Abstract
ITmD37E, a unique class II transposable element (TE) with an ancient origin, appears to have been involved in multiple horizontal transfers in mosquitoes as ITmD37E sequences from 10 mosquito species of five genera share high nucleotide (nt) identities. For example, ITmD37E sequences from Aedes aegypti and Anopheles gambiae, which have an estimated common ancestor of 145–200 million years ago, display 92% nt identity. The comparison of ITmD37E and host mosquito phylogenies shows a lack of congruence. The wide distribution of conserved ITmD37Es in mosquitoes and the presence of intact copies suggest that this element may have been recently active.
TRANSPOSABLE elements (TEs) are mobile genetic elements that are able to replicate and increase their copy number in a genome (Doolittle and Sapienza 1980). They are divided into RNA-mediated (class I) and DNA-mediated (class II) elements on the basis of their mechanism of transposition. Class I elements include long terminal repeat (LTR) retrotransposons, non-LTR retrotransposons, and SINEs, all retrotransposing via an RNA intermediate. Class II elements include Tc1, mariner, and the P element, which transpose by a “cut-and-paste” mechanism.
Horizontal transfer has been reported for many class II elements in insects (Kidwell 1992; Robertson 1993, 2002; Bonnivard et al. 2000; Handler and McCombs 2000; Silva et al. 2004). We have previously described a DNA TE named ITmD37E in mosquitoes, which has a unique DD37E catalytic triad (Shao and Tu 2001). Here we report the ancient origin, evolution, and horizontal transfer of ITmD37E elements in mosquitoes.
ITmD37E in mosquitoes:
A representative ITmD37E element from An. gambiae is 1.3 kb in length and contains a 1008-bp open reading frame (ORF) and imperfect 27-bp terminal inverted repeats. Insertions are flanked by a TA dinucleotide target-site duplication. A genomic database search revealed 18 and 3 elements, respectively, in the Ae. aegypti and An. gambiae genomes. Full-length copies with intact ORFs could be found only in An. gambiae. Additional ITmD37E element copies were obtained from other mosquito species using genomic library screening and polymerase chain reaction and submitted to NCBI (see supplemental File S2 at http://www.genetics.org/supplemental/ for information about sequences used in this study). Interestingly, no representatives could be found in the Culex pipiens EST database or in the newly released genomic assembly (http://www.vectorbase.org).
ITmD37E TEs found in divergent taxa suggest an ancient origin:
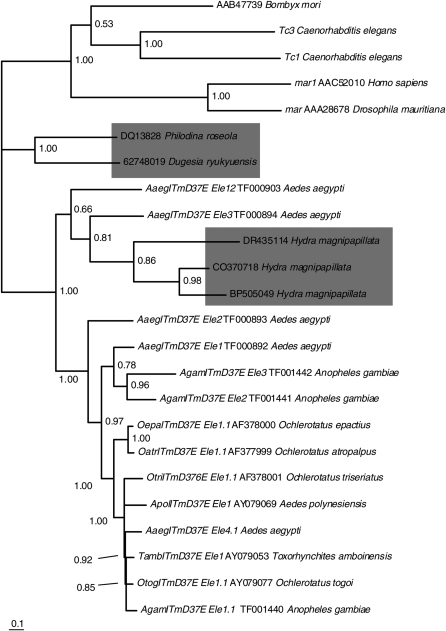
Database searches (NCBI) revealed the existence of ITmD37E representatives in divergent freshwater invertebrates such as Philodina roseola (Arkhipova and Meselson 2005), Dugesia ryukyuensis, and Hydra magnipapillata (Figure 1). All three H. magnipapillata sequences were found in the EST database and are short sequences. Two of the three sequences include the region spanning the second D and E residues that are a part of the DD37E catalytic triad motif. The only D. ryukyuensis sequence that could be found was from an EST database and this sequence contains all three residues of the triad. The H. magnipapillata sequences group with mosquito ITmD37E sequences, having strong phylogenetic support. The P. roseola and D. ryukyuensis sequences are more distantly related to the mosquito ITmD37E sequences, although they have the DD37E motif. They may simply represent a sampling from a divergent paralogous lineage. In summary, this analysis suggests that the ITmD37E TEs are a longstanding group.
