Abstract
We determined the number and functional status of CD4+CD25high regulatory T cells (Treg) in blood samples from patients with metastatic carcinoma, and evaluated their sensitivity to a single intravenous infusion of cyclophosphamide. Treg numbers were significantly higher in 49 patients with metastatic cancer (9·2% of CD4+ T cells) compared to 24 healthy donors (7·1%). These cells expressed the transcription factor forkhead box P3 (FoxP3), glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) and intracellular CD152, and demonstrated a suppressive activity in vitro against CD4+CD25− autologous proliferation. At a single intravenous infusion, cyclophosphamide failed, in association with a non-specific immunotherapy by intratumoral bacille Calmette–Guérin (BCG), to modulate significantly Treg numbers or function. Metastatic cancer is associated with an expansion of peripheral blood CD4+CD25highFoxP3+GITR+CD152+Treg cells whose immunosuppressive properties do not differ from those of healthy subjects. Moreover, cyclophosphamide administration may not represent an optimal therapy to eliminate Treg, which further underlines the need to identify specific agents that would selectively deplete these cells.
Keywords: immunotherapy, metastatic carcinoma, regulatory T cells
Introduction
CD4+CD25+ regulatory T cells (Treg) play a critical role in the regulation of self-tolerance, preventing the occurrence of autoimmune diseases [1, 2]. In humans, Treg represent 5–10% of circulating CD4+ T cells. They are defined functionally by their capability to suppress the activation and proliferation of CD4+ and CD8+ effector T cells [3, 4]. Despite the identification of several antigens, the identification of a specific Treg cell surface marker is still missing, and the transcription factor forkhead box P3 (FoxP3), involved in the development and the inhibitory functions of these cells, remains to date their only specific molecular signature. As well as their role in autoimmunity, Tregs participate in the control of infection, transplantation tolerance and tumour immunity.
It has been shown that Treg numbers were increased in tumour-bearing animals [5–7]. It has also been demonstrated that CD4+CD25+ T cell elimination, obtained with monoclonal antibodies or cyclophosphamide treatment, fostered a tumour-specific immune response in mice [7–11]. In various animal models, Treg depletion prevented the growth or triggered the regression of tumours when performed before or very early after tumour cell injection [6, 9, 10, 12–14]. Treg depletion also triggered tumour regression when associated with anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) antibody [15] or with CD4+CD25− T cell transfer [16]. In a rat model of syngeneic colon carcinoma, we have shown that cyclophosphamide-induced depletion of Tregs followed by a specific immunotherapy cured all animals bearing established and progressive tumours [7].
In humans, an increase in CD4+CD25+ T cell numbers has been detected in the blood of patients with cancers of diverse nature, including oesophageal and gastric [17], colon [18], pancreas and breast [19], melanomas [20], hepatocarcinoma [21], cervical and endometrial [22], ovarian and lung carcinomas [4, 23, 24] and Hodgkin's disease [25]. CD4+CD25+ T cells may also accumulate within the tumour site or at its vicinity and in the draining lymph nodes [19, 22, 25]. The percentage of Treg cells among peripheral blood T cells was shown to correlate with prognosis [18, 26].
The current study was designed to further explore CD4+CD25+Foxp3+ Treg population in patients with advanced and metastatic cancers from a variety of primary sites. Based on impressive results obtained in tumour-bearing rats by using a single injection of cyclophosphamide to deplete Treg cells [7], we evaluated in a Phase I trial the ability of a single infusion of cyclophosphamide (tested at three different doses) to deplete Treg cells from peripheral blood.
Patients and methods
Patient characteristics
Between September 2002 and December 2005, all patients with metastatic cancer admitted to the internal medicine, pneumology or medical oncology departments (Hôpital du Bocage, Dijon, France) were asked to participate in our study. They were excluded if they had received chemotherapy, radiation therapy or immunotherapy within the previous 12 weeks. After consent was obtained, blood samples were collected from 24 healthy volunteers and 49 patients with advanced cancer. The Institutional Review Board of the Ethics Committee of Burgundy (France) approved the study protocol (No. 2003/19). The entry criteria for the Phase I clinical trial were the same as above. All the patients included in this assay had extensive metastatic disease and had failed to respond to at least two prior chemotherapeutic regimens.
