Abstract
Peptide YY (3-36) [PYY(3-36)] inhibits feeding in rodents, nonhuman primates and humans, yet the neural circuits underlying this action remain to be determined. Here we assessed whether PYY(3-36) inhibits feeding by activating neurons in forebrain and hindbrain sites containing Y2 receptors and linked to control of food intake, or in hindbrain sites immediately downstream of vagal afferent neurons. Rats received an anorexigenic dose of PYY(3-36), and the number of neurons expressing Fos, an indicator of neural activation, was determined in anterior hypothalamus (AH), arcuate nucleus (ARC), dorsomedial hypothalamus (DMH), lateral hypothalamus (LH), ventromedial hypothalamus (VMH), central nucleus of the amygdala (CeA), area postrema (AP), and caudal medial nucleus tractus solitarius (cmNTS), commissural NTS (cNTS), and gelatinosus NTS (gNTS). Expression of tyrosine hydroxylase (TH), an indicator of catecholamine synthesis, was also measured in the cmNTS. PYY(3-36) increased Fos in ARC, cmNTS, gNTS and AP. Approximately 10% of Fos (+) neurons in the cmNTS were TH (+). These results suggest that PYY(3-36) inhibits feeding through direct activation of ARC neurons, and direct and/or indirect activation via vagal afferent nerves of cmNTS, gNTS and AP, including some catecholaminergic neurons in the cmNTS.
Keywords: PYY(3-36), satiety, forebrain, hindbrain, Fos, tyrosine hydroxylase
1. Introduction
Peptide YY (PYY), neuropeptide Y (NPY), and pancreatic polypeptide comprise a family of structurally-related brain-gut peptides with diverse actions mediated by five known receptors [6]. Endocrine cells of the distal gut provide a major source of PYY. Food intake releases at least two major forms of PYY into the circulation, PYY(1-36) and PYY(3-36); other predicted/detected isoforms include Ser13-phosphorylated PYY(1-36) and PYY(3-36) and glycine-extended carboxyl termini of both the phosphorylated and non-phosphorylated forms [12,15,18]. PYY(3-36) is a high affinity ligand for Y2 receptors, whereas PYY(1-36) has affinity for Y1, Y2, Y4 and Y5 receptors [6]. Systemic administration of PYY(3-36) potently inhibits food intake in rodents, monkeys, and humans [5,11,14,22–24,35], while PYY(1-36) is less effective in rats [11] and humans [34]. Obese humans appear to have a blunted plasma PYY response to food intake [4,23], yet PYY(3-36) appears to decrease food intake similarly in lean and obese humans [4]. These results suggest that PYY(3-36) may act physiologically to reduce food intake and body adiposity, and that insufficient production of PYY(3-36) may promote obesity. However, the central and peripheral targets that mediate the anorexic effects of PYY(3-36) remain to be determined.
Several lines of evidence suggest that circulating PYY(3-36) may inhibit food intake through a direct action at Y2 receptors in specific brain sites linked to control of food intake. First, PYY(3-36) has been shown to cross the blood-brain barrier in mice [25]. Second, high levels of Y2 receptor mRNA expression, moderate to high densities of PYY(3-36) binding, and activation of Y2 receptors by agonist-stimulated binding of [35S]GTPγ have been detected in the hypothalamus, amygdala, hippocampus, thalamus, and hindbrain in rats [16,28,33]. Third, many forebrain and hindbrain sites containing Y2 receptors are linked to the control of food intake, including anterior hypothalamus (AH), arcuate nucleus (ARC), dorsomedial hypothalamus (DMH), lateral hypothalamus (LH), paraventricular nucleus (PVN), ventromedial hypothalamus (VMH), central nucleus of the amygdala (CeA), ventral tegmental area, area postrema (AP), and nucleus tractus solitarius (NTS) [16,28,33]. Lastly, direct injections of PYY(3-36) into the ARC have been shown to inhibit food intake at a dose as low as 100 fmol [5].
Other evidence suggests that circulating PYY(3-36) may also inhibit food intake through a direct action at Y2 receptors on abdominal vagal sensory nerves. Vagal neurons have Y2 receptors [21], peripheral administration of PYY(3-36) stimulates vagal afferent nerve activity [21], and vagal denervation has been reported to block the anorexic response to peripheral administration of PYY(3-36) in rats [1,21] but not mice [19]. Rinaman and colleagues [30,31] have provided evidence that the prototypical intestinal satiety peptide cholecystokinin inhibits food intake by a mechanism involving downstream catecholaminergic neurons in the caudal NTS, an area that receives and integrates incoming meal-related satiety signals transmitted by vagal sensory neurons from the gut [17,26,32,38]. It remains to be determined whether PYY(3-36) acts in part through a similar mechanism.