Figure 1.—
ITmD37E TEs are of an ancient origin. Consensus tree (>50%) based on conceptual translations constructed using MrBayes version 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003). ITmD37E sequences from freshwater invertebrates are in shaded boxes. Tc1, mariner, and DD37D (Bmmar1) TEs are used to root the tree. Clade credibility values are shown at each node. The scale represents substitutions per site. All mosquito elements have intact ORFs except for AaegITmD37E_Ele4.1, AgamITmD37E_Ele2, and AgamITmD37E_Ele3, which were obtained from whole-genome sequence projects. Element name, accession number, and species name are given when applicable. See supplemental File S1 (http://www.genetics.org/supplemental/) for methods. Information pertaining to sequences can be found in supplemental File S2. See supplemental File S3 for alignment.
High nucleotide identities between copies from divergent hosts suggest horizontal transfer:
Several species from five genera have ITmD37E copies that share high nucleotide (nt) identities in their coding regions (Table 1). Sequences from Ae. aegypti and An. gambiae, which have an estimated common ancestor of 145–200 million years ago (MYA) (Krzywinski et al. 2006), have 93% identity in the coding region. The conservation is not restricted to the coding regions, as both 5′- and 3′-untranslated regions (UTRs) have a consistently high degree of conservation. The 162-bp 5′-UTR and the 125-bp 3′-UTR have 90 and 87% nt identity, respectively. Sequence comparisons between other highly divergent species such as Ae. aegypti and Toxorhynchites amboinensis also demonstrate high nt identities (>95%).
TABLE 1.
Pairwise nucleotide identities between select ITmD37E-coding sequences from species of five genera
| Ar. subalbatus | O. atropalpus | An. gambiae | T. amboinensis | O. epactius | O. togoi | Ae. aegypti | Ae. polynesiensis | |
|---|---|---|---|---|---|---|---|---|
| O. atropalpus | 79.8 | |||||||
| An. gambiae | 93.6 | 77.9 | ||||||
| T. amboinensis | 95.5 | 79.5 | 93.5 | |||||
| O. epactius | 80.3 | 97.6 | 78.4 | 79.9 | ||||
| O. togoi | 95.8 | 79.7 | 94.3 | 96.2 | 80.1 | |||
| Ae. aegypti | 95.2 | 78.9 | 93.2 | 95.3 | 79.0 | 95.4 | ||
| Ae. polynesiensis | 93.2 | 78.4 | 91.1 | 93.0 | 78.7 | 93.2 | 93.2 | |
| O. triseriatus | 94.6 | 80.6 | 92.1 | 94.2 | 81.1 | 94.6 | 93.9 | 92.0 |
Numbers are percentages. Only coding regions were compared because they were the only sequence available for some copies. Pairwise nt identities were determined by PAUP (Swofford 2002) after alignment with CLUSTAL_X (Thompson et al. 1997). Ae. aegypti, AaegITmD37E_Ele 4.1; Ae. polynesiensis, ApolITmD37E_Ele1; Ar. subalbatus, AsubITmD37E_Ele1.2; O. atropalpus, OatrITmD37E_Ele1.1; O. epactius, OepaITmD37E_Ele1.1; O. togoi, OtogITmD37E_Ele1.1; O. triseriatus, OtriITmD37E_Ele1.1; An. gambiae, AgamITmD37E_Ele1.1; T. amboinensis, TambITmD37E_Ele1. See supplemental File S1 at http://www.genetics.org/supplemental/ for methods. Refer to supplemental File S2 for sequence information.
It is important to note that of 300 class II elements from Ae. aegypti and An. gambiae, there are only 2 elements with copies sharing at least 80% nt identity for 500 bp or more between the two genomes. Two hundred forty-eight Ae. aegypti elements and 52 An. gambiae elements (http://tefam.biochem.vt.edu/tefam/index.php) were used as queries in BLASTN against Ae. aegypti or An. gambiae whole-genome sequences and then output was filtered for minimum 80% nt identity and minimum hit lengths of 500 bp. AaegITmD37E_Ele4 and AgamITmD37E_Ele1 have the highest identities, with 90% for 1300 bp [alignment by CLUSTAL_X (Thompson et al. 1997) and distance determination by PAUP (Swofford 2002) results in 92%]. The next highest identity match was between Tango elements (80% identity over a 500-bp fragment), which have been reported to be involved in horizontal transfer (Coy and Tu 2007).
