Abstract
Interleukin (IL)-22 is a T cell-derived cytokine that has been reported recently to induce cutaneous inflammation in an experimental murine model of psoriasis, and to induce in vitro an inflammatory-like phenotype. In the present study, we assessed the presence of IL-22 and the IL-22 receptor 1 (IL-22R1) in skin lesions, skin-derived T cells, as well as IL-22 levels in sera from patients with psoriasis. IL-22R1 and IL-10R2 transcripts are expressed at a similar level in psoriatic and healthy skin. In contrast, IL-22 mRNA expression was up-regulated in psoriatic skin lesions compared to normal skin, whereas IL-22 mRNA levels in peripheral blood mononuclear cells from psoriatic patients and normal subjects were similar. Circulating IL-22 levels were significantly higher in psoriatic patients than in normal subjects. T cells isolated from psoriatic skin produced higher levels of IL-22 in comparison to peripheral T cells isolated from the same patients. IL-10 was expressed at similar levels in skin biopsies and peripheral blood mononuclear cells of psoriatic patients and normal subjects. Finally, we show here that supernatants of lesional psoriatic skin-infiltrating T cells induce an inflammatory response by normal human epidermal keratinocytes, resembling that observed in psoriatic lesions. Taken together, the results reported in this study indicate that IL-22 is a cytokine produced by skin-infiltrating lymphocytes that is potentially involved in initiation and/or maintenance of the pathogenesis of psoriasis.
Keywords: cytokines, inflammation, psoriasis, skin disease, T cells
Introduction
Psoriasis is a common cutaneous inflammatory pathology affecting 2·5% of the world's population that results from genetic predispositions as well as environmental factors. Psoriasis is a T cell-mediated disease characterized by a thickened epidermis, hyperproliferation and abnormal differentiation of keratinocytes, accompanied by vascular hyperplasia and inflammatory immune cell infiltrates at the lesion site [1]. Immune cells infiltrated into psoriatic skin secrete large amounts of inflammatory cytokines, such as interferon (IFN)-γ, tumour necrosis factor (TNF)-α, oncostatin M (OSM), interleukin (IL)-12, IL-17 and IL-23, which have been shown to play an important role in the pathogenesis of psoriasis [2–4]. Although a reduced expression of IL-4 and IL-10 has been reported in psoriatic lesions [5, 6] the exact nature of the cytokines secreted by lesion-infiltrating T cells involved in keratinocyte alterations that are characteristic of psoriasis has not been established clearly.
IL-22 is a member of the IL-10 cytokine family, described as having proinflammatory activities on liver, pancreas, intestine and skin [7–11]. IL-22 is expressed mainly by activated T cells, mast cells and natural killer (NK) cells [12, 13] and acts through a heterodimeric receptor containing the IL-10R2 and IL-22R1 chains. Binding of IL-22 to its receptor leads to the activation of the Janus kinase and the signal transducer and activator of transcription (STAT), in particular STAT3, and of the mitogen-activating peptide kinase pathways [14–16]. IL-22 also binds the soluble IL-22 binding protein (IL-22BP), which prevents its interaction with the membrane receptor and antagonizes its activities [17–19]. We have shown previously that IL-22 up-regulates the expression of S100A7-psoriasin, S100A8, S100A9, platelet-derived growth factor (PDGF) and CXC chemokine ligand 5 (CXCL5) in normal human epidermal keratinocytes (NHEK) [10]. These proteins are known for their proinflammatory, chemotactic and/or anti-microbial properties and are up-regulated in psoriatic lesions [20, 21]. IL-22 also up-regulates the expression of human β-defensin 2 and β-defensin 3, and it strongly induces hyperplasia of in vitro reconstituted human epidermis, resulting in part from an inhibition of keratinocyte differentiation [10, 11]. These results show that the histological characteristics and the expression and secretory patterns of IL-22-treated keratinocytes resemble most of the features of psoriatic lesions [22]. Interestingly, very recent studies in mice have demonstrated that IL-22 is produced by the newly described Th17 cell subset [23] and that Th17 cells, through the production of IL-22 and IL-17, might have essential functions in the pathogenesis of psoriasis [24]. In the present study, we have investigated directly the expression of IL-22 and its receptor in cutaneous lesions of patients with psoriasis to evaluate its potential role in the initiation and/or maintenance of this pathology. We show that IL-22 production is up-regulated in psoriatic skin lesions and that lesion-infiltrating T cells are a major source of IL-22.
