Abstract
Schwann cells provide trophic support and in some cases, insulation to axons. After injury, Schwann cells undergo phenotypic modulation, acquiring the capacity to proliferate, migrate, and secrete soluble mediators that control Wallerian degeneration and regeneration. Amongst the soluble mediators are pro-inflammatory cytokines that function as chemoattractants but also may sensitize nociceptors. At the same time, Schwann cells produce factors that counterbalance the pro-inflammatory cytokines, including, for example, interleukin-10 and erythropoietin (Epo). Epo and its receptor, EpoR, are up-regulated in Schwann cells after peripheral nerve injury. EpoR-dependent cell signaling may limit production of TNF-α by Schwann cells within the first five days after injury. In addition, EpoR-dependent cell signaling may reduce axonal degeneration and facilitate recovery from chronic pain states. Other novel factors that regulate Schwann cell phenotype in nerve injury have been recently identified, including the low-density lipoprotein receptor related protein (LRP-1). Our recent studies indicate that LRP-1 may be essential for Schwann cell survival after peripheral nerve injury. To analyze the function of specific Schwann cell gene products in nerve injury and sensory function, conditional gene deletion and expression experiments in mice have been executed using promoters that are selectively activated in myelinating or non-myelinating Schwann cells. Blocking ErbB receptor-initiated cell-signaling in either myelinating or non-myelinating Schwann cells results in unique sensory dysfunctions. Data obtained in gene-targeted animals suggest that sensory alterations can result from changes in Schwann cell physiology without profound myelin degeneration or axonopathy. Aberrations in Schwann cell biology may lie at the foundation of neuropathic pain and represent an exciting target for therapeutic intervention.
Introduction
Peripheral sensitization may be manifested as a painful response to a non-painful stimulus or as an overly exuberant response to a painful stimulus. These abnormal responses have, at their foundation, changes in both the peripheral and central nervous system. Many central responses may occur due to extracellular mediators that are released at the injury site and transported retrogradely up the afferent neuron. When this transport is blocked, abnormal pain behaviors may be dampened (Yamamoto and Yaksh, 1993). Thus, the pathophysiology occurring in the injured nerve is of utmost importance in the generation of neuropathic pain states. It is well known that the nerve injury site is rich in extracellular mediators, including cytokines, growth factors and proteases. This microenvironment is highly regulated by the Schwann cell. In uninjured nerve, Schwann cells provide myelin encapsulation of axons and paracrine trophic support to nerve; however, in injured nerves, Schwann cells undergo dramatic phenotypic modulation, regaining capacity to proliferate, migrate, and secrete numerous factors that control Wallerian degeneration and nerve regeneration. In this review, I will discuss emerging evidence that Schwann cells play an important role in the development of neuropathic pain states. Understanding Schwann cell physiology, the response of Schwann cells to cytokines and other extracellular mediators, and the capacity of Schwann cells to express cytokines in a regulated manner emerges as a central problem in the pain field.
Schwann Cell Phenotypes
The endoneural space is composed of axons and nucleated cells, 90% of which are Schwann cells. Schwann cells wrap around peripheral axons, in a regular periodicity, from the root-entry zone (adjacent to the spinal cord) to the distal termination of the axon and form a continuous basal lamina. There are two major phenotypes of Schwann cells in the peripheral nervous system: the myelinating Schwann cell and the ensheathing (non-myelinating) Schwann cell. Both types of Schwann cells are derived from the neural crest (for review see Jessen and Mirsky, 2005). The myelinating Schwann cell produces myelin, a lipid rich membrane with specific proteins, such as protein zero (P0), which serves as an insulator for axons. Myelinating Schwann cells are found wrapped around medium and large diameter axons that include Aδ and Aβ primary afferent fibers, respectively. During formation and after injury, myelinating Schwann cells receive information by reciprocal axon-glia communication. Signals from the axon determine the proper formation of myelin, the periodic wrapping of Schwann cell cytoplasm, and the thickness and length of the myelin sheath (for review see Lemke, 2006). Signals from the Schwann cell determine radial sorting (Feltri et al., 2002), axonal diameter, axonal conduction velocity, and development of nodes of Ranvier (for review see Sherman and Brophy, 2005). Given the length of the axon in the peripheral nerve, it is probably not surprising that myelination is the result of a sequence of Schwann cells. These are spaced at approximately 1 mm intervals. The nodes of Ranvier, in between Schwann cells, are rich in sodium channels and play an important role in saltatory conduction. The structure of myelinated axons has been well described (Scherer and Arroyo, 2002). Figure 1 demonstrates the intimate association of myelinating Schwann cells and axons. Non-compact myelin in Schwann cell cytoplasm is located in molecular domains termed paranodes and Schmidt–Lanterman Incisures. These domains contain junctional specializations between the layers of the myelin sheath. The function of the incisures remains obscure, however, it is thought that they provide passage of molecules between the myelin sheath and the axon. Myelin associated glycoprotein (MAG) is used to identify these functional domains. In Figure 1, PINCH, an adapter protein that shuttles proteins from the cytoplasm to the nucleus in cultured myelinating Schwann cells (Campana et al., 2003), co-localizes with MAG in the myelinated fiber architecture, suggesting a role for axo-glial communication. Myelinating Schwann cells can be also be identified by S100 and by proteins in the myelin such as myelin protein zero (P0), peripheral myelin protein (PMP22) and to a lesser extent myelin basic protein (MBP).
