Abstract
The generation of regulatory T cells (Tregs) in vitro represents an attractive possibility to set up cellular therapies that could prevent and cure autoimmune disorders. Different methods have been proposed to generate Tregs in vitro and to evaluate their phenotype and function. Moreover, the overlap between generation of activated and regulatory cells could often be underestimated. We showed that in vitro treatment of CD4+ CD25− lymphocytes with different stimuli leads to a good expression of CD25 and forkhead box P3 (FoxP3) on most cells, but to a full Treg phenotype (including CD127 negativity) in only a minor percentage of cells, ranging from 17·38% of cells treated with phytohaemagglutinin (PHA) to 50·91% of cells treated with T cell receptor (TCR) stimulation in association with transforming growth factor (TGF)-β. Some suppressive activity was demonstrated for T cells activated with all the different stimuli. However, while suppression mediated by TCR/TGF-β treated T cells was associated with an inhibition of both interleukin (IL)-2 and interferon (IFN)-γ in the co-culture supernatant, the suppression observed for PHA-activated cells occurred in the presence of large amounts of these cytokines. In conclusion, also taking into account other recent publications, caution should be taken in interpretation of data in the field of regulatory T cells.
Keywords: activation, cytokines, proliferation, regulatory T cells
Introduction
Regulatory T cells (Tregs) represent the most important cell lineage responsible for active immune tolerance [1]. In a transgenic system, forced expression of the transcription factor forkhead box P3 (FoxP3) was able to convert murine effector T cells (CD4+ CD25−) into Tregs[2]. FoxP3 is now considered the most specific phenotypic marker of Tregs[3], even if the study of additional antigens, including glucocorticoid-induced tumour necrosis factor receptor-related gene (GITR), CD62 ligand (CD62L), CD103, cytotoxic T lymphocyte-associated protein antigen (CTLA)-4, human leucocyte antigen D-related (HLA-DR), chemokine (C-C motif) receptor 7 (CCR7), CD39, CD73 and CD127, may define these cells more clearly [4–7]. Moreover, the key role of FoxP3 in tolerance was confirmed further by the evidence that mutations in this protein are associated with a severe syndrome characterized by multiple autoimmune disorders and allergy both in humans and mice [X-linked autoimmunity–allergic dysregulation syndrome (XLAAD) or immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) or scurfy][8–10]. Since then, a defect in Tregs has been hypothesized and/or demonstrated in many autoimmune disorders, including autoimmune polyendocrinopathies, diabetes and multiple sclerosis [11–14]. As a consequence, methods to induce Tregs ex vivo or in vivo represent a very attractive opportunity in preventing or curing severe autoimmune disorders [15]. Cells with some Tregs features have been generated ex vivo by different authors, but it is still doubtful whether they represent similarly stable-lineage naturally occurring Tregs[16–23]. The induction of Tregs directly in vivo by means of super-agonist anti-CD28 antibodies [24, 25] seemed to be an even more promising approach but, unfortunately, a trial in human volunteers resulted in an almost fatal activation of lymphocytes and cytokine storm instead of allowing a tolerance increase [26].
In our study we tried to generate regulatory T cells in vitro. By means of different stimuli we obtained a mixture of cells with activated and regulatory phenotypes. These cells were not anergic, but exerted a suppressive function on both naive and memory T cells. These results strengthen the notion that methods for the generation of Tregs are still far from being standardized. As a consequence we suggest that, although some enthusiastic results have been published, great care should be taken when experimental data are brought from bench to bedside.
Materials and methods
Samples
For each experiment, blood samples were obtained from two unrelated donors 5 days apart from each other (first and second donor); informed consent was requested and obtained. For both samples, peripheral blood mononuclear cells were extracted by means of centrifugation on Histopaque 1077 (Sigma Aldrich, Milan, Italy) at 560 g for 30 min at room temperature. CD4+ cells were then separated by negative selection using magnetic sorting (Miltenyi Biotec, Bergisch Gladbach, Germany). CD25+ cells were depleted from the samples using anti-CD25-coated beads (Miltenyi Biotec) in order to obtain CD25− cells with a purity > 99%. CD25− cells were divided further into RA and R0 subpopulations using the memory CD4+ T cell kit (Miltenyi Biotec).
The study was approved by the local independent bioethical committee.
