Abstract
Evidence has emerged suggesting a role for the cannabinoid CB2 receptor in immune cell motility. This provides a rationale for a novel and generalized immunoregulatory role for cannabinoid CB2 receptor-specific compounds. In support of this possibility, we will review the biology of a class of cannabinoid CB2 receptor—specific inverse agonist, the triaryl bis-sulfones. We will show that one candidate, Sch.414319, is potent and selective for the cannabinoid CB2 receptor, based on profiling studies using biochemical assays for 45 enzymes and 80 G-protein coupled receptors and ion channels. We will describe initial mechanistic studies using this optimized triaryl bis-sulfone, showing that the compound exerts a broad effect on cellular protein phosphorylations in human monocytes. This profile includes the down regulation of a required phosphorylation of the monocyte-specific actin bundling protein L-plastin. We suggest that this observation may provide a mechanism for the observed activity of Sch.414319 in vivo. Our continued analysis of the in vivo efficacy of this compound in diverse disease models shows that Sch.414319 is a potent modulator of immune cell mobility in vivo, can modulate bone damage in antigen-induced mono-articular arthritis in the rat, and is uniquely potent at blocking experimental autoimmune encephalomyelitis in the rat.
Keywords: L-plastin, experimental autoimmune encephalomyelitis, cannabinoid CB2 receptor, inverse agonist, chemotaxis, chemokinesis
Introduction
Since its discovery in 1993, the cannabinoid CB2 receptor has been an appealing therapeutic target for novel immunomodulators. Initial studies suggested that the receptor was not expressed in the central nervous system (Munro et al., 1993), suggesting that cannabinoid compounds specific for the CB2 receptor would be free of the neural deficits seen in nonspecific cannabinoid compounds. The high-level expression within immune cells and the inducible expression of the receptor following inflammatory insult suggested that the receptor may serve to mediate the immune regulatory activities described for cannabinoids (Kaplan et al., 2003). Finally, studies showed that the primary active component in Cannabis, Δ9-tetrahydrocannabinol (THC), is a partial agonist at the cannabinoid receptor. This observation defined the pharmacology of a preferred therapeutic at the cannabinoid CB2 receptor.
However, this initial view of the cannabinoid system has proved to be simplistic. Detailed studies of the expression of the cannabinoid CB2 receptor have shown that functional receptors can be found in neural tissue (Van Sickle et al., 2005; Beltramo et al., 2006; Onaivi et al., 2006). This may alter the utility of cannabinoid CB2 receptor-specific compounds. In addition, selected animal models (Smith et al., 2000, 2001; Croci et al., 2003; Benamar et al., 2007) have suggested that cytokine levels can be mediated by manipulation of the cannabinoid CB1 receptor. This suggests that the cannabinoid CB2 receptor may not be the only cannabinoid-based mediator of the immune system, and may explain the fact that the cannabinoid CB2 receptor-deficient mice fail to show significant immune deficit under standard conditions (Buckley et al., 2000; Ofek et al., 2006). Finally, other components of Cannabis, including cannabidiol, have proved to be potent immunomodulatory compounds (Malfait et al., 2001; Costa et al., 2004; Giudice et al., 2007). Recent evidence has shown that this compound behaves as an inverse agonist to the cannabinoid CB2 receptor in standard biochemical assays (Thomas et al., 2007).
Taken together, these observations may suggest the value of adopting a broad view when considering the desired pharmacology of an immune therapeutic based on the cannabinoid CB2 receptor.
Into this arena enters cannabinoid CB2 receptor-specific triaryl bis-sulfones. In this review, we will discuss the biology of this novel class of cannabinoid CB2 receptor-specific inverse agonists. We will further document the specificity of a member of this class, and describe a biology based on the ability of these compounds to modulate immune cell migration. We will provide initial data providing a potential mechanism for the activity of these compounds, based on controlling the phosphorylation required for activity of an actin bundling protein. Finally, we will describe the activity of an optimized triaryl bissulphone, Sch.414319, in arthritis and in experimental autoimmune encephalomyelitis (EAE). The ability of these compounds to modulate EAE mimics a diminished disease observed in animals lacking the cannabinoid CB2 receptor. We conclude that cannabinoid CB2 inverse agonists may play a part in the therapeutic pharmacopoeia that targets this class of receptors.
Identification and initial biochemistry of triaryl bis-sulphones
Triaryl bis-sulphones were identified from a compound library screening campaign, in which compounds were tested for a selective ability to block binding of [3H]-CP55,940 to membrane preparations expressing recombinant human cannabinoid CB2 receptor versus membrane preparations expressing recombinant human CB1 receptor (Lunn et al., 2006a).
The ability of the identified triaryl bis-sulphones to decrease basal binding of [35S]-GTPγS to recombinant cell membranes expressing cannabinoid CB2 (and CB1) receptors, to bind with an increased potency to recombinant cell membranes in the presence of 100 μM GTPγS, and to decrease forskolin-stimulated cAMP levels in cells expressing the recombinant receptor are consistent with an inverse agonist pharmacology (Greasley and Clapham, 2006). This pharmacology has been maintained throughout an extensive medicinal chemistry effort designed to improve the pharmacological characteristics of triaryl bis-sulphones (Lavey et al., 2005, 2007; Shankar et al., 2005). An optimized compound, designated Sch.4141319, was engineered to contain a terminal trifluoromethane sulphonamide to permit salt formation and optimize solubility. This resulted in a compound that exhibited superior blood levels when dosed orally in rats (Table 1).
Table 1.
Binding selectivity and pharmacokinetic profile of selected triaryl bissulphones
| CB2 Ki | CB1 Ki | Ratio (CB1/CB2) | Rat AUC | |
|---|---|---|---|---|
| Sch.225336 | 0.4 nM | 905 nM | 2262 | 235 nM h@10 mg kg−1 |
| Sch.356036 | 1.3 nM | 4387 nM | 3375 | 3160 nM h@10 mg kg−1 |
| Sch.414319 | 2 nM | 6785 nM | 3393 | 27270 nM h@10 mg kg−1 |
Competitive binding experiments were carried out versus [3H] CP55,940, as described (Lunn et al, 2006a, 2006b). The pharmacokinetic profiles of orally administered drug candidates were approximated by monitoring the plasma concentration versus time after dosing and calculating the area under the curve (AUC). For these determinations, rats were orally dosed at 10 mg kg−1 (in 0.4% w/v methylcellulose). Plasma levels were sampled (3 times per day, n=3) and drug levels determined using standard quantitative HPLC methodologies (Cox et al., 1999).