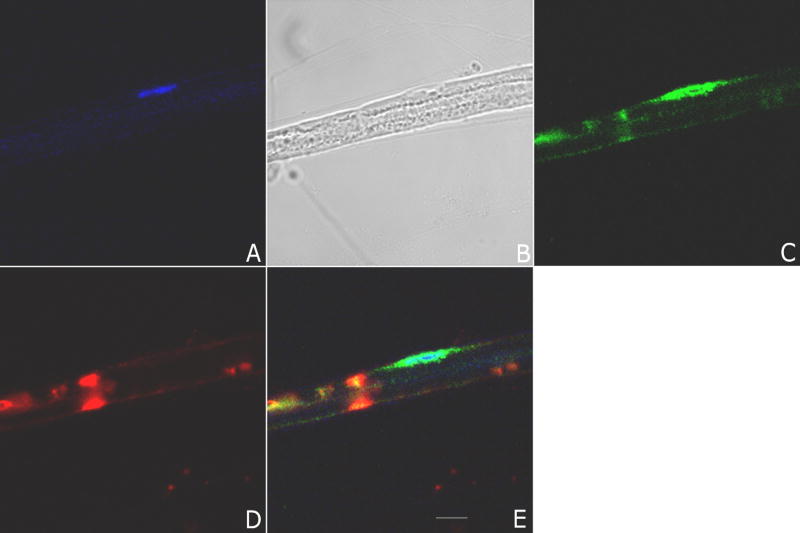
Figure 1. Confocal microscopy of myelinated teased fiber preparations from rat sciatic nerve.
Panel A shows TOPRO-3, a nuclear stain (blue), and the presence of one Schwann cell nucleus; Panel B shows a phase contrast image of the teased fiber; Panel C shows immunofluorescence with polyclonal antibody to a particularly interesting new cysteine histidine protein (PINCH) (Alexa 488, green); Panel D shows immunofluorescence using anti-myelin associated glycoprotein (MAG) (Alexa 564, red), a marker of Schwann cell cytoplasm where myelin is not compact; Panel E shows colocalization of MAG, PINCH and TOPRO-3. Note the peri-nuclear staining of PINCH and localization in compartments of non-compact myelin. Images represent at least 10 separate fibers analyzed. Scale bar 10 μm.
Schwann cells that ensheath small diameter axons (C-fibers) do not myelinate. One Schwann cell encompasses several small fibers that are separated by Schwann cell cytoplasm. This is called a Remak bundle. Recent evidence indicates that the number of unmyelinated axons in a Remak bundle varies depending upon its anatomical location (Murinson and Griffin, 2004). When axons exit the L5 dorsal root ganglion (DRG), more than half of the Remak bundles consist of greater than 20 axons. By contrast, unmyelinated axons in the hindpaw plantar nerve form bundles in which the ratio to the Schwann cell is 3:1. Qualitative differences in unmyelinated axons within Remak bundles are also evident; those closer to the DRG, which are grouped together in greater numbers, also show more substantial axonal contact with less intervening Schwann cell cytoplasm. The significance of this anatomical observation remains unclear; however, C-fibers are extremely important in pain states. Thus, deviations in this complex structure may indicate abnormalities in nociception. Furthermore, as suggested by Murinson and Griffin (Murinson and Griffin, 2004), a complex array of trophic factors may be necessary to support this complex intercellular interplay.