Phase I design
At day 1, patients received a single intravenous injection of cyclophosphamide. Three different dose cohorts, 250, 500, 750 mg/m2, with three patients in each group, were studied. At days 7 and 14, patients received a bacille Calmette–Guérin (BCG) injection into an easily accessible metastasis (cutaneous or lymph nodes). During this protocol CD4+CD25+ T cells were monitored: percentage, absolute number, FoxP3 expression and immunosuppressive function. Histology of the injected metastasis was performed at days 0 and 28 to evaluate immune cell activation and infiltration by dendritic cells (DCs), macrophages and T cells. Clinical activity was assessed by applying the response evaluation criteria in solid tumours (RECIST) criteria to computed tomography or magnetic resonance imaging scans obtained before and after treatment.
Peripheral blood mononuclear cell (PBMC) isolation
Whole blood was collected into heparin-containing Vacutainer tubes and processed within 2 h. PBMCs were isolated by Ficoll density gradient centrifugation for 20 min at 22°C and 800 g.
Phenotypic characterization of Tregs
To determine the frequency and the phenotype of Treg cells in PBMCs, multi-colour fluorescence-activated cell sorting analysis was performed using the following antibodies: anti-CD4, anti-CD25, anti-human leucocyte antigen D-related (HLA-DR), anti-CD45RA, anti-CD45RO, anti-CD152 (BD Pharmingen, Le Pont de Claix, France) and anti-glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) (R&D Systems, Lille, France). Expression of intracellular CD152, membrane GITR and CD25 were also assessed after cell sorting on the negative and positive fractions. Flow cytometry analysis was achieved on a Becton Dickinson LSRII using Facsdiva software (BD Biosciences; Erembodegen, Belgium). Isotype-matched antibodies were used as controls.
Functional characterization of Tregs
CD4+ T cells were first separated using a human CD4 T cell enrichment kit (StemSep; StemCell Technologies, Grenoble, France) according to the manufacturer's instructions. Selected cell purity was > 90% as determined by flow cytometry. Using CD25+ microbeads (Miltenyi Biotech, Paris, France), CD4+ T cells were then sorted into CD4+CD25+ and CD4+CD25− cells. The purity of the cells after sorting was 90–99%. T cell proliferation of both fractions was induced by two different methods: co-culture with allogeneic in vitro granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 differentiated DCs, or stimulation with anti-CD3 and anti-CD28 microbeads (Dynal, Invitrogen, Cergy Pontoise, France) in 96-well round-bottomed plates for 96 h at 37°C and 5% CO2. To determine the suppressive ability of CD4+CD25+ T cells, stimulated CD4+CD25− cells (100 000 per wells) were co-cultured with or without autologous CD4+CD25+ cells (50 000 per wells). Proliferation was measured by [3H]-thymidine incorporation (Amersham, GE Healthcare, Saclay, France) added for the last 18 h. Incorporated radioactivity was measured using a scintillation counter (Wallac, Turku, Finland). All experiments were performed in triplicate.
Foxp3 expression by real time reverse transcription–polymerase chain reaction (RT–PCR)
Total cellular RNA was extracted using TRIzol (Invitrogene, Cergy Pontoise, France) RNA lysis buffer according the manufacturer's instructions. Real-time quantification of FoxP3 gene expression (GenBank accession number: AF277993) and of the reference gene Abl was performed using gene-specific fluorogenic hydrolysis probes in a final volume of 20 μl with 1X universal master mix (Qiagen, Courtaboeuf, France), 10 μM of each primer and 5 μM of bifluorescent probe. Quantitative PCRs were run as a standard two-step cycling reaction in an iCycler (BioRad; Marnes-la-Coquette, France). Primer sequences were as follows: forward (spanning exons 1 and 2): 5′-CCCACAAGCCAGGCTGAT-3′; reverse: 5′-CATCGGGTCCTTGTCCAA3-′; Probe: 5′-FamTTTCTGTCAGTCCACTTCACCAAGCCT-Tamra-3′. Mean duplicate measurements were normalized and expressed as a ratio of FoxP3 mRNA copies/Abl mRNA copies.
