Abstract
Prior work has consistently revealed a relationship between antisocial behavior and reduced P300 amplitude. Fewer studies have directly evaluated behavioral indices of aggression and P300, and those that have generally do not account for potential mediating variables such as age, intelligence, and behavioral task performance. The current study assessed the relationship between the total number of convicted violent and non-violent offenses and P300 in a sample of inmates from a medium security state prison. Violent offenses evidenced a robust negative relationship with P300 amplitude, whereas non-violent offenses did not. Additional analyses evaluated age, intelligence, and behavioral task performance as potential mediating variables. Only reaction time significantly predicted P300 amplitude, and mediational analyses showed that this relationship did not account for the violent-offense/P300 relationship. Findings are discussed in terms of personality correlates and neurobiological process related to aggression.
There is long-standing interest in the notion that antisocial behavior, and aggression in particular, involves neurobiologically-based deficits in information processing. Neuropsychological research has revealed that antisocial behavior is associated with impaired executive function (c.f. Morgan & Lilienfeld, 2000), and neuroimaging studies have consistently identified frontal lobe abnormalities among violent offenders (Goyer, Andreason, Semple, & Clayton, 1994; Raine, Buchsbaum, & LaCasse, 1997; Raine, Lencz, Bihrle, LaCasse, Colletti, 2000; Volkow, Tancredi, Lawrence, Grant, Gillespie, & Hampton, 1995). Furthermore, research using event-related brain potentials has indicated that antisocial behavior is associated with reduced P300 responses to task-relevant stimuli in target detection tasks (e.g., Bauer, O'Connor, & Hesselbrock, 1994; Iacono, Malone, & McGue, 2003). These deficits may reflect inefficient neural processing of salient environmental stimuli (Donchin & Coles, 1988), which could potentially contribute to risk for antisocial deviance.
Notably, antisocial behavior encompasses both violent and nonviolent transgressions. One unresolved issue is whether P300 amplitude is associated with both violent and non-violent forms of antisocial behavior, or more predominantly with aggressive forms of acting out. Some research has examined P300 response in aggressive individuals specifically (e.g., Barratt, Stanford, Kent, & Felthous, 1997); this work has revealed that reduced P300 amplitude is selectively related to impulsive, but not instrumental, aggression. However, this work has not compared associations for aggressive versus non-aggressive offending behavior. Thus, the aim of the present study was to replicate and extend prior research on antisocial behavior and brain response by examining relations between violent and non-violent offenses and P300 amplitude in a sample of adult male inmates. Our prediction was that reduced P300 response would be associated more predominantly with a history of violent offending, and that this effect would be independent of age, intelligence, and task performance effects.
Aggression and P300
Prior work has consistently revealed links between antisocial behavior and reduced P300 amplitude. These investigations have largely focused on individuals with diagnoses of antisocial personality disorder (APD) or its developmental precursor, conduct disorder (CD), as determined by the Diagnostic and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association, 1994). Bauer and colleagues have shown that adult males with APD exhibit reduced P300 amplitude during visual oddball tasks (Bauer, 1997; Bauer, et al., 1994; O'Connor, Bauer, Tasman, & Hesselbrock, 1994). Furthermore, P300 amplitude correlates negatively with APD symptom counts and retrospectively assessed CD symptoms (Costa et al., 2000). Reductions in P300 amplitude have also been reported among adolescents with CD in both auditory (Bauer & Hesselbrock, 2003) and visual oddball tasks (Bauer & Hesselbrock, 1999a; Iacono, Carlson, Malone, & McGue, 2002; Iacono, Malone, & McGue, 2003) as well as the Stroop task (Bauer & Hesselbrock, 1999b). Recent work has demonstrated that the latent externalizing factor representing the covariance (shared or overlapping variance) among APD, CD, and substance use disorders is also related to reduced P300 amplitude (Patrick, Bernat, Malone, Iacono, Krueger, & McGue, 2006).