Here we assessed whether PYY(3-36) inhibits feeding by activating neurons in forebrain and hindbrain sites containing Y2 receptors and linked to control of food intake, or catecholaminergic neurons immediately downstream of vagal afferent neurons in the hindbrain. We examined the effects of intravenous (IV) infusion of an anorexigenic dose of PYY(3-36) (30 pmol/kg/min) on induction of Fos, an indicator of neural activation, in (i) AH, ARC, DMH, LH, PVN, VMH, CeA, AP, caudal medial NTS (cmNTS), commissural NTS (cNTS), and gelatinosus NTS (gNTS), and in (ii) cmNTS neurons either containing or not containing tyrosine hydroxylase (TH), a marker of catecholamine synthesis
2. Materials and Methods
2.1. Synthesis and purification of PYY(3-36)
Rat PYY(3-36) was synthesized manually by utilizing Fmoc batch-wise solid-phase methodology [3]. Purification was accomplished by reverse phase high performance liquid chromatography. Proof of structure was provided by coelution with a known sample and by electro-spray mass spectrometry. PYY(3-36) stock was prepared by dissolving the purified peptide in 0.15 M NaCl, 0.1% BSA to a concentration of 100 nmol/ml. Single-use aliquots were stored at −70°C.
2.2. Experimental Animals
Male Sprague-Dawley rats (Sasco, Charles River, Portage, MI; initially weighing 310–550 g) were housed in plastic shoe-box cages in a room with controlled temperature (19–21° C) under a 12/12 h light-dark cycle (lights off at 1800 h). Rats were provided pelleted rat chow (Labdiet®, 5001 Rodent diet, PMI® Nutrition International, MO) and water ad libitum for about a week before being subjected to experimental procedures. The Animal Studies Subcommittee of the Omaha Veterans Affairs Medical Center approved the experimental protocol.
2.3. Surgical procedures
The procedure for implantation of a jugular vein catheter for peptide infusion has been described previously [37]. Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg), a catheter composed of tygon and silastic segments was inserted into the right jugular vein, and the catheter was exteriorized in the dorsal neck region. Catheters were plugged with stainless steel wire and kept patent by flushing with 0.2 ml of 50% dextrose on alternate days. The animals were allowed at least 1 week to recover from surgery before being subjected to experimental procedures.
2.4. PYY(3-36) administration and tissue collection for immunostaining
Animals were adapted to experimental conditions for at least 1 week before the start of experiments. During the adaptation period, the animals were fasted overnight (~ 16 h) and subjected to mild restraint in Bollman cages for ~ 3 h (0900–1200 h). We previously showed that IV infusion of PYY(3-36) inhibits real and sham feeding under these conditions [11]. At the end of the adaptation period, the patency of the jugular vein catheters was determined by IV infusion of 0.2 ml of the short-acting anesthetic propofol (Abbott Laboratories). Catheters were considered patent if rats lost consciousness immediately on injection of the anesthetic; only such propofol-positive rats were included in subsequent experiments. Rats were divided into two treatment groups (n=9 each) to receive a 3-h IV infusion (0900–1200h) of vehicle (0.15 M NaCl, 0.1% BSA, 3 ml/h) or 30 pmol/kg/min of PYY(3-36). Previously, we showed that 3-h IV infusion of PYY(3-36) (5 to 50 pmol/kg/min) from the same stock used in this study dose-dependently decreased food intake in rats of the same size and strain from the same vendor [10,11]. Immediately following infusion of vehicle or PYY(3-36), rats were anesthetized with isoflurane and transcardially exsanguinated with saline followed by perfusion with 4% paraformaldehyde in 10 mM phosphate buffer (PBS), pH 7.2. Brains were removed and stored for 3–4 h in fresh fixative at 4°C and then transferred to 10 mM phosphate buffer containing 25% sucrose for 48 h. Brains were frozen by submerging for 10–15 seconds in isopentane and placed under crushed dry ice. Coronal cryostat sections (14 μm) through the AH, AP, ARC, CeA, DMH, LH, NTS, PVN and VMH from the same animals were mounted on slides and stored at −80° C.
2.5. Immunocytochemical staining for Fos in all brain sites
Slides were washed with 10 mM PBS at room temperature followed by blocking buffer (5% normal goat serum in 10 mM PBS) for 90 min and additional buffer washes. The primary antibody used in all brain sites was rabbit polyclonal anti-Fos, 1:5000 dilution (Ab-5, Oncogene, San Diego, CA ) [7]. Ab-5 was diluted in 10 mM PBS. Following an overnight incubation of Ab-5 at 4° C, slides were washed in 10 mM PBS followed by 1 h in a fluorescent second antibody (goat anti-rabbit IgG-Alexa 488) (Molecular Probes, Eugene, OR) diluted at 1:200 in 10 mM PBS to detect Fos antibodies. Slides were washed in 10mM PBS and coverslipped using an anti-fading glycerol-based mounting media. Immunostaining specificity controls included replacement of the primary antibody with normal rabbit serum at the same dilution as the primary antibody.
