Abstract
Metabotropic glutamate receptors (mGluRs) control intracellular signaling cascades through activation of G proteins. The inwardly rectifying K+ channel, GIRK, is activated by the βγ subunits of Gi proteins and is widely expressed in the brain. We investigated whether an interaction between mGluRs and GIRK is possible, using Xenopus oocytes expressing mGluRs and a cardiac/brain subunit of GIRK, GIRK1, with or without another brain subunit, GIRK2. mGluRs known to inhibit adenylyl cyclase (types 2, 3, 4, 6, and 7) activated the GIRK channel. The strongest response was observed with mGluR2; it was inhibited by pertussis toxin (PTX). This is consistent with the activation of GIRK by Gi/Go-coupled receptors. In contrast, mGluR1a and mGluR5 receptors known to activate phospholipase C, presumably via G proteins of the Gq class, inhibited the channel's activity. The inhibition was preceded by an initial weak activation, which was more prominent at higher levels of mGluR1a expression. The inhibition of GIRK activity by mGluR1a was suppressed by a broad-specificity protein kinase inhibitor, staurosporine, and by a specific protein kinase C (PKC) inhibitor, bis-indolylmaleimide, but not by PTX, Ca2+ chelation, or calphostin C. Thus, mGluR1a inhibits the GIRK channel primarily via a pathway involving activation of a PTX-insensitive G protein and, eventually, of a subtype of PKC, possibly PKC-μ. In contrast, the initial activation of GIRK1 caused by mGluR1a was suppressed by PTX but not by the protein kinase inhibitors. Thus, this activation probably results from a promiscuous coupling of mGluR1a to a Gi/Go protein. The observed modulations may be involved in the mGluRs' effects on neuronal excitability in the brain. Inhibition of GIRK by phospholipase C–activating mGluRs bears upon the problem of specificity of G protein (GIRK interaction) helping to explain why receptors coupled to Gq are inefficient in activating GIRK.
Keywords: G proteins, ion channel, modulation, phosphorylation, protein kinase C
introduction
Glutamate is a major excitatory neurotransmitter in the brain. It acts on receptors of two major types: (a) ionotropic glutamate receptors, which are ligand-gated ion channels and include the AMPA/kainate and NMDA receptors, and (b) metabotropic glutamate receptors (mGluRs)1 that activate G proteins (for reviews see Hollman and Heinemann, 1994; Nakanishi, 1994; Pin and Duvoisin, 1995). mGluRs participate in synaptic plasticity phenomena, playing a role both in long-term potentiation (LTP) and long-term depression (LTD), as well as in neurotoxicity and neuroprotection (for reviews see Bliss and Collingridge, 1993; Bear and Malenka, 1994; Nakanishi, 1994; Pin and Duvoisin, 1995). mGluRs form a family of eight genes cloned to-date, mGluR1– mGluR8 (Masu et al., 1991; Houamed et al., 1991; Tanabe et al., 1992; Abe et al., 1992; Tanabe et al., 1993; Nakajima et al., 1993; Okamato et al., 1994; Saugstad et al., 1994; Duvoisin et al., 1995) that encode proteins with structural similarity but low sequence homology to members of the seven-transmembrane domain receptors superfamily; thus, mGluRs may belong to a novel superfamily of G protein–coupled receptors (Nakanishi, 1994; Gomeza et al., 1996).
mGluRs are subdivided into groups according to their signal transduction mechanisms, pharmacological properties, and levels of sequence homology between the genes. Group I includes mGluR1 (the longest splice variant is called mGluR1a) and mGluR5, group II includes mGluR2 and mGluR3, and group III includes the rest (Nakanishi, 1994; Pin and Duvoisin, 1995). Group I mGluRs activate phospholipase C (PLC) and are thus believed to couple to the Gq class of G proteins. When expressed in Xenopus oocytes, these receptors activate a large endogenous Ca2+-dependent chloride current, a fact that enabled molecular cloning by functional expression of the first mGluR, mGluR1 (Masu et al., 1991; Houamed et al., 1991). Group II and group III receptors inhibit adenylyl cyclase (AC) activity, suggesting that they couple to G proteins of the Gi/Go class (Gilman, 1987).
The molecular mechanisms by which mGluRs exert their physiological effects are not yet fully understood. Their known effects include direct mediation of glutamatergic synaptic transmission at some synapses, both hyperpolarizing and depolarizing. Presynaptic group II and III autoreceptors inhibit transmitter release. All three groups have been shown to inhibit L-type voltage-gated Ca2+ channels, and groups I and II also inhibit N-type channels. mGluRs also modulate the ionotropic AMPA, NMDA, and GABA-A receptors (reviewed by Nakanishi, 1994; Pin and Duvoisin, 1995). mGluRs inhibit several types of K+ currents: the voltage-dependent M-type current, the Ca2+-activated current (IKAHP), a voltage-dependent K+ current IK,slow, and resting K+ currents (Schwartz, 1993; Guerineau et al., 1994; Ikeda et al., 1995; Luthi et al., 1996). Activation of K+ currents by mGluRs has been shown in cerebellar granule cells (Fagni et al., 1991).
GIRK1 (KGA, Kir3.1; Kubo et al., 1993; Dascal et al., 1993b) is a member of the GIRK, or Kir 3, family of G protein–activated inward rectifying K+ channels (reviewed by Doupnik et al., 1995), which also includes GIRK2 and GIRK3 cloned from brain (Lesage et al., 1994; Duprat et al., 1995), GIRK4 (CIR) from atrium (Krapivinsky et al., 1995a ; Lesage et al., 1995), and GIRK5 (XIR) present in Xenopus oocytes (Hedin et al., 1996). Functional inward rectifier channels are believed to be heterooligimers formed by GIRK1 with the other subunits (Lesage et al., 1995; Kofuji et al., 1995; Krapivinsky et al., 1995a ; Hedin et al., 1996). In the heart, acetylcholine (ACh) released from the vagus nerve binds to the muscarinic type 2 receptor (m2R) and activates the GIRK channel, mediating the parasympathetic negative chronotropic effect. Similar channels, most probably of the GIRK family, are activated in the brain by serotonin via 5HT1A receptors, GABA via GABAB receptors, opioids, etc., and presumably participate in regulation of neuronal excitability (for review, see North, 1989; Hille, 1992a ,b; Wickman and Clapham, 1995). The channel is activated via a membrane-delimited pathway, by direct binding of the βγ subunits released from heterotrimeric G proteins containing pertussis toxin (PTX)-sensitive Gα subunits (Gi/Go family; Breitwieser and Szabo, 1985; Pfaffinger et al., 1985; Logothetis et al., 1987; Ito et al., 1992; Huang et al., 1995; Inabobe et al., 1995; Krapivinsky et al., 1995b ; Kunkel and Peralta, 1995).
The fact that GIRK is activated by receptors coupled to Gi/Go proteins makes it feasible that the Gi-coupled mGluRs are capable of activating the channel. Such activation may be relevant to brain function. Extensive expression of GIRK1 and GIRK2 RNA and protein in the brain was revealed by in situ hybridization and immunohistochemical methods (Karschin et al., 1994, 1996; Bausch et al., 1995; Ponce et al., 1996). mGluR expression in the brain is wide, varying from receptor to receptor (see Nakanishi, 1994; Pin and Duvoisin, 1995), and there are areas in which expression of mGluRs and GIRK1 overlaps.
Our study shows that, indeed, the Gi-coupled mGluRs (types 2, 3, 4, 6, and 7) activate the GIRK channel when coexpressed in Xenopus oocytes. In addition, a negative coupling exists between the PLC-coupled mGluRs (types 1 and 5) and GIRK, most probably mediated by activation of the Gq–phospholipase C pathway and a PKC subtype.
materials and methods
Preparation of RNAs and Oocytes
DNA plasmids containing the various clones were linearized with the appropriate restriction enzymes using a standard protocol (Dascal and Lotan, 1992): GIRK1 (Dascal et al., 1993b) with XhoI, mGluR3 and mGluR4 (Tanabe et al., 1992) with HindIII, the other mGluRs (Masu et al., 1991; Tanabe et al., 1992; Abe et al., 1992; Nakajima et al., 1993; Okamoto et al., 1994) with NotI. Gβ1 (Fong et al., 1986) and Gγ2 (Gautam et al., 1989) were subcloned into the EcoRI site of pGEMHE vector (Liman et al., 1992) and linearized with NheI. cDNA for GIRK2 was prepared as described (Kofuji et al., 1995). The linearized plasmids were transcribed in vitro by SP6 (mGluR1a), T3 (mGluR2 and mGluR4) or T7 (the other clones) by RNA polymerase using standard procedures (Dascal and Lotan, 1992). The cDNAs were kindly provided by Drs. S. Nakanishi (all mGluRs, Kyoto University, Japan), E. Peralta (m2R, Harvard University, Cambridge, MA), P. Kofuji (GIRK2, Caltech, Pasadena, CA), M. Simon (G protein subunits, Caltech, Pasadena, CA), E. Liman (pGEMHE, Harvard University, Boston, MA).