However, while aggressive tendencies are featured prominently in the diagnostic criteria for APD and CD, and are central to the construct of externalizing (e.g., Krueger, Caspi, Moffitt, Silva, & McGee, 1996), relatively few studies have examined P300 amplitude in relation to more direct indices of aggressive behavior. Harmon-Jones, Barratt, and Wigg (1997) found aggressive behaviors and attitudes to be inversely correlated with P300 amplitude in a sample of adolescents. In a study involving a sample of undergraduates assessed for aggression via interview, Gerstle, Mathias, and Stanford (1998) found that aggression was related to reduced P300 amplitude in an auditory oddball task. Similar effects were reported for both standard and three-stimulus visual oddball tasks in a subsequent study from the same research group (Mathias & Stanford, 1999). Branchey and colleagues (Branchey, Buydens-Branchey, & Horvath, 1993; Branchey, Buydens-Branchey, & Lieber, 1988) have found reduced P300 amplitude among alcoholic and abstinent substance-abusing men with documented histories of aggressive behavior, with the lowest amplitudes recorded among those subjects who had histories of incarceration for violent crimes (Branchey et al., 1988). Barratt et al. (1997) examined differences in P300 amplitude between non-aggressive community controls and male inmates classified via interview as impulsively or instrumentally aggressive. Impulsively aggressive inmates demonstrated the lowest amplitude P300 responses relative to the other two groups in this study. Furthermore, P300 amplitude was negatively correlated with self-report indices of trait anger and impulsivity.
The Current Study
In the present study we investigated the relationship between P300 amplitude and both violent and non-violent criminal offenses. Additionally, mediating effects of age, IQ, and task performance data were evaluated. These relationships were explored in a large (N = 141) sample of adult male inmates incarcerated in a medium-security state correctional facility. Our experimental paradigm consisted of a standard visual oddball task in which subjects were instructed to ignore frequent non-target stimuli, and make button-press responses to infrequent target stimuli. It was hypothesized that violent criminal offenses would relate more strongly to reduced P300 amplitude than non-violent offenses. In addition, we predicted that while age, IQ, and task performance data might each individually predict P300 amplitude, violent offenses would still have unique predictive power with regard to P300 amplitude after controlling for these other factors.
Methods
Participants
Participants were 141 male residents (57.1% White, 28.6 % Black, 9.3% Hispanic, 5.0% other; mean age presented in Table 2) of a medium security correctional facility located near Minneapolis, Minnesota, selected from a larger group of volunteers (N=274) assessed for purposes of a larger study involving psychopathy assessment. Data from three subjects were excluded due to responses exceeding 3 SD from the group mean, one for excessive P300 amplitude and 2 for low mean accuracy (24% and 50% correct responses in the task). Thus, 138 participants were available for the primary analysis. Ns lower than this for the mediational variables were due either to either behavioral response recording equipment failure (three) or measures that were not collected for some participants (i.e., age for 1 participant and IQ for 18 participants). WAIS-IQ scores were estimated for each participant using the Shipley Institute of Living Scale (Zachary, 1994). All participants were convicted felons who were still under sentence at the time of the study. Volunteers were randomly selected from the institutional roster and received $20 each to participate. Individuals with visual or hearing impairments, as assessed via a screening questionnaire, were excluded from the study.
Table 2.
Means, Standard Deviations, and Intercorrelations for the Study Variables
| Correlations | M (SD) | N | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 1. Violent | 2.77 (2.35) | 138 | ||||||
| 2. Non-Violent | .22* | 8.59 (7.63) | 138 | |||||
| 3. Accuracy | -.03 | .14 | .96 (.05) | 135 | ||||
| 4. Reaction Time | .31*** | .03 | .01 | 929.62 (216.20) | 135 | |||
| 5. Age | .14 | .13 | -.05 | .32** | 32.09 (8.68) | 137 | ||
| 6. Predicted WAIS-R | -.22* | -.06 | .27** | -.20** | .14 | 94.16 (13.80) | 120 | |
| 7. P300 amplitude | -.22** | .08 | .01 | -.24** | -.06 | .08 | 12.13 (3.39) | 138 |
p<.05
p<.01
p<.001
Note: P300 amplitude is averaged across Fz, Cz, Pz electrodes.
