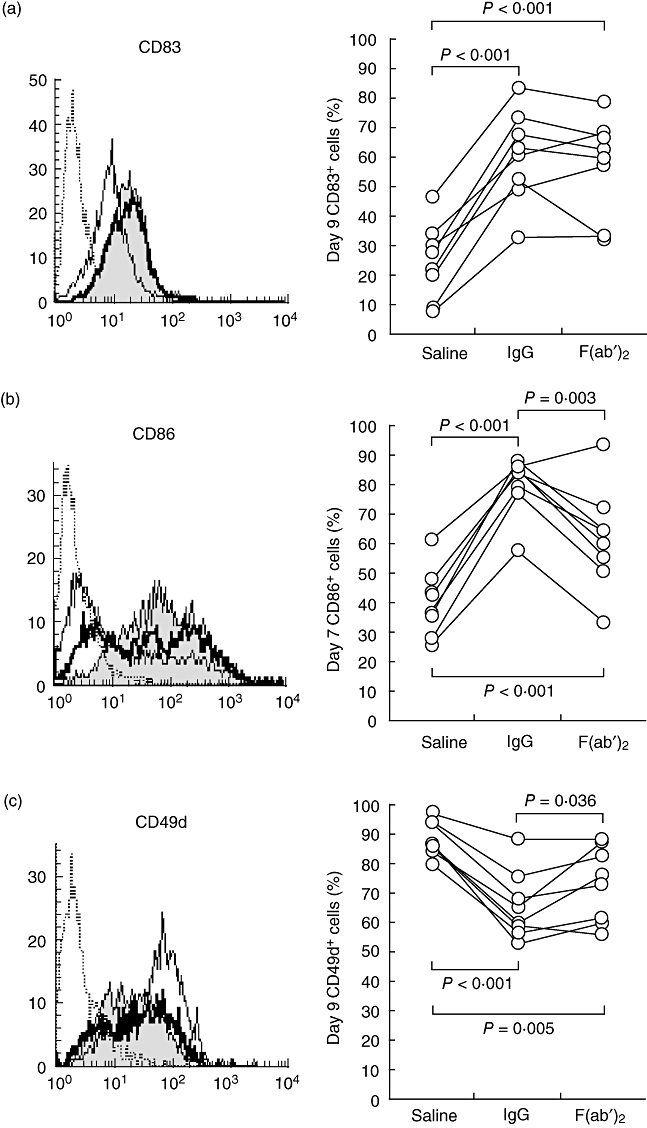
Effects of F(ab′)2 on the expression of CD83, CD86 and CD49d. We divided samples from healthy controls (n = 8) into three groups, one to which immunoglobulin G (IgG) was added at the beginning of culture, one treated with vehicle alone (saline) and one to which pepsin-treated IgG [F(ab′)2] was added. We then induced the differentiation of monocytes into mature dendritic cells (mDCs) as described in Materials and methods. This figure shows the results of flow cytometry analysis of (a) CD83 on day 9, (b) CD86 on day 7 and (c) CD49d on day 9. The left panel shows one typical result of each cell surface molecule analysis for each group [IgG sample (filled histograms), F(ab′)2 sample (bold-lined histograms), no IgG sample (thin-lined histograms) and an isotype control antibody (dotted-lined histograms)]. On the right, we used a paired t-test to calibrate the differences between the corresponding averages.
With samples from both healthy controls and MS patients, we confirmed that the generation of IL-12-producing cells is inhibited by IgG treatment. In contrast, we confirmed that IgG treatment did not affect, but rather enhanced, the generation of IL-10-producing cells. This result suggests that IgG inhibits the differentiation of Th0 cells into Th1 cells, while the differentiation to Th2 cells is enhanced. In animals with experimental autoimmune encephalomyelitis (EAE), an animal model of MS, Th17 cells that produce IL-17 are involved in tissue injury in the CNS [45, 46]. The differentiation of Th1 and Th17 cells requires the transcription factor T-bet; expression of T-bet requires IL-12R-mediated signalling [47, 48]. Therefore, the inhibitory effect of IgG treatment on IL-12-producing cells generation may affect not only the differentiation of Th1 cells, but also that of Th17 cells. To confirm this hypothesis, it will be necessary to perform mixed lymphocyte cultures of mDCs and Th0 cells to examine the effect of IgG on Th1, Th2 and Th17 cell differentiation. In future, we plan to focus on the relationship of DC differentiation and Th17 cell production in human cell systems.
We evaluated the effect of IgG on the expression of DC surface molecules using samples from MS patients in remission to determine if the effect seen in control cells also occurs in Mo-DCs from MS patients. We obtained results in MS-derived cells similar to those seen in healthy controls, suggesting that IgG similarly affects the monocytes of both healthy controls and MS patients in remission. Meanwhile, analyses using samples from relapsing MS patients might show different IgG effects compared to those of MS patients in remission, as the differentiation of Mo-DCs was presumably accelerated in relapsing MS patients. Therefore, we attempted to analyse the relapsing MS patient samples; however, we were unable to obtain enough samples for investigation during this study.