Histological study of the tumour cell injection site
Biopsies were embedded in Tissue-Tek (Sakura Finetechnical; Tokyo, Japan) and snap-frozen in methylbutane cooled in liquid nitrogen. An immunohistochemical study of tumour-infiltrating inflammatory cells was performed on acetone-fixed 5 μM cryostat sections. Mouse monoclonal antibodies (mAb) to human cells, including DCs and monocytes (CD11c), macrophages (CD68), activated/regulatory T cell (CD25) and CD8+ T cell (CD8) (all from Serotec, Oxford, UK), were used. After incubation with specific mAbs, sections were incubated with biotinylated sheep anti-mouse immunoglobulin (IgG) antibody (Amersham, Little Chalfont, UK), then with streptavidin–peroxidase, and stained with aminoethylcarbazole.
Statistical analysis
Data are expressed as mean and standard deviation (s.d.) for percentages. Statistical analysis was performed using Student's t-test to assess differences between the different study groups. P < 0·05 was considered statistically significant. A mixed model of statistical analysis was used to compare the proliferation curves.
Results
Patients with metastatic cancers demonstrate an increased frequency of blood CD4+CD25+ T cells
The prevalence of CD4+CD25high T cells was determined in the peripheral blood of 49 patients with metastatic cancer and 24 healthy donors. Cancer patient characteristics are described in Table 1. We analysed the CD4+ cells with the highest level of CD25 expression (CD4+CD25high), which protrudes as a tail on flow cytometry from the major population of CD4+CD25low cells in cancer patients (Fig. 1a) and healthy donors (Fig. 1b). Individual frequencies of CD4+CD25high to total CD4+ T cells as well as the cumulative data for all the cancer patients and healthy donors are represented as scatter plots (Fig. 1c). As depicted in Fig. 1c, the frequency of CD4+CD25high T cells was significantly higher in patients with metastatic cancer (9·22 ± 2·6%) than in healthy donors (7·08 ± 1·77%) (P< 0·01, Student's t-test). No correlation was found between the percentage of CD4+CD25high T cells and the type of cancer (breast, lung or kidney) (Table 1).
Table 1.
Patient characteristics.
| Sex | |||||
|---|---|---|---|---|---|
| Tumor type | Age (years) | Female | Male | Number of patients | Tregs (%) |
| Breast | 63 ± 7 | 3 | 3 | 8·0 ± 1·0 | |
| Lung | 68 ± 8 | 5 | 21 | 26 | 8·8 ± 2·8 |
| Kidney | 66 ± 14 | 2 | 5 | 7 | 9·8 ± 1·0 |
| Stomach | 40 ± 0 | 3 | 3 | 9·3 ± 1·2 | |
| Colorectal | 70 ± 6 | 2 | 2 | 4 | 8·3 ± 1·0 |
| Bladder | 65 ± 11 | 3 | 3 | 11·3 ± 3·2 | |
| Prostate | 64 ± 12 | 2 | 2 | 8·5 ± 2·1 | |
| Unknown | 58 ± 0 | 1 | 1 | 16 | |
| Healthy donors | 58·5 ± 15 | 9 | 15 | 24 | 7·08 ± 1·8 |
Fig. 1.

Increase of circulating CD4+CD25high T lymphocytes in cancer patients. Representative flow cytometry analysis of CD4+CD25+ T cells from a healthy donor (a) or a patient with metastatic cancer (b). (c) Percentage of circulating CD4+CD25high cells from patients with metastatic cancer (n = 49) and healthy donors (n = 24). CD4+CD25high cells are presented as a percentage of total CD4+ cells. Each plot represents an individual patient. P < 0·01 using Student's t-test.