Our initial screening studies of Sch.414319 showed that the compound was specific for the CB2 receptor over the CB1 receptor. However, an optimized therapeutic candidate requires data demonstrating significant selectivity over additional enzymes and receptors separate from the target receptor. To this end, we carried out a generalized evaluation to investigate whether any activity identified for the optimized triaryl bissulphone Sch.414319 could result from a high-affinity interaction with some off-target effectors. Sch.414319 was submitted to MDS Pharma Services for testing its ability to modulate the activity of a broad collection of receptors and enzymes. The selected panel of proteins and receptors (Table 2) included 45 enzymes (including 11 kinases), 80 G-protein-coupled receptors and ion channels, and three recombinant cell-based adhesion inhibition assays mediated by fibronectin, VCAM-1 and ICAM-1. Eight of these biochemical screening assays were judged to be affected by the addition of 10 μM Sch.414319, showing greater than 50% inhibition (Table 2). These selected assays were retested using a four-point logarithmic dilution series of Sch.414319 to generate an estimation of compound potency in eight identified assay systems. As expected, Sch.414319 blocked binding of [3H]-WIN55,212-2 to the human cannabinoid CB2 receptor (Ki≈70 nM). The compound also blocked binding to the L-type calcium channels that measured using radiolabelled dihydropyridine (Ki≈0.44 μM), phenylalkylamine (Ki≈2.43 μM) and benzothiazepine (Ki≈6.07 μM). The compound also bound with modest affinity to several other receptor preparations, including the thromboxane A2 receptor (Ki≈1.44 μM), the non-selective sigma receptor (Ki≈2.05 μM) and the cholecystokinin B type (CCKB) receptor (Ki≈5.7 μM). Although this analysis is not exhaustive, it provides additional evidence that the biology of Sch.414319 is mediated primarily by the cannabinoid CB2 receptor.
Table 2.
Characterizing selectivity of Sch.414319 for the human cannabinoid CB2 receptor
| ID | Name | Type | % lnhbt @ 10 μM | Protocol reference |
|---|---|---|---|---|
| 10730 | Angiotensin-converting enzyme | Enz | 8 | Bunning P et al. Biochemistry. 22:103, 1983 |
| 10800 | Protease calpain | Enz | 19 | Murachi T et al. Adv Enzyme Regul. 19: 407, 1980 |
| 11200 | Carbonic anhydrase | Enz | 3 | Eveloff J et al. Biochem Pharmacol. 28: 1434, 1979 |
| 11250 | Protease cathepsin G | Enz | 1 | Mehdi S et al. Biochem Biophys Res Commun. 166: 595, 1990 |
| 11300 | Choline acetyltranferase | Enz | 3 | Maderdrut JL. Neurochem Res. 20: 69, 1995 |
| 11501 | Cyclooxygenase COX-1 | Enz | −14 | Warner TD et al. Proc Natl Acad Sci USA. 96: 7563, 1999 |
| 11601 | Cyclooxygenase COX-2 | Enz | 18 | Warner TD et al. Proc Natl Acad Sci USA. 96(13): 7563, 1999 |
| 12100 | Myeloperoxidase | Enz | −7 | Syensson BE. Biochem J. 242: 673, 1987 |
| 12400 | HMG-CoA reductase | Enz | −1 | Kubo M and Strott CA. Endocrinology. 120: 214, 1987 |
| 12800 | Leukotriene A4 hydrolase | Enz | 20 | laumi T et al. Biochem Biophys Res Commun. 135: 139, 1986 |
| 13200 | Leukotriene C4 synthetase | Enz | −11 | Bach MK et al. Biochem Pharmacol. 34: 2695, 1985 |
| 13400 | Lipid peroxidase | Enz | 20 | Mansuy D et al. Biochem Biophys Res Commun. 135: 1015, 1986 |
| 13800 | Lipoxygenase 15-LO | Enz | 2 | Averbach BJ et al. Anal Biochem. 201: 375, 1992 |
| 14000 | Monoamine oxidase MAOA | Enz | 1 | Medvedev AE et al. Biochem Pharmacol. 47: 303, 1994 |
| 14010 | Monoamine oxidase MAOB | Enz | −11 | Egashira T et al. Biochem Pharmacol. 25: 2583, 1976 |
| 14200 | Nitric oxide sythase constitutive (cNOS) | Enz | 3 | Lowenstein CJ and Snyder SH. Cell. 70: 705, 1992 |
| 14400 | Nitric oxide sythase inducible (iNOS) | Enz | 3 | Nathan C. FASEB J. 6: 3051, 1992 |
| 14600 | Phosphodiesterase PDE1 | Enz | 65 | Nicholsen CD et al. Trends Pharmacol Sci. 12: 19, 1991 |
| 14800 | Phosphodiesterase PDE2 | Enz | 25 | Hidaka H and Asano T. Biochem Biophys Acta. 429: 485, 1976 |
| 15200 | Phosphodiesterase PDE3 | Enz | 12 | Nicholsen CD et al. Trends Pharmacol Sci. 12: 19, 1991 |
| 15400 | Phosphodiesterase PDE4 | Enz | 27 | Cortijo J et al. Br J Pharmacol. 108: 562, 1993 |
