Abstract
Background and purpose:
Vascular smooth muscle proliferation and migration triggered by inflammatory stimuli are involved in the development and progression of atherosclerosis and restenosis. Cannabinoids may modulate cell proliferation in various cell types through cannabinoid 2 (CB2) receptors. Here, we investigated the effects of CB2 receptor agonists on TNF-α-induced proliferation, migration and signal transduction in human coronary artery smooth muscle cells (HCASMCs).
Experimental approach:
HCASMCs were stimulated with TNF-α. Smooth muscle proliferation was determined by the extent of BrdU incorporation and the migration was assayed by modified Boyden chamber. CB2 and/or CB1 receptor expressions were determined by immunofluorescence staining, western blotting, RT-PCR, real-time PCR and flow cytometry.
Key results:
Low levels of CB2 and CB1 receptors were detectable in HCASMCs compared to the high levels of CB2 receptors expressed in THP-1 monocytes. TNF-α triggered up to ∼80% increase (depending on the method used) in CB2 receptor mRNA and/or protein expression in HCASMCs, and induced Ras, p38 MAPK, ERK 1/2, SAPK/JNK and Akt activation, while increasing proliferation and migration. The CB2 agonists, JWH-133 and HU-308, dose-dependently attenuated these effects of TNF-α.
Conclusions and implications:
Since the above-mentioned TNF-α-induced phenotypic changes are critical in the initiation and progression of atherosclerosis and restenosis, our findings suggest that CB2 agonists may offer a novel approach in the treatment of these pathologies by decreasing vascular smooth muscle proliferation and migration.
Keywords: cannabinoids, cannabinoid 2 receptor, smooth muscle, proliferation, migration, antibodies
Introduction
Vascular smooth muscle proliferation and migration are pivotal events in the pathogenesis of atherosclerosis and are directly implicated in the failure of clinical interventions used to treat patients with coronary heart disease. For example, percutaneous transluminal angioplasty (an invasive procedure aimed to repair a stenotic blood vessel by inflating a balloon-tipped catheter at the site of the vascular narrowing followed by an insertion of an expandable wire mesh or hollow perforated tube (stent) into the reconstructed/dilated blood vessel to protect against re-narrowing), often fails with the time due to the development of restenosis (Tanaka et al., 1996; Zimmerman et al., 2003; Cai, 2006; Tedgui and Mallat, 2006). Vascular smooth muscle cells are the principal cell type in both restenotic and atherosclerotic lesions (Tanaka et al., 1996; Zimmerman et al., 2003; Tedgui and Mallat, 2006), which are formed as a result of numerous pathological processes involving generation of reactive oxygen and nitrogen species (Pacher et al., 2006b, 2007), and the accumulation of inflammatory cells and the release of cytokines such as tumour necrosis factor alpha (TNF-α) (Patel et al., 2000; Harrison et al., 2003; Hansson and Libby, 2006; Pacher et al., 2007). The proinflammatory cytokine TNF-α is considered to play a key role in increasing the expression of endothelial-cell adhesion molecules and promoting the release of various chemokines recruiting monocytes to the site of injury and enhancing their adhesiveness to the endothelium. TNF-α also stimulates the release of variety of factors that induce smooth muscle migration and proliferation (from the media into the intima), which then synthesize and deposit extracellular matrix (Ip et al., 1990; Osterud and Bjorklid, 2003; Hansson, 2005; Tedgui and Mallat, 2006; Gerthoffer, 2007).
Endocannabinoids, which include arachidonoyl ethanolamide or anandamide (AEA) and 2-arachidonoylglycerol (2-AG), phytocannabinoids and their synthetic analogues exert various cardiovascular, metabolic, central nervous system and anti-inflammatory effects, predominantly, through CB1 and CB2 receptors (Mechoulam et al., 1998; Klein, 2005; Pacher et al., 2006a). The CB1 receptor is highly expressed in the brain, but lower levels are also detectable in peripheral tissues including heart, vascular endothelium and liver (reviewed in Mackie, 2006; Pacher et al., 2006a). Initially, the CB2 receptor was considered to be expressed primarily by immune and haematopoietic cells, however, recent studies have also intriguingly demonstrated these receptors in brain (Van Sickle et al., 2005), myocardium (Mukhopadhyay et al., 2007a), cardiomyoblasts (Shmist et al., 2006; Mukhopadhyay et al., 2007a) and endothelial cells of various origins (Blazquez et al., 2003; Zoratti et al., 2003; Golech et al., 2004; Mestre et al., 2006) (reviewed in Klein, 2005; Mackie, 2006; Pacher et al., 2006b).
Delta9-tetrahydrocannabinol (THC), a cannabis constituent, has recently been demonstrated to inhibit atherosclerosis progression in a mouse model of the disease, presumably by CB2 receptor stimulation in inflammatory cells (Steffens et al., 2005). However, the role of CB2 receptor in vascular smooth muscle cell activation in this phenomenon remains unexplored. There is considerable evidence that endocannabinoids and their synthetic analogues may differentially modulate cell proliferation in various cell types through CB2 receptor-dependent and -independent mechanisms (Lopez-Rodriguez et al., 2005; McAllister et al., 2005; Blazquez et al., 2006; Ofek et al., 2006; Lombard et al., 2007; Maresz et al., 2007; Wilkinson and Williamson, 2007). Thus, we aimed to evaluate the effects of selective CB2 receptor agonists on TNF-α-induced proliferation, migration and signal transduction of human coronary artery smooth muscle cells (HCASMCs). Given that the vascular smooth muscle proliferation and migration triggered by inflammatory stimuli are pivotal events in the pathogenesis and progression of atherosclerosis and restenosis, our findings may have important clinical implications. Since these are critical tools for assessing the role of CB2 in any process, we will discuss some limitations of the presently available CB2 antibodies and emphasize the importance of the use of proper controls and multiple methods to confirm the presence of CB2 receptors.