Stimulation of CD4+ CD25− cells
CD4+ CD25− from the first donor were incubated in X-vivo 15 (Cambrex Bio Science, Milan, Italy). In order to use standardized conditions, for each stimulus 1 × 106 cells were split into 10 wells of a U-bottomed 96-well plate (Corning, NY, USA) with the following stimuli: (i) not stimulated (n.s.); (ii) 1 μg/ml of coated anti-CD3 (cCD3) plus 1 μg/ml of soluble anti-CD28 (sCD28) (Caltag Laboratories, Burlingame, CA, USA); (iii) 1 μg/ml of cCD3 plus 1 μg/ml of sCD28 plus 10 ng/ml of transforming growth factor (TGF)-β (Euroclone, Milan, Italy); (iv) 5 μg/ml phytohaemagglutinin (PHA) (Sigma Aldrich, Milan, Italy). After 5 days, all cells treated using the same set of stimuli were put into a single vial and 1 × 105 cells were analysed by flow cytometry. CD4 TRI-COLOR® (TC) (Caltag Laboratories, Burlingame, CA, USA), CD25 allophycocyanin-cyanin 7 (APC-Cy7) (BioLegend, San Diego, CA, USA), CTLA4 phycoerythrin (PE) (Pharmingen, BD Biosciences, San Jose, CA, USA), CD39 PE (abcam, Cambridge, UK), CD127 Alexa Fluor 647 (BioLegend), CCR7 fluorescein (R&D Systems Inc., Minneapolis, MN, USA) and FoxP3 fluorescein isothiocyanate (FITC) (human regulatory T cell staining kit; eBioscience, San Diego, CA, USA) were used to evaluate the phenotype of these cells.
Evaluation of the proliferation of stimulated T cells upon restimulation
For the proliferation assay, stimulated cells were labelled with carboxy fluorescein diacetate succinimide ester (CFSE, Molecular Probes, Eugene, OR, USA) according to the Aids Clinical Trials Group (ACTG) protocol (http://aactg.s-3.com/labmanual.htm) and their proliferation was evaluated by means of the dye dilution at each cell division. The number of cells for each generation was measured using FlowJo software (Tree Star Inc., Ashland, OR, USA). The percentage of proliferated precursors was calculated using the following formula: % = (G1/2 + G2/4 + G3/8)/(G0 + G1/2 + G2/4 + G3/8). Results expressed the average of two independent experiments.
Evaluation of the suppression activity of stimulated T cells
The above-mentioned pretreated cells were then incubated with CD4+ CD25− target cells from the second donor at different ratios (first/second 1 : 0, 2 : 1, 1 : 1, 1 : 2, 0 : 1) in a 96-well plate coated with 1 μg/ml anti-CD3 (cCD3) plus 1 μg/ml soluble anti-CD28 (sCD28) for 5 days. Labelling of target cells with CFSE and calculation of the percentage of proliferated precursors were performed as described above. Proliferation of target cells in co-culture was expressed as a percentage of the target cells alone. Results expressed the average of two independent experiments.
Cytokine production by stimulated T cells and in co-culture
Cell culture supernatants were collected from each experiment and the production of interleukin (IL)-2 and interferon (IFN)-γ was measured by means of the cytometric bead array (CBA, Beckton Dickinson, San Diego, CA, USA) according to the manufacturer's instructions.
Results
Expression of Treg phenotype after stimulation in vitro of CD4+ CD25− cells
Stimulated cells showed positive expression of CD25, CTLA4, CD62L, CCR7, CD39 and FoxP3 irrespective of the received stimulus (Fig. 1a). PHA-stimulated cells showed higher mean fluorescence intensity (MFI) for CD25 (MFI = 138·91) and FoxP3 (MFI = 11·61) compared with cCD3/sCD28-stimulated cells (CD25 = 24·42, FoxP3 = 8·6) and with cCD3/sCD28-TGF-β-stimulated cells (CD25 = 14·65, FoxP3 = 9·60). On the other hand, expression of the activation marker CD127 was higher in PHA-stimulated cells (MFI = 58·97) than in the other two groups which showed, respectively, a CD127 MFI of 15·48 and 4·61. Among CD25+ cells, a combination of high expression of FoxP3 and low expression of CD127 was more common in cells treated with cCD3/sCD28-TGF-β (50·91%) compared with cells stimulated with cCD3/sCD28 (29·60%) or with PHA (17·38%) (Fig. 1b).
Fig. 1.