Phenotypic analysis of CD4+CD25high T cells
The phenotypic characteristics of CD4+CD25high and CD4+CD25− T lymphocytes from cancer patients and healthy donors were then analysed further by flow cytometry. GITR, a member of the tumour necrosis factor receptor superfamily reported to be expressed predominantly on CD4+CD25+ regulatory T cells [27], was detected on up to 100% of CD4+CD25high cells. Similarly, 70% of the purified CD4+CD25high cells expressed intracellular CD152 (Fig. 2a). Furthermore, consistent with previous reports [19], CD45RO, a marker for T cell responses to recall antigens was detected on 96% of CD4+CD25high cells (data not shown). In contrast, the CD45RA marker (a marker for naive T cells) was expressed by only 17% of CD4+CD25high cells (data not shown). The transcription factor FoxP3 has been described as the most specific molecular marker for regulatory T cells [28, 29]. We therefore analysed FoxP3 expression in CD4+CD25− and CD4+CD25high T cells isolated from cancer patients and healthy donors using real-time PCR. As depicted in Fig. 2b, contrasting with their CD4+CD25− counterparts, CD4+CD25high T lymphocytes from both cancer patients and healthy donors expressed FoxP3. Together, these results indicate that circulating CD4+CD25high T cell isolated from patients demonstrate specific phenotypic features of immunosuppressive regulatory T cells. Furthermore, no phenotypical difference was observed between CD4+CD25high T cells from metastatic cancer patients and healthy donors.
Fig. 2.

Phenotypic analysis of CD4+CD25high T cells and forkhead box P3 (FoxP3) expression. (a) CD4+ cells were sorted in CD4+CD25high and CD4+CD25− and then stained with anti-CD25, anti-glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) and anti-CD152 antibodies. CD4+CD25+ and CD4+CD25− cell populations were analysed separately. (b) cDNA obtained from purified populations of CD4+CD25− and CD4+CD25high cells from 49 cancer patients and 24 healthy donors were subjected to quantitative real-time polymerase chain reaction (PCR) analysis using primers specific for FoxP3 and Abelson (Abl), as described in Materials and Methods.
CD4+CD25high T cells from healthy donors and cancer patients exhibit a similar suppressive potential
Because the phenotypic definition of Tregs in the human population remains controversial, we sought to compare the functional status of sorted CD4+CD25high T cells from cancer patients and healthy donors. We first evaluated whether purified CD4+CD25high T cells were able to inhibit the proliferation of non-regulatory effector T cells induced by allogeneic dendritic cells from healthy patients. The results were highly variable, depending upon the allogeneic pair (data not shown), and allogeneic proliferation was never inhibited completely by CD4+CD25high cells from cancer patients or healthy donors (data not shown).
Then, autologous CD4+CD25− cells were stimulated with anti-CD3 and anti-CD28 microbeads in the presence or absence of CD4+CD25high cells. The proliferation of CD4+CD25− T cells induced by anti-CD3 and anti-CD28 was reduced in the presence of CD4+CD25high T lymphocytes (Fig. 3a). This suppressive effect was not significantly different between Tregs from cancer patients and those from healthy donors (respectively, 50% ± 28% and 58% ± 18%; P = 0·28, Fig. 3a). Inhibition of effector T cell proliferation by Tregs was extremely variable from patient to patient within each group (cancer patients and healthy donors), ranging from 22 to 94%, and no significant correlation was observed between the type of cancer and the potency of Treg-mediated suppression (Fig. 3b). These data suggest that CD4+CD25high T cells from patients with metastatic cancer are as capable as those from healthy donors of inhibiting the proliferation of autologous CD4+CD25− cells upon T cell receptor stimulation.
Fig. 3.