Materials and methods
Materials
The CB2 receptor agonist JWH-133 was either purchased from Tocris Bioscience (Ellisville, MO, USA) or synthesized as described earlier (Huffman et al., 1999). The selective CB2 receptor agonist HU-308 (Hanus et al., 1999) was from Cayman Europe (Tallinn, Estonia). CB2 antagonist AM-630 was purchased from Tocris Bioscience (Ellisville, MO, USA). SR141716A (SR1) and SR144528 (SR2) were purchased from NIDA Drug Supply (NC, USA). Human recombinant TNF-α was obtained from R&D Systems, (MN, USA). Sources of all the other reagents are mentioned in the text where appropriate.
Cell culture
Human coronary artery smooth muscle cells were obtained from Cambrex (MD, USA) and were cultured in smooth muscle growth medium (SmGM-2) (Cambrex). HCASMCs in passages 3–6 were used for the experiments. Human coronary artery endothelial cells and its growth medium were obtained from Cell Applications (CA, USA). Human monocytic cell line (THP-1) was obtained from American Type Culture Collection (ATCC, VA, USA) and was grown in RPMI 1640 medium supplemented with 2 mM L-glutamine, 10 mM HEPES, 10% fetal bovine serum, 100 U of penicillin and 100 μg of streptomycin per ml, respectively (Invitrogen, CA, USA).
Immunofluorescence staining
Human coronary artery smooth muscle cells were grown to confluence in chamber slides (Lab-Tek, Nalgene-Nunc Inc., New York, NY, USA). The growth medium was aspirated and cells were washed twice with phosphate buffered saline (PBS) and then were fixed with 4% paraformaldehyde for 20 min at 4 °C. After washing with PBS, cells were permeabilized in 0.2% Triton X-100 in PBS for 15 min at room temperature (RT). Following washing, the CB1 and CB2 expressions in the human vascular smooth muscle cells were determined by immunofluorescence staining technique using anti-CB1 (rabbit polyclonal, Cayman Chemical, MI, USA) or CB2 (rabbit polyclonal, Cayman Chemicals) respectively, used at 1:100 dilution for 5 h at 4 °C. Next, the cells were probed with goat-anti-rabbit FITC (1:250, Pierce, IL, USA) for 1 h at RT. After rinsing cells with PBS, the cells were probed with goat-anti-rabbit FITC (1:250, Pierce) for 1 h at RT. The nucleus was counterstained with DAPI (Molecular Probes, CA, USA). Images were obtained using fluorescent microscope (Olympus IX 81) at × 20 objective with × 1.5 optical zoom. To rule out the non-specific staining, the primary antibodies were preabsorbed with the corresponding blocking peptides for CB1 (Cayman, Cat #10006591) or CB2 (Cayman, Cat #301550), according to the protocol provided with the peptides. In brief, the blocking peptides were mixed with corresponding antibodies in 1:1 ratio and were incubated for 1 h at RT. Later, this preabsorbed antibody was used for the staining. This procedure essentially blocks the antibody–antigen (protein) formation during the immunofluorescence staining and thus aids in confirming/evaluating specificity of the CB1/CB2 receptor staining.
Conventional RT-PCR and real-time PCR
Total RNA was isolated from the cells using Trizol LS reagent (Invitrogen) according to the manufacturer's instruction. The RNA was treated with RNase-free DNase (Ambion, TX, USA) to remove traces of genomic DNA contamination. Total RNA was then reverse-transcribed to cDNA using the Super-Script II (Invitrogen) and the target genes were amplified using the standard PCR kit (BioRad, CA, USA). The PCR conditions were as follows: after initial denaturation at 95 °C for 2 min, 35 cycles were performed at 95 °C for 30 s and at 60 °C for 30 s. Primers used were as follows:
CB1
5′-TTCCCTCTTGTGAAGGCACTG-3′ (forward)
5′-TCTTGACCGTGCTCTTGATGC-3′ (reverse)
CB2
5′-TTTGCTTTCTGCTCCATGCTG-3′ (forward)
5′-TTCTTTTGCCTCTGACCCAAG-3′ (reverse)
β-Actin
5′-ATTGCCGACAGGATGCAGAAG-3′ (forward)
5′-TAGAAGCATTTGCGGTGGACG-3′ (reverse).
The amplified products were separated on 1.5% agarose gels stained with ethidium bromide and documented using phosphor-imaging system (GE Healthcare, NJ, USA).
In a separate set of experiments, HCASMCs were treated with TNF-α (50 ng ml−1) for 0−6 h and real-time PCR was performed with identical conditions except the amplification and quantification of the target gene expression was carried out using iTaq Syber green mix (BioRad) using BioRad chromo4/opticon system. Relative quantification was carried out using the relative (comparative) CT method.