Oocytes were isolated, incubated, and injected with RNA as described (Dascal and Lotan, 1992). The incubation solution, NDE96, contained 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES (pH = 7.5), 2.5 mM Na+-pyruvate, and 50 μg/ml gentamycin. The amounts of RNA injected per oocyte were as follows: GIRK1 and GIRK2, 0.2–1 ng; mGluR1a, 25–5,000 pg; mGluR2, 1–2 ng; mGluR3, 0.4–5 ng; mGluR4, 40 ng; mGluR5, 5 ng; mGluR6, 2–5 ng; mGluR7, 40 ng; Gβ1, 1 ng; Gγ2, 1 ng; m2R, 100 pg. Electrophysiological experiments were performed 3–7 d after RNA injection.
Electrophysiological Measurements
For two-electrode whole-cell experiments oocytes were voltage-clamped at −80 mV using OC-725B oocyte clamp (Warner Instruments, Hamden, CT). Agarose cushion electrodes (Schreibmayer et al., 1994) were used to ensure stable long-lasting recording. Data acquisition and analysis were performed with pCLAMP software (Axon Instruments, Foster City, CA). The oocyte was transferred from the incubation solution, NDE96, to the recording chamber, containing ND96 solution (identical to NDE96 besides not containing pyruvate and gentamycin). The responses to neurotransmitters were recorded in a high K+ solution (hK, identical to ND96 except for NaCl and KCl concentrations, which are reversed; Dascal et al., 1993a). Glutamate (100 μM, or for mGluR7 experiments, 1 mM) was added to this solution, and in some experiments Ba2+ followed, to block GIRK (Dascal et al., 1993a). A ∼90–95% block of GIRK was produced by 300 (GIRK1/GIRK5) or 1,000 (GIRK1/GIRK2) μM Ba2+. Current-voltage curves were obtained at various stages by applying a voltage ramp, from −120 mV to +50 mV during 0.5–1 s, and subtracting the voltage ramp performed in Ba2+-containing hK. Ba2+ does not significantly block the endogenous (“native”) oocyte's K+ currents at these concentrations (Dascal et al., 1993a). These curves, therefore, represent exclusively GIRK activity at the specified time.
In patch-clamp experiments, single channel currents (at −80 mV) were filtered at 2 kHz, sampled at 4 kHz with the Axotape software (Axon Instruments), and analyzed using the pCLAMP software. Pipette solution contained: 144 mM KCl, 2 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.1–1 mM GdCl3, 10 mM HEPES/ KOH, pH 7.5. Bath (500 μl) solution contained: 140 mM KCl, 6 mM NaCl, 4 mM MgCl2, 1 mM EGTA, 10 mM HEPES/KOH, pH 7.5. For calculating the open probability of a single channel, an idealized record was divided into 5-s bins, and then Po was averaged over the desired time periods (e.g. 3-min stretches as in Fig. 4). The number of channels in a patch was estimated from the maximal number of overlapping openings, and records with more than four channels were excluded from the study.
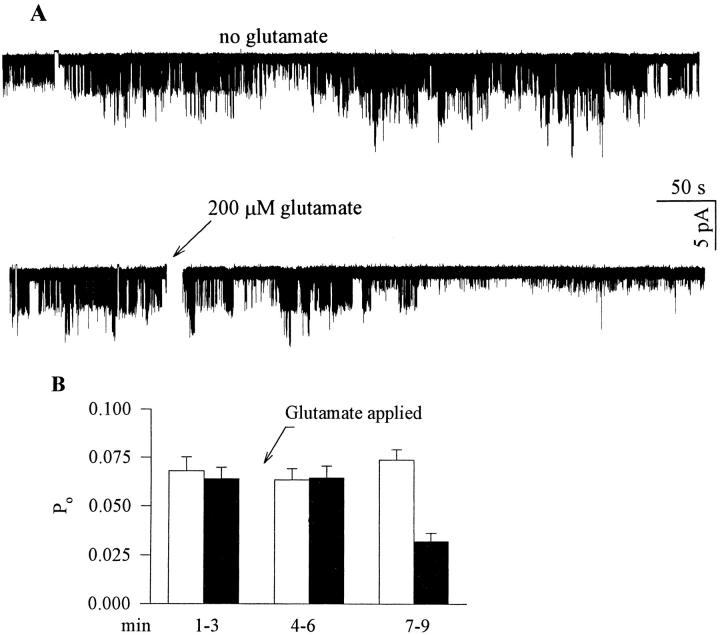
Figure 4.
Glutamate applied outside the patch pipette blocks GIRK activity in cell- attached patch clamp recordings from cells expressing mGluR1a, GIRK1, Gβ1, and Gγ2. (A) Top : a trace from a cell unexposed to glutamate, which shows steady activity of GIRK1. Bottom: a trace from a cell exposed to glutamate at the time indicated by the arrow, showing inhibition of channel activity. The time period during which glutamate was added to the bath is blanked out. (B) Glutamate reduces open probability (Po) in cells exposed to glutamate. Filled bars, Po in cells exposed to glutamate (n = 5). Empty bars, Po in cells unexposed to glutamate (n = 5). Po was averaged over periods of 3 min. The abscissa shows time after the start of the record. Glutamate was added at t = 3 min (arrow).
Treatment of Oocytes with EGTA, PTX, and with PKC Blockers
Oocytes were injected with 20 nl of 50 mM K+-EGTA (pH = 7.6) 2–8 h before the experiment; this corresponds to ∼1 mM EGTA in the oocyte. The A-protomer of PTX (List Biological Laboratories, product #182; kindly provided by Dr. M. Cohen-Armon, Tel Aviv University) was dissolved in sterile water at 20 ng/μl according to manufacturer's instructions; intermediate concentrations were made by further dilution in water to 0.18–7 ng/μl (depending on the batch of PTX), and 30 nl were injected into oocytes 10–20 h before the recording session. The activity of PTX was tested independently for its ability to cause a ∼70–90% block of the response to acetylcholine in oocytes injected with GIRK1, GIRK2, and m2R (data not shown).
Staurosporine and calphostin C (Sigma Chemical Co., St. Louis, MO) were dissolved in DMSO at 3 mM and stored in 2–3 μl aliquots at −20°C. 1 μl of the stock solution of staurosporine or calphostin C was added to 1 ml of the standard NDE96 medium in which the oocytes were incubated (the solutions were exposed to light) for at least 2 h before electrophysiological recording. Control (untreated) oocytes were incubated in 0.1% solution of DMSO in NDE96. In one experiment, 20 nl of 30 or 300 μM calphostin C were injected into the oocytes 2–6 h before recording the currents. Bis-indolylmaleimide (BIS; Calbiochem Corp., La Jolla, CA) was dissolved in water at 5 mM and stored in aliquots at −20°C. Oocytes were injected with 30 nl of a water solution of BIS at 150 μM (this corresponds to ∼5 μM BIS in the oocyte) and, in addition, incubated in 5 μM BIS 2–4 h before recording. Since BIS was usually tested in the same experiments as staurosporine, the incubation solution also contained 0.1% DMSO. The recording was performed in solutions free of blockers, no later than 5 min after the removal of the oocytes from the blocker-containing incubation medium. Although staurosporine has been reported to block directly the G protein-activated K+ channel (Lo and Breitwieser, 1994), this effect occurs at higher concentrations (>10 μM) than used by us (3 μM) and is promptly reversed upon the washout of the drug.
4β-phorbol-12-myristate 13-acetate (PMA; Sigma Chemical Co.) was dissolved in DMSO at 100 μM and stored in aliquots at −20°C. All solutions containing PMA were protected from light.
Statistics and Presentation of Data
The results in the text and in the figures are presented as mean ± SEM, n = number of cells tested. Comparisons between two groups were done using two-tailed Student's t test. Comparisons between more than two groups were done using one-way nonparametric ANOVA followed by Dunn's test, using the SigmaStat software (Jandel Scientific, Corte Madera, CA).
results
Gi/Go-coupled mGluRs Activate GIRK via PTX-sensitive G Proteins
The GIRK channels were expressed by injecting RNA of GIRK1 alone or with RNA of GIRK2. In oocytes injected with GIRK1 RNA alone, the channels are most probably formed by GIRK1 and the endogenous subunit, GIRK5 (Hedin et al., 1996), and they will be termed GIRK1/GIRK5 channels. In oocytes injected with RNAs of GIRK1 and GIRK2 (a combination especially relevant to GIRK composition in the brain), the amplitude of GIRK currents was increased five- to tenfold as compared with the injection of GIRK1 RNA alone; therefore, a majority of channels probably represented GIRK1/GIRK2 heterooligomers (cf. Kofuji et al., 1995).
