Abstract
Engagement of Fas (APO-1, CD95), a member of the tumor necrosis factor receptor superfamily, can induce apoptotic cell death. However, Fas engagement also can costimulate lymphocyte proliferation. The physiologic regulation of these two outcomes is poorly understood. Here, we have used two systems, the first in vitro and the second in vivo, to demonstrate that naïve and memory CD4+ T cells display dichotomous responses to Fas ligation. Naïve CD4+ T cells (CD44lo, CD45RB+, CD62L+) die as a consequence of Fas ligation in the presence of anti-CD3 antibody, whereas memory T cells (CD44hi, CD45RB−, CD62L−), freshly isolated from the same starting population and subjected to the same stimulation conditions, are costimulated to proliferate by Fas ligation. In vitro, we demonstrate that CD28-mediated signals or T helper 1 and T helper 2 differentiation cytokines alter the response of naïve T cells, but not of memory T cells, to Fas ligation. In vivo experiments in hen egg lysozyme (HEL) T cell receptor transgenic mice show that CD4+ T cells from HEL-naïve mice are killed by Fas ligation, but CD4+ T cells from long-term HEL-exposed mice are costimulated by Fas ligation. Thus, the physiological outcome of Fas ligation in CD4+ T cells is determined primarily by the antigenic history of the T cell.
The peripheral T cell pool is highly heterogeneous, consisting of multiple subpopulations distinguished by differences in phenotype and function. Lymphocytes in the periphery may be in one of three states, naïve, effector, or memory, depending on their history of exposure to antigen (1–3). Naïve cells have never been exposed to specific antigens and are characterized by expression of CD62L (MEL-14, l-selectin) and the CD45 high-molecular-weight isoforms (CD45RA/B/C), and by low CD44 expression. Effector lymphocytes are recently antigen-activated cells and express activation markers including CD69, CD25 (IL-2 receptor α), and high CD44. Memory cells are antigen-exposed cells that persist long after the primary immune response. Phenotypically, memory cells are CD62L− CD45RA/B/Clo CD44hi and have lost the expression of activation markers such as CD25 and CD69, but have increased expression of the intracellular survival molecules Bcl-2 and Bcl-xL. Functionally, memory cells accumulate with age and antigen exposure, are present at higher precursor frequencies, have a lower activation threshold, and may express differentiated cytokine patterns when reactivated (1–3). CD4+ memory cells may require the continuous presence of low amounts of antigen to persist and remain in a continuous, subproliferative activation state (4).
Some effector cells and other T cells with activated phenotypes, such as many clones and hybridomas, are sensitive to Fas-induced death (5–7). Fas is a member of the tumor necrosis factor receptor/nerve growth factor receptor superfamily, whose members are implicated in cell proliferation, differentiation, and death (8). The cytoplasmic tail of Fas contains a region termed the death domain, which, when coupled to a caspase cascade by the adapter protein FADD (MORT-1), can transduce signals leading to apoptosis (9–11). During an antigen-specific immune response, Fas is up-regulated and FasL is induced on activated T cells, which then may undergo Fas/FasL-mediated cell autonomous death (12, 13). Fas/FasL-mediated cell death has been suggested as a mechanism for the disappearance of effector cells after an immune response, a phenomenon termed activation-induced cell death (AICD) (12, 14, 15). Mice bearing a mutation in the Fas gene (lpr mice) and children with defective Fas function develop massive lymphadenopathy and autoimmune symptoms, demonstrating a role for Fas in lymphocyte homeostasis and peripheral tolerance (16–23). Paradoxically, ligation of Fas also can costimulate the proliferation of anti-CD3-activated human peripheral blood T cells (24–26). Thus, Fas engagement can both induce death and promote proliferation in T cells. Little is known regarding what regulates the outcome of Fas signaling. The effects of Fas engagement on naïve and memory T cells, which constitute the majority of the peripheral T cell pool, have not been compared.
To investigate the outcome of Fas signaling in naïve and memory T cells, we examined the effects of Fas engagement within two systems of freshly isolated mouse T cells. We found that under identical stimulation conditions, naïve and memory CD4+ T cells, defined by well established surface phenotypes and isolated from the same starting population, undergo opposite responses when subjected to Fas ligation. Fas engagement induced apoptosis in naïve cells, but costimulated the proliferation of memory T cells. Furthermore, CD28-mediated costimulation or T helper (Th)1 and Th2 differentiation cytokines altered the response of naïve T cells, allowing them to be costimulated by Fas ligation. We used a T cell receptor (TCR) transgenic model to study an in vivo system of antigen-specific T cell memory. We examined the peripheral CD4+ population in mice bearing a transgenic TCR specific for hen egg lysozyme (HEL) peptide (27), to investigate changes in Fas responsiveness induced by antigen-driven generation of a memory population. The results confirmed that the physiological response of CD4+ T cells to Fas is determined by previous antigenic history and availability of costimulation. Our findings suggest a model for Fas regulation of peripheral tolerance, as well as mechanisms for failure of peripheral tolerance and autoreactivity.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old C57BL/6 (B6) and B6.MRL-Faslpr (B6-lpr) mice were obtained from The Jackson Laboratory. 3A9 transgenic mice (B10BR animals transgenic for an αβ-TCR specific for HEL in the context of I-Ak) (27) were bred and maintained in our colony at Dartmouth College in compliance with state, federal, and institutional guidelines.