| 15600 | Phosphodiesterase PDE5 | Enz | 5 | Hidaka H and Asano T. Biochem Biophys Acta. 429: 485, 1976 |
| 16000 | Phospholipase PLA2-1 | Enz | 19 | Katsumata M et al. Anal Biochem. 154: 676, 1986 |
| 16400 | Protease neutral endopeptidase | Enz | −2 | Erdos EG and Skidgel RA. FASEB J. 3: 145, 1989 |
| 16600 | Protease elastase | Enz | 1 | Baugh J and Travis J Biochemistry. 15(4): 836, 1976 |
| 16800 | Protein kinase II, Ca2+/calmodulin-dep. | Enz | 18 | Lai Y et al. Proc Natl Acad Sci USA. 83: 4253, 1986. |
| 17000 | EGF receptor tyrosine kinase | Enz | −9 | Geissler JF et al. J Biol Chem. 265: 22255, 1990 |
| 17100 | ERK1 serine/threonine kinase | Enz | 10 | Dudley DT et al. Proc Natl Acad Sci USA. 32: 7686, 1995 |
| 17200 | Fya (p59fya) tyrosine kinase | Enz | −6 | Appleby MW et al. Cell. 70: 751, 1992 |
| 17400 | HER2 tyrosine kinase | Enz | 12 | Bargmann CI et al. Cell. 45: 649, 1986 |
| 17600 | Lck (p56lck) tyrosine | Enz | 6 | Caron L et al. Mol Cell Biol. 12: 2720, 1992 |
| 17700 | PKA non-selective | Enz | 5 | Quick J et al. Biochem Biophys Res Commun. 187: 657, 1992 |
| 17800 | Protein kinase C, non-selective | Enz | 1 | Jeng AY et al. Cancer Res. 46: 1966, 1986 |
| 18001 | Protein Kinase C alpha | Enz | 1 | Tamaki T et al. Biochem Biophys Res Commun. 135: 397, 1986 |
| 18200 | Protein kinase C- (I and II) | Enz | 17 | Woodgett JR and Hunter T. J Biol Chem. 262: 4836, 1987 |
| 18400 | Protein kinase C- | Enz | −14 | Woodgett JR and Hunter T. J Biol Chem. 262: 4836, 1987 |
| 18600 | Calcineurin PP2B phosphatase | Enz | 5 | Klee CB et al. Methods Enzymol. 102: 227, 1983 |
| 19000 | CD45 tyrosine phosphatase | Enz | 5 | Zhang ZY et al. Proc Natl Acad Sci USA. 90: 4446, 1993 |
| 19200 | PTP1B tyrosine phosphatase | Enz | −2 | Wiener JR et al. J Natl Cancer last. 86: 372, 1994 |
| 19220 | PTP1C tyrosine phosphatase | Enz | 13 | D'Ambrosio D et al. Science. 268: 293, 1995 |
| 19300 | T-cell tyrosine phosphatase | Enz | −2 | Yakura H Crit Rev Immunol. 14: 311, 1994 |
| 19350 | Free radical scavenger, SOD mimetic | Enz | 29 | Sun Y et al. Clin Chem. 34: 467, 1988 |
| 19400 | Thromboxane synthetase | Enz | 48 | Fiddler Gl and Lumley P. Circulation. 81: 169, 1990 |
| 19500 | Tyrosine hydroxylase | Enz | 19 | Roskoshi R Jr et al. J Biochem. 218: 363, 1993 |
| 19800 | Xanthine oxidase | Enz | −1 | Hatano T et al. Chem Pharm Bull. 38: 1224, 1990 |
| 20031 | Adenosine A1 | Bind | 22 | Libert F et al. Biochem Biophys Res Commun. 187: 919, 1992 |
| 20061 | Adenosine A2A | Bind | 47 | Jarvis MF et al. J Pharmacol Exp Ther. 251: 888, 1989 |
| 20071 | Adenosine A3 | Bind | 46 | Olah ME et al. Mol Pharmacol. 45: 978, 1994 |
| 20080 | Adenosine A2B | Bind | 38 | Brackett LE and Daly JW. Biochem Pharmacol. 47: 801, 1994 |
| 20340 | Adrenergic-1D | Bind | 27 | Kenny BA et al. Br. J Pharmacol. 115: 981, 1995 |
| 20362 | Adrenergic-2A | Bind | 3 | Uhlen S et al. J Pharmacol Exp Ther. 271: 1558, 1994 |
| 20380 | Adrenergic-2C | Bind | 13 | Uhlen S et al. J Pharmacol Exp Ther. 271: 1558, 1994 |
| 20401 | Adrenergic 1 | Bind | 3 | Feve B et al. Proc Natl Acad Sci USA. 91: 5677, 1994 |
| 20411 | Adrenergic-2 | Bind | −5 | McCrea KE and Hill SJ. Br. J Pharmacol. 110: 619, 1993 |
| 20420 | Adrenergic-3 | Bind | 36 | Feve B et al. Proc Natl Acad Sci USA. 91: 5677, 1994 |
| 20441 | Adrenergic norepinephrine transporter | Bind | 38 | Galli A et al. J Exp Biol. 198: 2197, 1995 |
| 21001 | Angiotensin AT1 | Bind | 22 | Dudley DT et al. Mol Pharmacol. 38: 370, 1990 |
| 21011 | Angiotensin AT2 | Bind | 7 | Whitebread SE et al. Biochem Biophys Res Commun. 181: 1365, 1991 |
| 21261 | Bradykinin B2 | Bind | 10 | Eggerickx D et al. Biochem Biophys Res Commun. 187(3): 1306, 1992 |
| 21361 | Calcitonin | Bind | 9 | Findlay DM et al. Cancer Res. 40: 1311, 1980 |
| 21401 | Calcitonin gene-related peptide (CGRP) | Bind | −5 | Zimmermann U et al. Peptide. 16(3): 421, 1995 |
| 21450 | Calcium channel type l, benzothiazepine | Bind | 69 | Schoemaker H and Langer SZ. Eur J Pharmacol. 111: 273, 1985 |
| 21460 | Calcium channel type l, dihydropyridine | Bind | 92 | Gould RJ et al. Proc Natl Acad Sci USA. 79: 3656, 1982 |
| 21500 | Calcium channel type l, phenylalkylamine | Bind | 72 | Reynolds IJ et al. J Pharmacol Exp Ther. 237: 731, 1986 |