Methods
Reagents
Oncostatin M (OSM), IL-22BP/Fc chimera and anti-OSM monoclonal antibody (mAb) (clone 17001) were purchased from R&D Systems (Oxon, UK). IL-22 was generously provided by Dr W. Ouyang from Genentech (San Francisco, CA, USA).
Patient studies
The study included 37 adult patients (29 male and eight female, age range 23–84 years) with moderate to severe chronic plaque-type psoriasis. Patients included had not received any topical or systemic therapy before biopsy. All the studies involving human tissues were approved by the institutional ethics committee on human experimentation, the Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale (CCPPRB) of the Région Poitou-Charentes. The study was conducted according to the Declaration of Helsinki principles, and participants gave their written informed consent. Six-mm punch biopsies were taken from surgical samples of healthy skin or from lesional skin of psoriatic patients. They were frozen in RNA later (Qiagen, Courtaboeuf, France) for RNA extraction, or cultured for 40 h in maintenance medium (Skinethic Laboratories, Nice, France) to analyse IL-22 concentration in supernatants.
T cell cultures
Skin-infiltrating or peripheral blood T cells were expanded using Expander beads® (Invitrogen, Cergy Pontoise, France), as described previously [25, 26]. Briefly, skin-infiltrating T cells were generated from 6-mm punch biopsies of psoriatic lesions in the presence of Expander beads® in RPMI-1640 medium, supplemented with 10% fetal calf serum and 10 ng/ml IL-2 (provided by Eurocetus, Amsterdam, the Netherlands). Peripheral blood mononuclear cells (PBMC) were isolated via Ficoll Hypaque density centrifugation, and 105 cells were cultured in the presence of Expander beads®, as described above. After 3 days, fresh culture medium containing 10 ng/ml IL-2 was added to the cultures, and growing T cells were collected after 10–14 days of culture for use in subsequent experiments. To preserve the functional and phenotypic properties of the skin-infiltrating and peripheral blood T lymphocytes, cells were stimulated with Expander beads® only once and not restimulated for further propagation. Two million T cells/ml were activated with immobilized anti-CD3 (mAb) (SPV-T3b, Beckman-Coulter, Marseille, France), anti-CD28 mAb (L293, BD Biosciences, San Jose, CA, USA) and IL-2 for 24 h for cytokine production or for 2 h for mRNA expression.
NHEK cultures
NHEK were obtained from surgical samples of healthy breast skin, as described previously [27]. NHEK were stimulated for 24 h with or without 20% culture supernatants of activated T cells derived from psoriatic skin, 10 ng/ml of IL-22, 10 ng/ml of OSM, 1·5 μg/ml of IL-22BP or 40 μg/ml of anti-OSM mAb.
Real-time reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total cellular RNA was isolated using Trizol reagent (Invitrogen) and treated with DNase I (0·1 U/μl; Promega, Madison, WI, USA). Four μg of total RNA were reverse-transcribed, using 1 μl of Improm-IITM reverse transcriptase (Promega) and 160 ng/μl of pd(N)6 random hexamer (Amersham Biosciences, Piscataway, NJ, USA), according to the manufacturer's instructions (Promega). Quantitative PCR was carried out using the LightCycler-FastStart DNA MasterPLUS SYBR Green I kit (Roche, Mannheim, Germany) and 0·5 μM of forward and reverse primers for IL-22 (forward 5′-CCAGCCTTATATGCAGGAGG-3′ and reverse 5′-TTTCAGCTTTGCTCTGGTCA-3′), IL-10 (forward 5′-AATAAGGTTTCTCAAGGGGCT-3′ and reverse 5′-AGAACCAAGACCCAGACATCAA-3′), IL-22R1 (forward 5′-TACGGAGAGAGGGACTGGG-3′ and reverse 5′-GGTGGCTTGAGGGTAGTGTG-3′), IL-10R2 (forward 5′-CACCTTCTGTCCTGTGGATG-3′ and reverse 5′-CCGTTTTTCCAGTATTGCAC-3′), IL-10R1 [10] and β-2 microglobulin (β2M) (forward 5′-TATCCAGCGTACTCCAAAGA-3′ and reverse 5′-GACAAGTCTGAATGCTCCAC-3′) as a housekeeping gene. A calibration curve was performed with purified PCR products of target genes. The cycling conditions comprised 10-min polymerase activation at 95°C and 45 cycles at 95°C for 10 s, 64°C for 5 s and 72°C for 18 s, with a single fluorescence measurement. Melting curve analysis, obtained by increasing temperature from 60°C to 95°C with a heating rate of 0·1°C per second and a continuous fluorescence measurement, revealed a single narrow peak of suspected fusion temperature. A mathematical model was used to determine the relative quantification of target genes compared to β2M reference gene [28].