Purification of Naïve and Memory CD4+ T Cell Populations.
T cell populations were purified from single-cell suspensions of pooled murine lymphoid tissues (spleens and cervical, axillary, and inguinal lymph nodes). Naïve cells were obtained by negative selection on CD4 T cell columns (Biotex Laboratories, Edmonton, Canada), using the manufacturer’s directions modified by adding anti-CD44 antibody (supernatant from clone IM7.8.1, diluted 1:15) to the antibodies supplied with the kit. Anti-CD44 antibodies selectively bind to CD44hi memory/activated cells and allow them to be retained in the column and removed from the suspension. Memory cells were purified by adding 5 μg/ml of sodium azide-free anti-CD62L antibodies (PharMingen) to those supplied with the CD4 column kit. CD62L (MEL 14, l-selectin) is expressed selectively on naïve cells, and this marker was used to remove the CD62L+ naïve cells from the suspension. Naïve and memory cells were prepared in parallel from the same starting population. These negative-selection purification procedures resulted in cell populations that were >90% CD4+ T cells. In both populations, contaminating cells could be accounted for by CD8− γδ T cells (2–3%) and natural killer cells (5–7%). B cells and monocytes were present at less than 1%.
Cell Culture, Antibodies, and Cytokines.
The wells of 96-well flat-bottomed tissue culture plates were coated with antibodies by incubating 100 μl/well of PBS containing 1 μg/ml of anti-CD3, with or without anti-Fas and/or anti-CD28 antibodies at the indicated concentrations, for 1 hr at 37°C, as described (28). The wells then were washed and the T cells were plated immediately into the coated wells, at 5 × 104 cells per well, in complete RPMI 1640 medium with 5% FCS. Recombinant mouse IL-2 (10 ng/ml) (PharMingen), recombinant mouse IL-4 (100 ng/ml) (PharMingen), or mouse IL-12 (3.5 ng/ml) (generously provided by M. Rincón), were added with the cells at the time of plating, only where indicated. In all figures, data points and error bars represent the average and SD of triplicate wells, and data shown are representative of a minimum of three experiments.
Flow Cytometry and Cell Cycle Analyses.
Cell phenotypes were determined by staining cells with anti-CD4, anti-Fas, anti-CD25, anti-CD69, anti-CD44, anti-CD45RB, and anti-CD62L antibodies (PharMingen). Expression of the transgenic TCR in the 3A9 mice was verified by staining with FITC-conjugated anti-Vβ8.2. Cell cycle analysis was performed by permeabilizing and staining the cells with Bauer DNA-staining solutions containing 0.1% Triton X-100 and 50 μg/ml of propidium iodide (29). Data were acquired on a Coulter Elite Epics flow cytometer (Coulter) and analyzed with cellquest software (Becton Dickinson).
Proliferation Assays.
Cells were cultured for 48 or 72 hr, as described above, and then were pulsed with [3H]thymidine (1 μCi/well) and cultured for an additional 18 hr. The wells were harvested onto filters, and the incorporated radiation was quantitated by a Packard scintillation counter.
Immunization of Transgenic Mice.
3A9 mice were injected with 50 μg HEL peptide emulsified in 200 μl incomplete Freund’s adjuvant s.c. on the back. Draining lymph nodes (axillary and cervical) were collected 3–4 weeks postinjection.
RESULTS
Characterization of CD4+ T Cell Naïve and Memory Populations in Normal Mice.