Coinjection of GIRK1 or GIRK1+GIRK2 RNAs with mGluR2 RNA into Xenopus oocytes gave rise to a glutamate-activated inwardly rectifying K+ current, which was not present in oocytes injected with the channel RNA alone, or in uninjected oocytes. Fig. 1 A depicts a typical two electrode voltage-clamp experiment with an oocyte coexpressing mGluR2 and GIRK1. Since the channel is an inward rectifier, the bathing solution is first changed from the standard incubation medium, ND96, to a high K+ solution, hK (containing 96 mM K+), permitting an inward K+ current at a holding potential of −80 mV. Application of glutamate in the hK solution induced a large increase in the current, designated Iglu. The GIRK current was blocked by 300– 1,000 μM Ba2+, leaving mainly a K+ current endogenous to the oocyte, denoted In and ranging between 50 and 300 nA (Dascal et al., 1993a, b). The current in hK alone consists of both In and the basal GIRK activity, designated IhK, and the application of Ba2+ enables to distinguish between them. Unless stated otherwise, In (revealed following Ba2+ block of GIRK in each cell) was always subtracted from the total current; thus, all reported values pertain to net GIRK currents. In oocytes injected with GIRK1, IhK was usually between 100 and 600 nA, and Iglu between 0.4 and 1.5 μA (depending on the oocyte batch, amount of injected RNA, and incubation time). In oocytes injected with GIRK1 and GIRK2 (200-500 pg RNA/oocyte), IhK was usually between 0.8 and 5 μA, and Iglu was between 2 and 10 μA.
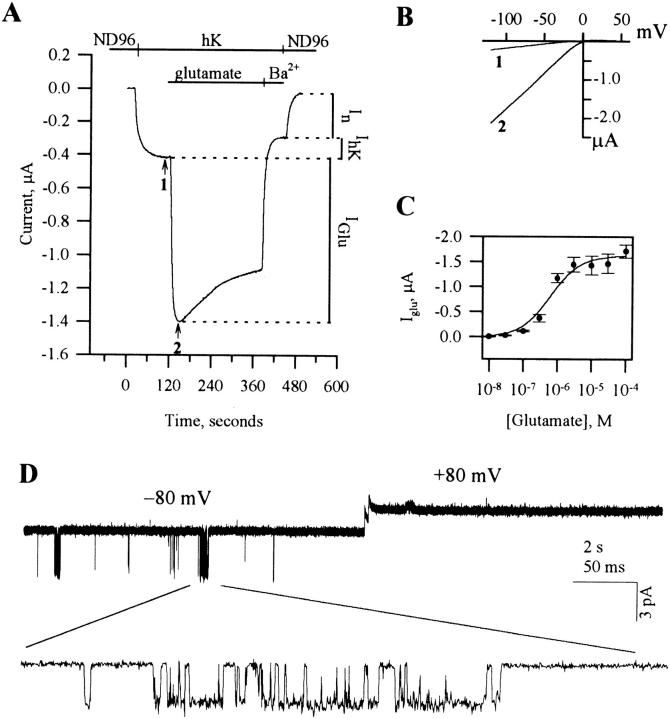
Figure 1.
GIRK activation by mGluR2. (A) Two-electrode voltage-clamp recording from a cell expressing mGluR2 and GIRK1. Bars above trace show solutions applied. (B) Current-voltage relationships of the GIRK currents at the times indicated by arrows in A: in hK alone (1) and in glutamate (2). Each net GIRK1 I-V curve was obtained by subtraction of the I-V curve recorded after addition of Ba2+. (C) Gluta-mate dose-response curve, with apparent K d = 0.63 μM, fitted to the standard Michaelis-Menten equation. n = 3 or 4 cells for each glutamate concentration. (D) A cell-attached patch clamp record of the activity of the GIRK channel with 10 μM K-glutamate in the pipette, from an oocyte injected with RNAs of GIRK1 and mGluR2.
Fig. 1 B shows the net GIRK1/GIRK5 current-voltage relationship before and after application of glutamate and demonstrates the inward rectification of the current induced by glutamate. The glutamate dose-response curve showed an EC50 of 0.63 μM in oocytes injected with mGluR2 and GIRK1 (Fig. 1 C). This is somewhat lower than the 4 μM value reported by Tanabe et al. (1992) in fibroblasts expressing mGluR2, and may reflect the existence of spare receptors in the oocytes under the conditions utilized here.
The channel activated by 10–100 μM glutamate in oocytes expressing GIRK1 and mGluR2 displayed the expected characteristics (Dascal et al., 1993b; Kubo et al., 1993) in cell-attached patches: inward rectification (note that openings were observed at −80 but not at +80 mV; Fig. 1 D), current of 2.65 ± 0.05 pA at −80 mV with 154 mM K+ in the pipette (n = 6), and a slope conductance of 37 ± 1.5 pS (n = 3) between −80 and −20 mV.
GIRK currents activated by glutamate via mGluR2 were strongly blocked by the injection of the catalytic subunit (A-protomer) of PTX (we found this treatment much more efficient than a 16–24 h incubation of the cells in heteromeric PTX; data not shown). In oocyte from two donors injected with RNAs of GIRK1, GIRK2, and mGluR2, PTX treatment reduced IhK by 55.1 ± 7.2% (n = 9, P < 0.01), and Iglu by 71.4 ± 4.7% (n = 8, P < 0.01). The inhibition of IhK by PTX suggests that at least a part of the basal activity of GIRK is due to activation by Gβγ released due to a basally occurring GTPase cycle of PTX-sensitive G proteins (see Okabe et al., 1991). The same PTX treatment did not affect a voltage-dependent Shaker H4 current (data not shown) or the Ca2+-activated Cl− current evoked by glutamate via mGluR1a (see below).
In oocytes injected with GIRK1 RNA, the GIRK current evoked by activating mGluR3 and mGluR6 was smaller than the mGluR2-activated current, ranging from <100 to 200–300 nA for the same RNA amounts. Visible activation of GIRK1/GIRK5 by mGluR4 and mGluR7 required tenfold higher RNA amounts, and the current was in the range of 50–70 nA. The fact that different Gi/Go-coupled mGluRs gave different response amplitudes to agonist could be due either to different levels of protein expression, or to different levels of activation of the appropriate G proteins.
Gq-coupled mGluRs Inhibit GIRK1 via a Pathway Involving a PTX-insensitive G Protein
Oocytes coinjected with RNAs of GIRK1 (or GIRK1 and GIRK2) and the PLC-coupled mGluR1a or mGluR5 were exposed to glutamate in the hK solution. This caused a large transient Cl− current (Fig. 2 A), as described previously for oocytes injected with mGluR1a or mGluR5 alone (Masu et al., 1991; Abe et al., 1992). In line with previous reports (Houamed et al., 1991; Masu et al., 1991), the mGluR1a-activated Ca2+-dependent Cl− current was not suppressed by the PTX treatment: it was 1,700 ± 530 nA in control and 2,050 ± 725 nA in PTX-treated oocytes (n = 4 in each group, oocytes of one donor; P > 0.7). This is consistent with activation of the PLC cascade via Gq/11 (Simon et al., 1991; Birnbaumer, 1992) followed by a rise in [Ca2+]in and activation of the Ca2+-dependent Cl− current endogenous to the oocyte (reviewed by Dascal, 1987).
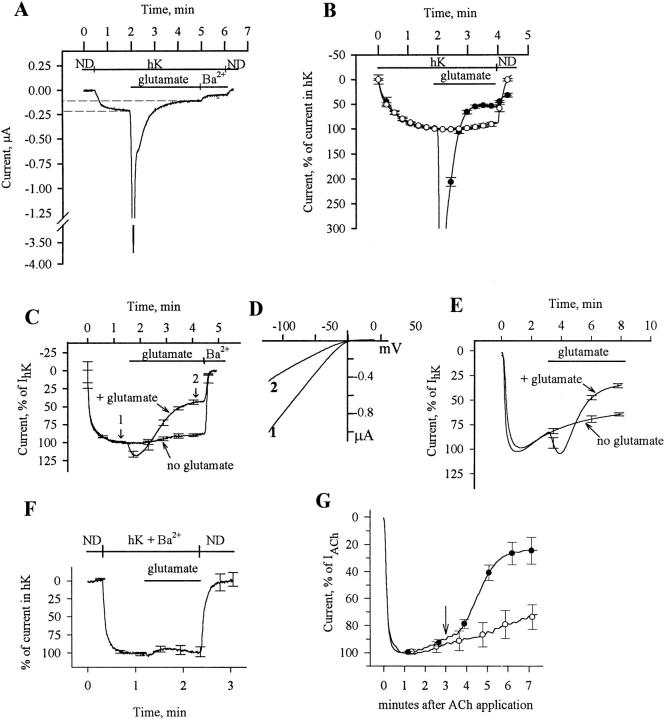
Figure 2.