Gene expression profiling using cDNA arrays
Total RNA was extracted as described for PCR studies and conventional 33P-cDNA target synthesis and hybridization were performed on custom Atlas array membranes displaying 154 cDNAs of potential interest for skin physiology, as described previously [4].
Cytokine measurement by enzyme-linked immunosorbent assay (ELISA)
Levels of IL-22, IL-4, IFN-γ and IL-10 were determined using the Quantikine human IL-22 immunoassay (R&D Systems), human IL-4 DuoSet (R&D Systems), IFN-γ and IL-10 Eli-Pair kit (Diaclone, Besançon, France), respectively.
Western blotting analysis
Antibodies used in this study were of rabbit anti-phospho-STAT3 (Tyr705) antibody, rabbit anti-phospho-STAT1 (Ser727) antibody (1/1000 dilution, all from Cell Signalling), rabbit anti-STAT3 sc-482 antibody and rabbit anti-STAT1 sc-346 antibody (0·2 μg/ml; all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cell lysis was performed according to the manufacturer's recommendations and 10 μg of protein were electrophoresed in 10% sodium dodecyl sulphate-polyacrylamide gels and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech). Proteins were detected with the relevant primary antibody and peroxidase-conjugated antibody (Sigma, Amersham Biosciences). Bound antibodies were revealed using chemiluminescence reaction (Amersham Pharmacia Biotech). Ponceau red staining was used to control loading homogeneity.
Results
IL-22 receptor transcripts are expressed at a similar level in psoriatic skin and normal skin
Using RT–PCR we quantified expression of the IL-22R1 and IL-10R2 transcripts in 10 normal skin and 14 psoriatic lesional skin biopsies. No significant differences were observed between healthy and psoriatic skin for the expression of the mRNA encoding the two chains of the IL-22 receptor (Fig. 1a, b). In addition, none of the normal skin samples expressed transcript for the IL-10R1 subunit of the IL-10 receptor, whereas the latter subunit was detected in two of six psoriatic skin biopsies (data not shown). As expected, the expression of S100A7 mRNA, used as a control for skin inflammation, was strongly up-regulated in psoriatic skin compared to normal skin (Fig. 1c).
Fig. 1.
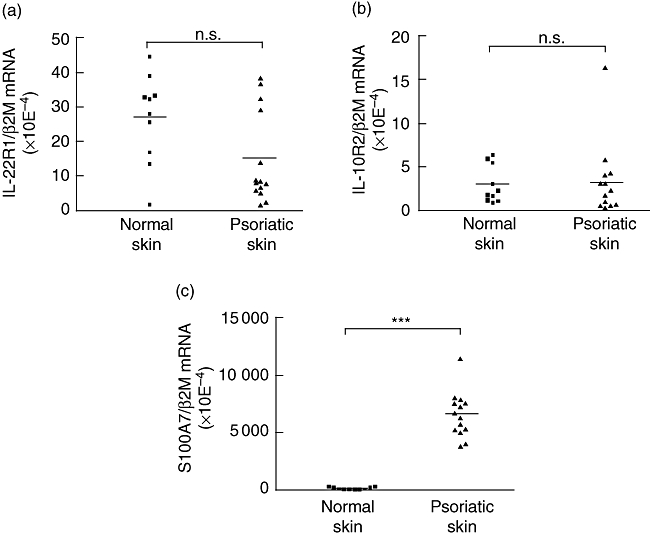
Expression of the interleukin (IL)-22 receptor subunits in normal or lesional psoriatic skin. Total RNA was extracted from normal and psoriatic skin, reverse-transcribed, and IL-22R1 (a), IL-10R2 (b) and S100A7 (c) mRNA relative expressions were quantified by real-time reverse transcription–polymerase chain reaction. β-2 microglobulin was used as a housekeeping gene to normalize gene expression. ***P < 0·001, n.s.: not significant, based on a Mann–Whitney test.