Schwann cells may also be resolved into two classes based on association with sensory or motor neurons (Hoke et al., 2006). Schwann cells that are coupled to regenerating axons in the sensory system, such as denervated and re-innervated cutaneous fibers, produce a high level of nerve growth factor-β (NGF-β), brain derived neurotrophic factor (BDNF), insulin like growth factor (IGF-1), and erythropoietin (Epo) (Hoke et al., 2006, Hammarberg et al., 1998, Li et al., 2005). By contrast, Schwann cells which associate with denervated and re-innervated ventral roots produce pleiotrophin and glial derived growth factor. These results suggest that Schwann cells may undergo phenotypic modulation to best support the axonal systems with which they are associated. The existence of this phenotypic modulation provides further evidence of the remarkable capacity of the Schwann cell to regulate expression and determine the composition of the extracellular microenvironment.
Overall, it is apparent that Schwann cells may adopt very different phenotypes depending on the nerve fibers with which they associate. Myelin production may occur or not, depending upon the type of the type of axon the Schwann cell encounters. The genetic expression profile of the cell is sensitive to the direction of axonal conduction. Finally, it is quite clear that Schwann cells undergo rapid changes in phenotype in response to injury (for review see Jessen and Mirsky, 2005). Myelin retraction occurs rapidly. The cells acquire increased capacity for phagocytosis and may be involved in the initial clearing of myelin debris. Furthermore, the cells express autocrine signals for survival and migration. In the injured nerve, retention of the Schwann cell basal lamina provides a conduit for axonal regrowth and is thus critical for axonal regeneration
Schwann Cell Production of Extracellular Mediators in Animal Models of Chronic Neuropathic Pain
The programmed series of events that occurs in peripheral nerve injury, Wallerian degeneration, and therapeutics in development to promote regeneration have been reviewed recently (Myers et al., 2006). Experimental therapeutics that prevent Schwann cell atrophy, loss of Schwann cell basal lamina, and promote Schwann cell survival may be effective in counteracting development of painful peripheral neuropathies (Hoke, 2006). In the peripheral nerve, the cellular composition of the endoneural space changes with predictable kinetics, reflecting proliferation of endoneural cells and infiltration with extraneural cells including macrophages and other inflammatory cells. Schwann cells predominate early on (within hours), secreting trophic factors and inflammatory cell chemoattractants that also, perhaps unfortunately, sensitize nociceptors (Watkins and Maier, 2003, Bergsteinsdottir et al., 1991, Wagner and Myers, 1996, Matsuoka et al., 1991). One cytokine that is up-regulated within hours in Schwann cells after peripheral nerve injury is the proinflammatory cytokine, tumor necrosis factor-α (TNF-α) (Campana et al., 2006). Schwann cell-derived TNF-α is a major mediator of hyperalgesia. In fact, direct injection of TNF-α induces many of the histological and molecular changes observed in the injured nerve (Wagner and Myers, 1996). Furthermore, animals receiving direct injection of TNF-α into uninjured nerves develop neuropathic pain states. TNF-α causes macrophage influx and further production of TNF-α in a positive-feedback loop. Similar genetic feedback programs result in increased expression of other inflammatory cytokines, such as IL-1β and IL-6 (Shamash et al., 2002). In addition to neuropathic pain states TNF-α also plays a role in other inflammatory pain states such as neuritis (Romero-Sandoval et al., 2005)
The central role of TNF-α in the pathophysiology of nerve injury suggests that regulating either expression or the activity of TNF-α may have a therapeutic advantage. Studies have been conducted in which management was attempted with neutralizing antibodies (Schafers et al., 2001) and with antagonists of TNF-α-initiated cell-signaling cascades (Myers et al., 2003). Each of these therapies demonstrated some advantages with regard to resolution of the injury with decreased pain. However, we now understand that TNF-α is also counteracted by endogenously produced agents in the injured peripheral nerve. The first is erythropoietin (Li et al., 2005). In various animal models in peripheral nerve injury, including crush injury and chronic constriction injury (CCI), the mRNAs for Epo and its receptor are rapidly up-regulated at the site of injury and distal to the injury site. The source of Epo production in the injured nerve is the activated Schwann cell. EpoR was identified in both axons and Schwann cells, establishing the context for both autocrine and paracrine EpoR-dependent cell signaling. In Schwann cells in culture, Epo stimulates phosphorylation of EpoR, activates JAK2, and activates ERK/MAP kinase (Li et al., 2005).