Inhibition of GIRK by mGluR1a. (A) Two-electrode voltage-clamp recording from a single cell injected with RNAs of GIRK1 (500 pg) and mGluR1a (25 pg). ND, stands for ND96. (B) Average recording from cells of one donor exposed to glutamate (•, n = 4) and unexposed to glutamate (○, n = 6). Ba2+ was not applied, and current was expressed as percent of the current in hK solution (IhK + In); I = 0 in ND96. (C) Inhibition of GIRK channel activity by mGluR1a in EGTA-treated oocytes: comparison of cells exposed to glutamate (n = 4) to cells unexposed to glutamate (n = 4). The cells were from the same donor, injected with 25 pg mGluR1a RNA and 500 pg GIRK1 RNA. The ND96 solution was changed to the hK solution at time t = 0. Currents in each oocyte were normalized to the peak basal GIRK current, IhK. Average traces from all 4 oocytes are shown. The mean IhK was 313 ± 29 nA (n = 8) in these oocytes. (D) Current-voltage relationships of GIRK currents at the times indicated by arrows in C, in a cell injected with EGTA: in hK (1) and in glutamate after the decay of the current (2). Net GIRK I-V curves were obtained as in Fig. 1 B. (E) Inhibition of GIRK1/GIRK2 channel activity by mGluR1a in EGTA-treated oocytes. Presentation as in C; n = 4 oocytes for each treatment. In this batch, oocytes were injected with 50 pg mGluR1a RNA and 250 pg of each of the GIRK1 and GIRK2 RNAs. Average IhK was 4,126 ± 707 nA (n = 8). (F) Absence of glutamate-activated inward current in oocytes incubated in 300 μM BaCl2. Oocytes were injected with RNAs of mGluR1a (25 pg) and GIRK1 (500 pg) and treated with EGTA. Ba2+ (300 μM) was added to all solutions. Currents in each cell were normalized to the peak inward current observed in the hK solution, which, because of the block of GIRK, equals to In (the average current in the hK solution was 86 ± 8 nA, n = 5). (G) Inhibition of m2R-induced activation of GIRK by mGluR1a. Oocytes expressing mGluR1a, m2R, and GIRK1, and injected with EGTA before recording, were placed in hK solution and then exposed to 10 μM ACh, which activated GIRK (the record starts at the time of addition of ACh). This current is denoted IACh. 3 min after exposure to ACh (at the time indicated by the arrow), one group of cells was exposed to glutamate (•, n = 3), which caused a decrease in the current compared with cells that were not exposed to glutamate (○, n = 3). The current is expressed as percent of IACh; records averaged from all oocytes in each group are shown.
After the large Cl− current transient subsided, the residual current was smaller than the initial current in hK (see Fig. 2 A), a decline that was not seen in oocytes injected with mGluR1a or mGluR5 alone (data not shown). This suggests that activation of these receptors inhibits the GIRK channel activity. The GIRK-related portion of the total current is shown in detail in Fig. 2 B, which illustrates a recording in an experiment similar to that shown in Fig. 2 A, averaged from several cells from one donor expressing mGluR1a and GIRK1. Glutamate caused a pronounced inhibition of the GIRK current (•). Since it has been previously shown that oocytes expressing GIRK1 show a slow spontaneous desensitization of IhK (Kovoor et al., 1995), we have recorded IhK without applying glutamate; cells unexposed to glutamate showed only a mild desensitization of the current (○). We estimated that IhK was inhibited by 30–60%; a more accurate estimate was difficult because of the existence of a small residual Cl− current in cells exposed to glutamate.
To observe the inhibition of GIRK by mGluR1a without the interference of the Ca2+-dependent Cl− current, we injected the calcium chelator, EGTA, into the oocytes before the recording (in these experiments, the oocytes were injected with 25–50 pg mGluR1a RNA and 500 pg GIRK1 RNA). This treatment abolished all of the transient and most of the sustained Ca2+-activated Cl− current (Boton et al., 1989) and allowed the holding current to return, after washout of glutamate and hK, close to its original value in the ND96 solution. As shown in Fig. 2 C, IhK decreased to less than half of its peak value three minutes after the application of glutamate (trace denoted: + glutamate). In the same oocyte batch as the above, in cells unexposed to glutamate IhK decreased by <15% after the same period of time in the hK solution (Fig. 2 C, no glutamate). Averaging over several oocyte batches showed that in cells exposed to glutamate the GIRK1/GIRK5 current was reduced to 43.9 ± 2.4% of the initial IhK (n = 21, from 5 donors), whereas in unexposed cells it decreased only to 81.9 ± 3.2% (n = 6, from 2 donors). Thus, the net inhibition of the GIRK current caused by glutamate via activation of mGluR1a was about 46%. Fig. 2 D shows the net GIRK current-voltage relationship before and after the application of glutamate and confirms that the current inhibited by glutamate is an inward rectifier. Similarly, activation of mGluR1a inhibited the GIRK1/GIRK2 current (Fig. 2 E) by 57.9 ± 3.9% 5 min after glutamate application (oocytes from 2 donors; see Fig. 5 D for details).
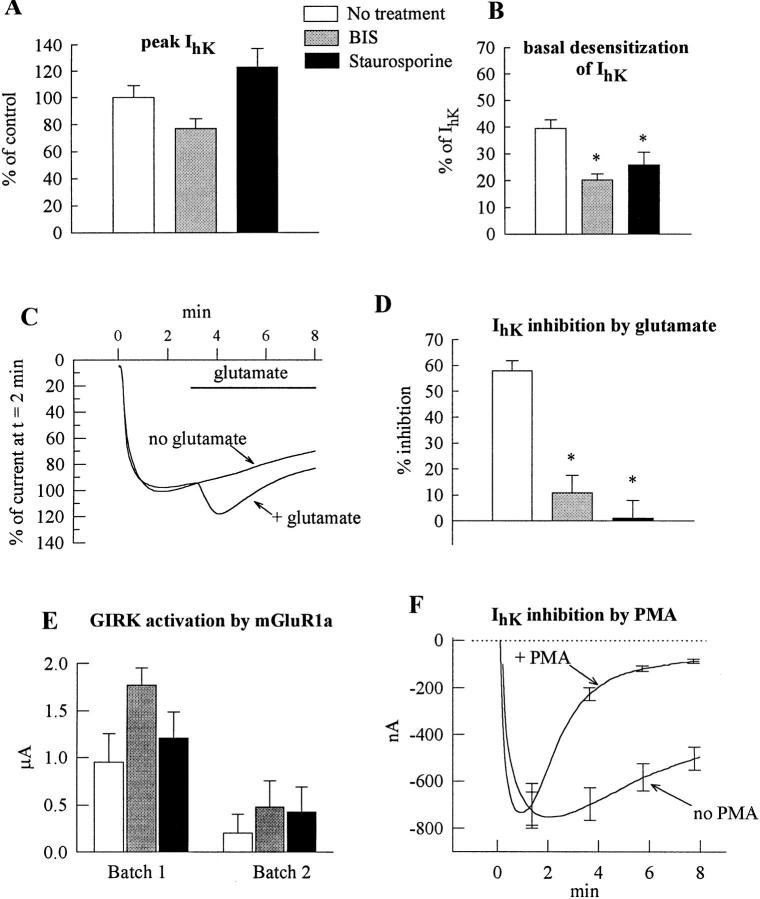
Figure 5.
mGluR1a-induced inhibition of GIRK is blocked by PKC inhibitors and mimicked by PMA. Oocytes expressing mGluR1a (30 or 50 pg/ oocyte), GIRK1 and GIRK2 (250 or 500 pg/oocyte) were injected with EGTA before recording (except in F). In A, B, D and E, the treatments are presented as follows: empty bars, no treatment (vehicle only); shaded bars, BIS; black bars, staurosporine. (A) Staurosporine and BIS do not significantly alter the amplitude of IhK. Summary of two oocyte batches, n = 8 for each treatment. In each oocyte, IhK was normalized to the average IhK amplitude in untreated cells of the same oocyte batch. (B) Staurosporine and BIS attenuate the basal desensitization of IhK. Same oocytes as in A. In each oocyte, the extent of desensitization was calculated as [1 − (IhK at the end of the 8 min exposure to hK)/ (peak IhK)] × 100%. (C) Gluta-mate (trace denoted: + gluta-mate) fails to cause inhibition of GIRK in a representative cell treated with staurosporine; the activation of GIRK remains unimpaired. For comparison, a re-cord is shown from an oocyte (from the same donor) treated with staurosporine but not exposed to glutamate (no gluta-mate). (D) The mGluR1a- induced inhibition of IhK is suppressed by BIS and staurosporine: a summary of two experiments. Same oocytes batches as in A and B. The extent of IhK inhibition was calculated as explained in Fig. 3 C. (E) mGluR1a-induced GIRK activation is not significantly changed by BIS and staurosporine. (F) PMA inhibits the basal GIRK current. Oocytes were incubated in ND96 as usual and, at t = 0, exposed either to the normal hK solution (no PMA; n = 5) or to hK containing 10 nM PMA (+ PMA; n = 5). Records averaged from all oocytes in each group are shown.
