Abstract
It is widely believed that Ba2+ currents carried through L-type Ca2+ channels inactivate by a voltage- dependent mechanism similar to that described for other voltage-dependent channels. Studying ionic and gating currents of rabbit cardiac Ca2+ channels expressed in different subunit combinations in tsA201 cells, we found a phase of Ba2+ current decay with characteristics of ion-dependent inactivation. Upon a long duration (20 s) depolarizing pulse, IBa decayed as the sum of two exponentials. The slow phase (τ ≈ 6 s, 21°C) was parallel to a reduction of gating charge mobile at positive voltages, which was determined in the same cells. The fast phase of current decay (τ ≈ 600 ms), involving about 50% of total decay, was not accompanied by decrease of gating currents. Its amplitude depended on voltage with a characteristic U-shape, reflecting reduction of inactivation at positive voltages. When Na+ was used as the charge carrier, decay of ionic current followed a single exponential, of rate similar to that of the slow decay of Ba2+ current. The reduction of Ba2+ current during a depolarizing pulse was not due to changes in the concentration gradients driving ion movement, because Ba2+ entry during the pulse did not change the reversal potential for Ba2+. A simple model of Ca2+-dependent inactivation (Shirokov, R., R. Levis, N. Shirokova, and E. Ríos. 1993. J. Gen. Physiol. 102:1005–1030) robustly accounts for fast Ba2+ current decay assuming the affinity of the inactivation site on the α1 subunit to be 100 times lower for Ba2+ than Ca2+.
Keywords: gating current, signal transduction, cardiac muscle, heterologous expression
introduction
Brief openings of L-type Ca2+ channels cause the increases in intracellular Ca2+ concentration required to effect rapid metabolic switching in a variety of cellular processes (Tsien and Tsien, 1990). The Ca2+ signalling process is controlled and limited by multi-layered inactivation mechanisms, that affect the plasmalemmal as well as the intracellular release Ca2+ channels. Channel inactivation sharpens the kinetics and temporal precision of the signals, and prevents longer term increases in [Ca2+]i, which, among other problems, lead to stimulation of intracellular proteolysis (Turner et al., 1988).
In L-type and other Ca2+ channels (Tareilus et al., 1994; Cox and Dunlap, 1994), inactivation is produced by two mechanisms. One is mediated by the calcium ions that carry the current (Brehm and Eckert, 1978; Eckert and Chad, 1984), and it appears to involve binding to a site highly specific for Ca2+. In L-type channels the site is located near the channel mouth (Imredy and Yue, 1992, 1994; Shirokov et al., 1993), on the main (α1) channel protein (Neely et al., 1994), and has been equated with an EF hand-like portion of the cytoplasmic COOH terminus (De León et al., 1995).
The other mechanism of inactivation does not require the passage of current (Fox, 1981). It is linked to the change in transmembrane potential, and is accompanied by changes in gating currents, which are broadly termed inactivation of charge (Shirokov et al., 1992). The two mechanisms function independently, as demonstrated by the fact that changes in [Ca2+]i (Hadley and Lederer, 1991) and Ca2+ current through the channels (Shirokov et al., 1993) do not affect gating currents.
Parallel recording of gating currents and ion currents helped elucidate voltage-dependent aspects of Ca2+ channel gating (Field et al., 1988; Bean and Ríos, 1989; Hadley and Lederer, 1989) but is complicated in native cells by the presence of substantial Na+ channel gating current. The present work overcomes this problem using tsA201 cells, an expression system virtually free of endogenous channels. With Ba2+ as the permeant ion, we compared inactivation of gating charge with decay of ion currents, under the prevailing hypothesis that inactivation of L-type channel current is exclusively voltage-dependent in the absence of Ca2+ (e.g., Kass and Sanguinetti, 1984). The results disproved this hypothesis, favoring instead a dual mechanism of inactivation.
methods
Preparation of Transfected and Native Cells
Experiments were performed in the large T antigen–transformed human embryonic kidney cells (tsA201 line) grown in DME medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% FBS (BioWhittaker, Walkersville, MD) and 1% penicillin/ streptomycin (Sigma) in 5% CO2. Cardiac rabbit α1C, rat brain β2a, and rabbit skeletal muscle α2δ cDNAs were cloned in pCR3, pCMV, and pMT2 plasmid vectors respectively. High purity (A260/A280 ≥ 1.95) large-scale plasmid preparations were obtained using standard protocols (Qiagen Inc., Chadworth, CA). Transfections were done with 30 μg of each expression plasmid in different combinations (α1C, α1C + β2a, and α1C + β2a + α2δ) using a modified calcium phosphate precipitation method (Chien et al., 1995) in 100-mm tissue culture dishes. Cells were incubated with DNA for 6–8 h and then were shocked with 10% DMSO for 8 min. After the shock, cells were transferred onto glass coverslips, fed with fresh media and incubated until further evaluation. Electrophysiological recordings were made within 24–48 h post-transfection on round nonclustered cells. No sizable ionic or gating currents were observed in tsA201 cells in the absence of transfection. After transfection, the fraction of cells selected and patched that had Ca2+ currents was 60–80%. Recording of native Ca2+ currents was carried out in single rabbit cardiac ventricular myocytes, which were obtained by an enzymatic dissociation method (modified from Mitra and Morad, 1985) from young (1,500 g) male mutt rabbits.
Electrophysiological Recording
Cells were placed in small chambers (<200 μl) and perfused by continuous flow of external solutions. Records were obtained by a standard whole-cell patch clamp procedure using an Axopatch 200A amplifier and a 16-bit A/D-D/A converter card (HSDAS 16; Analogic Corp., Peabody, MA) under a 486 PC computer. Patch electrodes had resistances of 1.5–4 MΩ. Whole-cell capacitance, calculated as the area under a linear capacitive transient elicited by a −10 mV pulse, was 6–40 pF. Capacitance and resistance compensations were routinely done, and the charging time constant was typically 100 μs. Ionic currents were sampled at 0.15–1 kHz and gating currents at 10–40 kHz, depending on pulse duration.
All the currents represented are asymmetric, obtained after subtraction of scaled control currents, and not corrected for baselines. The control currents were elicited by pulses from −90 to −110 mV before each depolarization and after holding the cell at −90 mV for 30 s. All experiments were carried out at room temperature (∼20°C).
Exponential Fitting and Statistics
Data are presented as averages ± SEM. Signficance of differences between mean values was evaluated by Student's t test. Time course of current decay was fitted (using a nonlinear least-squares routine) by the function Y1 = b + a exp (−t/τ) or the function Y2 = b + a slow exp (−t/τslow) + a fast exp (−t/τfast). A fast, A slow, and B are the best fit amplitude parameters, a fast, a slow, and b, normalized to their sum. A and B symbolize the normalized amplitudes in the single exponential fit. In all cases in which both fits converged, the statistical significance of the fit improvement provided by the two- exponential function Y2 was evaluated. The test was based on the likelihood ratio statistic, LRS = (ssr 1 − ssr 2)/σ2 (Hoel, 1971; Hui and Chandler, 1990), in which ssr 1 and ssr 2 are the sums of squares of the residuals for the two fits and σ2 is the variance of the record. LRS has a χ2 distribution with 2 degrees of freedom (the difference between the number of free parameters of Y2 and Y1). If the statistic exceeds 6.0, the improvement in fit provided by the two-exponential function is significant to better than 5%. σ2 was estimated by ssr 2/(N − 5), which, being probably in excess, gave an underestimate of LRS.
Solutions
The internal solution contained (in mM): 150 CsOH (or NMG), 110 glutamate, 20 HCl, 10 HEPES, 5 MgATP, and 10 EGTA (pH adjusted to 7.6 by addition of CsOH). Osmolality of the internal solution was routinely measured and adjusted to 300–320 mosmole/kg.