Western immunoblot assay
Human coronary artery smooth muscle cells were grown to confluence in 100 mm culture dishes coated with 0.2% gelatin and the cell lysates were prepared using lysis buffer (Pierce Biotechnology, IL, USA) supplemented with protease inhibitors (Roche, GmbH, IN, USA). The lysates were prepared by sonication (15k for 20 s) on ice. Later, the lysates were clarified to remove the cellular debris by centrifuging at 10 000 r.p.m. for 15 min at 4 °C. Protein content in the lysates was determined using the Lowry assay (BioRad). A measure of 25 μg protein was resolved in 12% SDS-PAGE and was transferred onto nitrocellulose membranes (GE Healthcare). Blocking was performed for 2 h at RT with 5% non-fat skimmed milk powder prepared in PBS containing 0.1% Tween 20 (Sigma, CA, USA). After washing with phosphate-buffered saline 0.1% Tween 20 (PBST), the membranes were probed with either rabbit polyclonal CB1 (Cayman Chemicals; 1:1000 dilution) or with an antibody raised against the last 15 residues of rat CB1 or CB2 antibody (Cayman Chemicals; 1:1000 dilution) overnight at 4 °C. After subsequent washing with PBST, the secondary antibody—goat-anti-rabbit was added to HRP (Pierce Biotechnology) was incubated at RT for 1 h. Similar experiments were also performed with CB1 antibody from Ken Mackie's laboratory (Figure 1d, lower blot). Later, the membranes were developed using chemiluminescence detection kit (Super Signal -West Pico Substrate, Pierce). To confirm uniform loading, the membranes were stripped and re-probed with β-actin (Chemicon, CA, USA). In a separate set of experiments, the cells were treated with TNF-α (50 ng ml−1) for different time periods (0–6 h) and CB2 expression was determined using immunoblot assays. The protein from mouse brain extract and human monocytic cell line (THP-1) lysate was used as appropriate positive controls for CB1 and CB2 receptors, respectively.
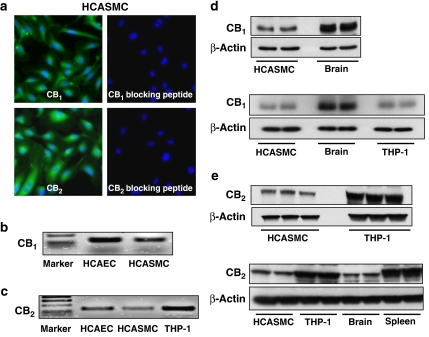
Figure 1.
CB1 and CB2 receptors expression in HCASMCs. (a) Expression of CB1 and CB2 receptors in HCASMCs demonstrated by immunofluorescence staining. CB1 and CB2 expression in the vascular smooth muscle cells was detected using rabbit-polyclonal anti-CB1 and -CB2 antibodies (Cayman Chemicals) and using secondary antibody goat-anti-rabbit FITC (Pierce), the nucleus was contrastained with 4′,6-diamidino-2-phenylindole (DAPI, Molecular Probes, Invitrogen). (b, c) RT-PCR analysis of CB1/2 receptor expression in human endothelial and smooth muscle cells. (d, e) Western immunoblot demonstrating expression of CB1 and CB2 receptors in HCASMCs. Here, representative images from three independent experiments with identical results are shown.
Flow cytometry
The surface expression of CB2 receptors was determined using flow cytometry technique. For surface staining, cells were incubated with CB2 antibody (rabbit-polyclonal, Cayman, (5–20 μg ml−1)) for 1 h at RT. THP-1 monocytes were used as a positive control. After blocking with 1%BSA (W/V) in Hank's buffered salt solution (HBSS) buffer (Invitrogen), the cells were incubated with CB2 antibody (rabbit-polyclonal, Cayman, (5–20 μg ml−1)) for 1 h at RT. After washing in HBSS, the cells were incubated with secondary anti-rabbit FITC-conjugate (1:100 dilution; Pierce Biotechnology) for 1 h. After washing two times with HBSS, the cells were analysed by FACS Calibur flow cytometer (BD, CA, USA) as described (Rajesh et al., 2007; Mukhopadhyay et al., 2007a, 2007b). To rule out the potential cross-reaction with immunoglobulin receptor (FcγII, CD32) while determining the surface expression of CB2 receptors in THP-1 monocytes, cells were blocked with/without CD32 antibody (5 μg ml−1; BD Biosciences) for 15 min followed by incubation with CB2 antibody (Cayman) as described above. After washing, cells were incubated with secondary antibody-antirabbit FITC (1:100 dilution, Pierce Biotechnology). Later, the analysis was performed in FACS Calibur System (Becton Dickinson, CA, USA) and the data were exported to and analysed using FlowJo version 8.5 (Ashland, OR, USA).
Detection of apoptosis by flow cytometry
Human coronary artery smooth muscle cells were grown in 12-well tissue-culture plates and then treated with/without TNF-α and CB2 receptor agonists/antagonists for 36 h. In separate experiments, smooth muscle cells were also treated with TNF-α and with/without CB1 antagonist for 36 h and apoptosis was determined by flow cytometry using the assay as described previously (Mukhopadhyay et al., 2007a, 2007b).
Ras activation assay
Ras activation in HCASMCs was determined using commercially available kit (Pierce). In brief, Ras activation assay involved the use of GTP-fusion protein containing Ras-binding domain (RBD) of Raf1, which specifically binds to and pulls down active Ras. The pulled-down active Ras was detected by western blot analysis using anti-Ras antibody.
Migration assay
The migration assays were performed in a modified, 24-well Boyden chamber as described earlier (Rajesh et al., 2006). In brief, 8 μm cell culture inserts (BD Biosciences) coated with 0.2% gelatin (Sigma) were placed on the bottom chamber with/without 50 ng ml–1 TNF-α. HCASMCs (3 × 104 cells) were suspended in 150 μl in growth-factor free medium containing 1% FBS. Later, the cells that were treated with CB2 agonists±CB2 antagonists for 90 min at 37 °C in 5% CO2 incubator. Later, the cell suspension was added to the upper chamber. After 8 h of incubation at 37 °C, the non-migrated cells on the upper surface of the filter were removed by gentle swabbing with cotton-tipped applicators. The cells that had migrated to the lower side of the chamber were fixed with 100% methanol for 15 min at RT. After complete drying, the inserts containing migrated cells were stained with 0.5% Giemsa solution (Sigma). Three to four fields per insert were counted using × 10 objective of Olympus IX81 microscope. The assays were performed in duplicates and the experiments were repeated three times.