To investigate whether the transient inward current appearing at the onset of exposure to glutamate in oocytes treated with EGTA (Fig. 2, C and E; compare with Fig. 2 A) is an activation of GIRK1 or a small residual Cl− current owing to incomplete chelation of intracellular Ca2+, cells expressing GIRK1 were perfused with Ba2+-containing hK. In this solution the GIRK channel (presumably GIRK1/GIRK5) is blocked by Ba2+ and cannot be activated, whereas the native Ca2+-activated Cl− current is not blocked by Ba2+ (Singer-Lahat et al., 1994). In cells given this treatment no inward current was evoked by glutamate (Fig. 2 F). Cells from the same donor but unexposed to Ba2+ showed the usual glutamate-activated inward current followed by inhibition (n = 4; data not shown). With 25 pg mGluR1 RNA/oocyte, the average glutamate-evoked GIRK1/GIRK5 current in oocytes treated with EGTA was 28.9 ± 16.3% of IhK (n = 16, from 5 donors. IhK in these cells was 346 ± 41 nA). The current-voltage relationships of the initial inward current evoked by glutamate in these oocytes showed clear inward rectification and a reversal potential of ∼0 mV (data not shown) and thus could not represent the Ca2+-activated Cl− current, which shows outward rectification and reverses at ∼−20 mV (Miledi and Parker, 1984). These results suggest that the glutamate-induced inward current in EGTA-treated cells expressing mGluR1a and GIRK1 is due to activation of the GIRK channel. Similar results were obtained with GIRK1/GIRK2 channels in EGTA-injected oocytes (e.g., Fig. 2 E and data not shown). With 50 pg mGluR1a RNA/oocyte, the mGluR1a-activated GIRK1/ GIRK2 current was 664 ± 121 nA (24 ± 6% of IhK, n = 7, oocytes from two donors).
It is possible to activate GIRK by ACh in oocytes expressing the muscarinic m2 receptor, m2R. We tested whether mGluR1a is able to inhibit the m2R-induced GIRK current in oocytes expressing mGluR1a, m2R and GIRK, and treated with EGTA (Fig. 2 G). ACh caused the expected agonist-induced activation of the channel, while applying glutamate 3 min later caused a decrease of this current (•) which was clearly larger than the desensitization in cells exposed only to ACh (○). The fact that application of glutamate did not evoke an additional inward current strengthens the conclusion reached above, that in oocytes expressing mGluR1a and GIRK1 and injected with EGTA, glutamate does not induce a Ca2+-activated Cl− current. In this experiment GIRK was not activated in response to glutamate, presumably because it was already activated by ACh.
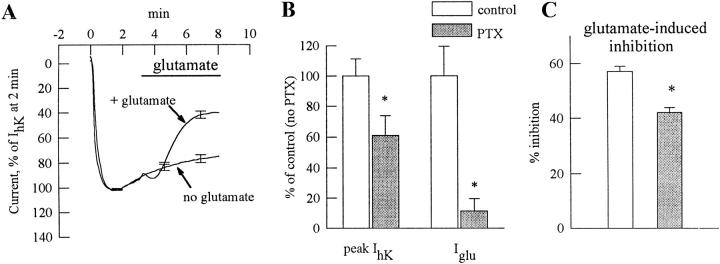
PTX was used to examine which G proteins are involved in activation and inhibition of GIRK by mGluR1a (Fig. 3). In oocytes of one donor expressing GIRK1, GIRK 2, and mGluR1a (50 pg receptor RNA/oocyte) and injected with EGTA, glutamate evoked an inward GIRK current, Iglu, of 775 ± 110 nA (n = 4), and the inhibition of IhK was ∼70% 5 min after application of glutamate (not shown). In PTX-treated oocytes of the same donor, the inward GIRK current was reduced to 32 ± 10 nA (a 96% reduction), whereas the inhibition was somewhat weaker than in untreated cells but still significant, ∼47% (Fig. 3 A). Fig. 3, B and C, summarizes the effects of PTX treatment in oocytes of two donors and confirm that the PTX almost completely (89%) suppressed the mGluR1a-evoked GIRK activation. IhK was reduced by ∼40% (Fig. 3 B), whereas the mGluR1a-induced inhibition was reduced from 57 to 42%, i.e. by ∼26% (Fig. 3 C).
Figure 3.
PTX suppresses mGluR1a-induced activation of GIRK but has little effect on mGluR1a-induced GIRK inhibition. (A) Inhibition of GIRK1/ GIRK2 channel activity by mGluR1a in EGTA-injected oocytes treated with PTX: comparison of cells exposed to glutamate (n = 4) to cells unexposed to glutamate (n = 4). The cells were injected with RNAs of mGluR1a (50 pg), GIRK1 (250 pg), and GIRK2 (250 pg). The ND96 solution was changed to the hK solution at time t = 0. Currents in each oocyte were normalized to the peak basal GIRK current, IhK. Records averaged from all oocytes in each group (n = 4) are shown. Average IhK was 833 ± 193 nA (n = 8) in these oocytes, and 1,993 ± 331 nA (n = 8) in PTX-untreated oocytes of the same donor. (B) The effect of PTX treatment on basal (IhK) and glutamate-evoked (Iglu) GIRK1/GIRK2 currents: a summary from oocytes of two donors (n = 8 in each group). IhK or Iglu in each cell was expressed as percent of control (i.e., average IhK or Iglu in the untreated group of oocytes of the same donor), and the results were averaged across all cells. Asterisks denote statistically significant differences, P < 0.05 or better. (C) The effect of PTX treatment on mGluR1a-induced GIRK1/GIRK2 inhibition. Same two oocyte batches as in B. The extent of glutamate-evoked inhibition was calculated as follows: first, in each cell, the current at the end of the record (t = 8 min in the hK solution) was expressed as percent of IhK measured at t = 2 min. In glutamate-exposed oocytes, this value was normalized to the average current in cells unexposed to glutamate at t = 8 min. This normalization procedure allowed averaging of the results despite the large difference in the absolute amplitudes of the currents recorded in oocytes of different donors.
Involvement of a Diffusible Second Messenger and of Protein Kinase C in mGluR1a-induced Inhibition of GIRK
Cell-attached patch-clamp experiments were exploited to examine whether the inhibition of GIRK channels by mGluR1a involves the production of a diffusible second messenger. Oocytes were injected with RNAs of mGluR1a and GIRK1, as well as of Gβ1 and Gγ2 subunits of heterotrimeric G proteins (to increase the basal GIRK1 activity; Reuveny et al., 1994). In Fig. 4 A, the upper panel shows the persistent activity of the channel in the absence of agonist. The lower panel demonstrates the reduction in this activity following application of glutamate, though this inhibition was delayed. Glutamate was applied outside the pipette in the cell-attached configuration, so its effect on the channel sealed in the pipette is likely to be mediated by a second messenger (Hille, 1992a ). Fig. 4 B shows the average open probability, Po, for cells exposed to glutamate, in comparison to unexposed cells. The inhibition of GIRK1 took roughly three minutes to develop, and was ∼50%. The decrease in Po appeared transient, but this has not been systematically studied.
Since mGluR1a activates PLC, which leads to activation of PKC, and since the PKC activator, PMA, has been reported to inhibit GIRK current in the oocytes (Chen and Yu, 1994), we tested whether protein phosphorylation is involved in the inhibition of GIRK by mGluR1a, by using a series of protein kinase inhibitors: a broad-specificity protein kinase inhibitor staurosporine acting at the protein kinase catalytic site, a specific PKC inhibitor bis-indolylmaleimide (BIS) acting at the PKC catalytic site, and a specific PKC inhibitor calphostin C acting at the diacylglycerol binding site (Hidaka and Kobayashi, 1992). Oocytes expressing mGluR1a and GIRK1/GIRK2 channels were incubated for 2–4 h in medium containing 3 μM staurosporine or 5 μM BIS; in addition, BIS was also injected into the oocytes (see materials and methods). Fig. 5 A shows that neither staurosporine nor BIS significantly altered the basal activity of GIRK. However, both blockers somewhat reduced the rate of the spontaneous decay (basal desensitization) of IhK (Fig. 5 B), suggesting that basal activity of PKC could account for part of the basal GIRK desensitization.