Offense Data
Before psychophysiological testing, institutional file data – consisting of case histories, criminal records, police and court reports, medical and psychiatric data, and daily summaries of institutional behavior – were used to assess the total number of offenses committed by each offender. Offenses considered violent were: murder/attempted murder, robbery, assault, possession of a weapon, sexual offenses, kidnapping/false imprisonment, arson, and criminal negligence with injury. Offenses considered non-violent were: theft-related (including burglary), trespassing/invading privacy, drug-related, fraud/forgery, parole or probation violation/escape/failure to appear, obstruction of justice/perjury, impaired or dangerous driving, and miscellaneous minor charges such as vandalism/mischief/willful damage. Only offenses that the offender was convicted and sentenced for were considered. The mean number of violent offenses for each inmate was 2.76 (SD = 2.33, Range = 0-12). The mean number of non-violent offenses for each inmate was 8.56 (SD = 7.58, Range = 0-30). Trained assessors, with at least a Bachelor's degree, completed assessments on each inmate.
Psychophysiological Assessment
ERP Task and Recording Procedure
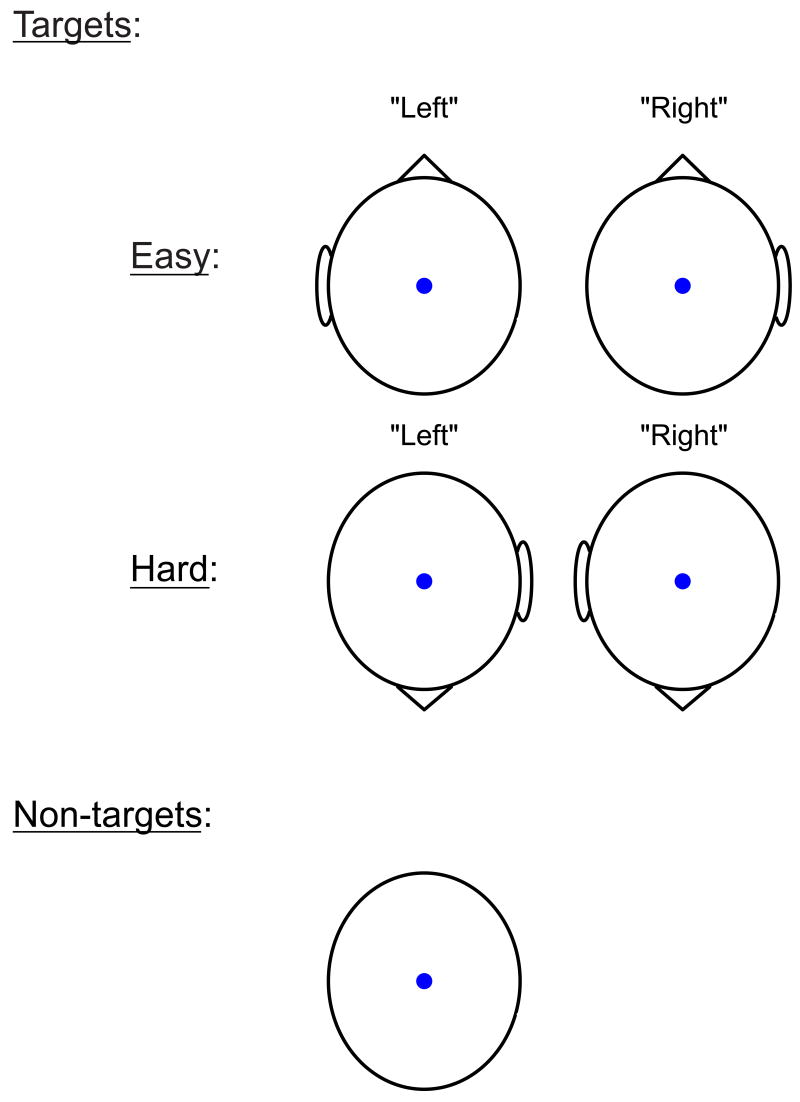
A rotated-heads oddball task (Begleiter, Porjesz, Bihari, and Kissin, 1984) was used to elicit P300 ERP responses. The implementation of the task was modeled after that of Malone, Iacono, and McGue (2001). Two hundred and forty trials were presented, consisting of 80 target and 160 non-target white stimuli presented on a black background. All stimuli were presented for 100ms, with a randomly varying ISI of 2500-3500 ms and a 1500 ms response window. Target stimuli consisted of schematic oval heads including a nose and one ear (Figure 1). The task was to press one of two buttons on a button-box situated on the participant's lap, with either the left or right hand respectively, to indicate whether the ear was on the left or right side of the head. For 40 of the targets (50% of target trials) the nose was pointed up, constituting the Easy condition. For the other 40 targets, the nose was pointed down, requiring that participants recognize, for example, that the ear was on the right side of the head even though it was on the left side of the screen, and vice versa. This constituted the Hard target condition. Non-target stimuli were ovals with no nose or ear. Participants completed a practice block of 20 trials (8 targets) before preceding to the main task. Participants who did not achieve 90% or greater accuracy during the first practice block completed additional blocks until 90% was achieved. During the experiment, participants sat in a padded recliner at a distance of 100 cm from a 21-in. computer monitor positioned directly in front of them. Physiological responses were recorded using a PC computer running Neuroscan acquisition software (Acquire version 4.2). A second PC running E-Prime software (MEL software, Inc.) was utilized for stimulus delivery and behavioral response collection.