ERK1/2 phosphorylation occurs downstream of JAK2, in Epo-treated Schwann cells, because pharmacologic antagonism of JAK2 with AG490 blocks ERK1/2 phosphorylation. These cell-signaling pathways result in Schwann cell proliferation in vitro and in vivo, and perhaps most importantly, from the standpoint of neuropathic pain, decreased expression of TNF-α (Campana et al., 2006). The ability of Epo to limit Schwann cell-produced TNF-α within the first five days after nerve injury correlates with reduced axonal degeneration and a facilitated rate of recovery from chronic pain states (Campana et al., 2006). We propose that Epo balances the inflammatory cytokine milieu and thereby protects axons and glia from further damage. This model is supported by the work of Keswani et al., (2004) who confirmed that Schwann cells produce Epo and that exogenously administered Epo prevents limb weakness and neuropathic pain behaviors in a rat model of distal axonopathy. Furthermore, Epo has been shown to downregulate production of inflammatory infiltrates in animal models of autoimmune encephalomyelitis (Savino et al., 2006). In this study, TNF-α and interleukin-6 (IL-6) expression were both reduced by greater than 50%. The extent to which neutralizing TNF-α production in the injured nerve is advantageous is unclear; however, these studies with Epo suggest that like in other forms of injury, excessive inflammation can be counterproductive. Anti-inflammatory therapy such as IL-10 decrease thermal hyperalgesia in rats (Wagner et al., 1998). Titrating the balance between inflammatory and anti-inflammatory cytokines may represent a therapeutic rheostat in the peripheral nervous system.
Understanding the changes in Schwann cell genetic expression that drive phenotypic modulation during injury is an important topic of research. Our laboratory is interested in receptors in the low density lipoprotein (LDL) receptor family because these receptors have been implicated in reactive and inflammatory states in the central nervous system. Low density lipoprotein receptor related protein (LRP-1) is a 600-kDa member of this receptor family which mediates the endocytosis of over forty distinct ligands, facilitates the internalization of other membrane proteins and participates as a cell-signaling receptor by binding signaling adaptor proteins (Gonias et al., 2004). My laboratory demonstrated that LRP-1 is expressed by both axons and Schwann cells in normal sciatic nerve (Campana et al., 2006a). After injury, Schwann cell LRP-1 expression is substantially increased. This is important because LRP-1 functions as a Schwann cell survival factor, based on its ability to increase the basal activation level of phosphoinositide-3-kinase (PI3K) and protein kinase b (Akt) and decrease caspase activation. When LRP-1 was antagonized in the injured nerve, Schwann cell death increased in response to nerve injury. The correlation between Schwann cell LRP-1 expression and cell survival raised important questions regarding the role of this receptor in the development of pain states. Included amongst the ligands for LRP-1 are a variety of growth factors that may be active in the injured endoneurium. Understanding the full spectrum of LRP-1 activities in the peripheral nerve is another future goal.
Genetic Targeting of the Myelinating Schwann Cell: Effects on Chronic Neuropathic Pain
Several approaches have been used for targeted expression or deletion of genes in myelinating Schwann cells in mice. Promoters for myelin-associated genes have been very useful because of their cell-specificity and because these genes are typically expressed only postnatally when myelin begins to develop. The promoter for the gene, CNPase, is active only in myelinating Schwann cells and has been used to generate conditional transgenic mice (Chen et al., 2006). CreLox technology also has been used successfully; exploiting the promoter for myelinating transcription factor Krox20 or P0 to drive expression of the enzyme Cre (Garratt et al., 2000, Feltri et al., 2002). Recent studies using these Schwann cell-targeting technologies have contributed to our understanding of peripheral sensitization on a molecular level.