Fig. 5 C shows an example of the effect of staurosporine on the glutamate response in a representative oocyte expressing mGluR1a, GIRK1, and GIRK2, and Fig. 5, D and E, provides a summary of experiments with BIS and staurosporine in oocytes of two donors. Both staurosporine and BIS almost fully suppressed glutamate-induced inhibition of the GIRK current (Fig. 5 D) and slightly enhanced the initial GIRK activation (Fig. 5 E). Similarly, with GIRK1/GIRK5 channels staurosporine almost completely suppressed the inhibition but did not significantly alter the activation phase (n = 3, one oocyte batch; data not shown). Similar results were obtained with mGluR5 (data not shown). However, neither a 4-h incubation with calphostin C nor injection of this blocker into the oocytes suppressed glutamate- induced inhibition of GIRK1/GIRK5 channels: the current was reduced to 35.3 ± 7.5% of the initial IhK (n = 7, from 2 donors), similar to the 43.9% observed in oocytes untreated with PKC blockers.
In view of the existing controversy concerning the inhibitory effect of phorbol esters on GIRK (cf. Kovoor et al., 1995 vs. Chen and Yu, 1994), we examined the effect of the phorbol ester, PMA, on the basal activity of the GIRK1/GIRK2 channels. Fig. 5 F demonstrates a clear suppression of the GIRK currents by 10 nM PMA. Similar results were obtained with GIRK1/GIRK5 channels (G. Levin, D. Vorobiov, and N. Dascal, unpublished results). Thus, it appears that the inhibition of GIRK channels caused by the activation mGluR1a is mediated by a subtype of PKC.
Since the glutamate-induced activation in EGTA-treated oocytes expressing mGluR1a and GIRK1 is transient, even when the inhibition is abolished by staurosporine (see Fig. 5), we tested whether the inhibition of the basal GIRK current by glutamate is also transient. After reaching peak inhibition, all cells tested slowly began to show recovery of IhK. In the constant presence of glutamate, 10 min after peak inhibition, 68.7 ± 21.4% (n = 7, from 3 donors) of the inhibited current had recovered. After washout of glutamate and hK the holding current did not return to its baseline value, suggesting that at least some of the inward current that developed during recovery might be a chloride current or an enlarged leak current. If the total deflection of the holding current is considered to be a part of the apparent recovery (and is thus subtracted from the recovered current), a recovery of 49.4 ± 22.1% is exhibited 10 min after peak inhibition in the same cells. These data suggest that not only activation but also inhibition of GIRK by mGluR1 are transient. One possibility is that mGluR1a and mGluR5 are desensitized shortly after being activated. The inhibition, however, diminishes at a slower rate than the activation. This is in accord with the activation being mediated by a fast membrane- delimited pathway and the inhibition being mediated by a slow process that involves phosphorylation, while dephosphorylation is probably required for recovery.
Effects of High mGluR1a RNA Concentrations
When 100 to 200-fold more RNA was injected into oocytes (5 ng per oocyte, instead of 25–50 pg in the inhibition experiments), two interesting results were obtained. The first is that in EGTA-treated cells, application of glutamate in the hK solution caused a large activation of GIRK1/GIRK5 channels, around 1 μA in amplitude (data not shown). This activation was not a Cl− current, as witnessed by the inward-rectifying current-voltage relationship it exhibited. The agonist- induced current rapidly inactivated, leaving a steady current of around half the peak amplitude. This “inactivation” was much faster than the basal GIRK desensitization or the desensitization of mGluR2-mediated responses of comparable amplitudes and possibly reflected a PKC-dependent inhibition of the GIRK activity.
The second observation associated with high mGluR1a concentrations (in oocytes injected with GIRK1 and 5 ng mGluR1a RNAs) was a reduction in basal GIRK activity (IhK) without the addition of the agonist. This effect was donor-dependent: in two batches the average IhK was 41.8 ± 16.5% of the average IhK in cells expressing GIRK1 alone, without the receptor (Student's t test, P < 0.01; n = 6 cells expressing GIRK1 alone, n = 11 cells expressing mGluR1a and GIRK1). The third batch showed no significant difference between the groups (n = 3 cells expressing GIRK1 alone, n = 4 cells expressing mGluR1a and GIRK1). In the fourth batch Ba2+ was not applied in the hK solution, and therefore it was impossible to distinguish the basal GIRK1 activity from the endogenous inward-rectifying current (In). However, the total inward-rectifying current in hK (IhK + In) was itself markedly reduced in mGluR1a- expressing cells (n = 3): 32.2 ± 9.7% of the average total inward-rectifying current in hK in cells expressing GIRK1 alone (n = 5). The inhibition of basal channel activity at high levels of receptor expression could be due to enough receptor molecules being spontaneously active without agonist, thereby continually activating the inhibitory cascade at a significant level (cf. Prezeau et al., 1996).
discussion
Positive and Negative Coupling of mGluRs to GIRK
This study shows that mGluRs couple to the GIRK channel in Xenopus oocytes. The Gi/Go-coupled mGluRs activate the channel in a PTX-sensitive manner, while the Gq-coupled mGluRs inhibit the channel, an effect which appears to be mediated by protein kinase C–catalyzed phosphorylation.
The fact that mGluR2, mGluR3, mGluR4, mGluR6, and mGluR7 activate the channel is consistent with previous findings of the channel being activated by Gi-coupled receptors. These data confirm and extend similar findings of Saugstad et al. (1996) published while this manuscript was under revision toward resubmission. Coupling to GIRK1 may provide a simple and convenient functional assay for studying the Gi-coupled mGluRs in the oocyte.
A somewhat unexpected observation was the inhibition of basal and ACh-evoked (via a coexpressed m2 receptor) GIRK activity by the PLC-coupled mGluR1 or mGluR5. The inhibitory effect of mGluR1 was observed with GIRK channels of GIRK1/GIRK2 and (presumably) GIRK1/GIRK5 composition. We also found that two additional PLC-coupled receptors, the serotonergic 5HT2C and the muscarinic m1 receptors, inhibit GIRK (data not shown). Like the mGluR1a-evoked Ca2+-dependent Cl− current, the inhibition of GIRK by activation of mGluR1a showed little PTX sensitivity, suggesting that both phenomena are mediated primarily by a PTX-insensitive G protein, probably of the Gq family.
The fact that some brain regions are common to both GIRK1 and mGluR expression makes it possible that the modulation of GIRK by mGluRs is an in vivo occurring phenomenon. Prominent among these regions, in regard to levels of expression and variety of mGluRs expressed, are the cerebral cortex, hippocampus (both Ammon's horn and granule cells in the dentate gyrus) and olfactory system (Masu et al., 1991; Tanabe et al., 1992; Abe et al., 1992; Tanabe et al., 1993; Okamato et al., 1994; Romano et al., 1995; Neki et al., 1996). Coupling of mGluRs to GIRK may be a means by which glutamate modulates the excitability of its target cell and could thus be involved in plasticity- related phenomena.
PKC Mediates the Inhibitory Effect of mGluR1a
Cell-attached patch-clamp recordings in which glutamate applied outside the pipette caused an inhibition of GIRK activity suggest that a second messenger mediates the effect of mGluR1a. The delay between application of glutamate and the onset of inhibition was <1 min in the whole-cell recordings (Fig. 2), as compared with ∼3 min in cell-attached patches (Fig. 4), in which the second messenger presumably had to diffuse to the vicinity of the channel sealed within the pipette. The slowness of diffusion indicates that it may occur in the plane of the membrane and/or is carried by a molecule whose diffusion is restricted (e.g., diacylglycerol), because small, water-soluble second messenger molecules such as Ca2+, cAMP or inositol trisphosphate evoke membrane responses within seconds when injected into Xenopus oocytes (see Dascal, 1987).
Normally, activation of mGluR1a elicited a large Ca2+-activated Cl− current evoked by the IP3-mediated Ca2+ release. Injection of EGTA efficiently suppressed the transient Cl− current but did not impair the ability of mGluR1a to inhibit GIRK currents. Thus, it is unlikely that the inhibition of GIRK is mediated by Ca2+ or by a protein that depends solely on Ca2+ level increase for activation (such as calmodulin or Ca2+/calmodulin-dependent protein kinase).
Involvement of PKC is implicated in the inhibitory effect of PLC-coupled mGluRs by two lines of evidence: (a) This effect is suppressed by two potent blockers, staurosporine (a wide-specificity blocker) and BIS (a specific PKC blocker). The fact that calphostin C did not block the mGluR1a-induced inhibition of GIRK may be related to an experimental problem, but it may also indicate the participation of a specific PKC subtype (see below). (b) The inhibitory action was mimicked by PMA, a specific PKC activator. PKC-mediated inhibition of GIRK may be physiologically relevant. The PLC-coupled neuropeptide, substance P, has been shown to inhibit a G protein–dependent K+ channel in nucleus basalis neurons via a PTX-insensitive G protein, and the involvement of PKC has been proposed (Velimerovic et al., 1995). There is no evidence thus far whether the phosphorylated protein is one of the GIRK subunits or a different protein exerting an effect on the channel (e.g., Gβγ).