1.
Schematic depiction of stimuli used in the rotated-heads visual oddball task. Non-targets (bottom) occurred on 160 trials and required no response. Each of the four target stimuli was presented on 20 trials; for these stimuli, the participant pressed a button with either the left or right hand to indicate the side of the head on which the ear was positioned. For easy head targets (top), the nose was pointed up and thus the correct button response (“left” or “right”) corresponded directly to the side of the screen on which the ear appeared. For hard targets (middle), the nose was pointed down and thus the correct button response was opposite to the side of the screen on which the ear appeared.
Physiological Measures and Data Preprocessing
ERP responses were recorded from scalp sites FP1, FP2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8 and Oz using a Neuroscan Quick-Cap (Ag/AgCl) with electrolyte paste. During online collection, the data were referenced to FCZ, and arithmetically re-referenced to the average of left and right mastoid electrodes offline. Data were collected at a sampling rate of 2,000 Hz with an online analog band pass filter of .05-500 Hz. Data epochs from −1000 ms to 2000 ms were extracted from the continuous recordings using Neuroscan EDIT software (version 4.2, Neuroscan Inc.) and corrected for eye movements using an algorithm defined in Semlitsch, Anderer, Schuster, and Presslich (1986), as implemented in the EDIT software. These epoched and corrected data were imported to Matlab (Mathworks, Inc.) for subsequent data processing. Data were subsampled to 256 Hz using the Matlab resample command (which applies a low pass antialiasing filter before downsampling), after applying a digital .5Hz high pass 3rd order Butterworth filter to reduce low frequency artifacts. Trials during which activity exceeded +/- 100 μV, relative to 500 ms baseline, were excluded from further processing. Data were averaged within each of the target stimulus conditions: easy-left, easy-right, hard-left, hard-right. Using these averages, P300 was scored as the maximum value between 250 and 600 ms post stimulus, relative to the median activity during a 500 ms baseline.
Data Analysis
The data analytic strategy was designed first to assess the relationship between P300 and offense history, and second to evaluate performance, age, and IQ as mediators of this relationship. For the first purpose, a mixed-model repeated measures general linear model (GLM, SPSS version 11, SPSS Inc.) was conducted, where both categorical and continuous predictors can be entered as within or between subjects predictors. P300 served as the continuous dependent measure, electrode site was a categorical within-subjects repeated measure (Fz, Cz, and Pz), and the numbers of violent and non-violent offenses were both entered as continuous between-subjects predictors. Separate analyses were initially conducted for both the amplitude and latency parameters of the P300, however, latency evidenced no significant relationships with any of the terms in the model, and for reasons of brevity, latency results are not presented. These two analyses were also run with task difficulty (easy/hard discrimination) included as a repeated measures factor, but it did not emerge as a significant predictor; again, for reasons of brevity, results of analyses including this factor are not presented. Thus, presented analyses are for P300 peaks averaged across these conditions.
To evaluate the impact of task performance, age, and IQ as possible mediators of the association between offense behavior and P300 amplitude, hierarchical regression analyses were performed. In all regressions, P300 amplitude served as the dependent measure, and the number of violent and non-violent offenses were entered as separate predictors in the first step of the two-step regression analyses. Next, in the second step of each regression, one of the four potential mediators was entered (i.e., one per regression). Using this method, the unique contribution of each potential mediator could be evaluated at the second step. Two measures are available in the second step. First, the change in R2 indicates whether the added variable significantly increases the overall variance accounted for by the model. Second, the significance of the added variable indicates whether the added variable uniquely predicts P300, beyond the offense variables. For a variable to qualify as a mediator, it must first relate to both the predictor and criterion variables (violent offenses and P300). Then, when entered into a regression together with the offense data to predict P300, violent offenses must become nonsignificant (Baron and Kenny, 1986).