2.6. Immunocytochemical staining for Fos and TH in cmNTS
cmNTS was chosen for analysis because this region exhibited the largest increase in Fos in response to PYY(3-36). Immunostaining for Fos and TH was accomplished by applying Ab-5 in combination with a mouse monoclonal anti-tyrosine hydroxylase, 1:1000 dilution (CHEMICON International, Inc, Temecula, CA) using the same protocol as described in 2.5. In addition to using the goat anti-rabbit IgG-Alexa 488 to detect Fos antibodies, a goat anti-mouse IgG-Cy3 (Jackson ImmunResearch, West Grove, PA) was used to detect TH antibodies. Under conditions used in the immunocytochemical staining protocol, immunoreactive Fos protein was concentrated in the nuclei of labeled cells, whereas immunoreactive TH was concentrated in the cytoplasm.
2.7. Immunocytochemical data analysis
Slides were analyzed with a Zeiss Axioplan fluorescence microscope and all measurements were made with a 20X objective lens. Identification of anatomic landmarks was assisted by staining cell nuclei with Hoechst 33258 (Sigma, St. Louis, MO), which was added to the mounting medium and observed with a conventional DAPI filter set. Digital RGB images of the fluorescent preparations were acquired with either a Hamamatsu (C4880; Tokyo, Japan) fast-cooled charge-coupled device camera and the MCID imaging system (Imaging Research, St. Catherines, ON, Canada) or a QImaging Retiga 1300i FAST 1394 Digital CCD camera (Burnaby, BC, Canada) and the Media Cybernetics, Inc., Image Pro Express imaging system (Bethesda, MD). All images were exported to Photoshop (Adobe, Tucson, AZ). Observation of Fos induction in brain regions was from sections separated by 280 μm (distance anterior (+) or posterior (−) to the interaural line [29] listed in parentheses as follows): AH (3 sections between AH levels +6.88 and +7.40 mm), AP (2 sections between AP levels −4.8 and −5.08 mm), ARC (4 sections between ARC levels +5.86 and +6.7 mm), CeA (two sections between CeA levels +6.88 and +7.2 mm), DMH (3 sections between DMH levels +5.86 and +6.44 mm), LH (6 sections between LH levels +5.86 and +7.4 mm), cNTS (2 sections between cNTS levels −4.8 and −5.08 mm), cmNTS (4 sections between cmNTS levels −4.24 and −5.08 mm), gNTS (2 sections between gNTS levels −4.24 and −4.52 mm), PVN (3 sections between PVN levels +6.88 and +7.20 mm), VMH (4 sections between +5.86 and +6.88 mm). In each section the number of neurons that had Fos immunofluorescence in the nucleus [Fos (+)] was recorded bilaterally (exception AP, cNTS) and the number of Fos (+) neurons were totaled across all sections analyzed.
Observation of Fos induction in neurons expressing TH in the cmNTS was across sections separated by 280 μm using identical hindbrain sections as described above. In each section analyzed, the cumulative number of neurons that had Fos immunofluorescence in the nucleus [Fos (+)] or TH immunofluorescence in the cytoplasm [TH (+)] was recorded bilaterally from anatomically matched sections and analyzed across the two treatment groups. A neuron was considered to be double labeled if the Fos (+) nucleus was located within the boundary of the TH (+) cytoplasm, as determined by focusing the microscope.
2.8. Statistics
All results are expressed as means ± SE. Comparisons were made using a one-tailed Student’s t-test to determine whether PYY(3-36) infusion increased the number of neurons in a brain site expressing Fos and/or TH.
3. Results
3.1. Effects of PYY(3-36) on number of Fos containing neurons in forebrain and hindbrain sites
PYY(3-36) at 30 pmol/kg/min significantly increased the number of Fos (+) neurons in the ARC by 46% [t(14)=1.72, P=0.053] (Figures 1 & 2), the AP by 220% [t(16)=2.52, P<0.05] (Figures 2 & 3), the cmNTS by 550% [t(16)=6.81, P<0.001] (Figures 2 & 3), and the gNTS by 545% [t(16)=5.19, P<0.001] (Figure 2), when compared to vehicle-induced Fos responses in these sites. PYY(3-36) did not increase the number of neurons expressing Fos in AH, DMH, LH, PVN, VMH, or CeA (all P > 0.05) (Figure 2).
Figure 1. Effects of 3-h IV infusion of PYY(3-36) at 30 pmol/kg/min on Fos induction within the ARC using immunocytochemistry.
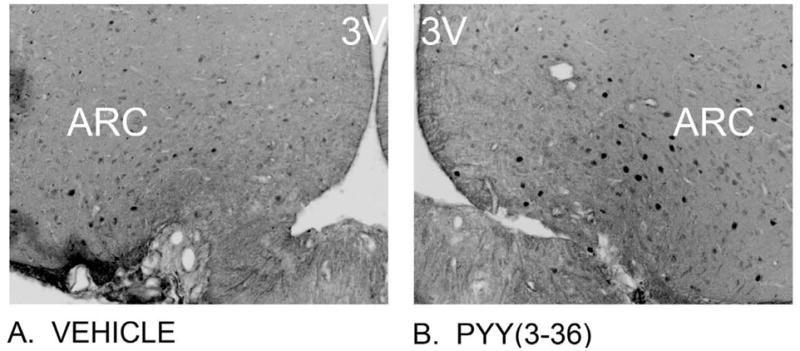
(A) Vehicle elicited a minimal effect on Fos induction in the ARC; (B) PYY(3-36) elicited a robust effect on Fos induction in the lateral ARC. Neural activation is revealed by concentration of immunoreactive Fos in cell nuclei [Fos (+) cells] in the ARC. Fos immunostaining was done by Cy3 fluorescence. Images were captured in grey scale and reversed in order to enhance clarity to allow better visualization of Fos (+) cells from these sections. Images in (A) and (B) are taken from one side of the ARC, with the 3V noted. A–B all visualized at 20X magnification.