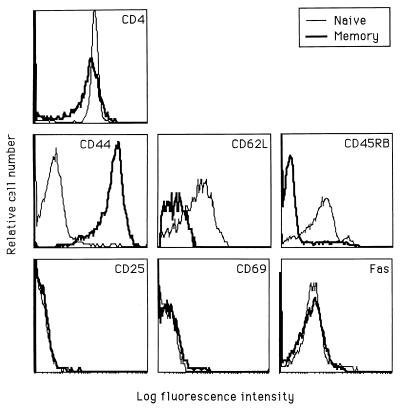
We investigated the effects of Fas ligation in mouse T cells purified from spleen or lymph nodes. Our initial findings showed that splenic T cells stimulated with anti-CD3 were costimulated by Fas ligation. In contrast, Fas ligation inhibited the proliferation of lymph node T cells (data not shown). Lymph nodes preferentially recruit naïve T cells from the peripheral circulation via the expression of l-selectin (MEL-14, CD62L) receptors on their specialized high endothelial venules (30, 31). Conversely, the spleen is relatively rich in memory cells, which lack l-selectin expression (3). We hypothesized that the opposite responses to Fas engagement could reflect the differential composition of memory vs. naïve cells in these two tissues. Accordingly, we isolated CD4+ T cell subpopulations from suspensions of pooled spleen and lymph node cells, using CD44+-depleted populations as naïve cells and CD62L+-depleted populations as memory cells. In both cases, we used negative selection techniques to avoid engaging any cell surface molecules before cell culture. We studied naïve and memory populations derived from purified CD4+ T cells to avoid the influence of different ratios of CD4+ to CD8+ cells. We have used a well characterized phenotypic definition of “naïve” and “memory” T cells, extensively correlated with function in the literature (reviewed in refs. 1–3). Naïve cells are uniformly CD62L+, CD44lo, and CD45RB+, whereas memory cells are CD62L−, CD44hi, and CD45RB− (Fig. 1). Activated effector T cells also express high levels of CD44, and recently activated cells are likely to respond differently from memory cells. Therefore, we examined both populations for the expression of the activation markers CD69 and CD25 (IL-2 receptor α) and found no differences in the percentage of activated cells in the naïve and memory populations (less than 1% activated cells in either population; Fig. 1). We also examined preculture cell surface Fas levels, because differences in Fas expression may affect the response to Fas engagement, and in human peripheral blood T cells, Fas expression is higher on memory than naïve T cells (32). We found that mean Fas expression was not significantly different on naïve and memory mouse T cell populations (Fig. 1), suggesting that differential responses to Fas ligation would not, in this instance, reflect different levels of cell surface Fas expression.
Figure 1.
Phenotypes of purified CD4+ naïve and memory cell populations. Naïve (thin line) and memory (thick line) CD4+ cells were prepared from pooled spleens and lymph nodes from B6 mice by negative selection, using T cell purification columns. Naïve cells were purified by removal of CD8+, Ig+, and CD44+ cells. Memory cells were prepared by removal of CD8+, Ig+, and CD62L+ cells.
Fas Ligation Inhibits the Proliferative Response of Naïve Cells, but Costimulates the Proliferation of Memory Cells.
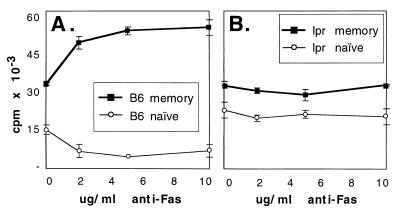
We stimulated freshly isolated naïve or memory peripheral T cells with anti-CD3, with or without exogenous Fas ligation as provided by plate-bound anti-Fas antibodies. Fas ligation inhibited the proliferation of naïve CD4+ T cells and costimulated the proliferation of memory cells in a dose-dependent manner (Fig. 2A). To verify that the effect of anti-Fas antibody was specific, we repeated the experiment with cells from young (predisease) B6-lpr mice, which lack cell surface Fas expression because of a mutation in the Fas gene (16). As expected, the proliferation of CD4+ naïve and memory cells from lpr mice was not affected by anti-Fas antibody (Fig. 2B). Both B6 and B6-lpr memory T cells responded more vigorously to anti-CD3 stimulation than did naïve cells. Interestingly, the baseline CD3-mediated proliferative response (in the absence of exogenous Fas ligation) of naïve cells from lpr mice was higher than that of naïve cells from B6 animals, whereas the baseline response of memory B6 cells from the two strains was identical. The cells were plated at very low density to prevent cell–cell contact in culture, suggesting that the inhibition of the naïve B6 cells compared with lpr cells may be mediated by cell autonomous death (Fas/FasL interactions occurring on a single cell). In contrast, because there is no difference between B6 and lpr memory cells, it is possible that Fas-mediated costimulation is not cell autonomous, but requires exogenous Fas ligation mediated by antibody (in this case) or cell–cell interactions (in vivo).
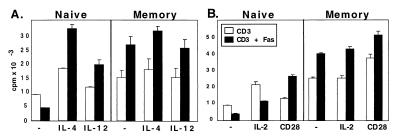
Figure 2.
Fas ligation inhibits naïve CD4+ T cell proliferation and augments memory CD4+ T cell proliferation in B6 (A) but not B6-lpr (B) mice. Naïve (thin lines) and memory (thick lines) CD4+ cell populations were purified as described in Fig. 1 from B6 (A) and B6-lpr (B) pooled peripheral lymphoid cells and stimulated with 1 μg/ml of anti-CD3 antibody and indicated concentrations of anti-Fas antibody for 72 hr. Incorporation of [3H]thymidine was measured as an indicator of proliferation. Engagement of Fas in the absence of anti-CD3 antibody produced no effect.
Fas and CD28 Have Opposite Effects on Naïve Cells but Identical Effects on Memory Cells During CD3-Induced Proliferation.