IL-22 transcripts are up-regulated in lesional psoriatic skin
We quantified IL-22 mRNA levels in normal and psoriatic skin. As shown in Fig. 2a, IL-22 transcripts were present in all psoriatic skin samples whereas they were not detected in eight of 10 normal skin samples (P < 0·001). As IL-10 has been reported to be clinically effective in the treatment of psoriasis [29–31], we also analysed its expression. IL-10 mRNA was detected at similar levels in five of 10 normal skin biopsies and in 10 of 14 psoriatic skin samples (data not shown). Interestingly, in PBMC, IL-22 and IL-10 mRNA levels were not significantly different between normal and psoriatic patients (Fig. 2b and data not shown). It is of note that IL-22 mRNA levels were approximately 300-fold higher in lesional skin than in PBMC of psoriatic patients.
Fig. 2.
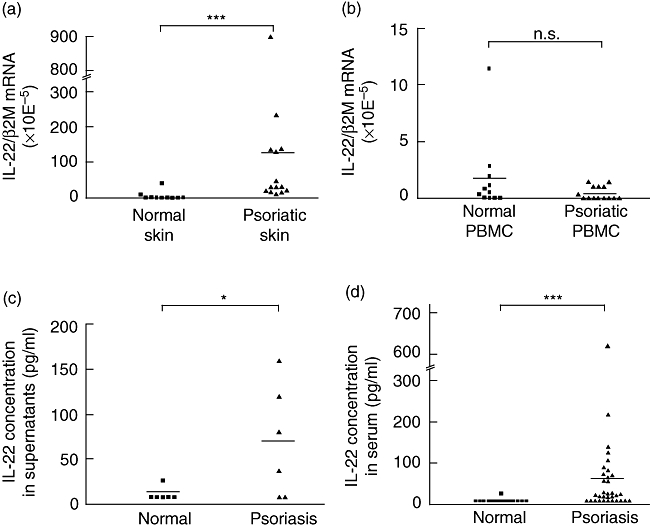
Interleukin (IL)-22 mRNA and protein detection in skin biopsies, peripheral blood mononuclear cells (PBMC) and sera of normal or psoriatic patients. Total RNA was extracted from skin biopsies (a) or PBMC (b) from normal and psoriatic patients and reverse-transcribed. IL-22 mRNA relative expression was quantified by real-time reverse transcription–polymerase chain reaction. β-2 microglobulin was used as a housekeeping gene to normalize gene expression. ***P < 0·001, n.s.: not significant, based on a Mann–Whitney test. (c) Biopsies from normal skin or lesional psoriatic skin were cultured for 40 h and IL-22 concentration was measured in culture supernatants as in (a). *P < 0·05 based on a Mann–Whitney test. (d) IL-22 concentration was determined in the sera of healthy and psoriatic patients by enzyme-linked immunosorbent assay. ***P < 0·001 based on a Mann–Whitney test.
IL-22 is detected in serum of psoriatic patients and in culture supernatants of lesional psoriatic skin biopsies
We measured systemic and local concentration of IL-22 in normal and psoriatic patients. Local production in the skin was assessed by measuring IL-22 concentrations in supernatants of skin biopsies from psoriatic patients or from normal subjects following a 40 h culture. As shown in Fig. 2c, IL-22 was detected in the 2 days' culture supernatants of four of six lesional psoriatic skin biopsies (69 ± 25 pg/ml), compared to one (25 pg/ml) of six normal skin biopsies supernatants (P < 0·05). IL-22 was detected in the serum of 22 of 33 psoriatic patients (59 ± 21 pg/ml), compared to only a single serum sample (27 pg/ml) of 20 normal subjects (P < 0·001) (Fig. 2d). The circulating IL-22 concentrations of patients were dispersed, but were found to correlate with levels of C-reactive protein (CRP; P < 0·05), a marker of inflammation, but not to the index of disease severity (data not shown).
T cells infiltrating psoriatic skin lesions produce high levels of IL-22
As IL-22 is produced by activated T cells, we tested the possibility of whether skin-infiltrating T cells might be a source of IL-22 in psoriatic lesions. T cells were isolated from psoriatic skin biopsies and expanded for 10–14 days in the presence of IL-2 and anti-CD3/anti-CD28 mAbs. Following subsequent activation with IL-2, anti-CD3 and anti-CD28 mAbs, these T cells contained about threefold higher levels of IL-22 mRNA than peripheral blood-derived T cells from the same patients or from healthy individuals, but this did not reach statistical significance due to scattered data (data not shown). Quantification of the protein by ELISA confirmed higher levels of IL-22 in the supernatants of psoriatic skin-derived T cells (13 ± 7 ng/ml, P < 0·05) compared to those from PBMC of psoriatic patients (1·6 ± 0·7 ng/ml) or of healthy individuals (1·2 ± 0·3 ng/ml) (Fig. 3a). IL-4 production by psoriatic skin-derived T cells was also higher than those from PBMC of psoriatic patients or of healthy individuals (Fig. 3b). However, IFN-γ production by psoriatic skin-derived T cells or PBMC of psoriatic patients were lower than production by PBMC from healthy individuals (Fig. 3c). Finally, IL-10 production by these T cells were comparable (Fig. 3d).