| 21600 | Calcium channel type N | Bind | −1 | Moresco RM et al. Neurobiol Aging. 11(4): 433, 1990 |
| 21701 | Cannabinoid CB1 | Bind | 10 | Felder CC et al. Mol Pharmacol. 48: 443, 1995 |
| 21710 | Cannabinoid CB2 | Bind | 85 | Munro S et al. Nature. 365: 61, 1993 |
| 21801 | Cholecystokinin CCKA | Bind | 10 | Jensen RT et al. Ann N Y Acad Sci. 713: 88, 1994 |
| 21811 | Cholecystokinin CCKB | Bind | 55 | Jensen RT et al. Ann N Y Acad Sci. 713: 88, 1994 |
| 21850 | Dopamine D1 | Bind | −1 | Dearry A et al. Nature. 347: 72, 1990 |
| 21860 | Dopamine D2L | Bind | 26 | Hayes G et al. Mol Endocrinol. 6: 920, 1992 |
| 21870 | Dopamine D2S | Bind | 18 | Grandy DK et al. Proc Natl Acad Sci USA. 86: 9762, 1989 |
| 21880 | Dopamine D3 | Bind | 30 | Sokoloff P et al. Nature. 347: 146, 1990 |
| 21890 | Dopamine D4-2 | Bind | 11 | Van Tol HHM et al. Nature. 358: 149, 1992 |
| 22000 | Dopamine D4-4 | Bind | −6 | Van Tol HHM et al. Nature. 350: 610, 1991 |
| 22010 | Dopamine D4-7 | Bind | −8 | Van Tol HHM et al. Nature. 358: 149, 1992 |
| 22020 | Dopamine D5 | Bind | 20 | Sunahara RK et al. Nature. 350: 614, 1991 |
| 22032 | Dopamine transporter | Bind | 36 | Gu H et al. J. Biol Chem. 269: 7124, 1994 |
| 22410 | Endothelin ETB | Bind | −14 | Mihara S et al. J Pharmacol Exp Ther. 268: 1122, 1994 |
| 22550 | Epidermal growth factor (EGF) | Bind | 6 | Dittadi R et al. Clin Chem. 36: 849, 1990 |
| 22601 | Estrogen ER | Bind | 8 | Obourn JD et al. Biochem. 32: 6229, 1993 |
| 23131 | Galanin | Bind | −4 | Heullet E et al. Eur J Pharmacol. 269: 139, 1994 |
| 23170 | Glucagon-like peptide-1 (GLP-1) | Bind | 14 | Dillon JS et al. Endocrinology. 133: 1907, 1993 |
| 23201 | Glucocorticoid | Bind | 14 | Cidlowski JA and Cidlowski NB. Endocrinology. 109: 1975, 1981 |
| 23350 | Histamine H1, central | Bind | 4 | Hill SJ et al. J Neurochem. 31: 997, 1978 |
| 23360 | Histamine H1, peripheral | Bind | −14 | Dini S et al. Agents Actions. 33: 181, 1991 |
| 23370 | Histamine H2 | Bind | 23 | Traiffort E et al. Eur J Pharmacol. 207: 143, 1991 |
| 23380 | Histamine H3 | Bind | 11 | West RE et al. Mol Pharmacol. 38: 610, 1990 |
| 24410 | Laterleukin IL-6 | Bind | 9 | Cornfield LJ and sills MA. Eur J Pharmacol. 202: 113, 1991 |
| 24430 | Chemokine CXCR1/2 (IL-8, non-selective) | Bind | −17 | Moser B et al. J Biol Chem. 266: 10666, 1991 |
| 24440 | Chemokine CXCR1 | Bind | 3 | Ahuja SK et al. J Biol Chem. 271: 20545, 1996 |
| 24450 | Chemokine CXCR2 | Bind | 8 | Ahuja SK et al. J Biol Chem. 271: 20545, 1996 |
| 25051 | Leukotriene B4 | Bind | −10 | Winkler JD et al. J Pharmacol Exp Ther. 246: 204, 1988 |
| 25060 | Leukotriene D4 | Bind | 6 | Mong S et al. Eur J Pharmacol. 102: 1, 1984 |
| 25260 | Muscarinic M1 | Bind | −23 | Buckley NJ et al. Mol Pharmacol. 35: 469, 1989 |
| 25270 | Muscarinic M2 | Bind | 2 | Buckley NJ et al. Mol Pharmacol. 35: 469, 1989 |
| 25280 | Muscarinic M3 | Bind | −7 | Buckley NJ et al. Mol Pharmacol. 35: 469, 1989 |
| 25290 | Muscarinic M4 | Bind | −5 | Buckley NJ et al. Mol Pharmacol. 35: 469, 1989 |
| 25300 | Muscarinic M5 | Bind | −18 | Buckley NJ et al. Mol Pharmacol. 35: 469, 1989 |
| 25551 | Tachykinin NK1 | Bind | 14 | Patacchini R and Maggi CA. Arch Int Pharmacodyn Ther. 329: 161, 1995 |
| 25560 | Tachykinin NK2 | Bind | 1 | Patacchini R and Maggi CA. Arch Int Pharmacodyn Ther. 329: 161, 1995 |
| 25570 | Tachykinin NK3 | Bind | 0 | Patacchini R and Maggi CA. Arch Int Pharmacodyn Ther. 329: 161, 1995 |
| 25700 | Neuropeptide Y1 | Bind | 22 | Fuhlendorff J et al. Proc Natl Acad Sci USA. 87: 182, 1990 |
| 25711 | Neuropeptide Y2 | Bind | −11 | Rose PM et al. J Biol Chem. 270(39): 22661, 1995 |
| 26011 | Opiate delta | Bind | 9 | Simonin F et al. Mol Pharmacol. 46: 1015, 1994 |
| 26021 | Opiate- | Bind | 8 | Simonin F et al. Proc Natl Acad Sci USA. 92(15): 7006, 1995 |
| 26041 | Opiate- | Bind | 23 | Wang JB et al. FEBS Lett. 338: 217, 1994 |
| 26060 | Orphanin (ORL1) | Bind | 7 | Ardati A et al. Mol Pharmacol. 51: 816, 1997 |
| 26330 | Potassium channel [KA] | Bind | 23 | Rehm H and Laadunski M. Proc Natl Acad Sci USA. 85: 4919, 1988 |
| 26370 | Potassium channel [KV] | Bind | −5 | Vazquez J et al. J Biol Chem. 264: 20902, 1989 |
| 26560 | Potassium channel [SKCA] | Bind | 2 | Mourre C et al. Brain Res. 382: 239, 1986 |