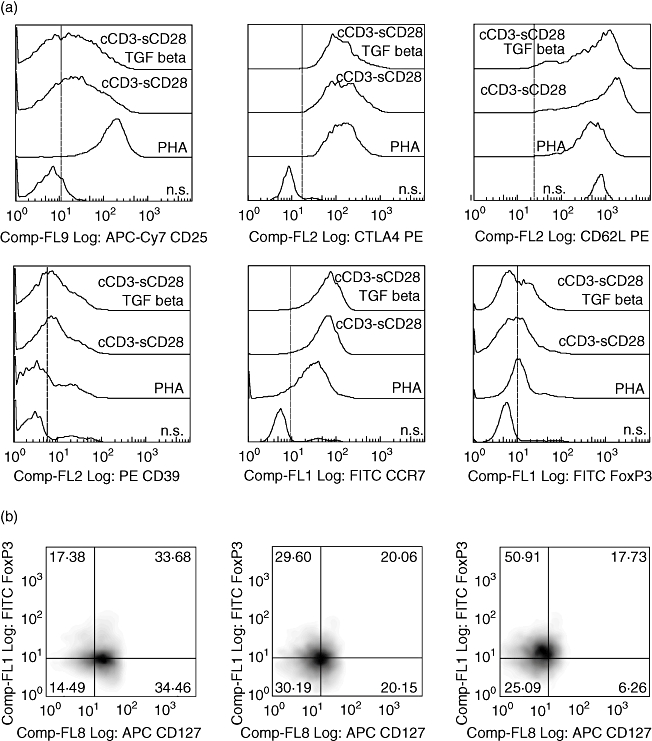
Evaluation of T regulatory (Treg) immunophenotype on T lymphocytes after 5 days of culture with different stimuli: (a) histograms of expression of CD25, cytotoxic T lymphocyte-associated protein antigen-4 (CTLA4), CD62 ligand (CD62L), CD39, chemokine (C-C motif) receptor 7 (CCR7) and forkhead box P3 (FoxP3); (b) percentage of FoxP3+ CD127− cells in a CD25 positive gate in the different groups. All dot plots were obtained after lymphocyte scatter and CD4+ gating.
Proliferation and cytokine production after restimulation
Cells prestimulated with PHA or with cCD3/sCD28 showed a good proliferative response when restimulated with cCD3/sCD28 antibodies (41·25% and 38·77%, respectively), while the proliferative response of TGF-β-treated cells was clearly lower (13·95%). Non-prestimulated cells responded very poorly to stimulation (1·45%) (Fig. 2a). The production of IFN-γ upon restimulation was higher with cCD3/sCD28 stimulus (13660 pg/ml) compared with PHA (5109 pg/ml) and cCD3/sCD28-TGFβ (925 pg/ml). Larger amounts of IL-2 were produced with PHA (4371 pg/ml) than with cCD3/sCD28 stimulus (105 pg/ml) and cCD3/sCD28-TGFβ (80 pg/ml) (Fig. 2b, c).
Fig. 2.
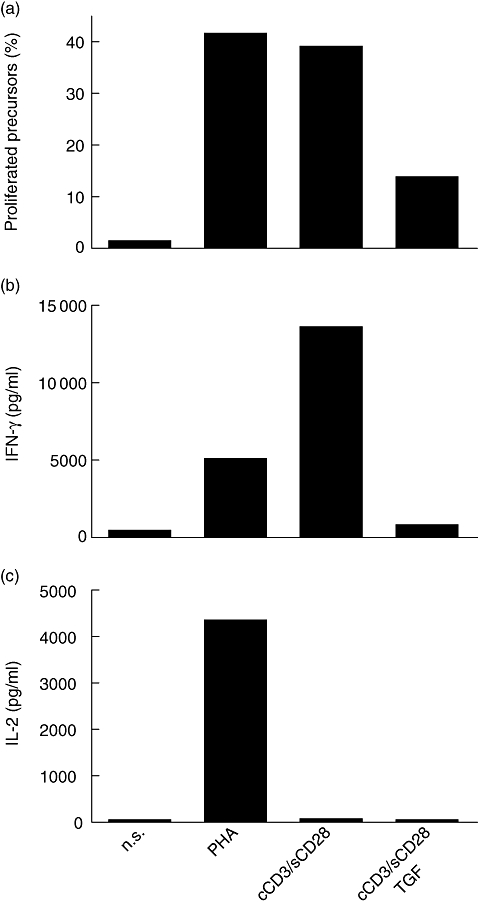
Behaviour of stimulated cells upon restimulation with cCD3/sCD28: percentage of proliferated precursors (a); production of interferon-γ (IFN-γ) (b); production of interleukin-2 (IL-2) (c).
Suppressive function of stimulated cells on heterologous CD4+CD25− cells
The proliferation of CFSE-labelled target cells was suppressed to various extents by the addition of prestimulated T cells in co-culture. At a ratio of 2 : 1 (prestimulated to target cells), the proliferation of target cells diminished to 68%, 47%, 21% and 27% in co-culture, respectively, with n.s., PHA-stimulated, cCD3/sCD28-stimulated and cCD3/sCD28-TGF-β-stimulated cells. The effect was similar on naive and memory T cells, apart from the PHA-stimulated cells which appeared to be more effective in suppressing naive T cells. The production of IL-2 was increased strongly in co-culture with PHA-stimulated T cells (Fig. 3).