Results
P300 Amplitude and Number of Offenses
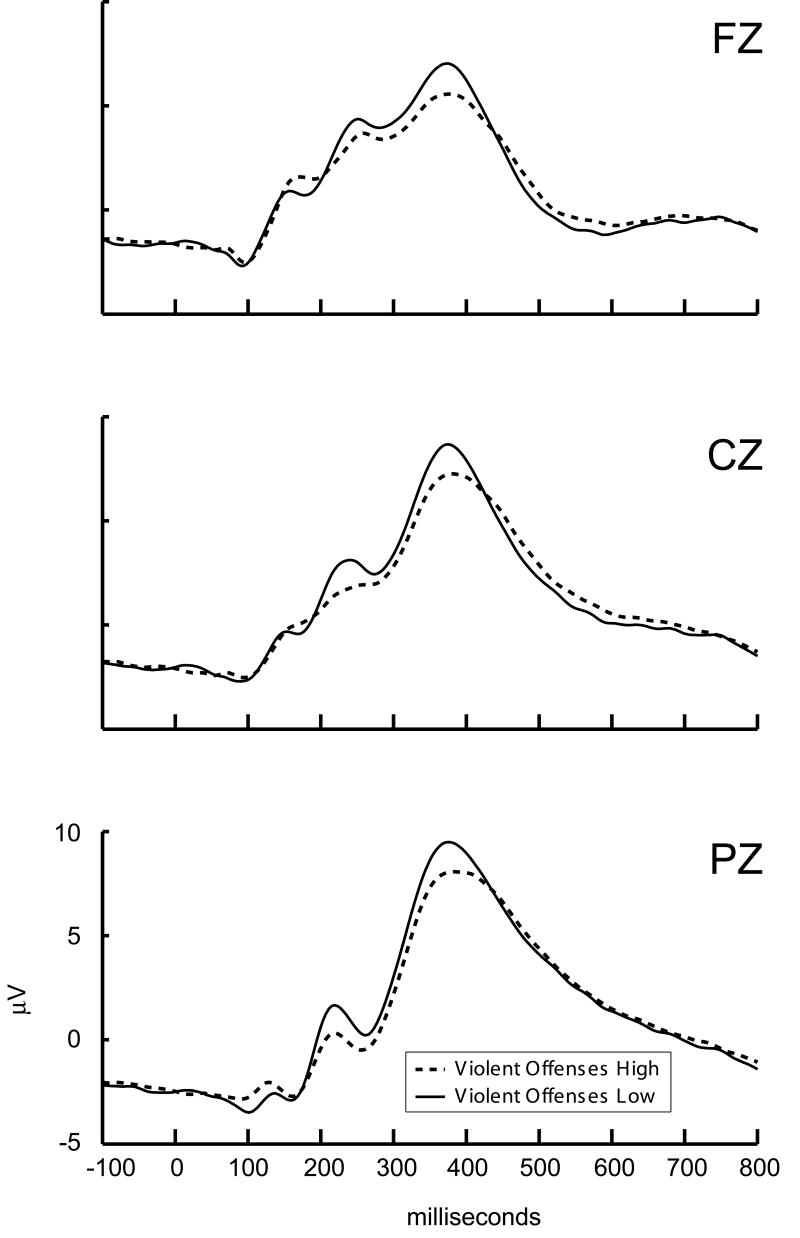
Results from this GLM are presented in Table 1. For the offense variables, significant effects were specific to the violent offenses: violent offenses significantly predicted reduced P300 amplitude, whereas non-violent offenses did not. There was also a main effect of electrode site, reflecting the fact that P300 responses were largest at the parietal, next at central, and smallest at the frontal sites, as expected in a standard oddball task. Figure 2 depicts P300 waveforms based on a median split for the number of violent offenses; the reduction in P300 amplitude is readily apparent.
Table 1.
GLM Including Offense Data and Electrode
| df | F | p | Partial eta2 | |
|---|---|---|---|---|
| Violent | 1,135 | 8.87 | .003 | .062 |
| Non-Violent | 1,135 | 2.61 | ns | |
| Electrode | 2,134 | 14.40 | .001 | .177 |
| Electrode X Violent | 2,134 | <1 | ||
| Electrode X Non-Violent | 2,134 | <1 |
Note: Electrode = Fz, Cz, Pz
2.