An interesting example concerns the epidermal growth factor family of receptors and ligands. ErbBs are receptor tyrosine kinases expressed by Schwann cells. ErbB-dependent cell signaling controls many aspects of Schwann cell development, proliferation, and differentiation (Lemke, 2006). Recent work by Taveggia et al., (2005) suggests that a ligand for ErbB receptors, Nrg-1, which is derived from axons, controls whether Schwann cells myelinate or not. Chen et al. (2006) ablated ErbB-dependent cell-signaling in myelinating Schwann cells by generating transgenic mice expressing a dominant-negative ErbB4 receptor under the control of the CNPase promoter. The sciatic nerves of these mice demonstrated thinner myelin, shorter internodal length and delayed myelination. These results were correlated with changes in myelin-associated protein expression. Sensory testing to determine alterations in pain-related behaviors was performed using von Frey filaments and hot-plate analysis. Transgenic mice displayed an increased response to mechanical stimulation compared with wild type mice; on average, the force required to evoke a response in transgenic mice was significantly decreased compared with wild type mice. Interestingly, there were no changes in thermal nociception in the transgenic mice. This was not surprising because thermal nociception reflects the activity of unmyelinated fibers, which were normal in these mice. By contrast, the myelinating Schwann cells were dramatically altered. Thus, the allodynia is attributable to alterations in myelinating fiber function and/or the reactions of neighboring intact unmyelinated fibers. Spontaneous pain, both neuropathic and inflammatory, can be related to frequency of spontaneous firing in intact C-fiber nociceptors (Djouhri et al., 2006). Myelinated fibers have been shown to mediate >90% of withdrawal reactions to light touch stimulation (Shir and Seltzer, 1990) and to the mechanical hypersensitivity observed in tactile allodynia (Woolf et al., 1998).
Further evidence that alterations of ErbB2 signaling in myelinating Schwann cells lead to sensory alterations and chronic pain is observed in animal models of Leprosy. Leprosy induces neuropathic pain in both untreated and treated patients (Hietaharju et al., 2000). As an in vivo model of M. leprae infection, Rag1−/− mice were injected with M. leprae directly into the endoneurium (Rambukkana et al., 2002). Within 72 h, demyelination was observed; however, when M. leprae was introduced into the nerve in the presence of PKI 166, a small molecule inhibitor of ErbB2 receptor, demyelination was not observed. Thus, the demyelination induced by M. leprae seems to be dependent on the ErbB2 receptor. It is well established that demyelination is associated with the development of pain states. Thus, targeting this EGF receptor family member may represent a therapeutic opportunity for specific inducers of chronic neuropathic pain. Based on in vitro studies, it was established that M. leprae binds directly to ErbB2, causing activation of ERK1/2 (Tapinos et al., 2006). This response may be blocked by the ErbB2-targeting antibody, herceptin, which is approved for clinical use in cancer patients.
Other examples in which gene deletion studies have revealed correlations regarding molecular changes in myelinating Schwann cells and sensory function include the Schwann cell-targeted dystroglycan gene knock-out (Saito et al., 2003) and the periaxin gene knock-out (Gillespie et al., 2000). These studies have shown that even limited alterations in the composition of myelin or in the basal lamina produced by myelinating Schwann cells may contribute to the development of neuropathic pain states. In the absence of profound myelin degeneration or axonapthy, such as is observed in the heterozygous P0 gene knock-out mouse, sensory deficits exist. Samsam et al. (2002) showed that P0+/− mice have increased withdrawal thresholds to both thermal and mechanical stimuli. These results were not confounded by motor dysfunction as both P0+/− mice and wild type littermates responded to a painful stimulus (chronic constriction injury, CCI) similarly. There were subtle changes at the node of Ranvier including poorly developed Schwann cell microvilli. These studies demonstrate that biochemical and specific molecular domain alterations in myelinating Schwann cells induce a peripheral sensitization that may be a significant contributor to neuropathic pain.