| 26670 | Purinergic P2X | Bind | 7 | Ziganshin AU et al. Br J Pharmacol. 110: 1431, 1993 |
| 27111 | Serotonin 5-HT1A | Bind | 2 | Martin GR and Humphrey PPA. Neuropharmacol. 33: 261, 1994 |
| 27130 | Serotonin 5-HT1D | Bind | −2 | Domenech T et al. Naunyn Schmiedebergs Arch Pharmacol. 356: 328, 1997 |
| 27165 | Serotonin 5-HT2A | Bind | 31 | Saucier C and Albert PR. J Neurochem. 68: 1998, 1997 |
| 27210 | Serotonin 5-HT5A | Bind | 21 | Rees S et al. FEBS Lett. 355: 242, 1994 |
| 27220 | Serotonin 5-HT6 | Bind | 54 | Monsma FJ Jr et al. Mol Pharmacol. 43: 320, 1993 |
| 27230 | Serotonin 5-HT7 | Bind | 28 | Roth BL et al. J Pharmacol Exp Ther. 268: 1403, 1994 |
| 27402 | Serotonin transporter | Bind | −5 | Gu H et al. J Biol Chem. 269: 7124, 1994 |
| 27830 | Sigma, non-selective | Bind | 70 | Weber E et al. Proc Natl Acad Sci USA. 83: 8784, 1986 |
| 28550 | Thromboxane A2 [TXA2] | Bind | 59 | Hedberg A et al. J Pharmacol Exp Ther. 245: 786, 1988 |
| 28631 | Tumor hecrosis factor, non-selective | Bind | 11 | Baglioni C et al. J Biol Chem. 260: 13395, 1985 |
| 28680 | Vascular endothelinal growth factor | Bind | 13 | Gitay-Goren H et al. J Biol Chem. 271: 5519, 1996 |
| 28701 | Vasoactive intestinal peptide VIP1 | Bind | −12 | Couvineau A et al. Biochem J. 231: 139, 1985 |
| 28751 | Vasopressia VIA | Bind | 18 | Thibonnier M et al. J Biol Chem. 269: 3304, 1994 |
| 30500 | Adhesion, fibronectin-mediated | Cell | 5.9 | Nowlin D et al. J Biol Chem. 268: 20351, 1993 |
| 30510 | Adhesion, ICAM-1 mediated | Cell | −4.3 | Cobb RR et al. Biochem Biophys Res Commun. 185: 1022, 1992 |
| 30750 | Adhesion, VCAM-1-mediated antagonist | Cell | 26.3 | Stoltenborg JK et al. J Immunol Methods. 175: 59, 1994. |
Abbreviations: Bind, specific binding; EGF, epidermal growth factor; Enz, enzymatic activity; ERK1, extracellular signal-regulated kinase 1; HMG, 3-hydroxy-3-methyl-glutaryl-CoA reductase; PKA, protein kinase A; ICAM, intercellular adhesion molecule; SOD, superoxide dismutase; VCAM, vascular cell adhesion molecule.
The ability of Sch.414319 to affect the biochemical activity of selected assays is presented. Assay results are presented as the percent inhibition of specific binding (Bind), enzymatic activity (Enz) or cellular activity (Cell) according to experimental procedures listed in the references provided.
Cell biology of triaryl bis-sulphones
A number of biochemical and cellular activities associated with immune modulation by cannabinoids have been ascribed to the cannabinoid CB2 receptor (Kaplan et al., 2003). Some of the activities require high cannabinoid concentrations that are unexpected for cannabinoid receptor-mediated events. Indeed, several laboratories have reported cannabinoid effects that are mediated by such alternative mechanisms (Kraft et al., 2004; Curran et al., 2005; Kaplan et al., 2005; McCollum et al., 2007). We believe this set of observations speaks for the complexity of cannabinoid biology, and supports the value of considering novel pharmacological entities in targeting the cannabinoid CB2 receptor.
An initial set of experiments were planned to test whether triaryl bis-sulphones could function as antagonists in several cannabinoid-induced in vitro and in vivo cytokine production systems. We were unable to demonstrate an effect of the compounds on human peripheral blood mononuclear cell and murine T-cell proliferation, T-cell and monocyte cytokine production, and surface-marker upregulation—the compounds were either inactive or demonstrated limited activity at concentrations >5 μM, deemed inconsistent with a CB2 receptor-mediated response (data not shown; Lunn et al., 2006a, 2006b).
As part of this effort, we evaluated the effect of WIN 55212-2 and HU-210 on lipopolysaccharide-induced cytokines in Corynebacterium parvum-primed and unprimed mice. As hoped, the compounds modified inflammatory cytokine levels, decreasing serum tumour necrosis factor- and interleukin-12, and increasing interleukin-10 (Smith et al., 2000). However, the compounds appeared to function through the cannabinoid CB1 receptor. Consistent with this conclusion, the cannabinoids showed similar cytokine responses at significantly lower doses when administered intracerebroventricularly, (Smith et al., 2001).