External recording solutions contained (in mM): 160 TEA-Cl, 10 Tris, and either 10 CaCl2 or 10 BaCl2. Sodium external solution contained (in mM): 150 NaCl, 10 HEPES. In some experiments about 0.5 μM CaCl2 was added to this solution to stabilize the patch seal. In gating current experiments, 10–20 μM GdCl3 was added to the external solution with 10 mM Ca2+. All external solutions were adjusted to pH 7.2 and 300–320 mosmole/kg.
results
Inactivation of Ba2+ Currents Had Two Distinct Kinetic Phases
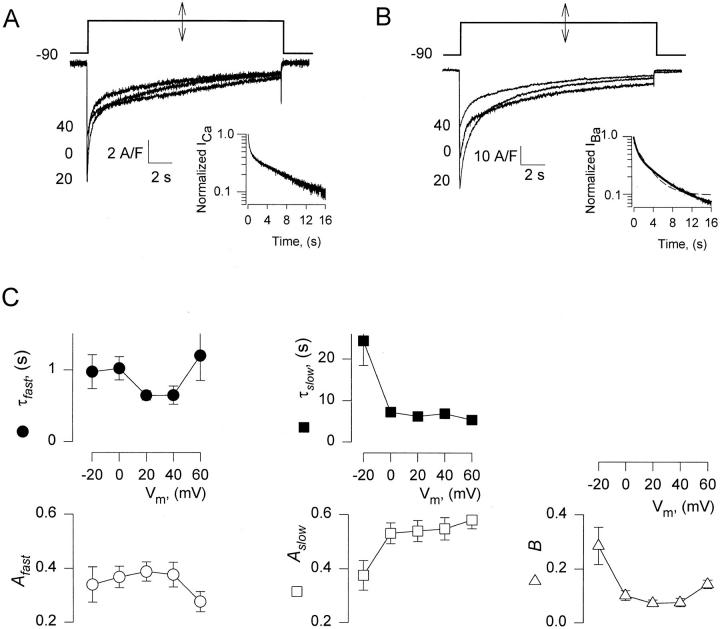
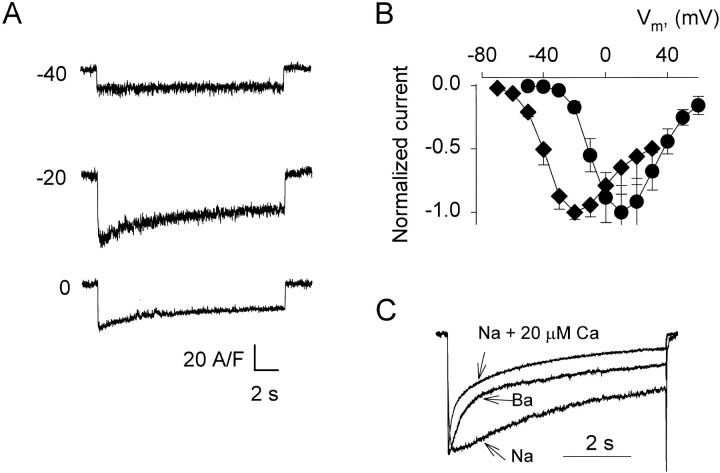
The lack of substantial endogenous voltage-dependent ion currents in tsA201 cells allowed us to study the time course of decay of Ba2+ currents (IBa) during very long pulses (up to 40 s). Ca2+ and Ba2+ currents in a cell transfected with α1C and β2a cDNAs are illustrated in Fig. 1, A and B. As shown in panel B, and as described often for ICa, the time course of IBa decay was well fitted by a sum of two exponentials plus a constant (Y2 in methods). The record and the best fit line are shown to overlap in the inset, where they are plotted semilogarithmically. The single exponential fit (dashed line) was poor.
Figure 1.
Kinetics of Ca2+ and Ba2+ currents. (A) Ca2+ currents elicited by depolarizing pulses of 16.5-s duration, from a holding potential of −90 mV to voltages indicated near the traces in mV. (inset) The current at +20 mV, normalized to peak value and plotted in semilog scale. (B) Ba2+ currents elicited by 16.5-s pulses. At membrane voltages from −20 to +60 mV IBa had a bi-exponential time course of decay. (inset) Semilog plot of IBa at +20 mV, normalized to its peak value. A sum of two exponentials fitted to IBa(t) is represented by a continuous line superimposed to the data. The dashed line represents the best fit single exponential. The LRS (defined in methods) was 354.3, indicating significant improvement by the two-exponential fit. (C) Parameters, defined in methods, of the two-exponential fit to IBa(t), (n = 19).
Families of records from 33 cells were fitted with one and two-exponential functions. At voltages positive to +20 mV, 12 cells were discarded because they had a small outward steady current. Two other cells, which had unusually slow activation kinetics, were discarded because the two-exponential fit was not significantly better than the single exponential fit, as judged by the LRS. The averages of parameters obtained from the remaining 19 cells are in Fig. 1 C. On average, the time constant of the fast component of IBa decay (τfast) was minimal at +20 to +40 mV and increased (at a P < 0.08) at +60 mV. The amplitudes of the two exponentials (A slow and A fast) were comparable. A fast went through a maximum at +20 mV, decaying at higher voltages. The slow component became faster and its amplitude greater at increasing depolarization. B decreased from 0.3 at −20 mV to 0.05 at +20 mV and increased to 0.15 at higher voltages. Because in the selected cells there was little if any outward current, even after 40 s pulses to +40 mV, and because neither current rundown nor substitution of NMG+ for intracellular Cs+ affected the decay parameters, these appear to represent kinetics of the expressed channels. The crisp separation of the two decay phases and the observed differences in the dependencies of their parameters on voltage suggest the presence of two independent mechanisms of inactivation.
Only the Slow Phase of IBa Decay Was Accompanied by Reduction of Gating Charge
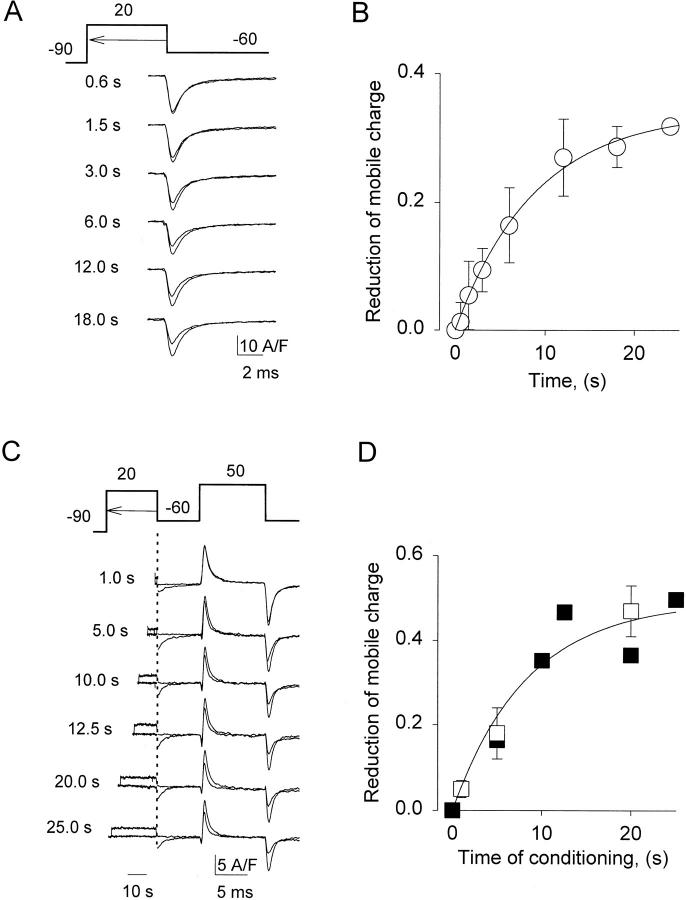
Using ionic current-blocking solutions we studied the effects of prolonged depolarization on the availability of gating currents associated with channel opening. The asymmetric (ON) charge transferred after the depolarizing transition of a 0.1 s pulse was equal to the (OFF) charge transferred upon repolarization. As expected from studies in native cells, prolonged depolarization reduced gating currents. This was demonstrated with one- and two-pulse protocols, to rule out errors due to ionic currents or fast recovery from inactivation of gating charge. Fig. 2 A shows the OFF transients obtained upon repolarization from +20 mV, after periods of depolarization indicated near the traces. The larger transient in each pair is the reference OFF after a 45-ms pulse (in every case preceding the prolonged depolarization). Surprisingly, reduction of charge transfer took much more time than decay of IBa. Fig. 2 B plots reduction of the OFF charge vs. pulse duration, averaged in nine cells. The curve is a single exponential fit (τ ≈ 8 s).