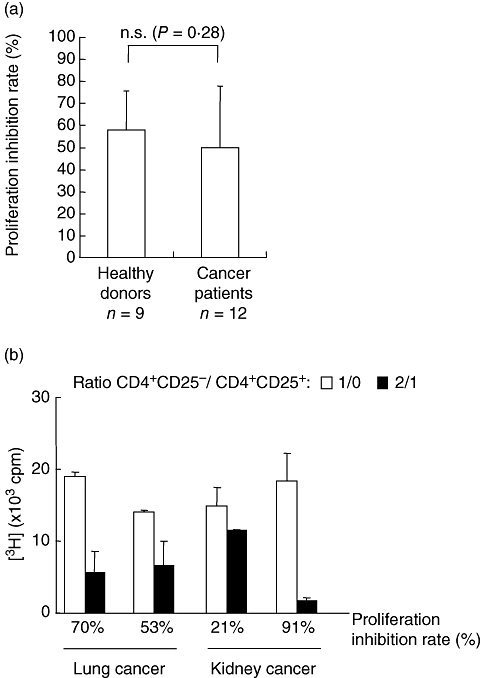
CD4+CD25high T cells from cancer patients and healthy donors exhibit a similar suppressive potential. (a) Sorted CD4+CD25high cells from nine healthy donors and 12 patients with metastatic cancer were cultured with autologous CD4+CD25− stimulated with microbeads-conjugated anti-CD3 and anti-CD28 antibodies. T cell proliferation was evaluated using [3H]-thymidine incorporation. Proliferation was determined in triplicate cultures. Inhibition rate of proliferation is expressed as a percentage of co-culture proliferations of 100 000 CD4+CD25− with CD4+CD25+ at a CD4+CD25−/CD4+CD25+ ratio of 2 : 1, compared to proliferation of CD4+CD25− alone. (b) Sorted CD4+CD25high cells from four representative cancer patients (kidney cancer: two, lung cancer: two) were cultured with autologous CD4+CD25− stimulated with anti-CD3 and anti-CD28 microbeads. Similar assay as described in (a).
Cyclophosphamide treatment and CD4+CD25high T cells in cancer patients
We have shown previously that, in a rat model of syngeneic colon cancer, cyclophosphamide-induced Treg depletion combined with a non-specific active immunotherapy was able to cure animals bearing established tumours [7]. These results prompted us to conduct a pilot clinical trial in patients with metastatic cancer who received a single intravenous injection of cyclophosphamide at day 1 and a BCG injection into an easily accessible metastasis (cutaneous or lymph nodes) at days 7 and 14. The characteristics of enrolled patients are described in Table 2. The regimens were well tolerated with only three grades 3/4 adverse events, such as pain at the injection site (two patients) and adenomegaly (one patient). Seven patients demonstrated progressive disease and died a few months later, one remained stable for 10 months and the remaining patient remains stable 4 years after treatment.
Table 2.
Summary of patient characteristics, clinical Phase I.
| Patients | Age (years) | Sex | Cancer type | Metastasis | Clinical outcome |
|---|---|---|---|---|---|
| 1 | 58 | F | Breast | Lymph nodes | PR (10 months) |
| 2 | 69 | F | Pancreatic | Lymph nodes, skin | PD |
| 3 | 79 | M | Mesothelioma | Peritoneum | PD |
| 4 | 54 | F | Uterine sarcoma | Bone, lungs | PR (4 years, stable) |
| 5 | 56 | M | Chordoma | Lymph nodes | PD |
| 6 | 58 | F | Colorectal | Liver | PD |
| 7 | 55 | F | Ovarian | Lymph nodes, peritoneum | PD |
| 8 | 53 | M | Neuroendocrine tumour | Bone | PD |
| 9 | 58 | F | Ovarian | Lymph nodes, peritoneum | PD |
PR, partial response; PD, progressive disease.
The number of Tregs were monitored weekly for the first 4 weeks, then monthly for 3 months after cyclophosphamide injection. Only a slight decrease in the absolute number (not shown) and the percentage of CD4+CD25high Treg cells to total CD4+ cells (Fig. 4a) was observed without significant differences between cyclophosphamide doses. In line with these data, FoxP3 expression was not modified significantly by cyclophosphamide treatment (Fig. 4b).
Fig. 4.