We next examined the ability of triaryl bis-sulphones to control immune cell movement. Modulating immune cells' migration to the site of inflammatory insult has been a powerful driving force behind campaigns to develop chemokine antagonists (De Clercq, 2003; Horuk, 2003; Chen et al., 2004). Such chemokine receptor antagonists have shown promise for the treatment of asthma (Varnes et al., 2004) and chronic obstructive pulmonary disease, arthritis and reperfusion injury (Widdowson et al., 2004). Other laboratories have suggested a link between the cannabinoid CB2 receptor and cellular movement. Cannabinoids have been demonstrated to mediate cell migration in a variety of cells, including myeloid leukaemic cell line 32D/G-CSF-R, isolated mouse splenocytes (Jorda et al., 2002), 1,25-(OH)2 vitamin D3-treated HL-60 cells (Kishimoto et al., 2006), EoL-1 human eosinophilic leukaemia cells and human peripheral blood eosinophils (Oka et al., 2004). However, the mechanism by which cannabinoids mediate this effect is complex. In many of the above papers, cannabinoids are shown to attract cells expressing the receptor. This suggests that cannabinoid agonists may be the therapeutically relevant class of compounds. However, cannabinoids upregulated chemokine expression in several cell types (Jbilo et al., 1999; Derocq et al., 2000). More recently, Coopman et al. (2007) have demonstrated an upregulation of chemokine receptors following T-lymphocyte activation. Adding to this complication, the behaviour of cells exposed to cannabinoid agonists did not mimic the behaviour of chemokines. Lopez-Cepero et al. (1986) showed that THC suppressed macrophage spreading. Sacerdote et al. (2000) argued that the nonspecific cannabinoid agonist CP55,940 blocked both chemokinesis (non-directed cell movement) and N-formyl-methionyl-leucyl-phenylalanine (fMLP)-induced cellular chemotaxis of rat peritoneal macrophages, with an EC50≈10–30 nM. Jorda et al. (2002) also argued that chemokinesis played a significant role in this biology—administration of the agonist 2-arachidonoyl glycerol on both sides of the membrane in a transwell chemotaxis chamber generated significant cellular motion to the distal surface. Gokoh (Gokoh et al., 2005) used differentiated HL-60 cells to show a time- and dose-dependent increase in F-actin staining (therefore actin polymerization) following 2-arachidonoyl glycerol treatment. This phenotype, abolished when cells were treated with SR144528, was accompanied by the extension of non-directed pseudopods. The investigators suggested the potential involvement of phosphoinositide 3-kinase, Rho-family small G-proteins and a tyrosine kinase in this process. Kurihara et al. (2006) observed a repeating random extension F-actin-containing pseudopods in HL60 cells treated with the cannabinoid CB2 receptor agonist JWH015 and 2-arachidonoyl glycerol. The activity of Rho-GTPase RhoA decreased, and Rac1, Rac2 and Cdc42 activity increased with cannabinoid treatment. These CB2 receptor-mediated changes appear to be similar to those observed using the Rho-dependent protein kinase (p160-ROCK) inhibitor Y27632. The authors concluded that the CB2 receptor might exert an immunomodulatory role by controlling the RhoA–ROCK pathway.
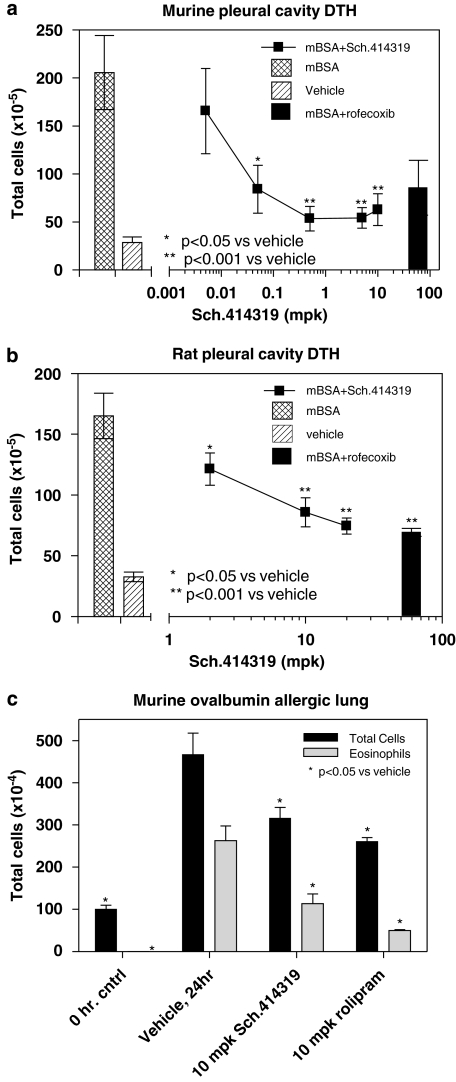
Our initial studies (Lunn et al., 2006) showed that the cannabinoid CB2 receptor inverse agonist Sch.225336 blocked recombinant cells chemotaxis to 2-arachidonoyl glycerol in vitro, and blocked migration of immune cells to a CC chemokine ligand (CCL)2-soaked gel foam sponge implanted intraperitoneally. We also showed that oral doses of Sch.225336 blocked cell migration into the peritoneal cavity following antigen challenge, and blocked cell migration to the lungs of sensitized mice following an aerosol antigen challenge. Further studies showed that oral administration of a cannabinoid CB2 receptor-specific triaryl bis-sulphone blocked cannabinoid-induced migration mediated by HU210, the protein immunogen methylated bovine serum albumin (mBSA), thioglycolate, zymosan and lipopolysaccharide (CA Lunn et al., in press). These results have extended to Sch.414319. Figure 1 shows that oral administration of Sch.414319 blocks accumulation of immune cells into the pleural cavity of sensitized CF1 outbred mice (panel A) and sensitized Sprague–Dawley female rats (panel B) challenged with 200 μg mBSA. In these models, the triaryl bis-sulphone is almost as effective as 10 mg kg−1 rofecoxib. Likewise, the figure (panel C) shows that oral administration of Sch.414319 blocks lung eosinophilia following aerosol administration of an ovalbumin antigen to ovalbumin/alum-sensitized male B6D2F1/J mice to an extent comparable to 10 mg kg−1 rolipram. In all cases, Sch.414319 and the control compound was dosed orally in 0.4% methylcellulose at days −2, −1, 1 h before challenge and 3 h post-challenge. Twenty-four hours after challenge, cells were collected from the pleural cavity or from a bronchial alveolar lavage and quantitated. We conclude from these studies and from the results in the literature that immune cell mobility can be modulated through the cannabinoid CB2 receptor.