In summary, our results suggest there is a mechanism of IgG which regulates the development of mDCs in vitro. Notably, we found that IgG down-regulates CD49d, which is a crucially important molecule in the pathogenesis of MS. The clinical efficacy of IVIg against MS is still controversial; however, IVIg is possibly effective against certain types of RR-MS, in which relapse is caused mainly by monocyte-derived DCs.
Acknowledgments
We would like to express our gratitude to Kaori Ogata-Michishita, Takako Inoue, Miho Shinmura and Fumiko Fukunaga for their assistance with the experiments performed. We would also like to express our gratitude to Fujio Matsuo for his advice regarding statistical analysis and to Toshihiro Nakashima for his helpful discussions. We gratefully acknowledge Professor Emeritus Kaoru Onoue (Kumamoto University), who sadly died while these experiments were under way, for his invaluable advice.
References
- 1.Bussel JB, Hilgartner MW. The use and mechanism of action of intravenous immunoglobulin in the treatment of immune haemotologic disease. Br J Haematol. 1984;56:1–7. doi: 10.1111/j.1365-2141.1984.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 2.Panto F, Giordano C, Amato MP, et al. The influence of high dose intravenous immunoglobulins on immunological and metabolic pattern in newly diagnosed type I diabetic patients. J Autoimmun. 1990;5:587–92. doi: 10.1016/s0896-8411(05)80025-3. [DOI] [PubMed] [Google Scholar]
- 3.Arsura EL, Bick A, Brunner NG, Grob D. Effects of repeated doses of intravenous immunoglobulin in myasthenia gravis. Am J Med Sci. 1988;295:438–43. doi: 10.1097/00000441-198805000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hsiao GR, Wolf RE, Kimpel DL. Intravenous immunoglobulin to prevent recurrent thrombosis in the antiphospholipid syndrome. J Clin Rheumatol. 2001;7:336–9. doi: 10.1097/00124743-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Akashi K, Nagasawa K, Mayumi T, Yokota E, Oochi N, Kusaba T. Successful treatment of refractory systemic lupus erythematosus with intravenous immunoglobulins. J Rheumatol. 1990;17:375–9. [PubMed] [Google Scholar]
- 6.Hilgartner MW, Bussel J. Use of intravenous gamma globulin for the treatment of autoimmune neutropenia of childhood and autoimmune hemolytic anemia. Am J Med. 1987;83:25–9. doi: 10.1016/0002-9343(87)90547-x. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas F, Deisenhammer F, Strasser-Fuchs S, Nahler G, Mamoli B. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Lancet. 1997;349:589–93. doi: 10.1016/s0140-6736(96)09377-4. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen PS, Wanscher B, Jensen CV, et al. Intravenous immunoglobulin G reduces MRI activity in relapsing multiple sclerosis. Neurology. 1998;50:1273–81. doi: 10.1212/wnl.50.5.1273. [DOI] [PubMed] [Google Scholar]
- 9.Achiron A, Gabbay U, Gilad R, et al. Intravenous immunoglobulin treatment in multiple sclerosis: effect on relapses. Neurology. 1998;50:398–402. doi: 10.1212/wnl.50.2.398. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen PS, Fazekas F, Lee M. Intravenous immunoglobulin G for the treatment of relapsing–remitting multiple sclerosis: a meta-analysis. Eur J Neurol. 2002;9:557–63. doi: 10.1046/j.1468-1331.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- 11.Rossi F, Kazatchkine MD. Antiidiotypes against autoantibodies in pooled normal human polyspecific Ig. J Immunol. 1989;143:4104–9. [PubMed] [Google Scholar]
- 12.Frank MM, Basta M, Fries LF. The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin Immunol Immunopathol. 1992;62:S82–6. doi: 10.1016/0090-1229(92)90045-p. [DOI] [PubMed] [Google Scholar]
- 13.Zhang G, Lopez PH, Li CY, et al. Anti-ganglioside antibody-mediated neuronal cytotoxicity and its protection by intravenous immunoglobulin: implications for immune neuropathies. Brain. 2004;127:1085–100. doi: 10.1093/brain/awh127. [DOI] [PubMed] [Google Scholar]
- 14.Sharief MK, Ingram DA, Swash M, Thompson EJ. I.v. immunoglobulin reduces circulating proinflammatory cytokines in Guillain–Barre syndrome. Neurology. 1999;52:1833–8. doi: 10.1212/wnl.52.9.1833. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda M, Zhang X, Petrosian A, Galera OA, Wang SJ, Jordan SC. Modulation of immunoglobulin production and cytokine mRNA expression in peripheral blood mononuclear cells by intravenous immunoglobulin. J Clin Immunol. 1994;14:178–89. doi: 10.1007/BF01533367. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 17.Bussel JB. Fc receptor blockade and immune thrombocytopenic purpura. Semin Hematol. 2000;37:261–6. doi: 10.1016/s0037-1963(00)90104-5. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–40. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouwenhoven M, Ozenci V, Tjernlund A, et al. Monocyte-derived dendritic cells express and secrete matrix-degrading metalloproteinases and their inhibitors and are imbalanced in multiple sclerosis. J Neuroimmunol. 2002;126:161–71. doi: 10.1016/s0165-5728(02)00054-1. [DOI] [PubMed] [Google Scholar]