Fig. 3.
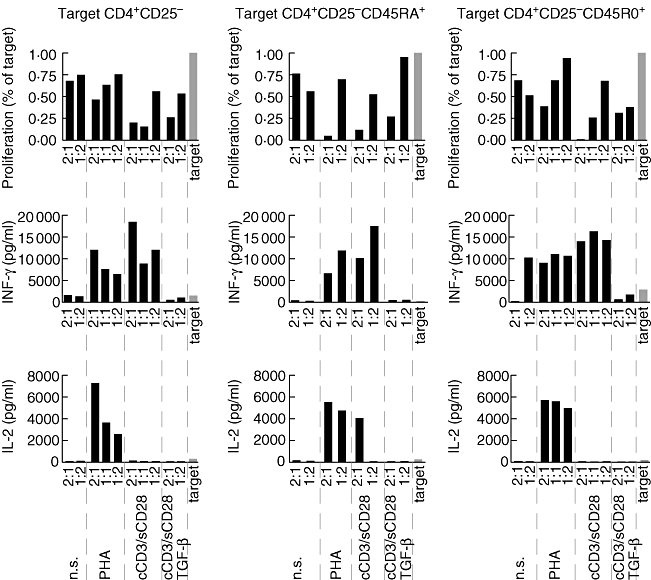
Proliferation of carboxy fluorescein diacetate succinimide ester-labelled target T cells co-cultured with prestimulated cells at different ratio (prestimulated : target) and production of cytokines in the co-culture supernatants. The results are compared with target cells alone (grey bar).
Discussion
The generation of regulatory T cells in vitro is an attractive opportunity in the prevention and cure of autoimmune disorders [15]. Unfortunately, different experimental studies for generation of Tregs in vitro obtained controversial results, due to slight differences in culture media, stimuli, time of incubation and cytokines used, and there is no agreement on the best method for generating Tregs in vitro. Only few researchers have pointed out the apparent paradox that the stimuli used to generate regulatory T cells are the same used to activate T cells, and that the expression of CD25 is not a prerogative of only regulatory T cells [27–29]. This apparent paradox continues when the generation of Tregs is also attempted in vivo, obtaining opposite results with the induction of regulatory T cells [30] or activated T cells [26]. We still do not know what is responsible for distinguishing the two cases. Moreover, recent data have suggested: (i) that a Treg phenotype is normally acquired transitorily by activated T cells [27] and (ii) that in vitro-generated Tregs require further stimulation to maintain their regulatory function [31]. Thus, in vitro-generated Tregs appear to be largely different from the naturally occurring Tregs, which represent a stable lineage.
We characterized the immune phenotype and the regulatory activity of lymphocytes after 5 days activation. After treatment with different stimuli, most cells expressed typical Treg markers, including CD25 and FoxP3. However, only minor percentages of cells showed a Treg phenotype as defined by the presence of CD25 and FoxP3 and the lack of CD127. These results are in agreement with the observations of Wan et al., who identified only a minority of FoxP3+ cells after TCR stimulation in the presence of TGF-β in a transgenic mouse model [32]. We suggest that, in vitro, T cell stimulation always leads to the generation of a mixture of activated and regulatory T cells, with higher percentages of the latter occurring after TCR stimulation in the presence of TGF-β. In fact, activated cells were not anergic after restimulation, even if the proliferative response appeared poorer for TCR/TGF-β-stimulated cells. These results are in partial agreement with recent data from Tran et al., who showed that TCR/TGF-β-stimulated naive T lymphocytes, in spite of the expression of CD25 and FoxP3, still retain the capacity of proliferating and secreting lymphotropic cytokines upon restimulation [33].
The high levels of lymphotropic cytokines produced (in particular after PHA stimulation) demonstrated that the mixture contains true activated T cells. The high production of cytokines in FoxP3+ cells is not surprising, as it has been shown recently that FoxP3 is also expressed transitorily in activated T cells [27, 28]. What is surprising is that some suppressive function could also be demonstrated for PHA-stimulated cells. This effect occurred with the production of increasing levels of cytokines, that tended to be even higher as the percentage of prestimulated cells in the co-culture increased. A possible explanation of this suppression at the presence of high levels of cytokines could reside in competition for nutrients and cytokines by prestimulated T cells. We cannot exclude that among prestimulated cells a small population of true regulatory T cells exist; however, this seems unlikely, as the PHA-stimulated cells did not show very high levels of FoxP3, while it has been argued recently that true regulatory cells display even higher levels of FoxP3 expression [28]. That the greater suppressive effect of PHA-prestimulated cells was on naive T cells has no clear explanation, but it could be consistent with a mechanism of suppression different from that of typical Tregs.