Figure 1.
Sch.414319 modulates cell migration in vivo. Murine delayed type hypersensitivity reaction was carried out using female CF1 mice as described (Fine et al., 2003). (a) Sensitized female CF1 outbred mice were challenged with vehicle or with mBSA (200 μg). Sch.414319 or rofecoxib (10 mg kg−1) was dosed orally in 0.4% methylcellulose at days −2, −1 and 1 h before challenge and 3 h post-challenge. Twenty-four hours after challenge, cells were collected from the pleural cavity and quantitated. (b) Sensitized Sprague–Dawley female rats were challenged with vehicle or with mBSA (200 μg), and then dosed as above. (c) B6D2F1/J mice sensitized with ovalbumin/alum were challenged with aerosolized ovalbumin after dosing with vehicle, Sch.414319 or rolipram, as described above. Twenty-four hours after challenge, total cells and eosinophils (using differential Wright–Giemsa staining) recovered from a broncheoalveolar lavage were quantitated. mBSA, methylated bovine serum albumin.
The mechanism by which the cannabinoid CB2 receptor is linked to cell motility, and the explanation for the effects of both cannabinoid agonists and inverse agonists on this process remain complex. Chemotaxis involves an interaction between both signalling elements (for example, receptors) and structural elements (linked to maintaining cell polarity), with numerous pharmacological strategies available for modulating the process. A number of neuronal receptor systems have been shown to desensitize chemokine receptors (Zhang and Oppenheim, 2005). For example, heterologous desensitization of chemokine receptors via opioid receptor signalling has also been documented (for review see Steele et al., 2002), a desensitization mediated by phosphorylation of the chemokine receptor via calcium-independent protein kinase C isotypes (Ali et al., 1999; Zhang et al., 2003). Zhang and coworkers have also shown that activation of the A2a adenosine receptors suppresses chemokine receptor function, based on protein kinase A-mediated heterologous desensitization (Zhang et al., 2006). This is believed responsible, in part, for the anti-inflammatory activity of adenosine.
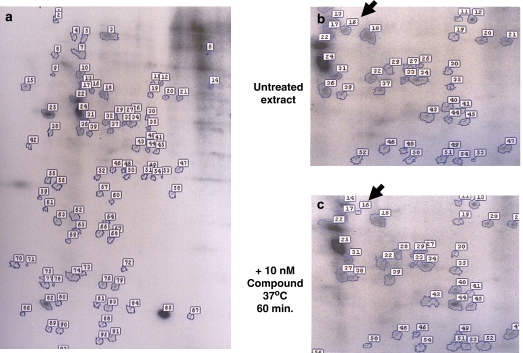
With these thoughts in mind, and the observation that the first cannabinoid CB2 receptor inverse agonist, the biarylpyrazole SR144528, was shown to alter p42/p44 MAPK signalling mediated by receptors to insulin and LPA (Bouaboula et al., 1999), we sought to investigate this problem by studying the effect of Sch.414319 on global pattern of protein phosphorylation in isolated immune cells. This decision was stimulated by kinetic experiments showing that initial inhibitory effects we observed on cellular chemotaxis appeared within 30 min of treatment with the cannabinoid receptor inverse agonist (L Bober, data not shown). We also were unable to detect significant changes in proteins synthesized by Jurkat cells treated with 10 nM Sch.414319, as measured by microarray expression profiling (D Lundell, personal communication). Based on these observations, we ruled out a role for new protein synthesis in the biology of Sch.414319 and speculated that the cannabinoid effect was the result of post-translational modification of existing proteins. As an initial test of this hypothesis, we determined the ability of Sch.414319 to modulate cellular protein phosphorylation in peripheral blood mononuclear cells. Peripheral blood mononuclear cells (1 × 107 cells ml−1) from normal human volunteers were resuspended in phosphate-free RPMI medium (Invitrogen Corporation, Carlsbad, CA, USA) containing 1% dialyzed foetal bovine serum (Invitrogen, Corporation, Carlsbad, CA, USA), and then treated with 0.5 mCi ml−1 [32P]orthophosphoric acid in the presence or absence of 10 nM Sch.414319 or 10 nM HU210. After incubation at 37 °C for 60 min, cell-free extracts were prepared for two-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis (O'Farrell, 1975), autoradiography (7-day exposure at −70 °C on Kodak XAR film with intensifier screen) and comparative digital imaging (Kendrick Laboratories Inc., Madison, WI, USA).
Figure 2 shows that we successfully visualized a number of phosphorylated proteins from the radiolabelled cell extracts. Comparative digital imaging of the gels detected 21 polypeptides that showed a difference in phosphorylation following cannabinoid treatment (Table 3). The cannabinoid agonist HU210 altered phosphorylation of five proteins—three of these proteins were also affected by the cannabinoid inverse agonist. However, there was no reciprocal relation between cannabinoid agonist treatment and the cannabinoid inverse agonist treatment (excepting protein no. 7). Two species (protein no. 71 and protein no. 1) showed equivalent changes with treatment by the agonist and inverse agonist. Two species (protein no. 69 and no. 96) were only affected by the agonist. The majority of the detected differences were seen only with Sch.414319 treatment. Because the cannabinoid inverse agonist does not reverse the phosphorylation pattern of the agonist, we question whether we should expect Sch.414319 to function as a classic inverse agonist—reversing the effects of an agonist. Similarly, the biarylpyrazole cannabinoid CB2 receptor inverse agonist SR144528 alone failed to induce significant pain hypersensitivity in model systems where cannabinoid CB2 receptor agonists clearly demonstrate decreased pain sensitivity (see Nackley et al., 2003; Elmes et al., 2004; Hohmann et al., 2004; Beltramo et al., 2006; La Rana et al., 2006; Gutierrez et al., 2007).
Figure 2.