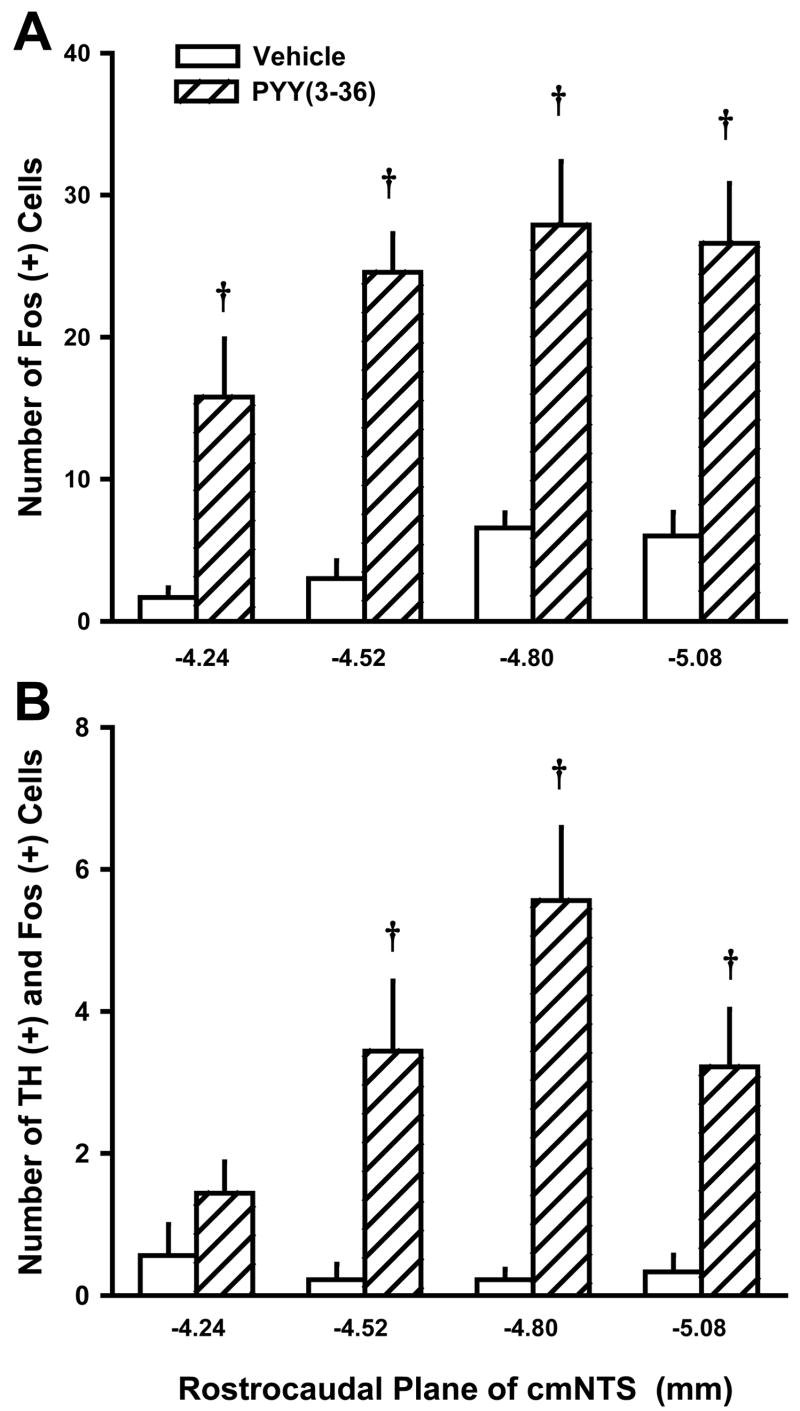
Figure 2. Quantification of Fos (+) neurons within forebrain and hindbrain sites following 3-h IV infusion of PYY(3-36) at 30 pmol/kg/min.