P300 target responses. High and low groups represent a median split of the total number of convicted violent offenses. Note that while median split groups are used for visual depiction, continuous counts of offenses were used in the analyses.
Role of Potential Mediators on P300/Violent-Offense Relationship
Correlations among the offense variables, task performance, age, and IQ measures, and P300 amplitude are presented in Table 2, along with means, standard deviations, and numbers of participant data for each variable. P300 amplitude in the correlations is average across electrodes, used because the offense data did not show significant interactions with electrode. P300 amplitude demonstrated significant correlations only with violent offenses and reaction time. Reaction time also evidenced a significant negative association with violent offenses. These results indicate that only reaction time qualified as a potential mediator (i.e., showed significant relations with both the predictor and the criterion; Baron and Kenny, 1986).
As noted earlier, hierarchical regressions were used to test for mediation. The offense variables were entered on the first step as predictors of P300, followed by the potential mediator (reaction time) on the second step. Inclusion of reaction time in the second step resulted in a significant change in R2 (step 1, offense data: ΔR2=.069, ΔF(2,132)=4.918, p<.009; step 2, reaction time: ΔR2=.030, ΔF(1,131)=4.390, p<.038). However, with offense data and reaction time both in the model, violent offenses and reaction time each uniquely and significantly predicted P300 amplitude reduction (violent offenses: t(1,131)=-2.214, p<.029; reaction time: t(1,131)=-2.095, p<.038), whereas non-violent offenses did not (t(1,131)=1.542, ns). Thus, while reaction time did relate to both violent offenses and P300, it did not mediate the relationship between the two. For the sake of thoroughness (even though they would not qualify as mediators) we performed this same analysis for accuracy, age, and IQ. As expected, none of these variables yielded a significant change in R2 when entered on the second step.
Discussion
Offense Data and P300 Amplitude
In the present study we investigated the role of violent and non-violent criminal convictions in the prediction of P300 brain response amplitude in a visual oddball task. We found that violent offenses were related to reduced P300 amplitude in response to target stimuli. This relationship remained significant even after accounting for the effects of age, intelligence, response accuracy, and reaction time. Furthermore, our data indicated a clear distinction between offense types, such that violent offenses were related to reduced P300 amplitude, whereas non-violent offenses were unrelated to P300. In fact, non-violent offenses were associated in the opposite direction with P300 amplitude, although this relationship failed to reach statistical significance. Nonetheless, the discrepant relationships of the two offense types with P300 is noteworthy, and suggests that unique neurobiological processes may be involved in these behaviors. Violent behavior, particularly aggression that is reactive (responding to threatening cues in the environment) rather than instrumental (involving perceived gain) likely reflects markedly deficient impulse control. At the neurobiological level, such shortcomings in behavioral regulation may be linked to deficits in cognitive-executive functioning, as reflected by P300 decrements. Even within a generally deviant sample such as that of the present study (i.e., adult male inmates) a violent history predicts incrementally more variance in electrocortical response deficits beyond the general antisocial deviance that characterizes all study participants. In contrast, non-violent offenses, which in this study included drug trafficking and property offenses, could reflect a form of antisocial behavior that is more closely associated with motivation for gain rather than deficient impulse control. Furthermore, those non-violent offenses that involve some degree of planning ability may be more prevalent among offenders with relatively intact neurobiological functioning. For example, individuals capable of planning elaborate burglaries or managing drug selling operations might be relatively high-functioning compared to individuals whose antisocial behavior manifests primarily in the form of impulsive aggression.