Figure 2.
Reduction of mobile charge takes seconds. (A) OFF gating current transients after increasing times at +20 mV. The records are asymmetric currents obtained as described in methods. Durations of the test depolarizations that preceded the transients are listed near each plot. Test OFF transients are shown superimposed with larger reference transients, obtained at the trailing edge of 0.045-s pulses applied before each test depolarization. (B) Difference between reference and test OFF charge transfer, normalized to reference, vs. duration of depolarization at +20 mV (n = 9). The continuous line represents ΔQ max(1 − exp(−t/τ)), with ΔQ max = 0.34 and τ = 8.7 s. (C) Gating currents elicited by a fixed test pulse after conditioning depolarizations of different durations (listed near each record). At left in the panel are the asymmetric currents during the conditioning depolarization, recorded at a slower sampling rate (up to the time indicated by the dotted line). Records obtained with and without conditioning pulse are shown superimposed. (D) Difference in ON charge transfer, reference minus conditioned, normalized to reference, vs. duration of the conditioning depolarization (open symbols, averages; n = 4; filled symbols, data from records in C). Continuous line generated as in B, with ΔQ max = 0.49 and τ = 8.5 s.
The reduction of the available charge after depolarization was also monitored by a double pulse protocol illustrated at top of Fig. 2 C. The membrane was conditioned at +20 mV, then it was repolarized to −60 mV for 7 ms and a test pulse to +50 mV was applied to move the charge available at voltages of channel opening. During conditioning depolarization currents were recorded at a low acquisition rate, which was increased at the repolarization swing. As in Fig. 2 A, the larger transient in each pair is the reference obtained before the corresponding conditioning pulse. The reduction in available charge is plotted vs. conditioning duration in 2 D. The reduction of gating charge at positive voltages had the same time course as the reduction of the OFF charge in the single pulse experiment.
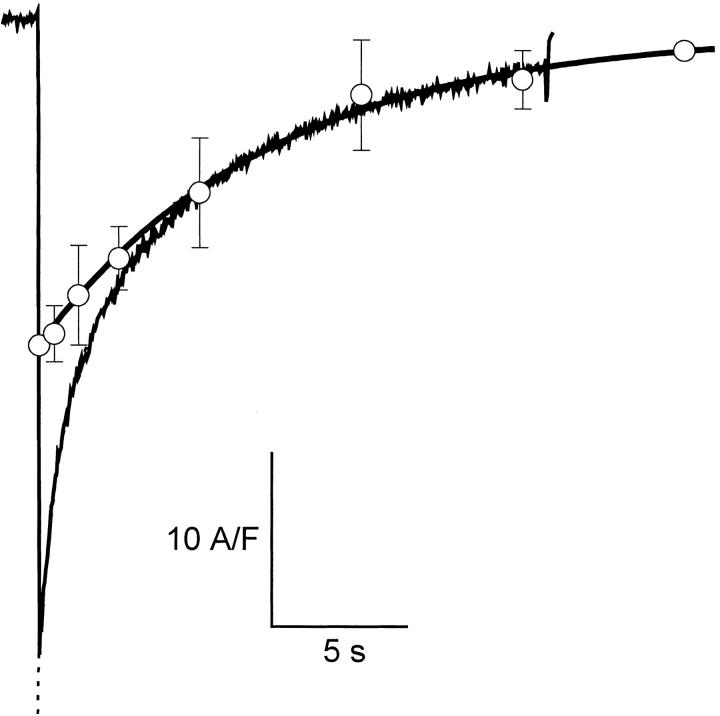
Fig. 3 compares the time course of IBa decay and that of gating charge reduction. In the figure we superimposed data and fit of charge availability (from Fig. 2 B) on a record of IBa elicited by a 16.5 s depolarization. The exponential decay of charge availability (solid curve) has a time constant similar to that of the slow decay of IBa, suggesting that these are due to a slow inactivation mechanism, distinct and voltage-dependent. On the other hand, the figure clearly shows that about half of the decay of IBa occurred much more rapidly than the process causing charge reduction. Indeed, as shown in Fig. 2 A, ON and OFF charges remained equal in single pulses of up to 1 s, a time at which ionic current had inactivated by ∼40%.
Figure 3.
Slow inactivation of IBa and reduction of mobile charge had the same kinetics. Ba2+ current evoked by a 16.5-s pulse to +20 mV from a holding potential of −90 mV. Shown superimposed are the average values and fit (thick line) of charge reduction at +20 mV (same as in Fig. 2 B). Parameters of the two- exponential fit to the Ba2+ current (dotted line): A fast = 0.38, τfast = 0.75 s, A slow = 0.57, τslow = 6.68 s, B = 0.05.
Fast Decay of IBa Is a Current-dependent Process
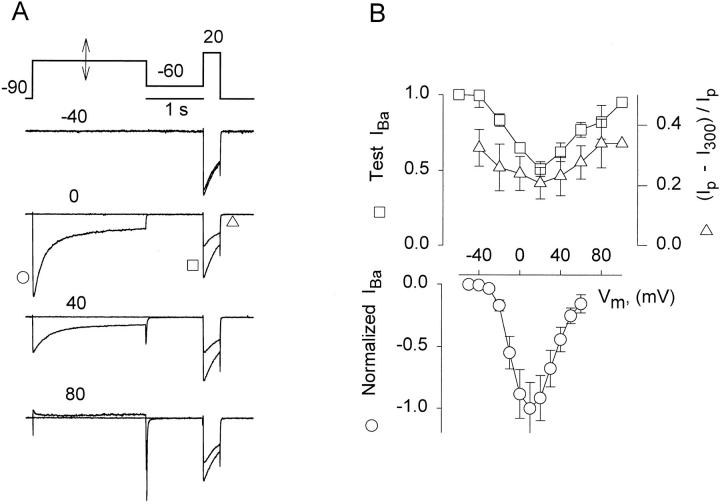
Fast inactivation of IBa was studied with brief (0.3 s) test pulses to +20 mV, following a 2 s conditioning pulse to different voltages (which should cause fast inactivation almost exclusively) and a 1 s interpulse to −60 mV (to ensure the return of all intramembrane charge). Each pair of pulses was preceded by a single test pulse for reference, and the sets were applied at 1-min intervals. Reference and conditioned currents are shown in pairs in Fig. 4 A, normalized to correct for rundown. A conditioning pulse to 0 mV (eliciting substantial IBa) reduced the magnitude and the rate of decay of the following test current. Conditioning at +80 mV evoked no inward current and had less effect on the test current. Averages in seven cells are plotted vs. conditioning voltage in Fig. 4 B. Circles represent peak conditioning current relative to the maximal value at +10 mV and squares represent peak test current (measuring channel availability after conditioning). IBa appeared at −30 mV, peaked at +10 mV, and decayed at higher voltages. The availability had a U-shaped voltage dependence that mimicked the voltage dependence of the conditioning current, as befits a current- (rather than voltage-) dependent inactivation process (Tillotson, 1979). This inactivation was not due to Ca2+ contamination, as [Ca2+] was measured in the external solutions with a Ca2+ electrode and found to be negligible (<5 μM).
Figure 4.
Availability of Ba2+ current after a brief conditioning pulse. (A) IBa elicited by a fixed test pulse after 2-s long conditioning pulses to different voltages (indicated in mV). Conditioned and reference current traces are shown superimposed. Within each pair, the currents are scaled to the peak of the reference current. Note symbols indicating measurements plotted in B. (B) Squares: peak of test IBa vs. conditioning voltage, (n = 7). Triangles : extent of inactivation of IBa during the test pulse, measured as (Ipeak − I (300 ms))/Ipeak, (n = 7). Circles : peak Ba2+ current during a pulse to the voltage in the abscissa, normalized in each cell to the maximum current (n = 19).