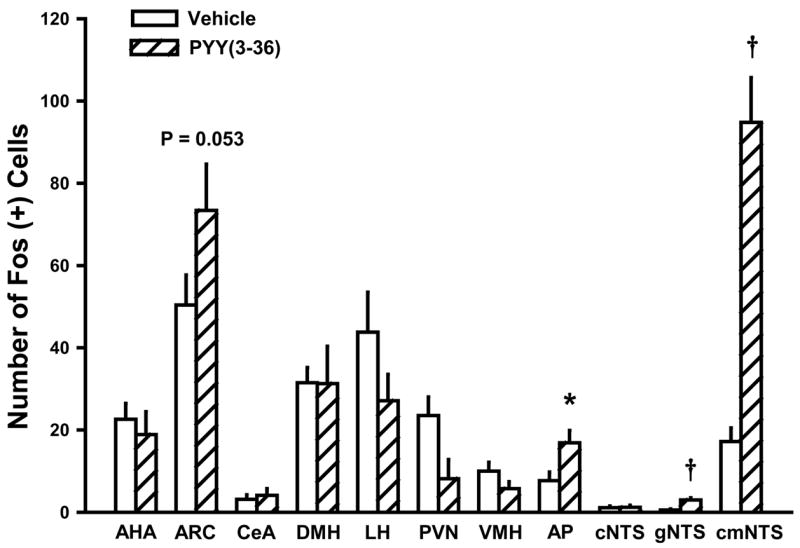
Data represent means ± SE (n = 8 or 9/dose). PYY(3-36) increased the number of Fos (+) neurons in the ARC, AP, cmNTS, and gNTS by 46, 220, 550, and 545% when compared to vehicle Fos (+) neurons in these sites. PYY(3-36) did not increase Fos (+) cells in AH, DMH, LH, PVN, VMH, CeA, or cNTS. *P<0.05, †P<0.01
Figure 3. Effects of 3-h IV infusion of PYY(3-36) at 30 pmol/kg/min on Fos induction within the cmNTS and AP using immunocytochemistry.
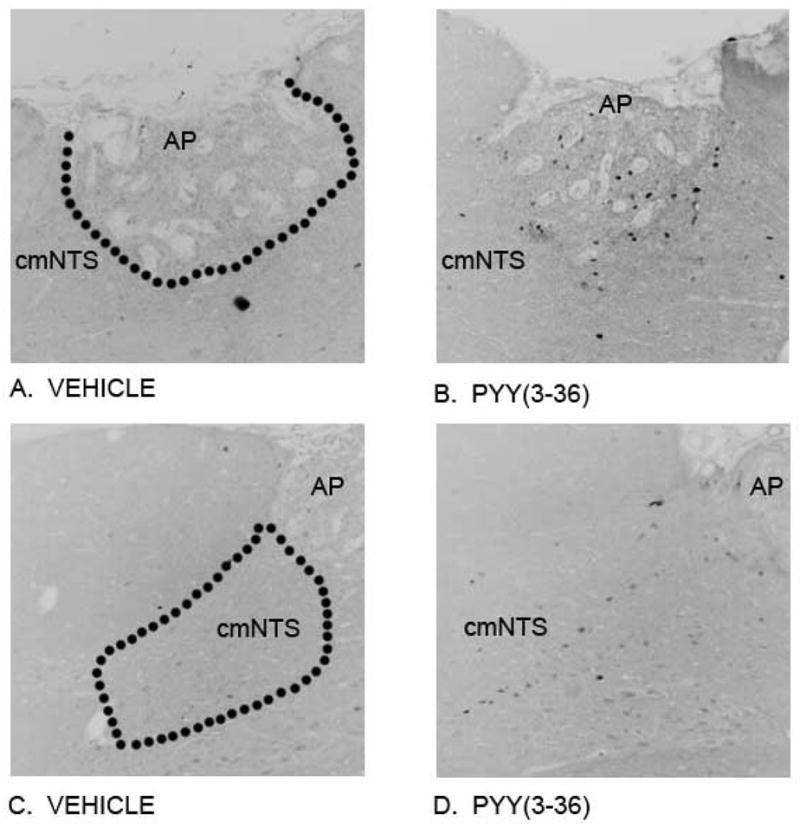
(A) Vehicle induced a minimal amount of Fos in the AP; (B) PYY(3-36) elicited a robust effect on Fos induction in the AP. (C) Vehicle elicited a minimal effect on Fos induction in the cmNTS; (D) PYY(3-36) elicited a robust effect on Fos induction in the cmNTS. Neural activation is revealed by concentration of immunoreactive Fos in cell nuclei [Fos (+)] in the cmNTS or the AP. Fos immunostaining was done by Cy3 fluorescence. Images were captured in grey scale and reversed to enhance clarity to allow better visualization of Fos (+) cells from these sections. Images in (A) and (B) are taken from one side of the cmNTS, with the AP shown in the upper left. (A–D) all visualized at 20X magnification. The dashed line indicates boundaries to indicate the AP (A) and the cmNTS (C) used in the analysis at level 5.08 mm posterior to the interaural line shown in this figure.
3.2. Effects of PYY(3-36) on expression of Fos in TH containing neurons in cmNTS
PYY(3-36) at 30 pmol/kg/min had no effect on the number of TH (+) neurons in the cmNTS (P > 0.05, data not shown), yet PYY(3-36) did increase the number of cmNTS TH (+) neurons that were Fos (+) (Figure 4), with the effect being most notable between 4.52 and 5.08 mm posterior to the interaural line, an area that encompasses the middle to caudal portion of the cmNTS (Figure 5). However, nearly 90% of the PYY(3-36) activated Fos (+) neurons were not TH (+) (Figure 5).