Proliferation assay
Human coronary artery smooth muscle cells (5 × 103) were suspended in 150 μl of growth factor free smooth muscle cell (SMC) medium containing 1% FBS and were plated on to 96-well plates and were allowed to adhere for 1 h. Later, the cells were treated with TNF-α (50 ng ml−1) ±CB2 agonists (4 μM)/antagonists (1 μM) and were allowed to proliferate for 24 h. After which, 100 μl of BrdU labelling solution (10 μM final concentration) was added to each well and incubated additionally for 12 h. At the end of incubation, the proliferation was determined by the extent of BrdU incorporated using an ELISA kit (Roche GmbH). Each treatment was performed in triplicates and the entire set of experiments was repeated three times.
Western blot analysis of signalling proteins in HCASMCs
The cells were grown in 100 mm dishes and after treatment with TNF-α±CB2 agonists/antagonists, the cell lysates were prepared using RIPA lysis buffer (Pierce). A measure of 25 μg protein was resolved in 12% SDS-PAGE and was transferred onto nitrocellulose membranes (Invitrogen) and the blots were probed with antihuman rabbit p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), ERK1/2, phosphor-ERK1/2, SAPK/JNK 1/2, phospho-SAPK/JNK (Thr183/Tyr185), Akt or pAkt (Ser 473) at 1:1000 dilution, respectively (Cell Signaling Technology, MA, USA) and the blots were developed using antirabbit HRP with a chemiluminescence detection kit (Super Signal -West Pico Substrate, Pierce). Protein content in the cell lysates was measured by Lowry protein assay (BioRad).
Statistical analysis
All the values were represented as mean±s.d. Statistical significance of the data was assessed by one-way ANOVA with Tukey's post hoc test (GraphPad-Prism4, CA, USA). P<0.05 was considered significant.
Results
CB2 receptors are expressed in HCASMCs
As shown in Figure 1, Figure 2, CB1 and CB2 receptors are expressed in cultured human vascular smooth muscle cells, at basal conditions as demonstrated by immunofluorescence assays (Figure 1a), conventional RT-PCR (Figures 1b and c), real-time PCR (Figure 2c), western blot (Figure 1d, e, Figure 2b) and flow cytometry (Figure 2a) assays, respectively. Protein extracts from mouse brain, spleen and THP-1 monocytes lysate or THP-1 monocytes were also used as appropriate positive controls for the detection of CB1 and/or CB2 receptors, respectively (Figure 1c, d, e, 2b–d). Preabsorbing either the CB1 or CB2, with the corresponding blocking peptides supplied with the primary antibodies, abrogated the detection of CB1 and CB2 in HCASMCs by immunofluorescence (Figures 1a and b) and western blot assays (data not shown).
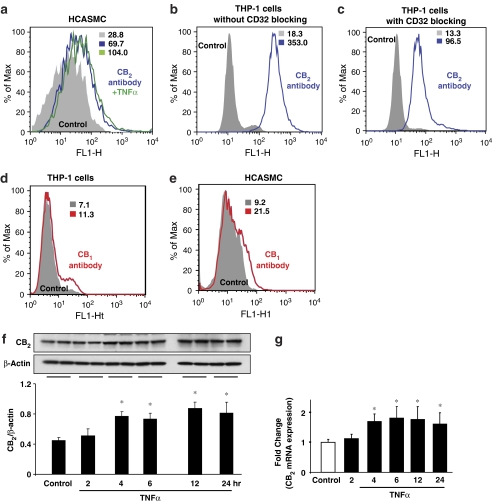
Figure 2.
CB2 receptor expression in HCASMCs and effect of TNF-α. Cells were treated with TNF-α (50 ng ml−1) for 6 h or indicated time intervals and flow cytometry (a–e), western blot (f) or quantitative real-time PCR (g) analyses were performed for CB2 and/or CB1 expression in HCASMCs and THP-1 monocytes, respectively. Panels a–c show surface expression of CB2 receptors in HCASMCs and THP1-monocytes (blue traces) and the effect of TNF-α on CB2 expression in HCASMCs (green trace, panel a). Panels d and e show surface expression of CB1 receptors in HCASMCs and THP1-monocytes (red traces). Panels f and g denote CB2 receptor expression in HCASMCs and the effect of TNF-α by western blot and real-time PCR, respectively. Data presented are representative of 5–7 independent experiments. *P<0.05 vs control.
We further studied the surface expression of CB2 using flow cytometry. As shown in Figure 2a, CB2 receptors are expressed in HCASMCs and TNFα pretreatment further augmented their expression by ∼30%. The mean intensities are provided in the respective panels. We used THP-1 monocyte cell line as a positive control for surface expression of CB2. To rule out the potential non-specific binding of CB2 antibody with immunoglobulin receptor (FcγII, CD32) while determining the surface expression of CB2 receptors in THP-1 monocytes, CD32 antibody (5 μg ml−1; BD Biosciences) was used for blocking. As shown in Figures 2b and c, indeed, CD32 blockade decreased CB2 receptor binding (353 mean intensity vs 96.5) emphasizing the importance of Fc receptor binding in these sorts of experiments.
TNF-α upregulates CB2 expression in HCASMCs
Human coronary artery smooth muscle cells were pre-treated with TNF-α (50 ng ml−1) for different time points as indicated in Figures 2f and g and then the CB2 expression was determined by western blot and quantitative real time-PCR and FACS assays. Results revealed that TNF-α treatment resulted in a time-dependent increase (up to ∼1.8 fold vs control, depending on the method used) in the CB2 receptor expression in HCASMCs (Figures 2f and g), respectively.