Host and ITmD37E phylogenies are incongruent:
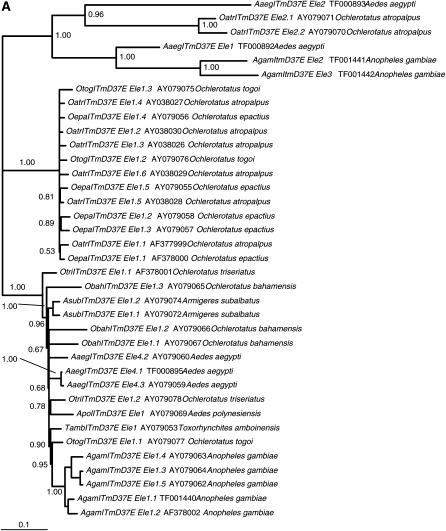
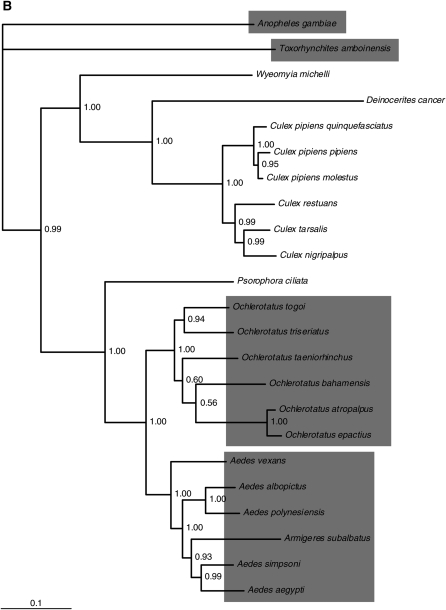
Comparison of the host species vitellogenin C (Vg-C) and ITmD37E phylogenies shows that there is a lack of congruence (Figure 2, A and B). There are three major groups in Figure 2A. The group at the bottom of Figure 2A shows a marked lack of congruence. The An. gambiae sequences do form a clade, but nevertheless the phylogeny is perplexing compared to the host phylogeny. For example, the relative distances between sequences of Ae. aegypti (from both the library screen and the genomic database) and several other species such as T. amboinensis and An. gambiae (distant relatives), Ae. polynesiensis, Ochlerotatus triseriatus, and O. bahamensis (closely related species) do not make sense when compared to the host phylogeny. The phylogeny involving these sequences is neither in agreement nor the same compared to the host phylogeny when the two are superimposed.
Figure 2.—
Comparison of host and ITmD37E phylogenies. Both trees shown are consensus trees (>50%) constructed using MrBayes version 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003). Clade credibility values are shown at each node. The scale represents substitutions per site. (A) ITmD37E phylogeny based on nt sequence from ORFs. Tree is rooted with divergent mosquito ITmD37E elements seen at the top of the tree. Element name, accession number, and species name are given when applicable. Information regarding sequences used in this study can be found in supplemental File S2 (http://www.genetics.org/supplemental/). (B) Mosquito host phylogeny based on Vg-C nt sequence. Tree is rooted with An. gambiae Vg-C. The five genera that have species containing ITmD37E sequences are shaded. The Armigeres subalbatus sequence is found within the Aedes group, which is consistent with previous analyses (Isoe 2000). Most Vg-C sequences were obtained from Isoe (2000). See supplemental File S1 for methods and supplemental File S3 for alignments.