Figure 4. Effects of 3-h IV infusion of PYY(3-36) at 30 pmol/kg/min on Fos induction in TH-containing neurons in the cmNTS using immunocytochemistry.
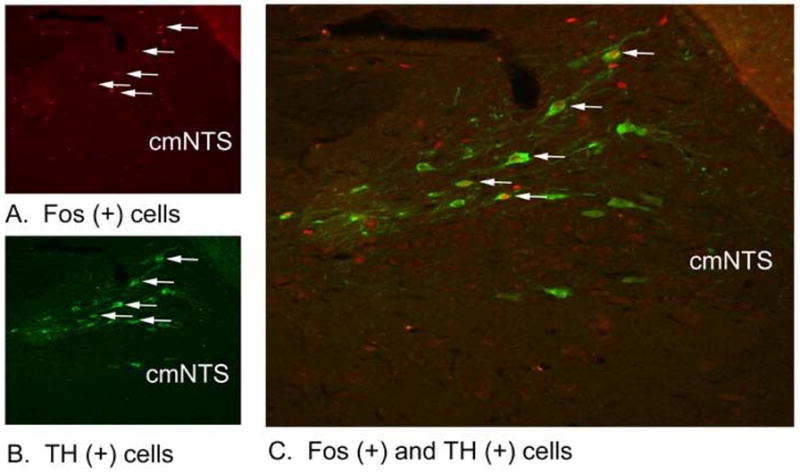
Neural activation is revealed by concentration of immunoreactive Fos in cell nuclei [Fos (+)] in the cmNTS. Fos- and TH-immunostaining were done by Cy3 fluorescence and Alexa 488 fluorescence, respectively. Images are taken from the whole AP or one side of the cmNTS. (A) Peripheral infusion of PYY(3-36) increased Fos (+) neurons in cmNTS. (B) TH staining in cmNTS from an animal infused with PYY(3-36). (C) Colocalization of TH (+) and Fos (+) neurons in cmNTS from a PYY(3-36) treated animal. (A–C) all visualized at 20X magnification.
Figure 5. Anatomical Resolution of Fos response in TH (+) neurons in cmNTS following 3-h IV infusion of PYY(3-36).
Data represent means ± SE. Data were grouped at each of the anatomically matched levels (n = 9/dose and level). Fos (+) and TH (+) neurons were analyzed at 280 μm intervals. PYY(3-36) increased the number of neurons in the cmNTS expressing both TH and Fos, with the effect being most notable between 4.52 and 5.08 mm posterior to the interaural line, along the middle to caudal portion of the cmNTS. †P<0.01
4. Discussion
Several lines of evidence suggest that PYY(3-36) inhibits food intake through direct action at Y2 receptors [1,21], yet the brain circuits underlying the anorexigenic response to systemic administration of PYY(3-36) remain to be determined. Our aim here was to detect neurons which express Fos, a marker of neural activation, in order to identify brain sites activated by an anorexigenic dose of PYY(3-36). Since PYY(3-36) has been reported both to penetrate the blood-brain barrier [25] and to activate vagal sensory nerves [21], we examined Fos-expressing neurons in forebrain and hindbrain sites containing Y2 receptors and linked to control of food intake (AH, ARC, DMH, LH, PVN, VMH, CeA, AP, NTS), and in hindbrain sites directly innervated by vagal sensory neurons (AP, NTS). Our results show that in adult, male rats, a 3-hour IV infusion of an anorexigenic 30 pmol/kg/min dose of PYY(3-36) increased the number of Fos (+) neurons in the forebrain ARC nucleus, and in several distinct areas in the hindbrain NTS and AP, especially within the cmNTS. No increase in Fos (+) neurons was observed in the AH, DMH, LH, PVN, VMH, or CeA. To determine the extent to which PYY(3-36) activates catecholaminergic neurons in the cmNTS, we double immunostained for both Fos and tyrosine hydroxylase (TH; a marker of catecholamine synthesis). PYY(3-36) increased the number of TH (+) cells containing Fos (+) nuclei in the cmNTS, yet nearly 90% of the neurons containing Fos did not contain TH. These results suggest that PYY(3-36) inhibits feeding through direct activation of forebrain ARC neurons, and direct and/or indirect activation of AP, cmNTS and gNTS neurons, including a relatively small number of catecholaminergic neurons in the cmNTS.