A U-shaped voltage dependence of the current availability could also be a manifestation of potentiation of channel activity by large positive pulses, as shown in snail neurons (Heyer and Lux, 1976), chromaffin cells (Hoshi et al., 1984), skeletal muscle (Sculptoreanu et al., 1993) and cardiac cells (Lee, 1987). However, the extent of current decay during the test pulses (triangles in Fig. 4 B) was minimal after conditioning at +10 mV and increased at more positive conditioning voltages. This implies that conditioning to a high voltage had a modest accelerating effect on the decay kinetics, which is against a major role of voltage-dependent potentiation, because in native cardiomyocytes potentiated currents had a much slower rate of decay (Lee, 1987). Also against voltage-dependent potentiation is the observation that the conditioned Ba2+ currents were never greater than reference currents, even after conditioning to +100 mV.
Fast Current Decay Was Absent when Na+ Was the Permeant Ion
Fig. 5 shows representative records of Na+ current (INa) through the α1C/β2a channels at submicromolar concentrations of extracellular divalent cations. The voltage dependence of INa (diamonds in Fig. 5 B) and its sensitivity to Ca2+ channel blockers were as described in native channels (Kostyuk et al., 1983; Hess et al., 1986), which proves that INa passed through the expressed L-type channels. In agreement with previous reports in isolated cardiac myocytes (Imoto et al., 1985; Hadley and Hume, 1987), INa activated at potentials about 30 mV more negative than IBa (circles). At all voltages INa decayed much more slowly than IBa. A scaled IBa trace plotted together with INa (Fig. 5 C) illustrates that the fast decay was absent when Ba2+ was removed. In fact, a fast component of decay was not resolvable even at very high densities of Na+ current (up to 54 A/F). Including Ca2+ in the external solution at a concentration of 20 μM led to the appearance of a phase of INa decay with kinetics similar to those of the fast inactivation of IBa (Fig. 5 C).
Figure 5.
The fast component of inactivation was absent when Na+ was the charge carrier. (A) Currents elicited in a Ca2+-free, 150 mM Na+ external solution by 16.5-s pulses to the voltages indicated in mV. (B) Diamonds: peak Na+ current, normalized to its maximum in each cell, vs. pulse voltage (n = 5). Circles : peak current in 10 mM Ba2+ (n = 19). Peak INa was 25.6 ± 4.3 A/F, whereas peak IBa was 36 ± 3.5 A/F. (C) Currents obtained in different cells, in either 150 mM Na+, 10 mM Ba2+, or 150 mM Na+ plus 20 μM Ca2+, at the voltages of maximal currents. Records were scaled to the same amplitude. Parameters of a single exponential fit to the Na+ current shown: A = 0.62, τ = 8.72 s, B = 0.38; two-exponential fits to Na+ + 20 μM Ca2+: A fast = 0.43; τfast = 0.35 s, A slow = 0.47, τslow = 5.84 s, B = 0.10; to Ba2+ current: A fast = 0.46; τfast = 0.93 s, A slow = 0.42, τslow = 12.63 s, B = 0.12. Note that the parameter values of the Na+ + 20 μM Ca2+ fit are not significantly different from the average values for IBa in α1/β cells (Table I).
Absence of Bulk Accumulation Effects
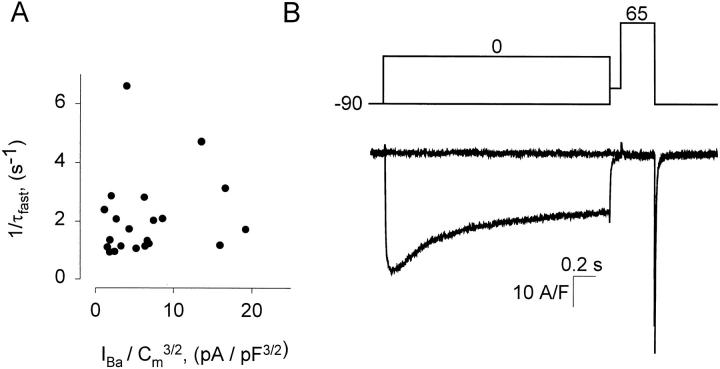
It is believed that the level of Ca2+ accumulation produced by typical membrane Ca2+ currents cannot significantly change the driving force that determines Ca2+ flux in a cell buffered with several mM of EGTA (Neher, 1986). The buffering capacity of such intracellular solutions will be lower for Ba2+ (because the affinity of EGTA for Ba2+ is about 100 times less than for Ca2+; Harafuji and Ogawa, 1980). It is therefore conceivable that the current dependent reduction of IBa is due to a reduction of driving force on the ion upon accumulation of Ba2+ inside the cell. A simple single pool model of accumulation of Ba2+ in the volume of the cell, in which unidirectional Ba2+ fluxes are assumed to be independent, predicts first order decay of current, with rate constant proportional to initial current amplitude and to the reciprocal of cell volume. The volume of round cells (a good approximation for transfected tsA201 cells) is proportional to Cm 3/2, where Cm is membrane capacitance. Fig. 6 A plots τfast −1 vs. (peak IBa/ Cm 3/2) at +20 mV for 21 cells, demonstrating no correlation between these variables.
Figure 6.
Two tests of Ba2+ accumulation. (A) Rate constant of fast decay of Ba2+ currents elicited at different voltages in different cells vs. the ratio (peak IBa/Cm 3/2). The correlation coefficient was r = 0.022. (B) IBa elicited by a test pulse to the reversal potential (+65 mV), following a 2-s conditioning pulse that elicited maximal IBa. The conditioned current is shown superimposed to the reference current, elicited without conditioning. Conditioned and reference currents were both nil during the test pulse.
The above test does not rule out depletion of Ba2+ ions outside the cell, or their accumulation in restricted spaces close to the conduction pathway. To test these possibilities we sought to demonstrate changes in the driving force for IBa, in experiments similar to those described in a study of Na+ accumulation (Hadley and Hume, 1987) and illustrated in Fig. 6 B. IBa was elicited with a double pulse protocol similar to that in Fig. 4. The test pulse took the membrane to the reversal potential and elicited no net ionic current. If a large current preceding the test determined substantial accumulation and a change in driving force for Ba2+, the conditioned pulse should elicit an outward current. Conditioning at 0 mV elicited near maximal IBa but did not result in the appearance of outward current during the test pulse. The decay in current can therefore be safely attributed to channel gating (inactivation) rather than a change in driving force.
Fast Decay Was Not due to Acidification
Ca2+ channel activity decreases at low intracellular pH (Kaibara and Kameyama, 1988) and EGTA is deprotonated when it chelates metal cations, with consequent acidification (Jong et al., 1993). Therefore, one possible mechanism of fast inactivation was channel closure due to acidification. Against this possibility we found that the fast phase of decay was present even when intracellular pH was buffered with 100 mM HEPES (data not shown). We also compared rates of IBa decay in cells dialyzed with 0.5, 1, 10, or 20 mM of EGTA. The rapidly decaying component was present in all and its kinetics changed only slightly (data not shown). These observations again rule out accumulation, as well as other possible mechanisms involving EGTA in the development of fast current decay.
Fast Inactivation of IBa Is a Property of the α1 Subunit
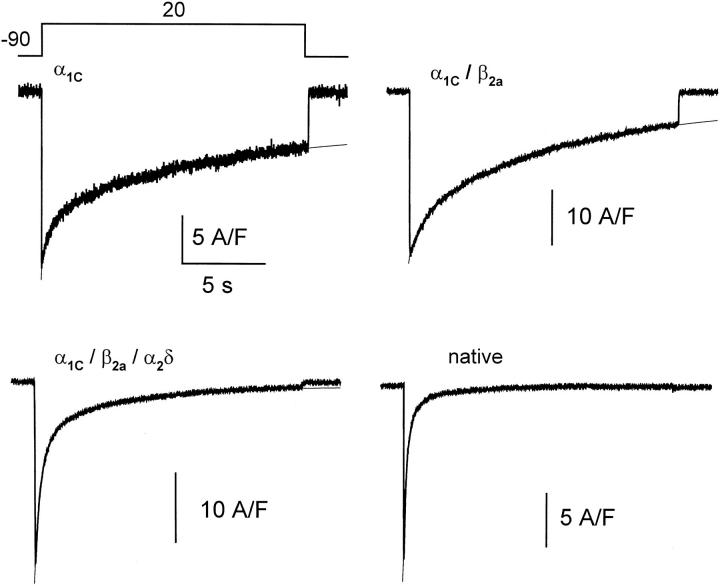
To test whether fast inactivation depended on certain auxiliary subunits or other properties of the expression system, experiments were carried out with different combinations of subunits α1C, β2a, and α2δ, and with native rabbit ventricular myocytes. As illustrated in Fig. 7, both phases of IBa decay were present in all cases, including cells transfected with α1 alone. The parameters of two-exponential fits and their averages are listed in Table I.