CB2 agonists/antagonists did not induce apoptosis in HCASMC
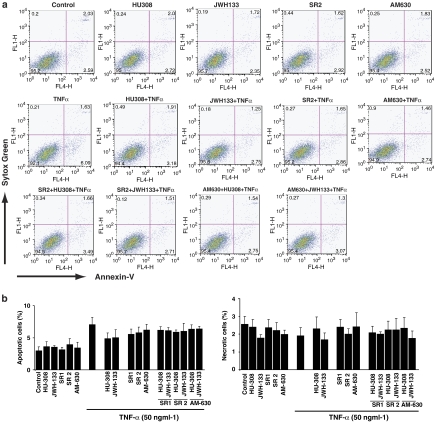
As shown in Figure 3, treatment of vascular smooth muscle cells with TNF-α (50 ng ml−1) alone for 36 h induced a moderate increase (∼2.5–3.5%) in early apoptotic (Annexin-V positive) but not late apoptotic/necrotic (Annexin-V and Sytox Green positive) cells, which was not significantly affected by either cannabinoid receptor agonists or antagonist (Figures 3a and b).
Figure 3.
Effect of TNF-α and/or CB2 agonists/antagonists on cell death in HCASMCs. Smooth muscle cells were grown in 12-well tissue-culture plates and then treated with agonists/antagonists, with or without TNF-α (50 ng ml−1) for 36 h. Agonists and antagonists were used at 4 and 1 μM, respectively. Later, apoptosis/necrosis was measured by FACS calibur flow cytometer (BD Biosciences, CA, USA) using Sytox Green and Annexin-V respectively. Panel (a) depicts representative FACS plots for the treatments indicated. Panel (b) shows the percentage of early apoptotic and late apoptoticnecrotic cells as indicated.
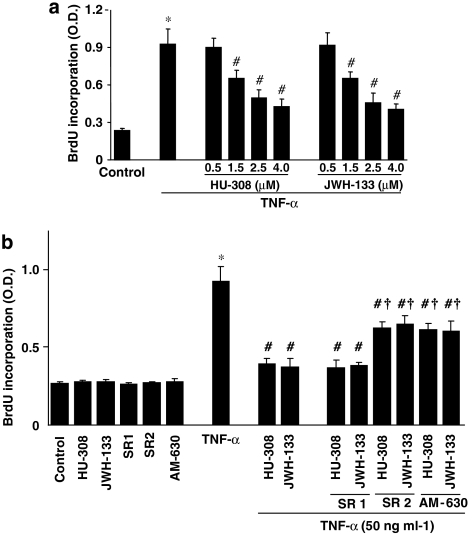
CB2 agonists inhibit TNF-α-induced HCASMCs proliferation
TNF-α-significantly stimulated the proliferation in vascular smooth muscle cells (∼3.5-fold increase vs untreated control cells; Figure 4a). Pretreatment of the cells with either JWH-133 or HU-308 (0.5–4 μM) dose-dependently inhibited proliferation of vascular smooth muscle cells (Figure 4a), which was attenuated by CB2 antagonists SR2/AM630 (1 μM; Figure 4b) but not by the CB1 antagonist, SR1 (Figure 4b). The CB2 agonists/antagonists or the CB1 antagonist SR1 alone did not affect the basal proliferation of smooth muscle cells (Figure 4b).
Figure 4.
Effect of CB2 agonists on TNF-α-induced human vascular smooth muscle cell proliferation. Panel (a) shows HCASMCs were treated as indicated and proliferation was determined by measuring the extent of BrdU incorporation using ELISA kit by absorbance at 450 nm. *P<0.05 vs control; #P<0.05 vs TNF-α; n=6. The concentration of TNF-α used in these experiments was 50 ng ml−1. In panel (b) HCASMCs were treated as shown, and proliferation was determined by ELISA. The CB2 agonists were used at concentration of 4 μM, while the antagonists were used at 1 μM, respectively. *P<0.05 vs control; #P<0.05 vs TNF-α; †P<0.05 vs TNF-α+HU-308/JWH-133, n=6.
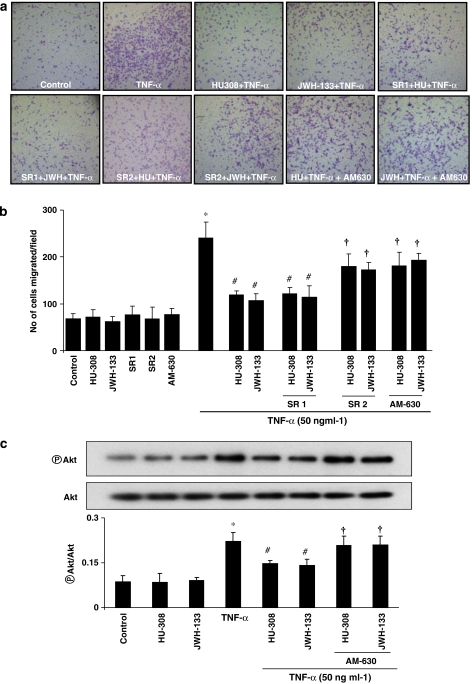
CB2 agonists attenuate TNF-α-induced HCASMCs migration
Tumour necrosis factor-α profoundly stimulated the migration of vascular smooth muscle cells (∼3.7-fold increase vs unstimulated control cells; Figure 5). HU-308/JWH-133 (4 μM) treatment (Figures 5a and b) abrogated TNF-α-induced migration. In turn, this was attenuated by AM630/SR2 but not by SR1 (Figures 5a and b). CB2 agonists/antagonists or CB1 antagonist SR1 alone did not affect the basal migration of smooth muscle cells (Figure 5b).
Figure 5.