Our findings are consistent with those of other studies suggesting that PYY(3-36) inhibits food intake through direct activation of Y2 receptors in the ARC. In rodents, IP injection of PYY(3-36) decreases NPY mRNA expression in the ARC [9], either has no effect [5] or increases POMC mRNA expression in the ARC [9], causes a nearly 2-fold increase in number of Fos (+) cells in the ARC [5], and produces a 13% [20] to 260% increase in Fos (+) POMC neurons in the ARC [5]. Furthermore, direct administration of Y2 agonists PYY(3-36) and Ac-[Leu28,Leu31] NPY(24-36) into the ARC dose-dependently inhibits food intake in rats [5], while PYY(3-36) administration into cerebral ventricles either has no effect or potently increases food intake [36]. Thus, Batterham et al. [5] initially postulated that the anorexigenic response to circulating PYY(3-36) is mediated by a Y2 receptor-mediated mechanism in the ARC. Our results provide further support for a central role for the ARC in mediating PYY(3-36)-induced suppression of food intake by showing that other forebrain sites containing Y2 receptors and linked to control of food intake, including the AH, DMH, LH, PVN, VMH, and CeA, are not activated by an anorexigenic dose of PYY(3-36) that activates ARC neurons.
Our findings are consistent with those of other studies suggesting that PYY(3-36) may also inhibit food intake through a Y2-receptor-mediated activation of abdominal vagal sensory nerves. In rodents, vagal neurons contain Y2 receptors [21], peripheral administration of PYY(3-36) stimulates vagal afferent nerve activity [21], and vagal denervation blocks the anorexic response to peripheral administration of PYY(3-36) in rats [1,21], but not mice [19]. Our findings extend these results by showing that systemic administration of an anorexigenic dose of PYY(3-36) activated neurons in hindbrain areas (cmNTS, gNTS, and AP) that receive and integrate incoming meal-related satiety signals transmitted by vagal sensory neurons from the gut [17,26,32,38]. These sites are similar to those activated by peripheral administration of the prototypic satiety peptide CCK-8, which is also postulated to inhibit food intake by activating vagal sensory nerves [7,31]. Results of the present study extend those of Tache et al., which showed that IP injection of porcine PYY(1-36) increases Fos in the mNTS, cNTS and AP [8]. The absence of PYY(3-36)-induced Fos in the cNTS in our study may be attributed to differences in host PYY administered (porcine vs rat), form of PYY administered [PYY(3-36) vs. PYY(1-36)], or mode and dose of peptide administration (3-h IV infusion vs. IP injection). More recently, PYY(3-36) was reported to increase Fos in the AP and the intermediate NTS (iNTS) of mice [19]. Our analysis did not differentiate between the cmNTS and iNTS due to the ambiguous boundaries separating these adjacent regions. Taken together, these results suggest that PYY(3-36) activates neurons in the NTS and AP by increasing the activity of vagal sensory neurons. However, because Y2 receptors are found in both the NTS and the AP, PYY(3-36) may directly activate neurons in these regions [28]. It remains to be determined whether blockade of vagal sensory neurons attenuates PYY(3-36)-induced activation of NTS and AP neurons.
Our finding that PYY(3-36) activates TH (+) neurons in the cmNTS are consistent with the hypothesis that PYY(3-36) inhibits food intake in part through direct activation of vagal afferent neurons and downstream catecholaminergic neurons in brain sites similar to those activated by CCK-8 and gastric distention. In the present study, the anatomical distribution of PYY(3-36)-induced activation of TH (+) cells in the cmNTS between levels 4.52 and 5.08 mm posterior to the interaural line is similar to the distribution pattern for CCK-8-induced activation of TH (+) cells in the cmNTS reported by Rinaman et al. [31]. Willing and Berthoud [38] also demonstrated that gastric distension increases Fos in TH (+) cells in the cmNTS. Thus, PYY(3-36), CCK-8, and gastric distension may inhibit food intake in part through activation of similar noradrenergic circuits in the hindbrain. However, 95% of the cmNTS neurons activated by gastric distention [38], 50% of the cmNTS neurons activated by CCK-8 [31], and 90% of the cmNTS neurons activated by PYY(3-36) (present study), are not catecholaminergic.
It is not clear from our findings whether PYY(3-36)-induced activation of the ARC precedes or follows activation of the NTS, or whether activation of the ARC causes activation of the NTS or vice versa. Descending pathways link the ARC to the NTS [2,27,39] and an ascending noradrenergic pathway links the NTS to the ARC [13]. The relative contribution of AP/NTS and ARC sites in mediating PYY(3-36)-induced suppression of food intake remains to be determined.
Acknowledgments
This research was supported by biomedical research core programs of the National Institutes of Health (NIH) Diabetes Endocrinology Research Center, and the Departments of Veterans Affairs Medical Center Research Services at the Seattle VA Puget Sound Health Care System and the Nebraska Western Iowa Health Care System. Additional support came from the Merit Review Research Program of the Department of Veterans Affairs, National Institutes of Health Grants DK-55830, DK-56805, DK-73152, RR-16469, and DK-41301, and Canadian Institutes of Health Research. This material is based upon work supported by the Office of Research and Development Medical Research Service, Department of Veterans Affairs. The authors would like to acknowledge the technical support of David Caldwell, Nishi Gill, Dean Heimann, and Jenny Kam, as well as the effort of Dr. Denis Baskin in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. E. Blevins, Email: jeblevin@u.washington.edu.