Figure 7.
Kinetics of IBa with different combinations of subunits and in ventricular myocytes. Ba2+ currents elicited by 16.5-s pulses to +20 mV, in tsA201 cells transfected with cDNA combinations listed in each panel, and in a rabbit ventricular myocyte (native).
Table I.
| Composition | Number of cells | A fast | A slow | B | τfast, (s) | τslow, (s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1C | 6 | 0.35 ± 0.05 | 0.45 ± 0.03 | 0.20 ± 0.03 | 0.57 ± 0.06 | 6.88 ± 0.66 | ||||||
| α1C/β2a | 19 | 0.54 ± 0.04 | 0.39 ± 0.04 | 0.07 ± 0.02 | 0.65 ± 0.07 | 6.21 ± 0.81 | ||||||
| α1C/β2a/α2δ | 16 | 0.58 ± 0.04 | 0.37 ± 0.03 | 0.05 ± 0.03 | 0.58 ± 0.06 | 5.15 ± 0.55 | ||||||
| native | 3 | 0.67 ± 0.06 | 0.28 ± 0.04 | 0.04 ± 0.01 | 0.21 ± 0.01 | 1.78 ± 0.18 |
Two-exponential fits to IBa decay in tsA201 cells transfected with different combinations of L-type Ca2+ channel subunits, and in rabbit ventricular myocytes. IBa was elicited by 16.5-s long pulse from the holding potential of −90 mV to +20 mV. The fit function and parameters are described in methods.
Although cells co-transfected with α1C + β2a had much higher current density than cells transfected with α1C alone, the rates of inactivation were similar in both groups, consistent with the previous observation that β2a, is the least potent among other cloned β subunits in acceleration of inactivation (De Waard and Campbell, 1995). A fast increased significantly with the number of subunits, being maximal for the native channels. The kinetics of fast inactivation did not depend on the composition of the expressed channels, being in all cases about threefold slower than in native channels. The rate of the slow component increased with the number of subunits. This change was marginally significant between α1C and α1C/β2a/α2δ cells (at a level P < 0.09) and not significant between α1C/β2a and α1C/β2a/ α2δ cells. (The histogram of slow time constants of the α1C/β2a/α2δ cells was bimodal, with approximately one quarter of the α1C/β2a/α2δ cells centered at about the same modal value as the α1C/β2a group. It is possible that a group of α1C/β2a/α2δ cells expressed little of the α2δ subunit or that the subunit did not incorporate stoi-chiometrically to the complex [Gurnett et al., 1996]. Therefore, the α2δ subunit may have had a greater effect than that reflected by our averaged measurements.)
The presence of both decay phases with every combination of subunits indicates that both inactivation mechanisms are determined by the α1C subunit. That the relative amplitudes and kinetics of the two phases varied with the subunit composition is consistent with a modulatory role of the auxiliary subunits (Catterall, 1995).
discussion
The present study was designed to compare inactivation of ionic and gating currents of L-type Ca2+ channels in the simplest possible situation. The tsA201 cells provided a system essentially devoid of other channels. Use of Ba2+ as current carrier was expected to eliminate the current-dependent inactivation mechanism. In spite of these conditions, inactivation of ionic current was found to follow two kinetic phases, of which only the slow one was co-temporal with the observed single exponential reduction of gating charge. The fast phase was current-dependent and did not correlate with the reduction of the gating charge. Because the two kinetic phases of inactivation of IBa were seen in cells transfected with the α1C subunit, alone, in combination with β2a or with β2a and α2δ, as well as in rabbit ventricular myocytes, they must be a property of the α1 subunit, depending on neither the auxiliary subunits nor the expression system. In this section we analyze the present findings, compare them with published observations and infer that the fast phase of decay is caused by Ba2+ binding to the channel's Ca2+-inactivation site.
The Population of Ca2+ Channels Was Homogeneous
Ba2+ currents through L-type Ca2+ channels have been observed to decay in two phases in many different preparations. In neurons, two components of decay were observed, but the significance of the observation was obscured by the existence of multiple types of high voltage-activated Ca2+ channels (Boland and Digledine, 1990; Kay, 1991). In cardiac myocytes, the presence of two components of inactivation of IBa was interpreted as due to two different processes of voltage-dependent inactivation, because inactivation of IBa was believed to be purely voltage dependent (Kass and Sanguinetti, 1984; Lee et al., 1985; Boyett et al., 1994). This view could not be tested rigorously because in cardiac myocytes it is difficult to correlate inactivation of IBa with inactivation of gating currents, due to the contribution of other channels to charge movement (Bean and Ríos, 1989; Shirokov et al., 1992). These problems may have been circumvented in the present work, provided that the Ca2+ channels expressed in a mammalian cell line after transfection were a single population, homogeneous in its biophysical properties. This seems to have been the case, for the three following reasons:
No sizable Ca2+ or Ba2+ currents were recorded in mock-transfected cells. In our experiments, amplitudes of endogenous currents (<0.5 A/F) were always one or two orders of magnitude smaller than those in transfected cells. Therefore, the bulk of the Ba2+ or Ca2+ current in transfected tsA201 cells was probably conducted through recombinant channels, and endogenous currents could not have been the reason for the multiplicity of inactivation phases.
In agreement with Kamp et al. (1996) and Josephson and Varadi (1996), we found a consistent correlation between the amount of intramembranous mobile charge and the ionic current density, suggesting that a major fraction of expressed channels function to carry current.
When rundown reduced ion current amplitude, the relative magnitudes of the two inactivation phases were conserved. Had the processes taken place in two different sets of channels, their proportions would not have been likely to remain constant during rundown.
Assuming that the population of channels was homogeneous, the question that then arises is if the kinetic phases of inactivation correspond to two different mechanisms.
Ba2+-dependent Inactivation Caused the Fast Decay of Current
The fast decay of current depended on the current through the channel, rather than the applied voltage. This was demonstrated by three observations:
In experiments with a double pulse protocol, current availability depended on the voltage of the conditioning pulse in a U-shaped manner, and inactivation reached a maximum at voltages that made inward current greatest, much as has been described for Ca2+-dependent inactivation (Eckert and Chad, 1984). In the present experiments the observation was free from many errors that may have obscured this property in previous studies with native cardiomyocytes. Although a U-shaped voltage dependence is expected in current-dependent processes, it could also arise from (voltage-dependent) potentiation, that offsets the inactivation effect at high voltages (Lee, 1987). Voltage-dependent potentiation cannot explain the present observations because current elicited by the test after a high voltage conditioning pulse was never greater than reference, and its kinetics remained the same. Our failure to observe voltage-dependent potentiation agrees with the report of Cens et al. (1996) that β2a failed to promote voltage-dependent potentiation, at variance with the effects of other types of β subunit.
Recovery of IBa from inactivation was slower after a conditioning to +20 mV than to +60 mV (data not shown). Much more Ba2+ enters the cell during a pulse to +20 than at +60 mV. The longer lasting inactivation induced at +20 mV may simply be a consequence of the persistence of inactivating concentrations of Ba2+ near the channels for a longer time (as argued by Kay, 1991, for Ca2+ currents in neurons).
There was no immobilization or voltage shift of gating current with kinetics corresponding to those of the fast decay of ionic current. In the current view, inactivation of Na+ and K+ channels acquires its voltage dependence indirectly, through a link to channel opening. In Na+ and K+ channels inactivation is more likely to happen when gating particles are in the activating position. In a microscopically reversible system, this implies that inactivation stabilizes the voltage sensor in its activating position, negatively shifting the transition voltage of gating currents and reducing the availability of charge mobile in the range of voltages that normally cause activation. This was not found in our case for the fast component of inactivation of IBa.