Fig. 3.
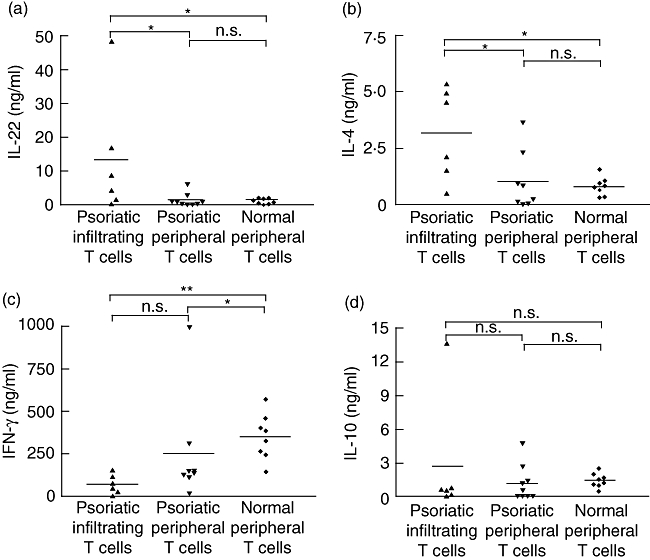
Production of interleukin (IL)-22, IL-4, interferon (IFN)-γ and IL-10 by lesional skin infiltrating T lymphocytes. T cells infiltrating lesional psoriatic skin and peripheral T cells of control or psoriatic patients were expanded for 10–14 days in the presence of Expander beads® and 10 ng/ml IL-2, and then activated for 24 h with IL-2, anti-CD3 and anti-CD28 monoclonal antibodies. IL-22 (a), IL-4 (b), IFN-γ (c) and IL-10 (d) concentrations were measured in culture supernatants by enzyme-linked immunosorbent assay. *P < 0·05, **P < 0·01, n.s.: not significant, based on a Mann–Whitney test.
Proinflammatory activities of psoriatic infiltrating T cells supernatants on NHEK
Using microarrays, we investigated the transcriptional profile induced by psoriatic skin-infiltrating T cell supernatants on NHEK. As shown in Fig. 4, the expression of 41 genes was increased and that of 19 genes decreased in response to these T cell supernatants. Most of the up-regulated genes encode proteins associated with inflammation and innate immune responses (β-defensins, S100A7, S100A8, S100A9 0ellip;), whereas the genes down-regulated by psoriatic T cell supernatants are implicated in keratinocyte differentiation [cytokeratin 10 (CK10), filagrin 0ellip;][32, 33]. For comparison, we analysed the effects of IL-22 and OSM, shown previously to have redundant proinflammatory activity on NHEK [4, 10]. The expression of only 14 and 12 genes, respectively, was modified in response to recombinant IL-22 and OSM (Fig. 4). Interestingly, almost all genes regulated by IL-22 or OSM were also modified to a larger extent by the supernatants of psoriatic skin-infiltrating T cells.
Fig. 4.