Selection pressure and codon bias are not responsible for the high conservation found between three ITmD37E copies from Ae. aegypti, T. amboinensis, and An. gambiae:
It is possible that selection pressure or codon bias could contribute to the high conservation of ITmD37E sequences. To investigate these possibilities, ITmD37E sequences from each of the three most divergent host species (Ae. aegypti, An. gambiae, and T. amboinensis) were analyzed for selection pressure (Table 2). dS/dN, a ratio of the synonymous and nonsynonymous substitutions between two sequences, was determined by SNAP (Nei and Gojobori 1986; Korber 2000). Values ranged from 1.0 to 3.6 for all pairwise comparisons, showing very low to no significant selection. There is no concern for substitution saturation in these cases, as ps and pn values are low. ps and pn are the proportion of observed synonymous and nonsynonymous substitutions, respectively. dS/dN values for Vg-C, a host gene known to be relatively rapidly evolving (Isoe 2000), were also obtained from the same three species. These values are significantly higher than that found for ITmD37E sequences. Because these Vg-C comparisons are possibly saturated with respect to synonymous substitution (ps is near or exceeding 0.75), we also compared Vg-C sequences from closely related species in the Aedes and Ochlerotatus genera, which have ps values similar to those of the above-mentioned ITmD37E sequence comparisons. These Vg-C comparisons also displayed high dS/dN values, ranging from 13.7 to 15.3. Therefore, the low dS/dN values from the ITmD37E comparisons suggest that the high sequence identity between the ITmD37E elements does not result from high selection pressure. The effective number of codons (Wright 1990) determined by PDA (http://dpdb.uab.es/pda/pda.asp) (Casillas and Barbadilla 2004) for all three ITmD37E sequences is 59–60, demonstrating no codon bias. Possible values range from 20 (one codon per aa—high bias) to 61 (all codons used equally—no bias). These analyses suggest that selection pressure and codon bias are not responsible for the observed high degree of conservation.
TABLE 2.
Selection pressure analysis of ITmD37E- and Vg-C-coding sequences
| Sequence 1 | Sequence 2 | ps | pn | dS | dN | dS/dN | % aa ID |
|---|---|---|---|---|---|---|---|
| AaegITmD37E_Ele4.1 | AgamITmD37E_Ele1.1 | 0.11 | 0.05 | 0.11 | 0.06 | 2.0 | 87.5 |
| AaegITmD37E_Ele4.1 | TambITmD37E_Ele1 | 0.05 | 0.05 | 0.05 | 0.05 | 1.0 | 89.6 |
| AgamITmD37E_Ele1.1 | TambITmD37E_Ele1 | 0.14 | 0.04 | 0.16 | 0.04 | 3.6 | 90.5 |
| AaegVg-C | AgamVg-C | 0.65 | 0.22 | 1.50 | 0.26 | 5.7 | 61.4 |
| AaegVg-C | TambVg-C | 0.72 | 0.21 | 2.52 | 0.24 | 10.5 | 64.4 |
| AgamVg-C | TambVg-C | 0.80 | 0.23 | NAa | 0.28 | NAa | 60.4 |
| AalbVg-C | ApolVg-C | 0.30 | 0.02 | 0.38 | 0.02 | 15.3 | 94.8 |
| OatrVg-C | OepaVg-C | 0.12 | 0.01 | 0.13 | 0.01 | 13.7 | 97.8 |
Manually codon-aligned sequences (see supplemental File S4 at http://www.genetics.org/supplemental/) were used to determine selection pressure by SNAP (http://hcv.lanl.gov/content/hcv-db/SNAP/SNAP.html) (Korber 2000), a program that uses the method of Nei and Gojobori (1986). AgamITmD37E_Ele1.1 and TambITmD37E_Ele1 have intact coding sequences. AaegITmD37E_Ele4.1 has multiple frameshifts that were corrected by codon alignment. Most Vg-C sequences were obtained from Isoe (2000). See supplemental File S1 for methods.
SNAP does not give an output for dS when ps > 0.75, indicating substitution saturation.
Discussion:
There are generally three lines of evidence used to make a case for horizontal transfer: the discovery of sequences from divergent taxa having high nt identities, incongruence between TE and host phylogenies, and a “patchy TE distribution” among related host taxa. We have shown the first two types of evidence here, and the support for the third is weak but worth mentioning (see below). First, high nt identities have been found between ITmD37E sequences from 10 species of five genera (Table 1, Figure 2A). Particularly noteworthy is the comparison of copies from Ae. aegypti and An. gambiae, species that are estimated to have diverged between 145 and 200 MYA. Second, host and TE phylogenies are clearly incongruent (Figure 2). Here we have many copies with such high nt identities that branch lengths are very short and phylogenetic resolution is low for some sequences. Finally, regarding patchy distribution, no ITmD37E representatives could be found in the C. pipiens genome assembly (http://www.vectorbase.org) by database search or by genomic library screen (not shown). While this is consistent with patchy distribution resulting from horizontal transfer, it is possible that ITmD37E was simply lost from this lineage.