Effect of CB2 agonists on TNF-α-induced human vascular smooth muscle cell migration and Akt activation. Panel (a) indicates the migration of smooth muscle cells as per the treatments indicated. Panel (b) shows quantification data for the migrated cells. *P<0.05 vs controls; #P<0.05 vs TNF-α; †P<0.05 vs TNF-α±HU-308/JWH-133 n=4. Panel (c) shows that cells were grown in 100 mm dishes and treated as indicated. In brief, cells were either treated with TNF-α (50 ng ml−1) alone for 6 h or TNF-α+ CB2 agonists (4 μM)/antagonists (1 μM). Later, cell lysates were subjected to western blot analysis for the determination of Akt activation. *P<0.05 vs controls; #P<0.05 vs TNF-α; †P<0.05 vs TNF-α±HU-308/JWH-133 n=3.
CB2 agonists attenuate TNF-α-induced Akt activation in HCASMCs
As shown in Figure 5c, TNF-α (50 ng ml−1) treatment for 6 h significantly (∼2.4. fold vs control) induced Akt activation, which was attenuated by the CB2 agonists HU-308 and JWH-133, whose actions were attenuated by AM630.
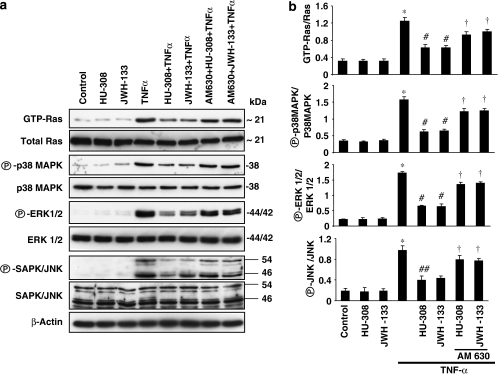
CB2 agonists inhibit TNF-α-induced Ras activation
Tumour necrosis factor-α has been shown to induce Ras activation resulting in proliferation and migration of human vascular smooth muscle cells, and Ras activation has also been shown to be involved in the pathogenesis of atherosclerosis (Mehrhof et al., 2005; Cai, 2006; Gerthoffer, 2007). Since CB2 agonists inhibited smooth muscle cells proliferation and migration, we tested the effect of HU-308 and JWH-133 on TNF-α-induced Ras activation in HCASMCs. We observed that TNF-α treatment led to marked Ras activation (∼3.0-fold increase vs control; Figures 6a and b). Treatment of the cells with HU-308/JWH-133 (4 μM) significantly decreased Ras activation by TNF-α, a decrease that could be attenuated by AM630.
Figure 6.
Effect of CB2 agonists on TNF-α-induced Ras-MAPK pathway activation in human vascular smooth muscle cells. Panel (a) shows cells were grown in 100 mm dishes and were treated as indicated. In brief, cells were either treated with TNF-α (50 ng ml−1) alone for 6 h or TNF-α+CB2 agonists (4 μM)/antagonists (1 μM). To determine Ras activation, 500 μg of protein was used for pull-down assays. For the rest of the signalling proteins, 25 μg, were resolved in 12% SDS-PAGE and blots were probed with antibodies as indicated. The representative blots from three identical experiments are also shown and panel (b) represents the densitometry quantification of protein expression. *P<0.05 vs controls; #P<0.05 vs TNF-α; †P<0.05 vs TNF-α±HU-308/JWH-133 n=3.
CB2 agonists mitigate TNF-α-induced MAPK pathway in HCASMCs
Tumour necrosis factor-α has been shown to induce MAPK kinase activation and to stimulate proliferation and migration of human vascular smooth muscle cells (Huang et al., 2004; Mehrhof et al., 2005; Cai, 2006; Gerthoffer, 2007). Since CB2 agonists inhibited proliferation and migration of HCASMCs, we studied the effects of CB2 activation on these signalling pathways. As shown in Figures 6a and b, TNF-α treatment of HCASMCs resulted in robust activation of p38 MAPK, ERK 1/2 and SAPK/JNK demonstrated by western blot assays with specific activation state antibodies. Pretreatment of cells with HU-308/JWH-133 blunted activation of MAPK pathway and these effects were attenuated by AM 630.
Discussion
In this study, we show, for the first time using multiple techniques that human coronary smooth muscle cells express CB2 receptor. Furthermore, we demonstrate that two selective CB2 receptor agonists attenuate TNF-α-triggered proliferation and migration of human coronary smooth muscle cells and the activation of various inter-related signalling pathways (Ras, p38 MAPK, ERK 1/2, SAPK/JNK and Akt).
It was previously held that CB2 receptors are mainly expressed by immune and haematopoietic cells (Klein, 2005; Mackie, 2006; Pacher et al., 2006a). Intriguingly, recent studies have provided evidence on the presence of CB2 receptors in brain (Van Sickle et al., 2005), myocardium (Mukhopadhyay et al., 2007a), endothelial cells (Blazquez et al., 2003; Zoratti et al., 2003; Golech et al., 2004; Mestre et al., 2006) and cardiomyoblasts (Shmist et al., 2006; Mukhopadhyay et al., 2007a). Here, we report expression of CB2 and/or CB1 receptors in human coronary smooth muscle cells under basal cell culture conditions by using immunofluorescence staining, western blotting, RT-PCR, real-time PCR and flow cytometry (Figure 1, Figure 2). Interestingly, the expression of CB2 receptors could be enhanced by the pro-inflammatory cytokine TNF-α both at mRNA and at protein levels in vascular smooth muscle cells. An analogous phenomenon has been observed during the microglia activation (Walter et al., 2003), and several recent studies have demonstrated markedly increased CB2 receptor expression in various inflammatory and other cell types under various pathological conditions (Zhang et al., 2003; Maresz et al., 2005; Mendez-Sanchez et al., 2007).