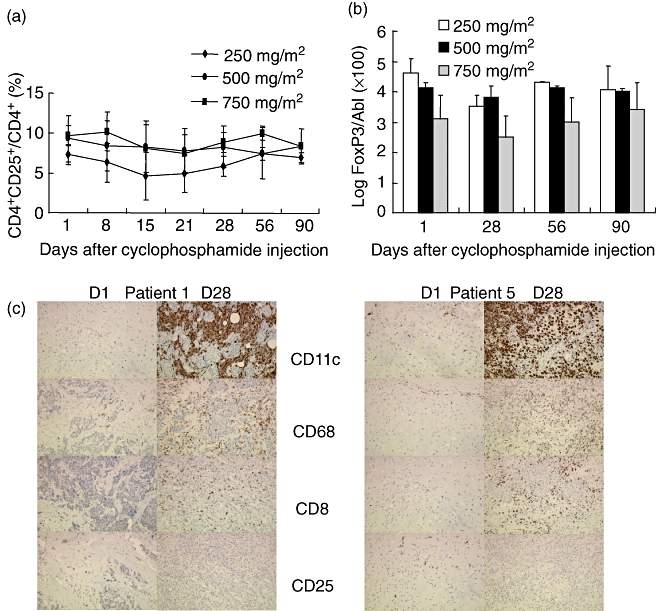
T regulatory (Treg) evolution following a single cyclophosphamide injection. (a) Determination of the percentage of CD4+CD25+ Treg to total CD4+ by flow cytometry at different time points after administration of the indicated doses of cyclophosphamide. The therapeutic protocol is described in Material and methods. (b) Evaluation of forkhead box P3 (FoxP3) expression in peripheral CD4+ T cells by real time reverse transcription–polymerase chain reaction (RT–PCR) at different time-points after administration of the indicated doses of cyclophosphamide. (c) Immunohistochemical analyses of metastatic tumours were performed at days 0 and at 28 of the therapeutic protocol on serial sections using monocloal antibodies: anti-CD68 that labels monocytes, anti-CD11c for myeloid dendritic cells, anti-CD8 and anti-CD25. Representative immunohistochemistry analyses of two patients (1 and 5) are shown.
Histological analysis performed in five patients before and after cyclophosphamide administration followed with BCG intratumoral injection demonstrated a dramatic infiltration of the tumour by myeloid cells, CD11c+ immature DCs, CD68+ macrophage cells and CD8+ T cells. Analysis of CD25+ T cells at the vicinity of the metastatic tumour showed variable degrees of tumour infiltration, and suggested a decrease in this infiltration following cyclophosphamide injection (Fig. 4c).
Discussion
In the current study we confirm that the prevalence of CD4+CD25high cells among CD4+ cells in peripheral blood was significantly higher in patients with metastatic cancer than in healthy subjects. Cell surface markers and FoxP3 expression analysis identified these cells phenotypically as Tregs. CD4+CD25high T cells from healthy donors and cancer patients were capable of inhibiting, to a similar extent, the proliferative response of autologous CD4+CD25− T cells. These results indicate that tumours do not modulate significantly the suppressive activity of peripheral blood Tregs, but rather increase their number. We did not find any correlation between the suppressive capacity of CD4+CD25high T cells and the type of cancer, and the inhibitory potency of Tregs was highly variable from one individual to another, in either healthy subjects or cancer patients. These results suggest that, unlike the reproducible Treg suppressive activity in rodents, the suppressive potential of human CD4+CD25high T cells is highly variable and depends on parameters that remain partly undefined. In cancer patients, the persistent inflammation induced by the tumour or growth factors and chemokines secreted by cancer cells create a microenvironment that may vary from patient to patient and consequently influence differentially the suppressive activity of Tregs. In our study, Tregs were able to inhibit T cell proliferation induced by anti-CD3 and anti-CD28, but not that induced by allogeneic DCs. This may be explained by the ability of anti-CD3 and anti-CD28 to not only stimulate effector cell proliferation, but to also promote and further enhance Treg immunosuppressive function.
As Treg elimination in cancer patients may foster the efficiency of anti-tumour vaccination [18, 21, 23, 26, 28], we adapted to the clinic an experimental protocol set up in rats [7]. This regimen was well tolerated and two patients demonstrated significant clinical improvement: one stable disease for 10 months and one disease that remains stable 4 years after treatment. However, no relationship was found between the percentage of the CD4+CD25high circulating T cells or the CD4+CD25high cells infiltrating the tumour and the clinical evolution of the disease.