Limitations
Some limitations of the present study should be noted. First, our primary independent measure was official crime data, the drawbacks of which have been pointed out elsewhere (Hood & Sparks, 1970; Klein, 1987). The most frequently cited critique of official crime data as a measure is that it underestimates the frequency of antisocial behavior, as many if not most crimes are either not reported or adjudicated. To the extent this is the case, however, the likely effect of this underestimation would be to attenuate correlations between violent offenses and physiological measures due to range restriction in the independent variable. Secondly, the measures used in the present study did not differentiate between impulsive/reactive and instrumental forms of aggression, as in prior research (Barratt et al., 1997; Gerstle et al., 1998; Mathias & Stanford, 1999). However, our results closely resemble those of previous studies, which have largely found P300 amplitude reductions to be selectively associated with impulsive aggression (Barratt et al., 1997), with reductions largest for individuals having violent criminal convictions (Branchey et al., 1988). It should also be noted that the present study did not include a comparison sample of non-offenders from the community. How would the responses of offenders in the current study have compared with non-offenders? Prior research suggests that incarcerated offenders (both violent and non-violent) exhibit reduced P300 amplitude relative to community controls (e.g. Barratt et al., 1997). The current study extends this prior work by demonstrating an association between P300 and violent offenses but not with non-violent offenses Finally, psychiatric diagnostic data would be helpful to better understand the specificity of the violent-offence relationships with P300. P300 has been shown to be reduced in relation to several disorders, most prominently including alcoholism (Porjesz, Rangaswamy, Kamarajan, Jones, Padmanabhapillai, and Begleiter, 2005), as well as depression and schizophrenia (Blackwood, Whalley, Chritine, Blackburn, St. Clair, and McInnes, 1987; Bruder, Tenke, Steward, Towey, Leite, Voglmaier, and Quitken, 1995; Roth and Cannon, 1972). The lack of such comparison data is a limitation in the current report, although the difference between violent and non-violent offenses remains discriminative in the current sample as a behavioral index.
Conclusions
Results from for the current large adult male inmate sample indicate that violent offenses, but not non-violent offenses, are related to reductions in P300 amplitude. These effects were independent of potential mediating factors such as age, intelligence, and task performance. This neurophysiological deficit may reflect inefficient deployment of neural resources to cognitively process task-relevant information (Donchin & Coles, 1988), which might contribute to risk for aggression. Our findings are consistent with previous studies demonstrating associations between reactive/impulsive aggression, APD/CD, and reduced P300 amplitude. However, it is widely known that reduced P300 in visual oddball tasks is not specific to aggression or even antisocial behavior in general; for instance, there is a substantial body of literature linking P300 to substance use disorders, as well as familial/genetic risk for such problems (c.f. Polich, Pollack, & Bloom, 1994). Epidemiological work has suggested that child and adult antisocial behavior, substance use disorders, and personality traits related to behavioral disinhibition are markers of a broad, highly heritable dimension of externalizing psychopathology (Krueger, 1999a; Krueger, Hicks, Patrick, Iacono, Carlson, & McGue, 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000). In a study utilizing the same visual oddball task employed here, Patrick et al. (2006) reported that the latent externalizing factor accounting for the co-variance among these disorders related to reduced P300 amplitude in a large community sample. Moreover, the effects of each individual diagnostic indicator became nonsignificant when the latent factor was accounted for, suggesting that this externalizing factor mediated the relationships between the observed indicators and P300. From this perspective, the present findings may be interpreted to suggest that, within such a high-externalizing sample, general antisocial behavior (i.e., non-violent offenses) may lack discriminatory power with regard to physiological measures such as P300. Violent offenses, on the other hand, may be indicative of a more severe level of externalizing psychopathology, and thus reveal evidence of neurobiological deficits. In the context of a less antisocially deviant sample (e.g., community adults, undergraduates), however, less extreme indicators may be more sensitive to P300 differences.
Future research should continue to explore the nature and role of information processing deficits in aggression. Accumulating research now suggests that antisocial behavior, and aggression in particular, is associated with the personality dimensions of negative emotionality and behavioral disinhibition (Krueger, 1999b; Krueger, Caspi, & Moffitt, 2000), and that these traits are in turn related to specific behavioral and physiological responses in laboratory tasks (e.g., Luu, Tucker, & Collins, 2000; Verona, Patrick, & Lang, 2002). Future studies might consider the role of these personality dimensions in the relationship between aggression and neurobiological deficits. Another priority for future research in this area will be to further examine the neurobiological basis of the broad externalizing dimension hypothesized to underlie a range of destructive and antisocial psychopathology, including aggression. One question raised by the present study is whether aggressive and non-aggressive externalizing behaviors are underpinned by separable neurobiological substrates, or whether they represent a shared diathesis that is simply more pronounced among more violent individuals. Further investigation on this topic will hopefully yield more refined insights regarding the neurological basis of aggression, and how it relates to other disorders of behavioral disinhibition.