Previous studies have documented that TNF-α can contribute to migration and proliferation of vascular smooth muscle cells (Warner and Libby, 1989; Goetze et al., 1999; Selzman et al., 1999; Wang et al., 2005; Gerthoffer, 2007). Smooth muscle migration and proliferation play important roles in the development of atherosclerosis and vascular remodelling that occurs during restenosis in patients who undergo balloon angioplasty (Tanaka et al., 1996; Zimmerman et al., 2003; Cai, 2006; Tedgui and Mallat, 2006). Numerous studies have demonstrated that endocannabinoids and their synthetic analogues may differentially modulate cell proliferation in various cell types through CB2 receptor-dependent and -independent mechanisms (McAllister et al., 2005; Blazquez et al., 2006; Ofek et al., 2006; Lombard et al., 2007; Maresz et al., 2007; Wilkinson and Williamson, 2007) (reviewed in Lopez-Rodriguez et al., 2005). Therefore, we hypothesized that CB2 receptors in vascular smooth muscle cells may play an important role in modulating TNF-α-induced cell migration and proliferation. Indeed, as shown in Figure 4, Figure 5, CB2 receptor stimulation with HU-308 and JWH-133 dose-dependently attenuated TNF-α-induced but not basal cell proliferation (measured by BrdU incorporation) as well as cell migration in a CB2-dependent fashion.
Next, to determine if CB2 agonists also influenced the TNF-α-induced signalling cascade leading to cell proliferation, additional experiments were conducted. Previous studies have suggested that TNF-α stimulates p21 GTP-loading and activates the MAPK cascade, SAPK/JNK leading to upregulation of nuclear regulatory factors. These ultimately upregulate cyclin-dependent kinases stimulating cell proliferation (Huang et al., 2004). Akt has also been shown to play an important role in the orchestrating cell migration and proliferation in various cell types including smooth muscle (Hsu et al., 2001; Shiojima and Walsh, 2002; Lee et al., 2007; Wang et al., 2007).
Our data also support a role for TNF-α in inducing cell migration and proliferation in normal human vascular smooth muscle. We now propose that CB2 receptor agonists may suppress the TNF-α-TNFR complex-triggered signalling cascade ultimately involved in cell proliferation and migration. Since vascular smooth muscle proliferation and migration triggered by inflammatory stimuli are crucial events in the pathogenesis and progression of atherosclerosis and restenosis our results suggest that selective CB2 receptor agonists may offer a novel approach in the treatment of these pathologies. Thus, the attenuation of TNF signalling, coupled with the absence of psychoactive effects associated with CB2 receptor stimulation, makes this a particularly encouraging therapeutic approach. Furthermore, implantable stents could also be coated with releasable CB2 agonists, and perhaps other cannabinergic ligands which decrease cell proliferation by other mechanisms, in order to decrease vascular smooth muscle proliferation/migration and thereby the chance of the consequent restenosis in the surgically reconstructed vessel.
Potential limitations of our study and currently available CB2 antibodies
In our current report, we have utilized a CB2 antibody that was previously reported to detect CB2 in numerous studies (for example, Blazquez et al., 2003; Casanova et al., 2003; Steffens et al., 2005 to list a few). Most probably, this antibody detects CB2 receptor in tissues/cells because: (a) it gives stronger signal in positive control THP-1 monocytes, both by western blot and flow cytometry techniques (Figure 1e, Figure 2b and c); (b) it also gives much stronger signal in THP-1 monocytes and spleen extracts by western blot than in brain extract, where the CB2 expression is low; (c) the signal could be abolished with its blocking peptide; (d) the receptor expression and changes in the pattern observed with the antibody were also confirmed with RT-PCR and real-time PCR (Figure 1c, Figure 2f and G). However, so far we have not been able to find any antibody, including the one used in the present study, which does not give signal in CB2-knockout mice by western blot. Similar observations have been reported by other investigators in the field (various personal communications, meeting: ‘CB2 Cannabinoid Receptors: A New Vista', May 31–June 2, 2007, Banff, Canada). The possible reason(s) for the above-mentioned phenomenon are varied and should be evaluated in further studies in order to design better and more specific antibodies. However, great care should be taken using the currently available CB2 antibodies to draw any conclusions based on using a single method (especially immunohistochemistry) without the proper, concurrent positive and negative controls. Furthermore, some of the commonly used positive controls should be re-evaluated. For example, we find surprisingly low level of CB2 receptor expression in normal mouse spleen by immunohistochemistry and flow cytometry (the latter from freshly isolated cells, where cells are not activated), consistently with a note in the manufacturer's product information ‘this antibody is not sensitive enough to detect CB2 from spleen homogenates'. However, using the same antibody, we see strong CB2 band from the spleen extract by western blot. In contrast, we find high-levels of CB2 receptors in human THP-1 monocytes by flow cytometry. Importantly, our recent observations also indicate that CD32 blockade should be performed in such experiments to avoid interactions with Fc receptors in cells that express this and related immunoglobulin binding proteins. Thus, we propose that human THP-1 monocytes could be more appropriate positive control for CB2 receptor detection (at least for flow cytometry experiments) than spleen homogenate.
Acknowledgments
This study was supported by the Intramural Research Program of NIH/NIAAA (to P.P.), NIDA DA11322 (to KM), NIDA Grant: DA03590 (to JWH).
Abbreviations
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- AM-630
(6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone
- CB1 and CB2 receptors
cannabinoid 1 and 2 receptors
- HCASMCs
human coronary artery smooth muscle cells
- HU-308
(+)-(1aH,3 H,5aH)-4-[2,6-dimethoxy-4-(1,1-dimethylheptyl) phenyl]-6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-carbinol
- JWH-133
3-(1,1-dimethylbutyl)-1-deoxy-Δ8-tetrahydrocannabinol
- MAPK
mitogen-activated protein kinase, SR2 or SR144528
- THC
delta9-tetrahydrocannabinol
- THP-1
human monocyte cell line
- TNF-α
tumour necrosis factor alpha
Conflict of interest
The authors state no conflict of interest.