P. K. Chelikani, Email: prasanth.chelikani@gmail.com.
A. C. Haver, Email: ahaver@juno.com.
R. D. Reidelberger, Email: roger.reidelberger@va.gov.
References
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Alessi NE, Quinlan P, Khachaturian H. MSG effects on beta-endorphin and alpha-MSH in the hypothalamus and caudal medulla. Peptides. 1988;9:689–95. doi: 10.1016/0196-9781(88)90108-8. [DOI] [PubMed] [Google Scholar]
- 3.Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase Peptide synthesis. Mol Biotechnol. 2006;33:239–54. doi: 10.1385/MB:33:3:239. [DOI] [PubMed] [Google Scholar]
- 4.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 5.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 6.Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med (Maywood) 2003;228:217–44. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- 7.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Bonaz B, Taylor I, Tache Y. Peripheral peptide YY induces c-fos-like immunoreactivity in the rat brain. Neurosci Lett. 1993;163:77–80. doi: 10.1016/0304-3940(93)90233-b. [DOI] [PubMed] [Google Scholar]
- 9.Challis BG, Pinnock SB, Coll AP, Carter RN, Dickson SL, O'Rahilly S. Acute effects of PYY3-36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun. 2003;311:915–9. doi: 10.1016/j.bbrc.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 10.Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R298–305. doi: 10.1152/ajpregu.00674.2005. [DOI] [PubMed] [Google Scholar]
- 11.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3-36) potently inhibits food intake in rats. Endocrinology. 2005;146:879–88. doi: 10.1210/en.2004-1138. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Eriste E, Jonsson AP, Norberg A, Nepomuceno D, Lovenberg TW, Bergman T, Efendic S, Jornvall H, Sillard R. Ser(13)-phosphorylated PYY from porcine intestine with a potent biological activity. FEBS Lett. 2001;492:119–22. doi: 10.1016/s0014-5793(01)02234-7. [DOI] [PubMed] [Google Scholar]
- 13.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–31. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology. 2005;129:1430–6. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Eberlein GA, Eysselein VE, Schaeffer M, Layer P, Grandt D, Goebell H, Niebel W, Davis M, Lee TD, Shively JE, et al. A new molecular form of PYY: structural characterization of human PYY(3-36) and PYY(1-36) Peptides. 1989;10:797–803. doi: 10.1016/0196-9781(89)90116-2. [DOI] [PubMed] [Google Scholar]
- 16.Fetissov SO, Byrne LC, Hassani H, Ernfors P, Hokfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J Comp Neurol. 2004;470:256–65. doi: 10.1002/cne.11047. [DOI] [PubMed] [Google Scholar]
- 17.Fraser KA, Raizada E, Davison JS. Oral-pharyngeal-esophageal and gastric cues contribute to meal-induced c-fos expression. Am J Physiol. 1995;268:R223–30. doi: 10.1152/ajpregu.1995.268.1.R223. [DOI] [PubMed] [Google Scholar]
- 18.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul Pept. 1994;51:151–9. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 19.Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab. 2005;1:159–68. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145:2585–90. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- 21.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 22.Koegler FH, Enriori PJ, Billes SK, Takahashi DL, Martin MS, Clark RL, Evans AE, Grove KL, Cameron JL, Cowley MA. Peptide YY(3-36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes. 2005;54:3198–204. doi: 10.2337/diabetes.54.11.3198. [DOI] [PubMed] [Google Scholar]
- 23.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 24.Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288:R384–8. doi: 10.1152/ajpregu.00535.2004. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Ther. 2003;306:948–53. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- 26.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci. 1993;4:93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- 27.Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res. 1987;436:323–38. doi: 10.1016/0006-8993(87)91676-3. [DOI] [PubMed] [Google Scholar]
- 28.Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–48. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Carrive P, Wang H, Wang P-Y. Chemo Architectonic Atlas of the Rat Brainstem. Orlando: Academic Press; 1999. [Google Scholar]
- 30.Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci. 2003;23:10084–92. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinaman L, Verbalis JG, Stricker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol. 1993;338:475–90. doi: 10.1002/cne.903380402. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–88. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 33.Shaw JL, Gackenheimer SL, Gehlert DR. Functional autoradiography of neuropeptide Y Y1 and Y2 receptor subtypes in rat brain using agonist stimulated [35S]GTPgammaS binding. J Chem Neuroanat. 2003;26:179–93. doi: 10.1016/j.jchemneu.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–8. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 35.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–56. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 36.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004;430:1. doi: 10.1038/nature02665. [DOI] [PubMed] [Google Scholar]
- 37.Weeks JR. Long-term intravenous infusion. In: Myers RD, editor. Methods in Psychobiology. London: Academic Press; 1972. pp. 155–68. [Google Scholar]
- 38.Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol. 1997;272:R59–67. doi: 10.1152/ajpregu.1997.272.1.R59. [DOI] [PubMed] [Google Scholar]
- 39.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–58. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]