Genetic Targeting of the Non-Myelinating Schwann Cell: Effects on Chronic Neuropathic Pain
The intimate association of non-myelinating Schwann cells with C-fibers is evident when cell-signaling is specifically antagonized in these cells as opposed to myelinating Schwann cells. In a second study by Chen and colleagues, dominant-negative ErbB4 was expressed this time under the control of the GFAP promoter so that EGF receptor family-dependent cell signaling was antagonized selectively in the non-myelinating Schwann cells (Chen et al., 2003). The phenotype involved 1) extensive Schwann cell proliferation and death; and 2) progressive loss of thermal sensitivity (hot and cold), without changes in mechanical sensitivity as might be anticipated, if this change in the physiology of the unmyelinating Schwann cell affected associated axons. Eventually, some of the DRG neurons died, perhaps due to lack of trophic support. Therefore, disruption of ErbB signaling in myelinating and non-myelinating Schwann cells have very different consequences.
Recently, a rodent model of HIV-associated sensory neuropathy was established by creating transgenic mice expressing the HIV-1 envelope glycoprotein gp120 under the GFAP promoter (Keswani et al., 2006). Thus, gp120 was expressed in non-myelinating Schwann cells. In this model, changes in sensory function (thermal sensation) were only noted when gp120 expressing mice were treated with didanosine (DDI), a reverse transcriptase inhibitor that is commonly used to treat HIV patients. The DDI-treated wild type mice had no changes in thermal sensation. The targeted effects of DDI treatment on non-myelinating Schwann cells indicates that biochemical changes within the Schwann cell impact axonal function and directly regulate nociception.
Conclusions
Overall, a model emerges in which Schwann cells regulate the function of the nerve by multiple mechanisms, which impact on response of neurons to stimuli. These regulatory activities include direct axonal contact and conditioning of the endoneurial microenvironment. In nerve injury, it is the Schwann cells that rapidly responds and orchestrates changes occurring within the nerve to optimize the potential for successful regeneration. Aberrations in this system may lie at the foundation of neuropathic pain. Alternatively, the Schwann cell and its physiology represent an exciting target for therapeutic intervention.
Acknowledgments
I would like to thank Dr. Andrew Mizisin for technical assistance with teased fiber preparations and Dr. Ann Rearden for providing the anti-PINCH polyclonal antibody. NIH R01 NS041983 to W.M.C has supported some of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergsteinsdottir K, Kingston A, Mirsky R, Jessen KR. Rat Schwann cells produce interleukin-1. J Neuroimmunol. 1991;34:15–23. doi: 10.1016/0165-5728(91)90094-n. [DOI] [PubMed] [Google Scholar]
- Campana WM, Li X, Shubayev VI, Angert M, Cai K, Myers RR. Erythropoietin reduces Schwann cell TNF-alpha, Wallerian degeneration and pain related behaviors after peripheral nerve injury. Eur J Neurosci. 2006;23:617–626. doi: 10.1111/j.1460-9568.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- Campana WM, Li XQ, Dragjovic N, Janes J, Gaultier A, Gonias SL. The low density lipoprotein related protein (LRP-1) is a pro-survival receptor in Schwann cells: Possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Myers RR, Rearden A. Identification of PINCH in Schwann cells and DRG neurons: Shuttling and signaling after nerve injury. Glia. 2003;41:213–223. doi: 10.1002/glia.10138. [DOI] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–92. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the Schwann cell precursor pool. J Cell Biol. 2000;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CS, Sherman DL, Fleetwood-Walker SM, Cottrell DF, Tait S, Garry EM, Wallace VC, Ure J, Griffiths IR, Smith A, Brophy PJ. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron. 2000;26:523–531. doi: 10.1016/s0896-6273(00)81184-8. [DOI] [PubMed] [Google Scholar]