Acknowledgments
This study was supported by grants MH52384 and MH65137 from the National Institute of Mental Health, and by funds from the Hathaway endowment at the University of Minnesota.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, D.C.: Author; 1994. [Google Scholar]
- Barratt ES, Stanford MS, Kent TA, Felthous A. Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biological Psychiatry. 1997;41:1045–1061. doi: 10.1016/s0006-3223(96)00175-8. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;31:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O'Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcohol Clin Exp Res. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug and Alcohol Dependence. 1997;44:1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biological Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: Effects on P300 during the Stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: Interactive effects of P300 amplitude and topography in male adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O'Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. British Journal of Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Branchey MH, Buydens-Branchey L, Horvath TB. Event-related potentials in substance-abusing individuals after long-term abstinence. American Journal on Addictions. 1993;2:141–148. [Google Scholar]
- Branchey MH, Buydens-Branchey L, Lieber CS. P3 in alcoholics with disordered regulation of aggression. Psychiatry Research. 1988;25:49–58. doi: 10.1016/0165-1781(88)90157-6. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O'Connor S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral & Brain Sciences. 1988;11:357–374. [Google Scholar]
- Gerstle JE, Mathias CW, Stanford MS. Auditory P300 and self-reported impulsive aggression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1998;22:575–583. doi: 10.1016/s0278-5846(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, Clayton AH. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Barratt ES, Wigg C. Impulsiveness, aggression, reading, and the P300 of the event-related potential. Personality and Individual Differences. 1997;22:439–445. [Google Scholar]
- Hood R, Sparks R. Key issues in criminology. Wallop, Hampshire, England: BAS Printers; 1970. [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Klein MW. Watch out for that last variable. In: Mednick S, Moffitt TE, Stack SA, editors. The causes of crime: New biological approaches. New York: Cambridge University Press; 1987. pp. 25–41. [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999a;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Personality traits in adolescence predict mental disorders in early adulthood: A prospective-epidemiological study. Journal of Personality. 1999b;67:39–65. doi: 10.1111/1467-6494.00047. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: The unifying role of personality in population-based research on problem behaviors. Journal of Personality. 2000;68:967–998. doi: 10.1111/1467-6494.00123. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differentially linked to mental disorders: A multi-trait/multi-diagnosis study of an adolescent birth cohort. Journal of Abnormal Psychology. 1996;105:299–312. doi: 10.1037//0021-843x.105.3.299. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Luu P, Tucker DM, Collins P. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Lynam D, Moffitt TE, Stouthammer M. Explaining the relation between IQ and delinquency: Class, race, test motivation, school failure, or self-control? Journal of Abnormal Psychology. 1993;102:187–196. doi: 10.1037//0021-843x.102.2.187. [DOI] [PubMed] [Google Scholar]
- Malone SM, Iacono WG, McGue M. Event-related potentials and comorbidity in alcohol-dependent adult mailes. Psychophysiology. 2001;38:367–376. [PubMed] [Google Scholar]
- Mathias CW, Stanford MS. P300 under standard and surprise conditions in self-reported impulsive aggression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23:1037–1051. doi: 10.1016/s0278-5846(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Bauer LO, Tasman A, Hesselbrock VM. Reduced P3 amplitudes of ERPs are associated with both a family history of alcoholism and antisocial personality disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:1307–1321. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43(1):84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones K, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biological Psychiatry. 1997;42:495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Roth WT, Cannon EH. Some features of the auditory evoked response in schizophrenics. Archives of General Psychiatry. 1972;27:466–471. doi: 10.1001/archpsyc.1972.01750280034007. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Verona E, Patrick CJ, Lang AR. A direct assessment of the role of state and trait negative emotion in aggressive behavior. Journal of Abnormal Psychology. 2002;111:249–258. doi: 10.1037//0021-843x.111.2.249. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tancredi LR, Grant C, Gilespie H. Brain glucose metabolism in violent psychiatric patients: A preliminary study. Psychiatry Research: Neuroimaging. 1995;61:243–253. doi: 10.1016/0925-4927(95)02671-j. [DOI] [PubMed] [Google Scholar]
- Wilson JQ, Herrnstein RJ. Crime and human nature. New York: Simon & Schuster; 1985. [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:684–695. [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale. Los Angeles, CA: Western Psychological Services; 1994. [Google Scholar]