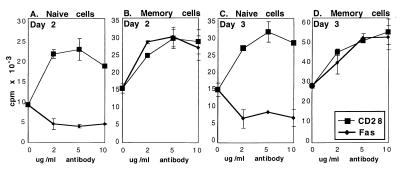
Engagement of the T cell surface molecule CD28 delivers potent costimulatory signals for T cell proliferation and cytokine production (33–35). We used CD28 ligation together with anti-CD3 stimulation as a positive control for costimulation in our system to verify that the naïve populations retained the ability to be costimulated after the purification procedure and to compare the levels of memory cell costimulation obtained via Fas and CD28. Naïve cells were extremely sensitive to CD28 costimulation and were inhibited by Fas ligation (Fig. 3 A and C). In contrast, memory cells were costimulated equally well by Fas and CD28 signals (Fig. 3 B and D). Results are shown after 2 (Fig. 3 A and B) and 3 days (Fig. 3 C and D) of stimulation; after 4 days in culture, the costimulated wells were overgrown. The costimulatory effects persisted throughout the period studied, and no outgrowth was observed in the cultures of naïve cells treated with anti-Fas antibodies. Instead, cells with apoptotic morphology accumulated in these wells (Fig. 4A). Thus, naïve cells displayed opposite responses to Fas and CD28 ligation, but memory cells responded indistinguishably in terms of costimulated proliferation.
Figure 3.
Fas and CD28 ligation have opposite effects on CD4+ naïve T cell proliferation (A and C) but identical costimulatory effects on CD4+ memory cells (B and D). Purified naïve (A and C) or memory (B and D) CD4+ T cells from B6 mice were cultured with 1 μg/ml of anti-CD3 antibody and indicated concentrations of anti-CD28 (thin line) or anti-Fas (thick line) antibody. Kinetics were determined by harvesting the cells after 48 hr (A and B) or 72 hr (C and D) of stimulation. Incorporation of [3H]thymidine was measured as an indicator of proliferation.
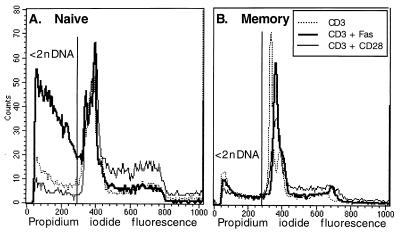
Figure 4.
Fas ligation induces apoptotic cell death in naïve but not in memory CD4+ T cells. Naïve (A) or memory (B) cells were stimulated with 1 μg/ml of anti-CD3 antibody alone (dotted line) or with anti-CD3 plus 5 μg/ml of anti-CD28 (solid, thin line) or anti-Fas antibody (thick line) for 48 hr. Cells were permeabilized and stained with DNA-binding solutions and analyzed by flow cytometry. Apoptotic (<2n DNA) cells are those to the left of the gate. For naïve cells (A), 29% of cells were apoptotic (<2n DNA) when stimulated with anti-CD3 alone, 8% were apoptotic when treated with anti-CD3 and anti-CD28 antibodies, and 57% were apoptotic when treated with anti-CD3 and anti-Fas antibodies. For memory cells (B), percentages were 23, 12, and 18% for anti-CD3 alone, anti-CD3 + anti-CD28, and anti-CD3 + anti-Fas treatments, respectively.
Fas Suppresses Naïve Cell Proliferation by Inducing Apoptotic Death.
Fas is known to induce cell autonomous death by apoptosis in T cell hybridomas and in human T cell clones, malignant T cells, and peripheral activated T cells (5–7). Inspection of the Fas-treated naïve cells by light microscopy revealed morphologically apoptotic cells. Conversely, memory cells cultured with anti-Fas proliferated rapidly and showed no evidence of increased numbers of dead cells. To determine whether the inhibition of proliferation in the CD3-stimulated naïve cells was accompanied by increased apoptotic death, we analyzed the cell cycle by flow cytometry (29). Apoptosis, determined by the percentage of cells with less than 2n DNA, was increased dramatically by Fas ligation in naïve cells (Fig. 4A; apoptosis was increased from 29 to 62% by Fas engagement). In contrast, CD28 ligation decreased the percentage of naïve apoptotic cells and increased the percentage of cycling cells (Fig. 4A; apoptosis was decreased from 29 to 8%). The costimulatory effect of Fas ligation on memory cells was not accompanied by increased cell death; in fact, in memory cells, Fas engagement slightly decreased apoptosis, from 23 to 18% (Fig. 4B). Fas ligation did not increase the percentage of memory cells in G2/S (Fig. 4B), although these cells demonstrate increased proliferation as determined by thymidine incorporation (Figs. 2A and 3 B and D). These results suggest that Fas ligation alters the rate of proliferation of memory cells, rather than the number of cells in cycle at any given time, and show that naïve cells, but not memory cells, undergo apoptosis in response to Fas ligation.
Differentiation Cytokines and Costimulation Rescue Naïve Cells from Fas-Induced Death and Render Them Receptive to Fas-Mediated Costimulation.