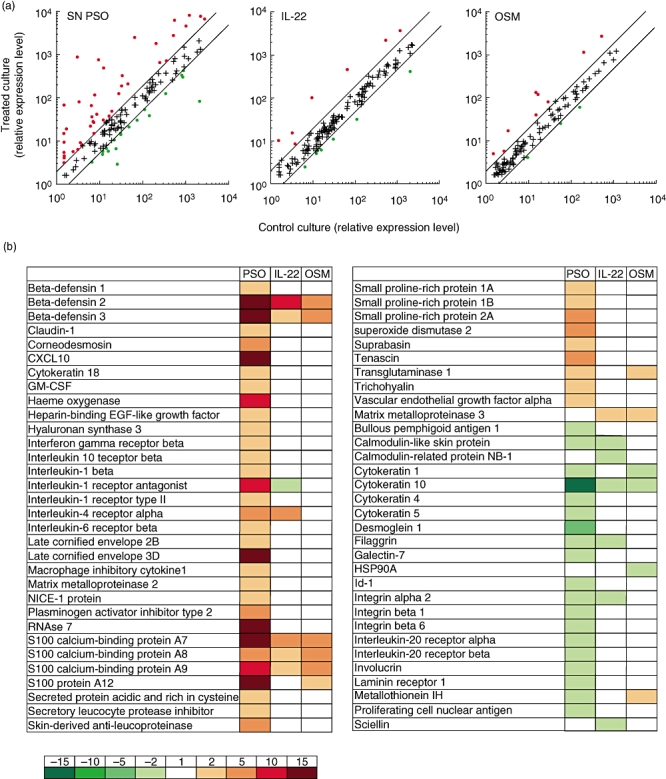
Proinflammatory activities of psoriatic skin infiltrating T cells supernatants on normal human epidermal keratinocytes (NHEK). NHEK were cultured for 24 h with or without 20% supernatants of activated psoriatic skin infiltrating T cells (SN PSO) or 10 ng/ml of IL-22 or oncostatin M (OSM). The expression of 154 genes of potential interest for skin physiology was compared using home-made cDNA microarrays [27]. (a) The relative expression level in treated culture versus control culture is plotted. (b) List of regulated genes. The induced modulation was expressed as the ratio of the signal intensities for treated cells over unstimulated cells. The induced genes are shown in red, the suppressed ones in green. Representative results from one of three independent experiments, performed with three different supernatants of activated psoriatic skin infiltrating T cells (PSD), are shown.
These findings were sustained further by quantitative RT–PCR analysis of S100A7, β-defensin 2 and CK10 gene expression, showing that the modulation of the gene expression profile induced by psoriatic skin-infiltrating T cell supernatants is higher than that obtained with OSM and IL-22, either alone or in combination (Fig. 5a). In addition, culture supernatants of psoriatic skin-infiltrating T cells were found to strongly induce STAT1 and STAT3 phosphorylation (Fig. 5b). Addition of IL-22BP and/or anti-OSM blocking mAb did not reduce the activity of psoriatic skin-infiltrating T cells supernatants, suggesting the presence of redundant cytokines (Fig. 5a).
Fig. 5.
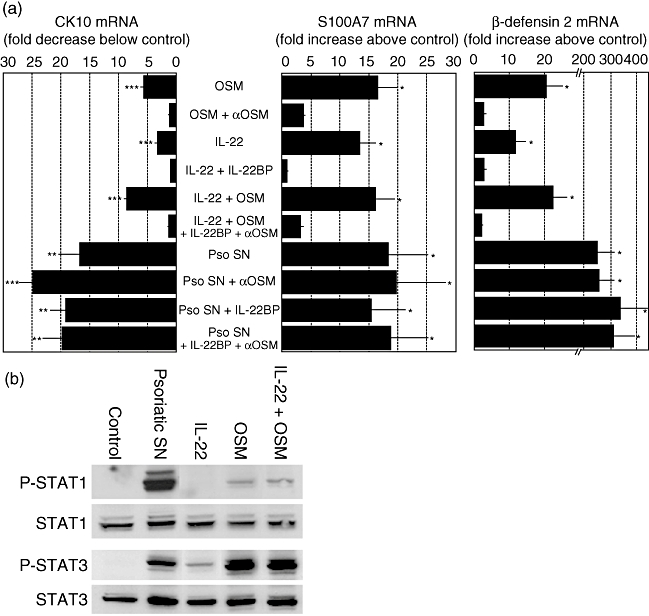
Modulation of S100A7, β-defensin 2 and cytokeratin 10 (CK10) mRNA expression by psoriatic skin infiltrating T cells supernatants. (a) Normal human epidermal keratinocytes (NHEK) were cultured for 24 h with or without 20% supernatants of activated psoriatic skin infiltrating T cells (Pso SN), 10 ng/ml of interleukin (IL)-22, 10 ng/ml of oncostatin M (OSM), 1·5 μg/ml of IL-22BP or 40 μg/ml of anti-OSM blocking monoclonal antibody. Total RNA was extracted and reverse-transcribed. S100A7, β-defensin 2 and cytokeratin 10 mRNA relative expressions were quantified by real-time reverse transcription–polymerase chain reaction. β-2 microglobulin was used as a housekeeping gene to normalize gene expression. Data are mean ± standard error of the mean of three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, based on a one-way analysis of variance and Newman–Keuls test. (b) NHEK were cultured 30 min with or without 20% supernatants of activated psoriatic skin infiltrating T cells or 10 ng/ml of IL-22 and/or OSM. Phospho-signal transducer and activator of transcription 1 (P-STAT1), P-STAT3, STAT1 and STAT3 protein levels were determined by Western blotting. Representative results from one of three independent experiments are shown.