The current data are in disagreement with a recent study which demonstrated cyclophosphamide-induced Treg depletion [30]. A single injection of cyclophosphamide may not be sufficient to impair significantly circulating Treg numbers. In fact, there is no clear evidence to suggest the mechanism for the induction of Tregs in cancer patients or Treg trafficking. The predominant location of these suppressive cells may be the tumour, where Tregs could have been attracted by CCL22 secreted by tumour cells or the myeloid cells infiltrating the tumour [26]. In accordance with this hypothesis, we demonstrated that an increase of myeloid cells infiltrating the tumour fosters the growth of rat colon carcinoma [31].
Taken together, our results confirm that CD4+CD25high Tregs are increased in peripheral blood of patients with metastatic cancer. Their immunosuppressive activity appears more heterogeneous than in animal models and cannot be inhibited by a single cyclophosphamide injection. Metronomic doses of cyclophosphamide combined with immunotherapy have shown a synergistic anti-tumour effect, but the mechanisms remain unclear [32]. Therefore an efficient strategy aiming to deplete Treg cells is still required to determine whether this depletion could favour tumour regression.
Acknowledgments
This work was supported by the French National League against Cancer (Burgundy and Nièvre committees) and by the Centre Hospitalier de Dijon and the French Ministry of Health − PHRC Régional: 2003-R-07-01. D. Cathelin received financial support from the Saône-et-Loire committee of the French National League against Cancer. N. Larmonier received support from the Leukaemia and Lymphoma Society Fellow Award 5188-07, the Tee Up for Tots and Raise a Racquet Funds. We thank A. Fromentin, P. Monin Baroille and F. Krzystanek, C. Parmeland, C. Serrée, C. Ridel for excellent technical assistance.
References
- 1.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 2.Morse MA, Clay TM, Mosca P, Lyerly HK. Immunoregulatory T cells in cancer immunotherapy. Expert Opin Biol Ther. 2002;2:827–34. doi: 10.1517/14712598.2.8.827. [DOI] [PubMed] [Google Scholar]
- 3.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 4.Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 5.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu T, Shen Y, Fujimoto S. Tumor-specific CD4(+) suppressor T-cell clone capable of inhibiting rejection of syngeneic sarcoma in A/J mice. Int J Cancer. 2000;87:680–7. [PubMed] [Google Scholar]
- 7.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 8.Tuve S, Chen BM, Cheng TL, et al. Combination of tumor site-located CTL-associated antigen-4 blockade and systemic regulatory T-cell depletion induces tumor-destructive immune responses. Cancer Res. 2007;67:5929–39. doi: 10.1158/0008-5472.CAN-06-4296. [DOI] [PubMed] [Google Scholar]
- 9.Jones E, Dahm-Vicker M, Simon AK, et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 10.Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207–17. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 13.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–18. [PubMed] [Google Scholar]
- 14.Tawara I, Take Y, Uenaka A, Noguchi Y, Nakayama E. Sequential involvement of two distinct CD4+ regulatory T cells during the course of transplantable tumor growth and protection from 3-methylcholanthrene-induced tumorigenesis by CD25-depletion. Jpn J Cancer Res. 2002;93:911–16. doi: 10.1111/j.1349-7006.2002.tb01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casares N, Arribillaga L, Sarobe P, et al. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–9. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 17.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–8. [PubMed] [Google Scholar]
- 18.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–99. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 19.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 20.Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–64. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 22.Fattorossi A, Battaglia A, Ferrandina G, et al. Lymphocyte composition of tumor draining lymph nodes from cervical and endometrial cancer patients. Gynecol Oncol. 2004;92:106–15. doi: 10.1016/j.ygyno.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 24.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 25.Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 26.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakagushi S. Stimulation of CD25(+)CD4(+) regulatory T cells through G1TR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 30.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnotte B, Crittenden M, Larmonier N, Gough M, Vile RG. MIP-3alpha transfection into a rodent tumor cell line increases intratumoral dendritic cell infiltration but enhances (facilitates) tumor growth and decreases immunogenicity. J Immunol. 2004;173:4929–35. doi: 10.4049/jimmunol.173.8.4929. [DOI] [PubMed] [Google Scholar]
- 32.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–13. [PubMed] [Google Scholar]