Clearly, a physiological mechanism is required to rescue newly stimulated naïve cells from Fas-mediated death or T cell responses to novel antigens would never develop. We studied the response of naïve and memory cells to Fas ligation in the presence of prototypical Th1 (36, 37) or Th2 (38, 39) differentiation cytokines to determine whether an imposed cytokine bias affected the outcome of Fas ligation. We found that IL-4 (Th2) or IL-12 (Th1) could shift naïve cells from being susceptible to Fas-mediated death to being responsive to Fas-dependent costimulation (Fig. 5A). In the presence of IL-4 and, to a lesser extent, of IL-12, naïve T cells were rescued from apoptosis and were costimulated by Fas ligation (Fig. 5A). Thus, the differentiation cytokines did not simply rescue the naïve cells from Fas-mediated death, but also conferred the capacity for Fas costimulation. In contrast, the response of memory cells to Fas ligation was not affected by exogenous IL-4 or IL-12.
Figure 5.
The cytokine environment modulates naïve but not memory CD4+ T cell response to Fas ligation. (A) Differentiation cytokines IL-4 (Th2) and IL-12 (Th1) reverse the Fas responsiveness of naïve cells but do not affect the Fas responsiveness of memory cells. Naïve or memory CD4+ T cells were stimulated with 1 μg/ml of anti-CD3 alone (open bars) or 5 μg/ml of anti-Fas antibody (solid bars) in the presence of optimal concentrations of IL-4 or IL-12. Proliferation was assessed by [3H]thymidine incorporation after 48 hr in culture. (B) CD28-mediated costimulation, but not IL-2, reverses the Fas responsiveness of naïve cells. Cells were cultured as in A except that the growth factor IL-2 (10 ng/ml) or anti-CD28 antibody (5 μg/ml) was added instead of differentiation cytokines.
IL-2 is required for T cell proliferation but has also been implicated in T cell apoptosis (40). In fact, addition of exogenous IL-2 to naïve T cells increased both their proliferation and Fas-mediated inhibition. Thus, exogenous IL-2, unlike IL-4 and IL-12, did not confer the capacity to be costimulated through Fas (Fig. 5B). Costimulation via CD28, however, mimicked the effect of the differentiation cytokines on naïve cells and synergized with Fas costimulation in memory cells (Fig. 5B). Thus, costimulation or an exogenous source of high levels of Th1/Th2 differentiation cytokines can shift Fas signals from death-inducing to costimulation-conferring in naïve CD4+ T cells.
CD4+ T Cells of TCR Transgenic Mice Shift from Sensitivity to Fas-Induced Apoptosis to Responsiveness to Fas-Mediated Costimulation, After Immunization with Peptide.
We used a TCR transgenic mouse model to investigate whether populations of naïve or memory cells, generated by in vivo exposure to specific antigen, also would display a dichotomous response to Fas ligation. Unimmunized 3A9 mice, transgenic for a TCR specific for HEL peptide 46–61, served as a source of “naïve” T cells (27). Age-matched 3A9 transgenic mice were immunized with a single dose of HEL 46–61 peptide in incomplete Freund’s adjuvant and served as a source of “memory” cells 4 weeks after immunization. In contrast to previous experiments, CD4+ T cells obtained from the transgenic mice were not further fractionated according to phenotype. The entire population of peripheral CD4+ T cells from the uninjected mice was considered “naïve,” and the entire population of CD4+ T cells from the immunized mice was considered “memory.” The phenotypes of the cell populations were not as homogenous as in previous experiments on normal (nontransgenic) mice, where stringent fractionation was employed (Fig. 6A; compare with Fig. 1). Although T cells, which lack the transgenic idiotype, are a small minority of the total T cell population in the transgenic mice (27), 11–13% of CD4+ T cells were Vβ8.2− (Fig. 6A). As much as 30% of TCR transgenic T cells can convert to memory phenotype by exposure to cross-reactive environmental antigens, in the absence of specific antigen (41). The “naïve” population in our TCR transgenic mice included memory phenotype cells, presumably through exposure to environmental antigens of cells bearing endogenous TCRs. The “memory” population also included cells with a naïve phenotype, probably because the injected antigen failed to reach the entire complement of peripheral T cells in the transgenic animal. However, a shift in expression of CD44 and CD45RB occurred postimmunization, and the cells no longer expressed activation markers by 24 days after immunization (Fig. 6A). Furthermore, Fas expression was comparable on the naïve and memory populations (Fig. 6A). Thus, exposure to antigen changed the expression of memory markers on the transgenic CD4+ T cells, but activation markers and Fas expression had returned to resting levels by 24 days after immunization (Fig. 6A).
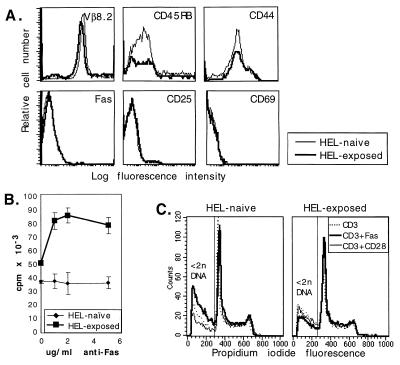
Figure 6.