Discussion
Psoriasis is one of the most common cutaneous inflammatory disorders. Although the pathogenesis of this disease is still unclear, the results from several studies suggest that it is associated with the cross-talk between immune-competent cells and keratinocytes and orchestrated by a particular set of cytokines produced by T cells that reside in the psoriatic lesions [1]. The presence of this cellular infiltrate, accompanied by a complex network of cytokines, chemokines and growth factors, leads to abnormal keratinocyte proliferation and differentiation as well as to altered angiogenesis. In particular, the production of Th1 cytokines involved in the activation and proliferation of keratinocytes is believed to result in perpetuation of the disease. The hypothesis implicating Th1 cells in the pathogenesis of psoriasis has been revisited recently with the discovery of the Th17 proinflammatory pathway underscoring a key role for Th17 cells in this disease [24, 34]. Independently of the Th1 and Th2 pathways, in mice the Th17 subset is induced by IL-6 and transforming growth factor (TGF)-β and expanded by IL-23; it produces large amounts of IL-17A, IL-17F and IL-22 [23]. Interestingly, IL-23 injected intradermally in mice mimics changes seen in psoriasis, and this effect is mediated in large part by IL-22 [24]. We have shown previously that IL-22, the more potent keratinocyte activator among the cytokines of the IL-10 family, is able to induce in vitro a skin phenotype resembling human lesional psoriatic skin [10, 27]. In the present study, we show that IL-22 is detected in sera from psoriatic patients but not in sera from normal subjects. Given that our data exclude an enhanced systemic production of IL-22 by PBMC, we hypothesize that the presence of IL-22 in sera from psoriatic patients may result from local synthesis. Indeed, IL-22 levels in culture supernatants of psoriatic lesional skin and IL-22 mRNA levels in psoriatic lesions were higher than in normal skin, demonstrating local production of the cytokine at the inflammatory site. Furthermore, we show that T cells infiltrating psoriatic lesions are an important source of IL-22, even if we cannot exclude production by other cells infiltrated in the lesion, such as polymorphonuclear cells, monocytes or dendritic cells. These results are in accordance with a recent study showing elevated IL-22 production in plasma and skin lesions of psoriatic patients [35]. These authors also show that mRNA levels of IL-22 are reduced in the skin by anti-psoriatic therapy. In contrast, IL-22R1 and IL-10R2 transcripts are expressed at a similar level in psoriatic and healthy skin, in accordance with quantitative RT–PCR studies reported recently [36].
Conventional anti-psoriatic treatments are associated with enhanced IL-10 and decreased IFN-γ production by PBMC, suggesting that IL-10 may have anti-psoriatic activity [29, 37]. Overall, clinical and biological data generated by clinical trials demonstrate that IL-10 therapy induces a marked reduction of the disease and reduces the production of type 1 cytokines, such as TNF-α and IL-12 [30, 31]. In this context, we analysed the expression of IL-10 in skin biopsies from healthy or psoriatic patients and did not observe any significant variation of IL-10 mRNA expression, excluding deficient production of IL-10 in psoriatic patients. IL-10R1 mRNA is not detected in healthy skin and is expressed in one-third of the lesional psoriatic skin samples, linked possibly to the presence of infiltrated immune cells in the lesions. We hypothesize that the effects of IL-10 therapy in psoriasis are likely to result from indirect action of IL-10 on keratinocytes mediated by immune cells.
The putative role of psoriatic infiltrating T cells in the psoriasis phenotype is reinforced by the demonstration that supernatants of these activated T cells strongly induce in keratinocytes the expression of genes involved in inflammation (S100A7, β-defensin 2 0ellip;) and down-regulate that of genes associated with differentiation (CK10, filaggrin, involucrin 0ellip;). This has been shown previously for IL-22 and OSM, as well as for proinflammatory cytokines combinations [4, 10]. Beside cytokines that do not activate STAT3 following interaction with their respective receptors, such as IL-1, IL-17 and TNF-α, we suggest that IL-22 is one of the STAT3-signalling cytokines produced by skin-infiltrating T cells involved in initiation and/or maintenance of psoriasis. Recent studies in mice demonstrating that Th17 lymphocytes, through the production of IL-17 and IL-22, might have essential functions in the pathogenesis of an autoimmune disease such as psoriasis strongly reinforced this hypothesis [24]. Our data also suggest the presence of other redundant cytokines beside IL-22 and OSM. Indeed, we have described recently the production of IL-17 by psoriatic skin-infiltrating T cells and showed its requirement for the induction of S100A7 and hBD2 expression by supernatants of psoriatic skin-infiltrating T cells [38]. In this context we show that these psoriatic skin-infiltrating T cells also produced a high quantity of IFN-γ, which remained significantly lower than the production by PBMC-derived T cells. This could be the reflection of the dichotomy between the IL-23/IL-17 and IL-12/IFN-γ cytokine axis, as Th17 cells produce less IFN-γ than Th1 cells [38, 39]. This lower IFN-γ production by psoriatic T cells should have negligible impact on keratinocytes as the transcriptional profile induced by IFN-γ is partially redundant with that induced by IL-22 or OSM (data not shown). However, the relatively higher production of IL-4 is surprising and is currently under investigation. The respective role of each cytokine, as well as the additive or synergistic effects of these T cell-derived cytokines, would have to be delineated carefully.