It is not clear which PKC subtype mediates the mGluR1a-induced inhibition, but candidates may be inferred from the available data. The PKC family is divided into three subfamilies (Newton, 1995; Nishizuka, 1995). The involvement of “classical” PKC subtypes (α, β, and γ) is unlikely, because they depend on Ca2+ in their activation (whereas mGluR1a-induced inhibition of GIRK is not impaired by Ca2+ chelation) and are sensitive to calphostin C. The “atypical” PKCs are not activated by PMA. This leaves one or more of the “new” PKC subtypes as viable candidates; notably, PKC-μ is insensitive to calphostin C (Johannes et al., 1995) and appears to be a plausible one. It remains to be clarified whether the mGluR1a inhibition is mediated by this PKC subtype because it is the most abundant PKC in the oocyte, or because it specifically affects the GIRK activation pathway. Interestingly, Shapiro et al. (1996) have reported recently that activation of a PKC subtype with an identical pharmacological profile disrupts the norepinephrine-induced, Gβγ-mediated (Herlitze et al., 1996; Ikeda, 1996) inhibition of a voltage-dependent Ca2+ channel in rat superior cervical ganglion neurons. It is tempting to speculate that, in both cases, the mechanisms of PKC action may be similar.
Activation and Inhibition of GIRK Are Mediated by Different G Proteins: The Problem of Specificity
The differential effects of PTX demonstrate that most of the activation of GIRK is mediated by a PTX-sensitive G protein; in contrast, a major part of the inhibitory effect of mGluR1a is mediated by a PTX-insensitive G protein. The coupling of mGluR1a to the inhibitory process was better than to the activation of GIRK. The extent of the inhibition of basal or acetylcholine-evoked GIRK activity was nearly identical (∼50% of peak current) with either 25, 30, or 50 pg mGluR1a RNA per oocyte; the large GIRK response in oocytes injected with 5 ng mGluR1a RNA also rapidly “inactivated” to ∼50% of total amplitude. This suggests that the inhibitory, Gq-mediated pathway was activated almost to saturation at the lowest levels of receptor expression employed in this work. In contrast, activation of GIRK by mGluR1a was far from maximal with 25–50 pg RNA (<30% of basal activity) and became comparable to the activation achieved by the Gi/Go-coupled mGluR2, i.e., several fold higher than the basal activity, only at higher levels of receptor expression. Activation of GIRK by mGluR1a at high expression levels (several tens of nanograms of receptor RNA were injected per oocyte) was also reported by Saugstad et al. (1996); this activation was also highly PTX-sensitive. These data indicate a specific coupling of mGluR1a to a PLC-coupled, PTX-insensitive G protein, most probably of the Gq class, as expected from its known properties. Coupling to Gi/Go proteins appears to be less efficient and occurs at high levels of expression of the receptor. Such promiscuous coupling to G proteins is well documented for many receptor types, but its physiological significance remains unclear (see Hedin et al., 1993; Gudermann et al, 1996). Thus, under physiological conditions, the main effect of activation of mGluR1a must be the inhibition of GIRK channels rather than their activation.
The inhibition of GIRK1 by Gq-coupled receptors bears upon the problem of specificity of G protein-effector coupling. Various combinations of Gβ and Gγ subtypes activate GIRK almost equipotently (Wickman et al., 1994; Krapivinsky et al., 1995b ), and it is unclear why βγ subunits released from G protein heterotrimers containing the PTX-insensitive Gαs or Gαq do not activate GIRK. One possible mechanism is co-localization of the relevant Gα-Gβγ heterotrimeric complexes with the effector, in our case GIRK (Simon et al., 1991; Huang et al., 1995; Neer et al., 1995). Another possibility is an antagonistic or synergistic interaction between Gα and Gβγ subunits, such as found for the various adenylyl cyclase subtypes (see Inigues-Lluhi et al., 1993; Neer, 1995; Conklin and Bourne, 1993). Indeed, we have found that two Gα subunits (Gαi1 and Gαs) inhibit GIRK in a membrane-delimited fashion, suggesting that antagonistic Gα–Gβγ interactions may play a role in determining the specificity of G protein activation of GIRK (Schreibmayer et al., 1996). Our present results indicate that, like Gαi1 and Gαs, proteins of the Gαq family counteract the activation of GIRK by Gβγ, in the latter case by activating PKC.
There is another open question related to the problem of specificity of interaction within the receptor–G protein–GIRK pathway: although both Gαq and Gβγ directly and independently activate PLC-β (Smrcka et al., 1991; Berstein et al., 1992; Camps et al., 1992; Katz et al., 1992), evidence has been presented that, in Xenopus oocytes, PLC is activated mainly by the Gβγ moiety of the Gαq-Gβγ heterotrimers (Stehno-Bittel et al., 1995). If this is so, the question arises as to why Gβγ released from other heterotrimers (e.g., Gi/Go) does not rapidly inhibit GIRK via the PLC pathway. This question may be, in fact, relevant to many cell types.
Acknowledgments
We are grateful to Dr. Shigetada Nakanishi for supplying the clones of mGluRs and for helpful discussions, to Gal Levin, Dr. Ilana Lotan, and Dr. Henry A. Lester for the critical reading of the manuscript, and to Michael Malca and Rachel Barzilay for excellent technical assistance.
This work was supported by the Human Frontiers Scientific Programme and by the USA-Israel Binational Science Foundation.
Footnotes
Abbreviations used in this paper: ACh, acetylcholine; mGluRs, metabotropic glutamate receptors; PTX, pertussis toxin.
references
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Bausch SB, Patterson TA, Ehrengruber M, Lester HA, Davidson N, Chavkin C. Colocalization of μ opioid receptors with GIRK1 potassium channels in the rat brain: an immunohistochemical study. Recept Chann. 1995;3:221–241. [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Berstein G, Blank JL, Smrcka AV, Higashijima T, Sternweis PC, Exton JH, Ross EM. Reconstitution of agonist-stimulated phosphatidylinositol 4,5 bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11and phospholipase C-β1. J Biol Chem. 1992;267:8081–8088. [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for βγ dimers as well as α subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature (Lond) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boton R, Dascal N, Gillo B, Lass Y. Two calcium-activated chloride conductances in Xenopus laevisoocytes permeabilized with the ionophore A23187. J Physiol (Lond) 1989;408:511–534. doi: 10.1113/jphysiol.1989.sp017473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser GE, Szabo G. Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by a guanine nucleotide anologue. Nature (Lond) 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschnik P. Isozyme-selective stimulation of phospholipase C-GbN2 by G protein βγ-subunits. Nature (Lond) 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu L. Differential regulation by cAMP-dependent protein kinase and protein kinase C of the μ opioid receptor coupling to a G protein-activated K+channel. J Biol Chem. 1994;269:7839–7842. [PubMed] [Google Scholar]
- Conklin BR, Bourne HR. Structural elements of Gα subunits that interact with Gβγ, receptors, and effectors. Cell. 1993;73:631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopusoocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22:317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Dascal N, Lim NF, Schreibmayer W, Wang W, Davidson N, Lester HA. Expression of an atrial G-protein-activated potassium channel in Xenopusoocytes. Proc Natl Acad Sci USA. 1993a;90:6596–6600. doi: 10.1073/pnas.90.14.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal, N., and I. Lotan. 1992. Expression of exogenous ion channels and neurotransmitter receptors in RNA-injected Xenopus oocytes. In Methods in Molecular Neurobiology, Vol. 13. Humana Press, Totowa, NJ. 205–225.