Fas responsiveness of TCR-transgenic CD4+ T cells shifts from Fas-death-sensitive to Fas-costimulated after in vivo exposure to HEL. Purified CD4+ T cells were prepared from cervical and axillary lymph nodes of HEL TCR-transgenic mice that were uninjected (HEL-naïve) or were injected with HEL peptide 24 days previously (HEL-exposed). (A) Cell surface phenotypes of HEL-naïve (thin line) and memory (24 days after HEL exposure; thick line) CD4+ TCR-transgenic T cells were determined by flow cytometry. Anti-Vβ8.2 antibodies recognize the transgenic TCR β chain; 87 and 89% of CD4+ cells were Vβ8.2+ in HEL-naïve and HEL-exposed mice, respectively. Differences in phenotype between memory and naive cells occur only in CD44 and CD45RB (memory markers) expression. (B) Memory cells generated by in vivo exposure to antigen are costimulated by Fas. HEL-naïve (thin line) and memory (thick line) cells were cultured with 1 μg/ml of anti-CD3 antibody plus increasing concentrations of anti-Fas antibody. (C) Fas ligation increases apoptotic death (<2n DNA) in cultures of naïve transgenic cells, but reduces apoptosis in memory cell cultures. CD4+ TCR-transgenic T cells were cultured for 48 hr with anti-CD3 alone (dotted line), anti-CD3 plus anti-CD28 (thin, solid line), or anti-CD3 plus anti-Fas (thick line).
Despite the overlap of cell surface phenotypes in the “naïve” and “memory” cells, the two populations manifested strikingly different responses to Fas ligation. CD4+ “memory” cells from antigen-exposed transgenic mice were costimulated by Fas in a dose-dependent manner and, in fact, appeared more sensitive to Fas costimulation than did nontransgenic memory cells (Fig. 6B). In contrast, the proliferative response of CD4+ “naïve” cells from unimmunized mice was not affected overall by Fas ligation (Fig. 6B). However, Fas ligation increased the amount of apoptotic cell death among the “naïve” cells and decreased apoptosis among the “memory” cells (Fig. 6C). This result suggests that the apparent lack of Fas-mediated inhibition of naïve cell proliferation (Fig. 6B) is due to increased naïve cell death, on the one hand, and increased proliferation of contaminating memory cells, on the other. In agreement with previously published data, when T cells were collected early after antigen administration (within 2 weeks), T cells still displayed activation markers and Fas ligation resulted in marked cell death of the activated T cells, presumably by the well characterized phenomenon of Fas-mediated AICD (5–7) (data not shown).
Thus, CD4+ T cells from TCR-transgenic mice reveal that preantigen-exposure naïve cells are susceptible to Fas-mediated death (Fig. 6C) whereas postexposure memory cells are costimulated by Fas (Fig. 6B). These results are consistent with experiments using stringently fractionated T cells from nontransgenic mice.
DISCUSSION
A Dichotomy in the CD4+ T Cell Response to Fas Ligation.
We have shown opposite outcomes of Fas ligation, namely, death vs. increased proliferation, in naïve versus memory CD4+ T cells. We have used two independent systems to confirm our results. In the first, naïve and memory CD4+ T cells were defined phenotypically and obtained from a common starting population of freshly isolated, normal lymphoid cells. Naïve and memory populations cultured under identical conditions displayed opposite responses to Fas ligation. In the second system, a functional definition of naïve and memory was employed, based on exposure to antigen in a TCR-transgenic mouse model. In this model, antigen was administered in vivo to produce a pool of physiologically generated, antigen-specific memory cells, and the transgenic CD4+ T cells were not subjected to phenotype-based fractionation before Fas ligation and culture. Results obtained in both systems confirm that the outcome of Fas ligation is fundamentally different between a naïve and a memory T cell. In the absence of exogenous rescue signals, the default response of a naïve CD4+ T cell to Fas ligation in conjunction with activation signals is apoptosis. In contrast, memory T cells expand more rapidly as a consequence of Fas ligation with an antigenic signal. Previous work has shown that human peripheral blood T cells are costimulated by Fas ligation during anti-CD3-induced proliferation (25). Unfractionated mouse T cells also can be costimulated by Fas. The findings presented here suggest that in both cases, the cells being costimulated and exhibiting increased proliferation are the antigen-experienced memory cells within the total peripheral T cell population.
Memory cells acquire altered phenotypic and functional characteristics as they leave the naïve (or activated cell) pool(s), including differential cell surface expression of numerous molecules, loss of dependence on costimulation for efficient and sustained proliferation, and decreased threshold for activation (3). Such functional differences likely reflect altered levels or activities of intracellular mediators, some of which may impinge upon the Fas-signaling cascades and allow the cell to respond to identical Fas ligation events with opposite physiological outcomes. In particular, FLIP (the FLICE-inhibitory protein), which blocks Fas-induced death by preventing coupling of Fas to the downstream caspase cascades (42), is differentially regulated in T cells during activation (40) and may be expressed at higher levels in memory cells.
A Model for Fas-Dependent Peripheral Tolerance.