References
- Blazquez C, Carracedo A, Barrado L, Real PJ, Fernandez-Luna JL, Velasco G, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Casanova ML, Planas A, Del Pulgar TG, Villanueva C, Fernandez-Acenero MJ, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17:529–531. doi: 10.1096/fj.02-0795fje. [DOI] [PubMed] [Google Scholar]
- Cai X. Regulation of smooth muscle cells in development and vascular disease: current therapeutic strategies. Exp Rev Cardiovas Ther. 2006;4:789–800. doi: 10.1586/14779072.4.6.789. [DOI] [PubMed] [Google Scholar]
- Casanova ML, Blazquez C, Martinez-Palacio J, Villanueva C, Fernandez-Acenero MJ, Huffman JW, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- Goetze S, Xi XP, Kawano Y, Kawano H, Fleck E, Hsueh WA, et al. TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent. Hypertension. 1999;33:183–189. doi: 10.1161/01.hyp.33.1.183. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- Hsu YM, Chiu CT, Wang CC, Chien CS, Luo SF, Hsiao LD, et al. Tumour necrosis factor-alpha enhances bradykinin-induced signal transduction via activation of Ras/Raf/MEK/MAPK in canine tracheal smooth muscle cells. Cell Signal. 2001;13:633–643. doi: 10.1016/s0898-6568(01)00182-6. [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1',1'-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorgan Med Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Ip JH, Fuster V, Badimon L, Badimon J, Taubman MB, Chesebro JH. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990;15:1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Lee CW, Lin CC, Lin WN, Liang KC, Luo SF, Wu CB, et al. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L799–812. doi: 10.1152/ajplung.00311.2006. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez ML, Viso A, Ortega-Gutierrez S, Diaz-Laviada I. Involvement of cannabinoids in cellular proliferation. Mini Rev Med Chem. 2005;5:97–106. doi: 10.2174/1389557053402819. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Ann Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Chan C, Taft RJ, Luu T, Abood ME, Moore DH, et al. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J Neuro Oncol. 2005;74:31–40. doi: 10.1007/s11060-004-5950-2. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Mehrhof FB, Schmidt-Ullrich R, Dietz R, Scheidereit C. Regulation of vascular smooth muscle cell proliferation: role of NF-kappaB revisited. Cir Res. 2005;96:958–964. doi: 10.1161/01.RES.0000166924.31219.49. [DOI] [PubMed] [Google Scholar]
- Mendez-Sanchez N, Zamora-Valdes D, Pichardo-Bahena R, Barredo-Prieto B, Ponciano-Rodriguez G, Bermejo-Martinez L, et al. Endocannabinoid receptor CB2 in nonalcoholic fatty liver disease. Liver Int. 2007;27:215–219. doi: 10.1111/j.1478-3231.2006.01401.x. [DOI] [PubMed] [Google Scholar]
- Mestre L, Correa F, Docagne F, Clemente D, Guaza C. The synthetic cannabinoid WIN 55,212-2 increases COX-2 expression and PGE2 release in murine brain-derived endothelial cells following Theiler's virus infection. Biochem Pharmacol. 2006;72:869–880. doi: 10.1016/j.bcp.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007a;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007b;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–1112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006a;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006b;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-Usmar VM. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Rad Biol Med. 2000;28:1780–1794. doi: 10.1016/s0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Godlewski G, Hasko G, Liaudet L, et al. Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem Biophys Res Commun. 2006;350:352–357. doi: 10.1016/j.bbrc.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293:H610–H619. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzman CH, Shames BD, Reznikov LL, Miller SA, Meng X, Barton HA, et al. Liposomal delivery of purified inhibitory-kappaBalpha inhibits tumor necrosis factor-alpha-induced human vascular smooth muscle proliferation. Cir Res. 1999;84:867–875. doi: 10.1161/01.res.84.8.867. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Cir Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, et al. Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production. Mol Cell Biochem. 2006;283:75–83. doi: 10.1007/s11010-006-2346-y. [DOI] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sukhova G, Schwartz D, Libby P. Proliferating arterial smooth muscle cells after balloon injury express TNF-alpha but not interleukin-1 or basic fibroblast growth factor. Arterioscler Thromb Vasc Biol. 1996;16:12–18. doi: 10.1161/01.atv.16.1.12. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AB, Li HL, Zhang R, She ZG, Chen HZ, Huang Y, et al. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/Akt singling in vitro and in vivo. J Biomed Sci. 2007;14:357–371. doi: 10.1007/s11373-007-9150-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Rao PJ, Castresana MR, Newman WH. TNF-alpha induces proliferation or apoptosis in human saphenous vein smooth muscle cells depending on phenotype. Am J Physiol Heart Circ Physiol. 2005;288:H293–H301. doi: 10.1152/ajpheart.00165.2004. [DOI] [PubMed] [Google Scholar]
- Warner SJ, Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989;142:100–109. [PubMed] [Google Scholar]
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87–92. doi: 10.1016/j.jdermsci.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman MA, Reznikov LL, Sorensen AC, Selzman CH. Relative contribution of the TNF-alpha receptors to murine intimal hyperplasia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1213–1218. doi: 10.1152/ajpregu.00434.2002. [DOI] [PubMed] [Google Scholar]
- Zoratti C, Kipmen-Korgun D, Osibow K, Malli R, Graier WF. Anandamide initiates Ca(2+) signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Brit J Pharmacol. 2003;140:1351–1362. doi: 10.1038/sj.bjp.0705529. [DOI] [PMC free article] [PubMed] [Google Scholar]