Taken together, our results suggest that IL-22 is one T cell-derived cytokine involved potentially in the pathogenesis of psoriasis and of potential interest for the treatment of the disease.
Acknowledgments
This study was supported by the ‘Région Poitou-Charentes’, ‘Programme Hospitalier de Recherche Clinique’, ‘Association pour la Recherche sur le Cancer’ and the innovations programme of the CHU de Poitiers. K. B and E. G. were supported by grants from the ‘Région Poitou-Charentes’ and URGO, respectively.
References
- 1.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and ‘Type 1’ inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Gearing AJ, Fincham NJ, Bird CR, et al. Cytokines in skin lesions of psoriasis. Cytokine. 1990;2:68–75. doi: 10.1016/1043-4666(90)90045-u. [DOI] [PubMed] [Google Scholar]
- 3.Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 2007;28:51–7. doi: 10.1016/j.it.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Boniface K, Diveu C, Morel F, et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol. 2007;178:4615–22. doi: 10.4049/jimmunol.178.7.4615. [DOI] [PubMed] [Google Scholar]
- 5.Schlaak JF, Buslau M, Jochum W, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–9. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 6.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 7.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA. 2000;97:10144–9. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–53. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 9.Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–91. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–19. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 13.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 14.Xie MH, Aggarwal S, Ho WH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–9. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 15.Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–32. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 16.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–82. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 17.Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–5. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- 18.Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol. 2001;166:7096–103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Presnell SR, Parrish-Novak J, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci USA. 2001;98:9511–16. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51:675–85. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaerli P, Britschgi M, Keller M, et al. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol. 2004;173:2151–8. doi: 10.4049/jimmunol.173.3.2151. [DOI] [PubMed] [Google Scholar]
- 22.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 23.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 25.Yssel H, Spits H. In vitro culture of subpopulations of human T lymphocytes. In: Strober W, editor. Current protocols in immunology. New York: Green and Wiley; 2001. pp. 7–19. [Google Scholar]
- 26.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275:251–5. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 27.Boniface K, Lecron JC, Bernard FX, et al. Keratinocytes as targets for interleukin-10-related cytokines: a putative role in the pathogenesis of psoriasis. Eur Cytokine Netw. 2005;16:309–19. [PubMed] [Google Scholar]
- 28.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT–PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asadullah K, Sterry W, Stephanek K, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–94. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asadullah K, Docke WD, Ebeling M, et al. Interleukin 10 treatment of psoriasis: clinical results of a phase 2 trial. Arch Dermatol. 1999;135:187–92. doi: 10.1001/archderm.135.2.187. [DOI] [PubMed] [Google Scholar]
- 31.Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319–29. doi: 10.1046/j.1523-1747.2001.01248.x. [DOI] [PubMed] [Google Scholar]
- 32.Bowcock AM, Shannon W, Du F, et al. Insights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Hum Mol Genet. 2001;10:1793–805. doi: 10.1093/hmg/10.17.1793. [DOI] [PubMed] [Google Scholar]
- 33.Harder J, Schroder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–86. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 34.Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–4. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- 35.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 36.Otkjaer K, Kragballe K, Funding AT, et al. The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br J Dermatol. 2005;153:911–18. doi: 10.1111/j.1365-2133.2005.06800.x. [DOI] [PubMed] [Google Scholar]
- 37.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. T cells in psoriatic lesional skin that survive conventional therapy with NB-UVB radiation display reduced IFN-gamma expression. Arch Dermatol Res. 2004;295:509–16. doi: 10.1007/s00403-004-0460-9. [DOI] [PubMed] [Google Scholar]
- 38.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 39.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]