Mice and humans with defective Fas expression or function are highly susceptible to autoimmune disease (16, 17, 21–23). In the absence of functional Fas, AICD occurs to a lesser extent and with delayed kinetics than in normal individuals (43–46). However, lymphoaccumulation resulting from defective AICD does not explain the increased frequency of self-reactive clones (15) and the development of overt autoimmunity. The Fas-dependent death of naïve cells described here is distinct from Fas-dependent AICD, which requires prior clonal expansion of effector cells (47) and is resistant to CD28-mediated costimulation (14). In contrast, CD28-mediated costimulation of naïve cells not only prevents Fas-induced apoptosis, but allows Fas engagement to augment the CD3-induced proliferative response. Our data suggest that Fas acts at an early “checkpoint” in clonal survival, before significant clonal expansion, by inducing death of naïve cells if these cells do not receive a costimulatory signal with the initial antigen signal. Our findings provide experimental evidence for Fas-mediated clonal abortion as a mechanism behind the “two-signal” hypothesis of peripheral tolerance. According to the two-signal hypothesis, CD4+ T cells fail to proliferate if they are activated by signal 1 (antigen) in the absence of signal 2 (a costimulatory signal), as occurs during T cell recognition of a self-antigen on a non-antigen-presenting cell (48). Our data provide experimental evidence of a mechanism for clonal abortion of naïve cells activated in the absence of costimulation and, hence, a mechanism for Fas-dependent peripheral tolerance. Furthermore, our findings demonstrate that memory cells are not susceptible to Fas-mediated death and, in fact, are costimulated by exogenous Fas ligation. Although Fas-mediated costimulation is not an absolute requirement for a primary immune response (14), Fas-mediated costimulation may contribute to the increased efficiency and accelerated kinetics displayed during memory responses.
Clearly, multiple mechanisms to ensure peripheral tolerance operate in normal individuals. Redundancy in peripheral tolerance mechanisms is suggested by the fact that not all children with mutations in Fas develop the autoimmune lymphoproliferative syndrome (ALPS), despite manifesting defective apoptosis (21–23). Similarly, the lpr mutation leads to overt autoimmunity only when expressed on susceptible genetic backgrounds (18). However, incorporating the notion that Fas controls an early checkpoint in clonal expansion of naïve T cells, as well as clonal downsizing by AICD, provides a mechanism for specific Fas-mediated deletion of potentially self-reactive cells.
Paths to Autoimmunity.
Our data suggest three mechanisms of Fas-dependent predisposition to autoimmunity. First, individuals with defective Fas expression or function, such as lpr mice and children with ALPS, not only manifest lymphadenopathy, which can be explained by defective AICD, but also develop a high frequency of autoreactive clones and clinical autoimmune symptoms (19, 21–23). In the absence of Fas, naïve cells activated without costimulation will not be eliminated. Clonal expansion of naïve cells with autoreactive potential will no longer be aborted selectively, resulting in an abnormally high frequency of activated, potentially autoreactive T cells, which could lead to overt disease in susceptible individuals. Second, in individuals with normal Fas expression and function, our data suggest that early Fas-mediated peripheral tolerance could be bypassed in the presence of high levels of IL-4 or IL-12. A chronic inflammatory lesion secreting differentiation cytokines therefore may provide an environment conducive to the outgrowth of potentially self-reactive clones, because naïve cells would lose sensitivity to Fas-mediated death and gain the capacity for Fas costimulation while in an environment rich in self-antigens released from damaged tissues. This may be a basis for epitope spreading during experimentally induced and clinical autoimmune diseases and, perhaps, also in the gradual development of multiple organ involvement during autoimmunity (49–52). Third, autoimmunity may arise through reactivation of memory cells, because they lack sensitivity to Fas-mediated death even in the absence of CD28 costimulation. Autoimmunity arising from antigen cross-reactivity has been examined extensively, but the mechanisms by which it occurs are poorly understood (53, 54). Damaging responses may arise when cross-reactivity between self- and foreign antigens allows a pool of memory cells, generated in response to foreign antigens, to expand efficiently in response to self-antigen, helped instead of hindered by Fas expression.
Acknowledgments
We thank Jeffrey E. Stone and Jeff Rogers for technical assistance, Colette Charland and Jami Kupperman for flow cytometry, Roberta Christie for secretarial assistance, and Dr. Karen Fortner for critical review of the manuscript. This work was supported by National Institutes of Health Grants A1–33470 (M.K.N.) and AG-14782 (W.F.W.). J.D. is the recipient of a postdoctoral fellowship from the Medical Research Council of Canada.