The observed decay of IBa could not have been due to bulk accumulation or cell loading by the permeating Ba2+, as the effect failed to correlate with the geometry of the cell (Fig. 6 A), and Ba2+ entry during long-lasting depolarization failed to change the reversal potential of the current (Fig. 6 B). This conclusion is also consistent with the finding (Cavalié et al., 1983) that the amplitude of single channel current remains the same during prolonged depolarization. The decay can therefore be attributed to an inactivation gating process, dependent on the local increase of [Ba2+], at levels that do not significantly change the driving force for IBa.
The evidence strongly favors a mechanism that requires binding of Ba2+ to a site on the channel molecule. Most importantly, the fast decay of Ca2+ channel current was absent when Na+ carried the current, even at high current densities. This fact is further evidence against an accumulation phenomenon, which would not be expected to work solely for Ba2+. Furthermore, because IBa in α1 cells exhibits the fast decay, the putative Ba2+ site has to be on the α1 polypeptide. That the fast decay was observed in currents carried by Ba2+, by Ca2+, and by Na+ in the presence of extracellular Ca2+, suggests that Ba2+ causes inactivation by binding to the Ca2+-inactivation site. That fast inactivation of Ba2+ current occurred without correlative changes in gating current is consistent with this hypothesis, considering that the voltage-dependent and the Ca2+-dependent mechanisms of inactivation do not interact (Hadley and Lederer, 1991; Shirokov et al., 1993).
Previous work has failed to clarify whether or not IBa inactivates by an ion-dependent mechanism. McDonald et al. (1986) did not find U-shaped voltage dependence for inactivation of Ba2+ currents in on-cell patches of guinea pig ventricular myocytes. On the same preparation, Imredy and Yue (1994) saw some reduction in the extent of decay of single channel IBa at voltages above +20 mV, and tentatively interpreted it as evidence of Ba2+-dependent inactivation. Mazzanti et al. (1991) found fast decay of Ba2+ current provided that there were multiple channels in a patch, and proposed a Ba2+-dependent mechanism of inactivation enhanced by channel clustering. The interpretation of single channel on-cell measurements is complicated by the possibility of inactivation by Ca2+ released from intracellular stores (Galli et al., 1994; Sham et al., 1995). Schneider et al. (1991) found that entry of Ba2+ into smooth muscle cells through ATP-activated channels reduced whole-cell L-type channel Ba2+ currents by about 20%. Perfusion of rabbit portal vein smooth muscle cells with 100 μM of Ba2+ in the patch pipette led to a 10% reduction of ICa (Ohya et al., 1987). The results are consistent with a finding of Brown et al. (1981) that perfusion of Helix aspersa neurons with 1 mM Ba2+ in the pipette reduced IBa by some 20%. In sum, the evidence with single channels is inconclusive, while in whole cells there are modest effects of perfusion with Ba2+. These results do not rule out inactivation by the high concentrations of Ba2+ that are likely to occur at the putative site, which is presumably located near the channel mouth (De León et al., 1995).
The main characteristics of Ca2+-dependent inactivation of ICa were previously reproduced with a simple three-gate model of the L-type channel (Shirokov et al., 1993). We now show that this model also accounts for the properties of IBa, assuming that Ba2+ substitutes for Ca2+ at the inactivation site. The model includes voltage-dependent properties, activation, and inactivation, which are independent of cation binding, and are represented in scheme I:kRI,RPkRP,RIRIRPAIAPkAP,AIkAI,APkAP,RPkRP,AP (scheme I)kAI,RIkRI,AI
Activation is the opening of a voltage-operated gate, whose states are resting (R) and activated (A). Voltage-dependent inactivation involves a second gate, with a permissive (P) and an inactivated state (I). The P → I transition is not intrinsically voltage-dependent, but is favored from channel state AP.1
Ion-dependent inactivation is pictured by a third gate, the states of which are represented in scheme II:
kC,CMekCMe,CC + Me2+C:Me2+U + Me2+U:Me2+kUMe,UkU,UMekUme, CMekCMe, UMe (scheme II)ku,ckc,u
The ion-controlled gate can be “covering” (C, channel closed) or “uncovering” (U). Me2+, normally Ca2+, inactivates the channel because its binding stabilizes state C, that is, the equilibrium between C:Me2+ and U:Me2+ is shifted toward C:Me2+. Accordingly, and to satisfy microscopic reversibility, the affinity of the site must be greater in state C than in state U. The channel state is described by the state of its three gates and is open only in state APU.2 The ion-dependent inactivation process does not affect the voltage-dependent processes in any way (implying that the evolution of states depicted in scheme I can be solved independently), while the states in scheme II are influenced by the voltage-dependent gates only because the concentration of the current-carrying ion changes when the channel opens. [Me2+] at the site was assumed to be an instantaneous function of the channel current i, defined as: [Me2+]bulk + i/ 4πFDr, where [Me2+]bulk is 50 nM, F is the faraday, the diffusion constant D is 10−6 cm2s−1, and the distance r from the channel mouth to the binding site is 1 nm.
In this model, dissociation constants (K d) for Ca2+ are supposed to be 1 μM in state C and 100 μM in state U. IBa was simulated assuming 100-fold higher K ds in both states (which was achieved by reducing the binding rate constants). The only other difference between the two ions was a two-times greater single channel current for Ba2+ at any given extracellular [Me2+].3
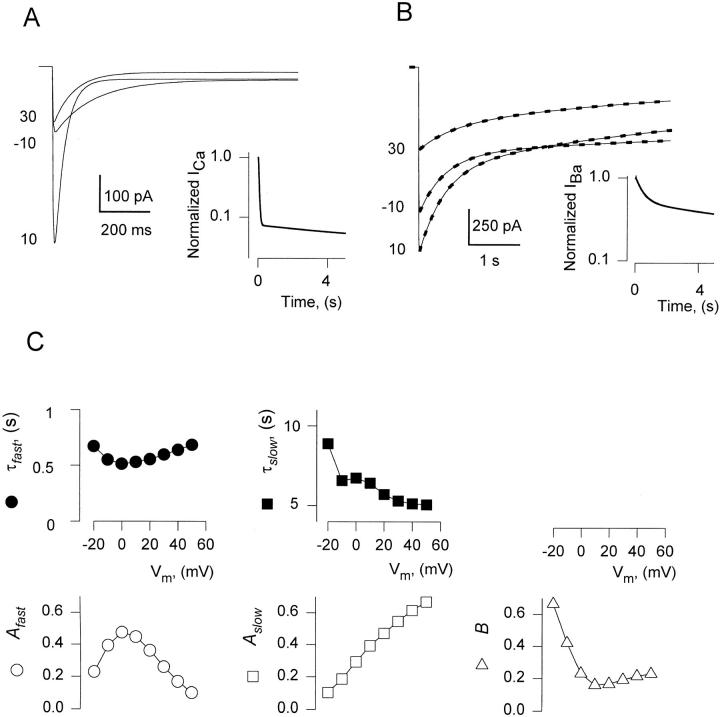
The simulations4 are illustrated in Fig. 8, where current records, exponential fits, and fit parameters are shown in a manner similar to Fig. 1. With this choice of parameters the model reproduced well the main properties of IBa, including: the presence of two kinetic phases of inactivation, the voltage dependence of τslow, the amplitudes of all components, and the fact that τfast was steeply voltage-dependent for ICa (Fig. 8 A) but not IBa (Fig. 8 B).
Figure 8.
A model of Ca2+-dependent inactivation can also describe Ba2+currents. Simulations with the model described in the text, presented and analyzed in the same order as the experimental records in Fig. 1. (A) Simulated ICa elicited by pulses from −90 mV to voltages indicated near the traces. (inset) Simulated ICa at 0 mV, normalized to the peak value and plotted in semilog scale. (B) Simulated IBa during long duration pulses to voltages indicated. Dashed lines: two-exponential fits. (inset) Simulated IBa at 0 mV, normalized to the peak value and plotted in semilog scale. (C) Parameters of two-exponential fits to the simulated IBa.