When all these evidences are taken together, the case for horizontal transfer of ITmD37E in mosquitoes is strong. While alternative explanations can be provided, they are not likely. It could be argued that ITmD37E copies have inserted into genomic regions having a low substitution rate. However, this argument is hard to make, given the nt identities of 92% between copies from Ae. aegypti and An. gambiae, species with a common ancestor from 145 to 200 MYA. This argument requires that conserved ITmD37E copies from all species be inserted into locations with low substitution rates. We have determined the location of AgamITmD37E_Ele1 copies because chromosomal assignment is available only for An. gambiae. Copies are present on all chromosome arms (determined by BLAST using Ensembl; not shown). Although we cannot completely rule out the low-substitution-rate hypothesis, it seems unlikely.
ITmD37E could have been “co-opted” for a host function and therefore be highly conserved. First, it is unlikely that such an indispensable function in so many divergent mosquitoes would have been lost from C. pipiens. Second, substitution analysis performed to detect selection pressure by SNAP (Nei and Gojobori 1986; Korber 2000) (http://hcv.lanl.gov/content/hcv-db/SNAP/SNAP.html) using sequences from the three divergent species Ae. aegypti, An. gambiae, and T. amboinensis shows a rather weak selection for sequences with this degree of conservation (Table 2). Even when we compare ITmD37E sequences to Vg-C, a gene under moderate selection, ITmD37E sequences still demonstrate a much lower selection pressure yet have much higher sequence conservation. Therefore, there is no indication that high conservation of ITmD37E sequences is due to selection. A low dS for ITmD37E sequences is consistent with the horizontal transfer hypothesis, as there has not been enough time for the accumulation of substitutions. This makes sense if horizontal transfer was recent and therefore not enough time had elapsed for selection to become evident by dS/dN analysis. Third, we have also shown that the conservation is not from codon bias, as the effective number of codons (Wright 1990) for these sequences is very high, demonstrating effectively no bias.
It is difficult to determine the direction of horizontal transfer, but the relatively high nt identities among copies in both divergent and closely related taxa suggest that ITmD37E has been introduced into mosquitoes recently in evolution. It is interesting that both An. gambiae and Ae. aegypti have long coexisted in parts of Africa, providing a possible ecological connection for horizontal transfer, although we can only speculate about the mechanism. A common virus is a likely candidate, where TE insertions that do not inactivate the viral copy are excised and inserted into a naive genome after infection. Although the piggybac TE from a lepidopteran cell line has been found to be responsible for insertional mutations in baculoviruses (Fraser et al. 1983), no direct evidence for viral transmission as a mechanism for horizontal transfer has been demonstrated.
The ITmD37E family has been very successful in mosquitoes evidenced by its widespread distribution in 10 species of five genera. Except for those found in a few other invertebrate species (Figure 1), to our knowledge no other ITmD37E representatives are known at this time. It will be interesting to see if this group is restricted to invertebrates.
The horizontal transfer of ITmD37E and the presence of several copies with intact ORFs indicate that ITmD37E has been recently active. Of the 39 mosquito copies used in this study, 9 from five species of four genera have intact ORFs. This may suggest that ITmD37E elements are good candidates for developing molecular tools for transgenesis, as there has been significant success in using class II elements as molecular tools in mosquitoes (Adelman et al. 2002; Perera et al. 2002; O'Brochta et al. 2003). Efforts to determine the transposition activity of ITmD37E are currently underway.
Acknowledgments
We thank Jun Isoe from the laboratory of Henry Hagedorn for providing genomic libraries and Vg-C sequences and Shirley Luckhart for providing the An. gambiae genomic library. This work was supported by a grant from the National Institutes of Health (AI42121) to Z.T.