It is noteworthy that the suppressive function of preactivated cells could be seen only if we use methods able to measure the proliferation of target cells in the co-culture (e.g. CFSE dilution assay), rather than those based on the measure of the whole co-culture proliferation (e.g. [3H]-thymidine). The fact that other authors failed to demonstrate a suppressive capacity of activated T cells may depend upon small differences in the experimental procedure (e.g. the stimulation of naive T cells in the work by Tran et al. [33]) or in the method used to measure the proliferation of target cells ([3H]-thymidine, instead of dye dilution methods).
Our results highlight the possible problems and pitfalls in in vitro generations of Tregs. Walker proposed that the timing after stimulation could be crucial for the choice between the fate of activated and regulatory T cells, the latter requiring as long as 10 days to be generated [18]; however, other authors argued later that regulatory T cells generated in vitro are not a stable lineage [31]. We still lack evidence on the possibility of generating stable regulatory T cells in vitro and it is possible for most attempts to result only in the description of a particular stage of lymphocyte activation and development. As a consequence, we suggest that we still need to be extremely cautious in translating transient results in vitro to experimentation in vivo.
Acknowledgments
This work was supported by the Italian Ministry of Health grant no. RF100/05 and by the Telethon Foundation, grant no. GGP04285.
References
- 1.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–18. [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Banham AH, Powrie FM, Suri-Payer E. FOXP3+ regulatory T cells: current controversies and future perspectives. Eur J Immunol. 2006;36:2832–6. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- 4.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses 1-selectin and high levels of CCR7. J Immunol. 2002;169:2461–5. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 6.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borsellino G, Kleinewietfeld M, Di MD, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 8.Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity–allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 11.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–91. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 13.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundsgaard D, Holm TL, Hornum L, Markholst H. In vivo control of diabetogenic T-cells by regulatory CD4+CD25+ T-cells expressing Foxp3. Diabetes. 2005;54:1040–7. doi: 10.2337/diabetes.54.4.1040. [DOI] [PubMed] [Google Scholar]
- 15.Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 16.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25– precursors. J Immunol. 2002;169:4183–9. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 17.Suciu-Foca N, Manavalan JS, Cortesini R. Generation and function of antigen-specific suppressor and regulatory T cells. Transpl Immunol. 2003;11:235–44. doi: 10.1016/S0966-3274(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 18.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+ Proc Natl Acad Sci USA. 2005;102:4103–8. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong Y, Konrad A, Iqbal N, Hatton RD, Weaver CT, Elson CO. Generation of antigen-specific, Foxp3-expressing CD4+ regulatory T cells by inhibition of APC proteosome function. J Immunol. 2005;174:2787–95. doi: 10.4049/jimmunol.174.5.2787. [DOI] [PubMed] [Google Scholar]
- 20.Tsang J, Jiang S, Tanriver Y, Leung E, Lombardi G, Lechler RI. In-vitro generation and characterisation of murine CD4+CD25+ regulatory T cells with indirect allospecificity. Int Immunopharmacol. 2006;6:1883–8. doi: 10.1016/j.intimp.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Taams LS, Curnow SJ, Vukmanovic-Stejic M, Akbar AN. The generation and antigen-specificity of CD4+CD25+ regulatory T cells. Inflamm Allergy Drug Targets. 2006;5:149–56. doi: 10.2174/187152806778256070. [DOI] [PubMed] [Google Scholar]
- 22.Rao PE, Petrone AL, Ponath PD. Differentiation and expansion of T cells with regulatory function from human peripheral lymphocytes by stimulation in the presence of TGF-{beta} J Immunol. 2005;174:1446–55. doi: 10.4049/jimmunol.174.3.1446. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CH, Hunig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–38. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 25.Beyersdorf N, Hanke T, Kerkau T, Hunig T. CD28 superagonists put a break on autoimmunity by preferentially activating CD4+CD25+ regulatory T cells. Autoimmun Rev. 2006;5:40–5. doi: 10.1016/j.autrev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Ioan-Facsinay A, van d V, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 28.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 29.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyersdorf N, Gaupp S, Balbach K, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–55. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–31. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3- T cells by T cell receptor stimulation is TG-dependent but does not confer a regulatory phenotype. Blood. 2007 doi: 10.1182/blood-2007-06-094656. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