- Gonias SL, Wu L, Salcioni AM. Low density lipoprotein receptor related protein:regulation of the plasma membrane proteome. 2004;91:1056–64. doi: 10.1160/TH04-01-0023. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Risling M, Hokfelt T, Cullheim S, Piehl F. Expression of insulin-like growth factors and corresponding binding proteins (IGFBP1–6) in rat spinal cord and peripheral nerve after axonal injuries. J Comp Neurol. 1998;400:57–72. [PubMed] [Google Scholar]
- Hietaharju A, Croft R, Alam R, Birch P, Mong A, Haanpaa M. Chronic neuropathic pain in treated leprosy. Lancet. 2000;356:1080–1081. doi: 10.1016/S0140-6736(00)02736-7. [DOI] [PubMed] [Google Scholar]
- Hoke A. Mechanisms of Disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory pehnotypes that regulated axon regeneration. J Neurosci. 2006;26:9646–55. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Buldanlioglu U, Fischer A, Reed N, Polley M, Liang H, Zhou C, Jack C, Leitz GJ, Hoke A. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. Neuregulin and myelination. Sci STKE. 2006;325:pe11. doi: 10.1126/stke.3252006pe11. [DOI] [PubMed] [Google Scholar]
- Li X, Gonias SL, Campana WM. Schwann cells express erythropoietin receptor and represent a major target for Epo in peripheral nerve injury. Glia. 2005;51:254–265. doi: 10.1002/glia.20202. [DOI] [PubMed] [Google Scholar]
- Matsuoka I, Meyer M, Thoenen H. Cell-type specific regulation of nerve growh factor (NGF) synthesis in non-neuronal cells: comparisons of Schwann cells with other cell types. J Neurosci. 1991;11:3165–3177. doi: 10.1523/JNEUROSCI.11-10-03165.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinson BB, Griffin JW. C-fiber structure varies with location in peripheral nerve. J Neuropathol Exp Neurol. 2004;63:246–254. doi: 10.1093/jnen/63.3.246. [DOI] [PubMed] [Google Scholar]
- Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006;11:8–20. doi: 10.1016/S1359-6446(05)03637-8. [DOI] [PubMed] [Google Scholar]
- Myers RR, Sekiguchi Y, Kikuchi S, Scott B, Medicherla S, Protter A, Campana WM. Inhibition of p38 MAP kinase activity enhances axonal regeneration. Exp Neurol. 2003;184:606–614. doi: 10.1016/S0014-4886(03)00297-8. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296:927–31. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval E, McCall C, Eisenach JC. A2-Adrenoceptor stimulation transforms immune responses in neuritis and blocks neuritis-induced pain. J Neurosci. 2005;25:8988–8994. doi: 10.1523/JNEUROSCI.2995-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Samsam M, Frei R, Marziniak M, Martini R, Sommer C. Impaired sensory function in heterozygous P0 knockout mice is associated with nodal changes in sensory nerves. J Neurosci Res. 2002;67:167–173. doi: 10.1002/jnr.10115. [DOI] [PubMed] [Google Scholar]
- Savino C, Pedotti R, Baggi F, Ubiali F, Gallo B, Nava S, Bigini P, Barbera S, Fumagalli E, Mennini T, Vezzani A, Rizzi M, Coleman T, Cerami A, Brines M, Ghezzi P, Bianchi R. Delayed administration of erythropoietin and its nonerythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172:27–37. doi: 10.1016/j.jneuroim.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Schafers M, Brinkhoff J, Neukirchen S, Marziniak M, Sommer C. Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci Lett. 2001;310:113–116. doi: 10.1016/s0304-3940(01)02077-8. [DOI] [PubMed] [Google Scholar]
- Scherer S, Arroyo EJ. Recent progress on the molecular organization of myelinated axons. J Periph Nerv Syst. 2002;7:1–12. doi: 10.1046/j.1529-8027.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Shir Y, Seltzer Z. A-fibers mediate mechanical hyperesthesia and allodynia and Cfibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci Lett. 1990;115:62–67. doi: 10.1016/0304-3940(90)90518-e. [DOI] [PubMed] [Google Scholar]
- Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12:961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment and endoneurial TNF-alpha expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myer RR. Endoneurial injection of TNF-alpha produces neuroapthic pain behaviors. NeuroReport. 1996;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R, Torebjork E. Towards a mechanism-based classification of pain? Pain. 1998;77:227–229. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Effects of colchicine applied to the peripheral nerve on the thermal hyperalgesia evoked with chronic nerve constriction. Pain. 1993;55:227–233. doi: 10.1016/0304-3959(93)90151-E. [DOI] [PubMed] [Google Scholar]