References
- Adelman, Z. N., N. Jasinskiene and A. A. James, 2002. Development and applications of transgenesis in the yellow fever mosquito, Aedes aegypti. Mol. Biochem. Parasitol. 121: 1–10. [DOI] [PubMed] [Google Scholar]
- Arkhipova, I. R., and M. Meselson, 2005. Diverse DNA transposons in rotifers of the class Bdelloidea. Proc. Natl. Acad. Sci. USA 102: 11781–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnivard, E., C. Bazin, B. Denis and D. Higuet, 2000. A scenario for the hobo transposable element invasion, deduced from the structure of natural populations of Drosophila melanogaster using tandem TPE repeats. Genet. Res. 75: 13–23. [DOI] [PubMed] [Google Scholar]
- Casillas, S., and A. Barbadilla, 2004. PDA: a pipeline to explore and estimate polymorphism in large DNA databases. Nucleic Acids Res. 32: W166–W169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy, M. R., and Z. Tu, 2007. Genomic and evolutionary analyses of Tango transposons in Aedes aegypti, Anopheles gambiae and other mosquito species. Insect Mol. Biol. 16: 411–421. [DOI] [PubMed] [Google Scholar]
- Doolittle, W. F., and C. Sapienza, 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603. [DOI] [PubMed] [Google Scholar]
- Fraser, M. J., G. E. Smith and M. D. Summers, 1983. Acquisition of host cell DNA sequences by baculoviruses: relationship between host DNA insertions and FP mutants of Autographa californica and Galleria mellonella nuclear polyhedrosis viruses. J. Virol. 47: 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler, A. M., and S. D. McCombs, 2000. The piggyBac transposon mediates germ-line transformation in the Oriental fruit fly and closely related elements exist in its genome. Insect Mol. Biol. 9: 605–612. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck, J. P., and F. Ronquist, 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Isoe, J., 2000. Comparative analysis of the vitellogenin genes of the Culicidae. Ph.D. Thesis, University of Arizona, Tucson, AZ.
- Kidwell, M. G., 1992. Horizontal transfer of P elements and other short inverted repeat transposons. Genetica 86: 275–286. [DOI] [PubMed] [Google Scholar]
- Korber, B., 2000. Computational analysis of HIV molecular sequences, pp. 55–72 in HIV Signature and Sequence Variation Analyisis, edited by A. G. Rodrigo and G. H. Learn. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- Krzywinski, J., O. G. Grushko and N. J. Besansky, 2006. Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol. Phylogenet. Evol. 39: 417–423. [DOI] [PubMed] [Google Scholar]
- Nei, M., and T. Gojobori, 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3: 418–426. [DOI] [PubMed] [Google Scholar]
- O'Brochta, D. A., N. Sethuraman, R. Wilson, R. H. Hice, A. C. Pinkerton et al., 2003. Gene vector and transposable element behavior in mosquitoes. J. Exp. Biol. 206: 3823–3834. [DOI] [PubMed] [Google Scholar]
- Perera, O. P., I. R. Harrell and A. M. Handler, 2002. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol. Biol. 11: 291–297. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., 1993. The mariner transposable element is widespread in insects. Nature 362: 241–245. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., 2002. Evolution of DNA transposons in Eukaryotes, pp. 1093–1110 in Mobile DNA II, edited by N. L. Craig, R. Craigie, M. Gellert and A. M. Lambowitz. American Society for Microbiology, Washington, DC.
- Ronquist, F., and J. P. Huelsenbeck, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Shao, H., and Z. Tu, 2001. Expanding the diversity of the IS630-Tc1-mariner superfamily: discovery of a unique DD37E transposon and reclassification of the DD37D and DD39D transposons. Genetics 159: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, J. C., E. L. Loreto and J. B. Clark, 2004. Factors that affect the horizontal transfer of transposable elements. Curr. Issues Mol. Biol. 6: 57–71. [PubMed] [Google Scholar]
- Swofford, D. L., 2002. Phylogenetic Analysis Using Parsimony (*and Other Methods). Sinauer Associates, Sunderland, MA.
- Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin and D. G. Higgins, 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, F., 1990. The ‘effective number of codons’ used in a gene. Gene 87: 23–29. [DOI] [PubMed] [Google Scholar]