- 21.Pashenkov M, Soderstrom M, Huang YM, Link H. Cerebrospinal fluid affects phenotype and functions of myeloid dendritic cells. Clin Exp Immunol. 2002;128:379–87. doi: 10.1046/j.1365-2249.2002.01850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duddy ME, Dickson G, Hawkins SA, Armstrong MA. Monocyte-derived dendritic cells: a potential target for therapy in multiple sclerosis (MS) Clin Exp Immunol. 2001;123:280–7. doi: 10.1046/j.1365-2249.2001.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Link H, Huang YM, Xiao BG. Dendritic cells in experimental allergic encephalomyelitis and multiple sclerosis. J Neuroimmunol. 1999;100:102–10. doi: 10.1016/s0165-5728(99)00197-6. [DOI] [PubMed] [Google Scholar]
- 24.Huang YM, Xiao BG, Ozenci V, et al. Multiple sclerosis is associated with high levels of circulating dendritic cells secreting pro-inflammatory cytokines. J Neuroimmunol. 1999;99:82–90. doi: 10.1016/s0165-5728(99)00106-x. [DOI] [PubMed] [Google Scholar]
- 25.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 26.de Jong EC, Vieira PL, Kalinski P, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704–9. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 27.Verbeek MM, Westphal JR, Ruiter DJ, de Waal RM. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J Immunol. 1995;154:5876–84. [PubMed] [Google Scholar]
- 28.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–42. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 29.Brown KA, Bedford P, Macey M, et al. Human blood dendritic cells: binding to vascular endothelium and expression of adhesion molecules. Clin Exp Immunol. 1997;107:601–7. doi: 10.1046/j.1365-2249.1997.d01-951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CC, Wright A, Punnonen J. Monocyte-derived CD1a+ and CD1a− dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J Immunol. 2000;165:3584–91. doi: 10.4049/jimmunol.165.7.3584. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 33.Fomsgaard A, Holder IA. Effect of a human IgG preparation rich in antibodies to a wide range of lipopolysaccharides on Gram-negative bacterial sepsis in burned mice. APMIS. 1993;101:229–34. doi: 10.1111/j.1699-0463.1993.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 34.Trautmann M, Held TK, Susa M, et al. Bacterial lipopolysaccharide (LPS)-specific antibodies in commercial human immunoglobulin preparations: superior antibody content of an IgM-enriched product. Clin Exp Immunol. 1998;111:81–90. doi: 10.1046/j.1365-2249.1998.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plumb J, Armstrong MA, Duddy M, Mirakhur M, McQuaid S. CD83-positive dendritic cells are present in occasional perivascular cuffs in multiple sclerosis lesions. Mult Scler. 2003;9:142–7. doi: 10.1191/1352458503ms890oa. [DOI] [PubMed] [Google Scholar]
- 36.Pashenkov M, Teleshova N, Link H. Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol. 2003;13:23–33. doi: 10.1111/j.1750-3639.2003.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayry J, Lacroix-Desmazes S, Carbonneil C, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–65. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 38.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–6. [PubMed] [Google Scholar]
- 39.Banki Z, Kacani L, Mullauer B, et al. Cross-linking of CD32 induces maturation of human monocyte-derived dendritic cells via NF-kappa B signaling pathway. J Immunol. 2003;170:3963–70. doi: 10.4049/jimmunol.170.8.3963. [DOI] [PubMed] [Google Scholar]
- 40.Renschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 41.Karandikar NJ, Eagar TN, Vanderlugt CL, Bluestone JA, Miller SD. CTLA-4 downregulates epitope spreading and mediates remission in relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;109:173–80. doi: 10.1016/s0165-5728(00)00322-2. [DOI] [PubMed] [Google Scholar]
- 42.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 43.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 44.Lapointe BM, Herx LM, Gill V, Metz LM, Kubes P. IVIg therapy in brain inflammation: etiology-dependent differential effects on leucocyte recruitment. Brain. 2004;127:2649–56. doi: 10.1093/brain/awh297. [DOI] [PubMed] [Google Scholar]
- 45.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gocke AR, Cravens PD, Ben LH, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–8. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 48.Ylikoski E, Lund R, Kylaniemi M, et al. IL-12 up-regulates T-bet independently of IFN-gamma in human CD4+ T cells. Eur J Immunol. 2005;35:3297–306. doi: 10.1002/eji.200526101. [DOI] [PubMed] [Google Scholar]