- Dascal N, Schreibmayer W, Lim NF, Chavkin C, DiMagno L, Labarca C, Keiffer BL, Gaveriaux-Ruff C, Trollinger D, Lester HA, Davidson N. Atrial G protein-activated K+channel: expression cloning and molecular properties. Proc Natl Acad Sci USA. 1993b;90:10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995;5:268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Guillemare E, Fink M, Hugnot J-P, Bigay J, Lazdunski M, Romey J, Barhanin J. Heterologous multimeric assembly is essential for K+channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun. 1995;2121:657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Zhang C, Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J Neurosci. 1995;15:3075–3083. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Bossu J-L, Bockaert J. Activation of a large-conductance Ca2+-dependent K+-channel by stimulation of glutamate phosphoinositide-coupled receptors in cultured cerebellar granule cells. Eur J Neurosci. 1991;3:778–789. doi: 10.1111/j.1460-9568.1991.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Fong HK, Hurley JB, Hopkins RS, Miake-Lye R, Johnson MS, Doolittle RF, Simon MI. Repetitive segmental structure of the transducin β subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci USA. 1986;83:2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Baetcher M, Aebersold R, Simon MI. A G protein γ subunit shares homology with ras proteins. Science (Wash DC) 1989;244:971–974. doi: 10.1126/science.2499046. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Joly C, Kuhn R, Knopfel T, Bockaert J, Pin JP. The second intracellular loop of metabotropic glutamate receptor 1 cooperates with the other intracellular domains to control coupling to G-proteins. J Biol Chem. 1996;271:2199–2205. doi: 10.1074/jbc.271.4.2199. [DOI] [PubMed] [Google Scholar]
- Gudermann T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G protein interaction. Annu Rev Pharmacol Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- Guerineau NC, Gahwiler BH, Gerber U. Reduction of resting K+current by metabotropic glutamate and muscarinic receptors in rat CA3 cells: mediation by G-proteins. J Physiol (Lond) 1994;474:27–33. doi: 10.1113/jphysiol.1994.sp019999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin KE, Duerson K, Clapham DE. Specificity of receptor-G protein interactions: searching for the structure behind the signal. Cell Signal. 1993;5:505–518. doi: 10.1016/0898-6568(93)90046-o. [DOI] [PubMed] [Google Scholar]
- Hedin KE, Lim NF, Clapham DE. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKAChcurrents in oocytes. Neuron. 1996;16:423–429. doi: 10.1016/s0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+channels by G-protein βγ subunits. Nature (Lond) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992a. Ionic channels of excitable membranes, 2d ed. Sinauer Associates, Inc., Sunderland, MA. pp. 607.
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992b;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Hollman M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O'Hara PJ. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science (Wash DC) 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Huang C-L, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature (Lond) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Lovinger DM, McCool BA, Lewis DL. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- Inabobe A, Morishige KI, Takahashi N, Ito H, Yamada M, Takumi T, Nishina H, Takahashi K, Kahano Y, Katada T, Kurachi Y. Gβγ directly binds to the carboxyl terminus of the G protein-gated muscarinic K+channel, GIRK1. Biochem Biophys Res Commun. 1995;212:1022–1028. doi: 10.1006/bbrc.1995.2072. [DOI] [PubMed] [Google Scholar]
- Inigues-Lluhi J, Kleuss C, Gilman AG. The importance of G-protein βγ subunits. Trends Cell Biol. 1993;3:230–236. doi: 10.1016/0962-8924(93)90122-h. [DOI] [PubMed] [Google Scholar]
- Ito H, Tung RT, Sugimoto T, Kobayashi I, Takahashi K, Katada T, Ui M, Kurachi Y. On the mechanism of G protein βγ subunit activation of the muscarinic K+channel in guinea pig atrial cell membrane. J Gen Physiol. 1992;99:961–983. doi: 10.1085/jgp.99.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F-J, Prestle J, Dietrich S, Oberhagemann P, Link G, Piezenmaier K. Characterization of activators and inhibitors of protein kinase Cμ. Eur J Biochem. 1995;227:303–307. doi: 10.1111/j.1432-1033.1995.tb20389.x. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK1-3 and GIRK1-4 inwardly rectifying K+channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C, Schreibmayer W, Dascal N, Lester HA, Davidson N, Karschin A. Distribution and localization of a G protein-coupled inwardly rectifying K+channel in the rat. FEBS Lett. 1994;348:139–144. doi: 10.1016/0014-5793(94)00590-7. [DOI] [PubMed] [Google Scholar]
- Katz A, Wu D, Simon MI. Subunits βγ of heterotrimeric G protein activate β2 isoform of phospholipase C. Nature (Lond) 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+channels are activated by Gβγ subunits and function as heteromultimers. Proc Natl Acad Sci USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Henry DJ, Chavkin C. Agonist-induced desensitization of the μ opioid receptor coupled potassium channel KGA. J Biol Chem. 1995;270:589–595. doi: 10.1074/jbc.270.2.589. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature (Lond) 1995a;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Wickman K, Clapham DE. Gβγ binds directly to the G protein-gated K+ channel, IKACh . J Biol Chem. 1995b;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Reuveni E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G protein-coupled muscarinic potassium channel. Nature (Lond) 1993;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kunkel MT, Peralta EG. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot J-P. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+channels in the brain. FEBS Lett. 1994;353:37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+channels. J Biol Chem. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lo CF, Breitwieser GE. Protein kinase-independent inhibition of muscarinic K+channels by staurosporine. Am J Physiol. 1994;266:C1128–C1132. doi: 10.1152/ajpcell.1994.266.4.C1128. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+channel in the heart. Nature (Lond) 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Luthi A, Gahwiler BH, Gerber U. A slowly inactivating potassium current in CA3 pyramidal cells of rat hippocampus in vitro. J Neurosci. 1996;16:586–594. doi: 10.1523/JNEUROSCI.16-02-00586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature (Lond) 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Miledi R, Parker I. Chloride current induced by injection of calcium into Xenopusoocytes. J Physiol (Lond) 1984;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signaling. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci Lett. 1996;202:197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;48:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- North AR. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K, Yatani A, Brown AM. The nature and origin of spontaneous noise in G protein-gated ion channels. J Gen Physiol. 1991;97:1279–1293. doi: 10.1085/jgp.97.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- Pfaffinger PG, Martin JM, Hunter DD, Nathanson N M, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature (Lond) 1985;317:538–540. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Ponce A, Bueno E, Kentros C, Demiera EVS, Chow A, Hillman D, Chen S, Zhu LX, Wu MB, Rudy B, Thornhill WB. G-protein-gated inward rectifier K+channel proteins GIRK1 are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J Neurosci. 1996;16:1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezeau L, Gomeza J, Ahern S, Mary S, Galvez T, Bockaert J, Pin JP. Changes in the carboxyl-terminal domain of metabotropic glutamate receptor 1 by alternative splicing generate receptors with differing agonist-independent activity. Mol Pharmacol. 1996;49:422–429. [PubMed] [Google Scholar]
- Reuveny E, Slesinger P, Inglese J, Morales JM, Inigues-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature (Lond) 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, Omalley K, Vandenpol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Kinzie JM, Mulvihill ER, Segerson TP, Westbrook GL. Cloning and expression of a new member of the L-2-amino-4-phosphonobutyric acid-class of metabotropic glutamate receptors. Mol Pharmacol. 1994;45:367–372. [PubMed] [Google Scholar]
- Saugstad JA, Segerson TP, Westbrook GL. Metabotropic glutamate receptors activate G-protein-coupled inwardly rectifying potassium channels in Xenopus oocytes. J Neurosci. 1996;16:5979–5985. doi: 10.1523/JNEUROSCI.16-19-05979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer W, Lester HA, Dascal N. Voltage clamp of Xenopus laevisoocytes utilizing agarose cushion electrodes. Pflüg Arch. 1994;426:453–458. doi: 10.1007/BF00388310. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N. Inhibition of an inwardly rectifying K+channel by G protein α subunits. Nature (Lond) 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. L-glutamate conditionally modulates the K+current of Muller glial cells. Neuron. 1993;10:1141–1149. doi: 10.1016/0896-6273(93)90062-v. [DOI] [PubMed] [Google Scholar]
- Shapiro MS, Zhou JY, Hille B. Selective disruption by protein kinases of G-protein-mediated Ca2+channel modulation. J Neurophysiol. 1996;76:311–320. doi: 10.1152/jn.1996.76.1.311. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science (Wash DC) 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Singer-Lahat D, Lotan I, Biel M, Flockerzi V, Hofmann F, Dascal N. Cardiac calcium channels expressed in Xenopusoocytes are modulated by dephosphorylation but not by cAMP-dependent phosphorylation. Recept Chann. 1994;2:215–226. [PubMed] [Google Scholar]
- Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Regulation of phosphoinositide-specific phospholipase C activity by purified Gq . Science (Wash DC) 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L, Krapivinsky G, Krapivinsky L, Perez-Terzic C, Clapham DE. The G protein βγ subunit transduces the muscarinic receptor signal for Ca2+ release in Xenopusoocytes. J Biol Chem. 1995;270:30068–30074. doi: 10.1074/jbc.270.50.30068. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992;8:169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velimerovic BM, Koyano K, Nakajima S, Nakajima Y. Opposing mechanisms of regulation of a G-protein-coupled inward rectifier K+channel in rat brain neurons. Proc Natl Acad Sci USA. 1995;92:1590–1594. doi: 10.1073/pnas.92.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Wickman KD, Iniguez-Lluhi JA, Davenport PD, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature (Lond) 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]