ABBREVIATIONS
- Th
T helper
- AICD
activation-induced cell death
- TCR
T cell receptor
- HEL
hen egg lysozyme
References
- 1.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 4.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, et al. Nature (London) 1995;373:342–345. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 6.Ju S T, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Nature (London) 1995;373:345–348. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 7.Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer P H. Nature (London) 1995;373:438–440. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 8.Smith C G, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 11.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 12.Renno T, Hahne M, Tschopp J, MacDonald H R. J Exp Med. 1996;183:431–437. doi: 10.1084/jem.183.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger R, Panka D J, Wang J K, Stanger B Z, Ju S T, Marshak-Rothstein A. J Immunol. 1995;154:4302–4308. [PubMed] [Google Scholar]
- 14.Parijs L V, Ibraghimov A, Abbas A. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 15.Parijs L V, Peterson D A, Abbas A. Immunity. 1998;8:265–274. doi: 10.1016/s1074-7613(00)80478-1. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 17.Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 18.Lenardo M J. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer G G, Carrera A C, Marshak-Rothstein A, Martinez-A C, Abbas A K. Curr Opin Immunol. 1994;6:913–920. doi: 10.1016/0952-7915(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 20.Itoh N, Itoh N, Imagawa A, Hanafusa T, Maguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Namba M, et al. J Exp Med. 1997;186:613–618. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sneller M C, S E, S, E S, J, Jaffe J S, Fleisher T A, Stetler-Stevenson M, Strober W. J Clin Invest. 1992;90:334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts I A, Deban K M, Fischer A, de Villartary J P. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 23.Fisher G H, Rosenberg F J, Straus S E, Dale J K, Middleton L A, Lin A Y, Stober W, Lenardo M J, Puck J M. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 24.Alderson M R, Tough T W, Braddy S, Davis-Smith T, Roux E, Schooley K, Miller R E, Lynch D H. Int Immunol. 1994;6:1799–1806. doi: 10.1093/intimm/6.11.1799. [DOI] [PubMed] [Google Scholar]
- 25.Alderson M R, Armitage R J, Maraskovsky E, Tough T W, Roux E, Schooley K, Ramsdell F, Lynch D H. J Exp Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch D H, Ramsdell F, Alderson M R. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 27.Ho W Y, Cooke M P, Goodnow C C, Davis M M. J Exp Med. 1994;179:1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desbarats J, Freed J H, Campbell P A, Newell M K. Proc Natl Acad Sci USA. 1996;93:11014–11018. doi: 10.1073/pnas.93.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 30.Mackay C R, Marston W L, Dudler L. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay C R. Immunol Today. 1991;12:189–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal S, Gupta S. J Immunol. 1998;160:1627–1637. [PubMed] [Google Scholar]
- 33.Krummel M F, Allison J P. J Exp Med. 1995;182:459–463. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linsley P, Ledbetter J A. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 35.Rudd C E. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 36.Seder R A, Gazzinelli R, Sher A, Paul W E. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 38.Le Gros G, Ben-Sasson S Z, Seder R, Finkelman F D, Paul W E. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swain S L, Weinberg A D, English M, Huston G. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 40.Rafaeli Y, Van Parijs L, London C A, Tschopp J, Abbas A K. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 41.Lee W T, Cole-Calkins J, Street N. J Immunol. 1996;157:5300–5307. [PubMed] [Google Scholar]
- 42.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, et al. Nature (London) 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 43.Scott D E, Kisch W J, Steinberg A D. J Immunol. 1993;150:664–672. [PubMed] [Google Scholar]
- 44.Kim C, Siminovitch K A, Ochi A. J Exp Med. 1991;174:1431–1437. doi: 10.1084/jem.174.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mixter P F, Russel J Q, Budd R C. J Autoimmunol. 1994;7:697–710. doi: 10.1006/jaut.1994.1055. [DOI] [PubMed] [Google Scholar]
- 46.Mogil R J, Radvanyi L, Gonzales-Quintial R, Miller R, Mills G, Theophilopoulos A N, Green D R. Int Immunol. 1995;7:1451–1458. doi: 10.1093/intimm/7.9.1451. [DOI] [PubMed] [Google Scholar]
- 47.Renno T, Hahne M, MacDonald H R. J Exp Med. 1995;181:2283–2287. doi: 10.1084/jem.181.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller D L, Jenkins M K, Schwartz R H. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 49.Vanderlugt C J, Miller S D. Curr Opin Immunol. 1996;8:831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan L S, Vanderlugt C J, Hashimoto T, Nishikawa T, Zone J J, Black M M, Wojnarowska F, Stevens S R, Chen M, Fairley J A, et al. J Invest Dermatol. 1998;110:103–109. doi: 10.1046/j.1523-1747.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 51.Miller S D, McRae B L, Vanderlugt C L, Nikcevich K M, Pope J G, Pope L, Karpus W J. Immunol Rev. 1995;144:225–244. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 52.Kouskoff V, Korganow A-S, Duchatelle V, Degott C, Benoist C, Mathis D. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 53.Rose N R. Semin Immunol. 1998;10:5–13. doi: 10.1006/smim.1997.0100. [DOI] [PubMed] [Google Scholar]
- 54.Karlsen A E, Dyrberg T. Semin Immunol. 1998;10:25–34. doi: 10.1006/smim.1997.0102. [DOI] [PubMed] [Google Scholar]