Voltage-dependent Inactivation Underlies the Slow Phase of Current Decay
The present results include a reduction in the amount of charge available to move above −60 mV, upon prolonged depolarization. This reduction, which depends exponentially on duration of the depolarizing pulse, is cotemporal with the slow phase of current decay, regardless of the ion that carries the current. This implies that the slow phase of decay of IBa is the result of voltage-dependent inactivation. The concomitant reduction in the availability of gating charge could be the consequence of two not mutually exclusive mechanisms: charge immobilization associated with inactivation (Armstrong and Bezanilla, 1977), or a negative shift in the voltage distribution of intramembrane mobile charge, similar to that accompanying slow inactivation of squid Na+ channels (Bezanilla et al., 1982), inactivation of the voltage sensor of skeletal muscle (Brum et al., 1988), and inactivation of L-type channels in guinea pig cardiomyocytes (Shirokov et al., 1992) and embryonic chick ventricular myocytes (Josephson, 1996).
Implications for Other Ca2+ Channel Studies
The present results define two kinetic components of decay of Ba2+ currents through Ca2+ channels, show that the slow phase, with characteristic times of seconds, is caused by voltage-dependent inactivation, and suggest binding of Ba2+ as probable cause for the fast phase. These findings call into question the widespread use of Ba2+ currents as tools to quantify purely voltage- dependent processes in Ca2+ channels. By showing the close association between gating current inactivation and a decay of current that takes place in seconds, it forces reconsideration of studies that attribute decay phases of hundreds of ms to voltage-dependent processes.
For example, Zong et al. (1994), in their study of L-type Ca2+ channels in HEK 293 cells, used the difference between the “slow” time constant (∼200 ms) in a two- exponential fit to ICa elicited by 400-ms pulses, and the time constant in a single exponential fit to IBa (∼300 ms) to argue that Ca2+ may make voltage-dependent inactivation faster. Clearly the time constants were too short (and so were the applied pulses) to significantly explore the voltage-dependent process, which we now show to be much slower.
Zhang et al. (1994) analyzed the kinetics of IBa through chimeric channels in Xenopus oocytes to localize determinants of voltage-dependent inactivation. In this study, an α1A (a putatively P/Q-type channel from brain) with relatively slow rate of IBa decay was chimerized with the rapidly inactivating α1E (doe-1, marine ray). The work unquestionably localized the determinants of subunit-specific differences in decay of IBa to segment IS6 and its vicinity. Because the inactivation of IBa in doe-1 is believed to be exclusively voltage dependent (Ellinor et al., 1993), the rates of decay found in the rapidly inactivating chimeras, 10–30 s−1, were naturally attributed to voltage-dependent inactivation. However, P-type channels are susceptible to Ca2+-dependent inactivation (Tareilus et al., 1994), and therefore the assertion that fast inactivation in the chimeras is voltage-dependent should be supported with the use of longer pulses or other tests.
Possible involvement of the first domain in determining the rate of IBa decay was also demonstrated by Parent et al. (1995) working with a chimera of α1C and α1S. Even though the demonstration was clear, the conclusion that the first repeat affects voltage-dependent inactivation can be criticized on similar grounds, because it relies on the assumption that IBa inactivation is exclusively voltage-dependent.
In conclusion, the identification of structural determinants of voltage-dependent inactivation in Ca2+ channels can not be based solely on the analysis of IBa, since the ability to induce inactivation of the L-type Ca2+ channel appears not to be a unique property of Ca2+ ions. The main consequence of the present work is to require careful reconsideration of the mechanisms attributed to inactivation in previous and future studies.
Acknowledgments
This work was supported in part by National Institutes of Health Grant AR 43113 and a Grant-in-Aid from the American Heart Association (to E. Ríos) and by grants from the American Heart Association of Metropolitan Chicago (to R. Shirokov).
Footnotes
The tsA201 cell lines were a gift of Dr. M.M. Hosey (Northwestern University, Chicago, IL). The L-type Ca2+ channels cDNA sequences were gifts of Drs. E. Perez-Reyes (Loyola University, Maywood, IL) and M.M. Hosey. We thank Andy Chien, Tipu Puri and M.M. Hosey (Northwestern) for supplying the rabbit cardiomyocytes.
The horizontal transitions in scheme i are voltage dependent, with rate constant kRP,AP = a 1exp{(V − V1)/2K − b[(V − V1)/2K]2}, where V1 is a transition voltage and K a steepness factor, the rate constant of the reverse transition is equal to kRP,AP exp{(V1 − V)/K}, and the rate constants between states RI and AI were calculated similarly, with a 2 and V2 substituted for a 1 and V1. a 1 = 100 s−1, a 2 = 33 s−1, V1 = 0 mV, V2 = −80 mV, K = 8 mV, and b = 0.1. The inactivation rate constants were: kAP,AI = 0.15 s−1, kAI,AP = 0.05 s−1. The rates of recovery from inactivation were set to satisfy kinetics of recovery, kRP,RI + kRI,RP = 20 s−1, and microscopic reversibility, kRI,RP/kRP,RI = (kAP,AI/kAI,AP)exp [(V1 − V2)/2K ].
Dr. Ferreira's permanent address is Dept. Biofisica, Facultad de Medicina, Montevideo, Uruguay. Dr. Shirokov's permanent address is A.A. Bogomoletz Institute of Physiology, Kiev, Ukraine.
Parameters of scheme ii were: kCMe,C = 1 s−1, kUMe,U = 0.01 s−1, kC,U = 100 s−1, kU,C = 1,000 s−1, kUMe,CMe = 1,000 s−1, kCMe,UMe = 1 s−1. kC,CMe = kU,UMe = 106 M−1s−1 for Ca2+ and 104 M−1s−1 for Ba2+.
Single channel currents in Ba2+ and Ca2+ were 0.6 and 0.3 pA, respectively, at 20 mV and saturating [Me2+]e. At other voltages and [Me2+]e = 10 mM, the amplitude of single channel current was calculated as XV{(exp[−V/12 mV])/(1 − exp[(−V/12 mV])} × {[Me2+]e/([Me2+]e + 14 mM)}, where X is 0.08 pA for Ca2+ and 0.16 pA for Ba2+.
Total current was calculated as N × P o × i, where P o is the probability that the three gates are in their permissive states. N was 125,000, corresponding to ≈25 channels/μm2 in a cell of Cm = 50 pF. The channels generated both ionic and gating currents, in parallel with Cm and in series with resistance Rs = 5 MΩ. (The computer program can be obtained by E-mail requests to rshiroko@rush.edu)
references
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Ríos E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na+ and Ca2+channel gating. J Gen Physiol. 1989;94:65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Taylor RE, Fernandez J. Distribution and kinetics of membrane dielectric polarization. I. Long-term inactivation of gating currents. J Gen Physiol. 1982;79:21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland L, Dingledine R. Multiple components of both transient and sustained barium currents in a rat dorsal root ganglion cell line. J Physiol (Lond) 1990;420:223–245. doi: 10.1113/jphysiol.1990.sp017909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR, Honjo H, Harrison SM, Zang W-J, Kirby MS. Ultra-slow voltage dependent inactivation of the calcium current in guinea-pig and ferret ventricular myocytes. Pflüg Arch. 1994;428:39–50. doi: 10.1007/BF00374750. [DOI] [PubMed] [Google Scholar]
- Brehm P, Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science (Wash DC) 1978;202:1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Brown AM, Morimoto K, Tsuda Y, Wilson DL. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. . J Physiol (Lond) 1981;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G, Fitts R, Pizarro G, Ríos E. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol (Lond) 1988;398:475–505. doi: 10.1113/jphysiol.1988.sp017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Cavalie A, Ochi R, Pelzer D, Trautwein W. Elementary currents through Ca2+channels in guinea-pig myocytes. Pflüg Arch. 1983;405:284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cens T, Mangoni M, Richard S, Nargeot J, Charnet P. Coexpression of the β2subunit does not induce voltage-dependent facilitation of the class C L-type Ca channel. Pflüg Arch. 1996;431:771–774. [PubMed] [Google Scholar]
- Cox DH, Dunlap K. Inactivation of N-type calcium current in chick sensory neurons: calcium and voltage dependence. J Gen Physiol. 1994;104:311–336. doi: 10.1085/jgp.104.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A, Zhao X, Shirokov R, Puri T, Chang CF, Sun D, Ríos E, Hosey M. Roles of membrane-localized β subunit in the formation and targeting of functional L-type Ca2+channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- De Leon M, Wang Y, Jones L, Perez-Reyes E, Wei X, Wah T, Soong, Snutch TP, Yue DT. Essential Ca2+-binding motif for Ca2+-sensitive inactivation of L-type Ca2+channels. Science (Wash DC) 1995;270:1502–1506. doi: 10.1126/science.270.5241.1502. [DOI] [PubMed] [Google Scholar]
- De Waard M, Campbell K. Subunit regulation of neuronal α1A Ca2+ channels expressed in Xenopusoocytes. J Physiol (Lond) 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R, Chad JE. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44:215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Zhang J-F, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature (Lond) 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Field A, Hill C, Lamb G. Asymmetric charge movement and calcium currents in ventricular myocytes of neonatal rat. J Physiol (Lond) 1988;406:277–297. doi: 10.1113/jphysiol.1988.sp017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AP. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci USA. 1981;78:953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Ferroni A, Bertollini L, Mazzanti M. Inactivation of single Ca2+ channels in rat sensory neurons by extracellular Ca2+ . J Physiol (Lond) 1994;477:15–26. doi: 10.1113/jphysiol.1994.sp020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett C, De Waard M, Campbell K. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Hadley RW, Hume JR. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol (Lond) 1987;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RW, Lederer WJ. Intramembrane charge movement in guinea-pig and rat ventricular myocytes. J Physiol (Lond) 1989;415:601–624. doi: 10.1113/jphysiol.1989.sp017738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley RW, Lederer WJ. Ca2+ and voltage inactivate Ca2+channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol (Lond) 1991;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harafuji H, Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(-aminoethyl ether)- N,N,N′,N′-tetraacetic acid with calcium around neutral pH. J Biochem. 1980;87:1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman J, Tsien RW. Calcium channel selectivity for divalent and monovalent carions. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer CB, Lux HD. Properties of a facilitating calcium current in pace-maker neurones of the snail Helix pomatia. . J Physiol (Lond) 1976;88:319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoel, P.G. 1971. Introduction to Mathematical Statistics. Fourth Edition. John Wiley & Sons, Inc. New York. 211–217.
- Hoshi T, Rothlein J, Smith SJ. Facilitation of Ca2+channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1984;81:5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CS, Chandler WK. Intramembranous charge movement in frog cut twitch fibers mounted in a double Vaseline-gap chamber. J Gen Physiol. 1990;96:257–298. doi: 10.1085/jgp.96.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto Y, Ehara T, Goto M. Calcium channel currents in isolated guinea-pig ventricular cells superfused with Ca-free EGTA solution. Jpn J Physiol. 1985;35:917–932. doi: 10.2170/jjphysiol.35.917. [DOI] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Submicroscopic Ca+2 diffusion mediates inhibitory coupling between individual Ca+2channels. Neuron. 1992;9:197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Imredy JP, Yue DT. Mechanism of Ca+2-sensitive inactivation of L-type Ca+2channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Jong D-S, Pape PC, Chandler WK, Baylor SM. Reduction of calcium inactivation of sarcoplasmic reticulum calcium release by fura-2 in voltage-clamped cut twitch fibers from frog muscle. J Gen Physiol. 1993;102:333–370. doi: 10.1085/jgp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. Depolarization shifts the voltage dependence of cardiac sodium channel and calcium channel gating charge movements. Pflüg Arch. 1996;431:895–904. doi: 10.1007/s004240050083. [DOI] [PubMed] [Google Scholar]
- Josephson I, Varadi G. The β subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+channels. Biophys J. 1996;70:1285–1293. doi: 10.1016/S0006-3495(96)79685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibara M, Kameyama M. Inhibition of the calcium channel by intracellular protons in single ventricular myocytes of the guinea-pig. J Physiol (Lond) 1988;403:621–640. doi: 10.1113/jphysiol.1988.sp017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp T, Perez-Garcia MT, Marban E. Enhancement of ionic current and charge movement by coexpression of calcium channel β1A subunit with α1Csubunit in a human embryonic kidney cell line. J Physiol (Lond) 1996;492:89–96. doi: 10.1113/jphysiol.1996.sp021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. J Gen Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Inactivation kinetics of calcium current of accutely dissociated CA1 pyramidal cells of the mature guinea-pig hippocampus. J Physiol (Lond) 1991;437:27–48. doi: 10.1113/jphysiol.1991.sp018581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk PG, Mironov SL, Shuba YM. Two ion selecting filters in the calcium channel of the somatic membrane of mollusc neurons. J Membr Biol. 1983;76:83–93. [Google Scholar]
- Lee KS. Potentiation of the calcium channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci USA. 1987;84:3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Marban E, Tsien RW. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol (Lond) 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, DeFelice LJ, Liu YM. Gating of L-type Ca2+channels in embrionic chick ventricle cell: dependence on voltage, current and channel density. J Physiol (Lond) 1991;443:307–344. doi: 10.1113/jphysiol.1991.sp018835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald TF, Cavalié A, Traurwein W, Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflüg Arch. 1986;406:437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Neely A, Olcese R, Wei X, Birnbaumer L, Stefani E. Ca2+-dependent inactivation of a cloned cardiac Ca2+ channel α1C subunit (α1C) expressed in Xenopusoocytes. Biophys J. 1994;66:1895–1903. doi: 10.1016/S0006-3495(94)80983-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Concentration profiles of intracellular Ca2+in the presence of diffusible chelator. Exp Brain Res Ser. 1986;14:80–96. [Google Scholar]
- Ohya Y, Kitamura K, Kuriyama H. Regulation of calcium current by intracellular calcium in smooth muscle cells of rabbit portal vein. Circ Res. 1987;62:375–383. doi: 10.1161/01.res.62.2.375. [DOI] [PubMed] [Google Scholar]
- Parent L, Gopalakrishnan M, Lacerda AE, Wei X, Perez-Reyes E. Voltage-dependent inactivation in a cardiac-skeletal chimeric calcium channel. FEBS Lett. 1995;360:144–150. doi: 10.1016/0014-5793(95)00090-v. [DOI] [PubMed] [Google Scholar]
- Schneider P, Hopp HH, Isenberg G. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICain myocytes from guinea-pig urinary bladder. J Physiol (Lond) 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Scheuer T, Catterall W. Voltage-dependent potentiation of L-type Ca2+channels due to phosphorylation by cAMP-dependent protein kinase. Nature (Lond) 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Sham J, Cleeman L, Morad M. Functional coupling of Ca2+channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci USA. 1995;92:121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokov R, Levis R, Shirokova N, Ríos E. Two classes of gating current from L-type Ca channels in guinea pig ventricular myocytes. J Gen Physiol. 1992;99:863–895. doi: 10.1085/jgp.99.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokov R, Levis R, Shirokova N, Ríos E. Ca2+-dependent inactivation of cardiac L-type Ca2+channels does not affect their voltage sensor. J Gen Physiol. 1993;102:1005–1030. doi: 10.1085/jgp.102.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareilus E, Schoch J, Breer H. Ca2+-dependent inactivation of P-type calcium channels in nerve terminals. J Neurochem. 1994;62:2283–2291. doi: 10.1046/j.1471-4159.1994.62062283.x. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci USA. 1979;77:1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Turner P, Westwood T, Regen C, Steinhardt R. Increased protein degradation results from elevated free calcium levels found in muscle from mdxmice. Nature (Lond) 1988;335:735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Zhang J-F, Ellinor P, Aldrich R, Tsien R. Molecular determinants of voltage-dependent inactivation in calcium channels. Nature (Lond) 1994;372:97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]
- Zong, S., J. Zhou, and T. Tanabe. 1994. Molecular determinants of calcium-dependent inactivation in cardiac L-type calcium channels. B.B.R.C. 201:117–123. [DOI] [PubMed]