The effect of Sch.414319 on phosphoproteins detected in human mononuclear cell preparations. Isolated human peripheral blood mononuclear cells were treated for 60 min in low phosphate medium containing 0.5 mCi ml−1 [32P]orthophosphoric acid in the absence or presence of 10 nM Sch.414319 or 10 nM HU210. Solubilized protein fractions from treated cells were submitted to Kendrick Laboratories for two-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis (O'Farrell, 1975) autoradiography and comparative digital imaging. (a) Autoradiogram of untreated extract. (b) Portion of a showing protein no. 16 (arrow). (c) Corresponding autoradiogram of extract from cells treated with 10 nM Sch.414319 at 37 °C for 60 min. Protein no. 16 is designated by an arrow.
Table 3.
Quantitating the effect of Sch.414319 on phosphoproteins detected in human mononuclear cell preparations
| Protein reference no. | Protein pI | Protein MWt | Sch.414319 Difference | HU210 Difference | |
|---|---|---|---|---|---|
| 23 | 8.34 | 64 541 | 2616 | — | |
| 35 | 6.62 | 56 000 | 301 | — | |
| 44 | 6.6 | 47 000 | 554 | — | |
| 53 | 6.81 | 40 833 | 6559 | — | |
| 71 | 4.9 | 25 814 | 4879 | 1423 | |
| 78 | 5.24 | 23 173 | 2138 | — | |
| 1 | ND | ND | −91 | −97 | |
| 2 | 5.28 | 194 711 | −88 | — | |
| 5 | 5.67 | 156 000 | −89 | — | |
| 16 | 5.77 | 71 000 | −98 | — | L-plastin |
| 52 | 5.84 | 41 000 | −78 | — | |
| 57 | 5.87 | 37 616 | −97 | — | |
| 61 | 5.15 | 35 000 | −69 | — | 14-3-3-ɛ |
| 69 | 5.46 | 28 647 | — | −91 | |
| 70 | 4.74 | 26 000 | −99 | — | |
| 72 | 6.24 | 25 057 | −72 | — | |
| 76 | 5.94 | 23 000 | −70 | — | |
| 77 | 5.13 | 23 424 | −96 | — | |
| 86 | 4.83 | 20 000 | −78 | — | |
| 96 | ND | ND | — | −91 | |
| 7 | 5.61 | 119 000 | −52 | 137 |
Abbreviations: MWt, molecualr weight; ND, not done.
Cell-free extracts from [32P] orthophosphoric acid-labelled human peripheral blood mononuclear cells plus/minus drug treatment (10 nM, 37 °C, 60 min) were analysed by two-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis, followed by autoradiography. Resulting films were digitally analysed and quantitated by Kendrick Laboratories (Madison, WI, USA). Differences in film darkening were quantitated according to the following formula:
Difference=(1−spot% sample × /spot % sample ref.)(−100).
Proteins in grey were selected for peptide sequencing by MALDI-MS (Protein Chemistry Core Facility, Columbia University).
Proteins present in sufficient quantities and showing significant changes in phosphorylation following drug treatment were excised from stained daughter gel sheet, digested with endoproteinase Lys-C and trypsin and the resulting peptides sequenced by matrix assisted laser desorption time-of-flight mass spectrometry (MALDI-MS; Protein Chemistry Core Facility, Columbia University). The majority of the proteins tested could not be resolved from background noise. However, two proteins were identified. Figure 3 shows that analysis of protein no. 61 generated seven peptide sequences consistent with human 14-3-3-ɛ isoform protein (accession number: AAD00026.1) and analysis of protein no. 16 generated eight peptide sequences, consistent with human L-plastin (accession number: P13796; also known as Lymphocyte cytosolic protein 1, LCP-1, LC64P). Examining the biology of these proteins may offer insight into the pathway by which triaryl bis-sulphones regulate cell motility. The first protein, 14-3-3-ɛ is a member of a conserved acidic protein family known to bind a number of signalling proteins, including kinases, phosphatases and transmembrane receptors. With such a broad distribution of activities, the reported association of 14-3-3 protein with the regulation of cell spreading (Rodriguez and Guan, 2005) may reflect the broad regulatory role of this protein rather than a specific role in cannabinoid CB2 receptor biology.
Figure 3.
Identification of phosphoproteins selectively modulated by treatment with Sch.414319. Phosphoproteins modulated by treatment of human mononuclear cell preparations with Sch.414319 were detected by two-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis, followed by autoradiography and quantitative digital imaging of the resulting autoradiogram. Proteins present in sufficient quantities and showing significant changes in phosphorylation following drug treatment were isolated from stained daughter gels, digested with endoproteinase Lys-C and trypsin and submitted for sequencing (Protein Chemistry Core Facility, Columbia University). Sequences and sequence alignments derived from the analysis of protein spot no. 61 (a) and protein spot no. 16 (b) are presented as lines under specific protein sequences.
Of more interest is the modulation of L-plastin phosphorylation by Sch.414319. Plastins are a set of actin-bundling proteins involved in the regulation of the actin cytoskeleton (Delanote et al., 2005). Three isoforms have been identified. T-plastin is constitutively expressed in epithelial and mesenchymal cells, I-plastin expression is restricted to absorptive intestinal and kidney cells and finally, L-plastin is expressed in hematopoetic cells, and is overexpressed in many solid tumours. Localization studies find L-plastin associated with structures involved in locomotion, adhesion and immune defense, including filopodia and immune complexes (Jones and Brown, 1996; Babb et al., 1997; Samstag et al., 2003). Recent studies have shown that phosphorylation of Ser5 increases F-actin-binding activity to the periphery of membrane protrusions in Vero cells (Janji et al., 2006). Boldt et al. (2006) showed that L-plastin is phosphorylated in polymorphonuclear neutrophils upon FPRL-1 activation with 0.1 μM W-peptide (Trp-Lys-Tyr-Met-Val-Met-NH2) or 1 μM sCKβ1-8 (amino acids 46–137 of the β-chemokine CKβ8, whereas other proteins associated with the cytoskeleton (moesin, cofilin and stathmin) are dephosphorylated. Finally, cells expressing a non-phosphorylatable ser5ala mutant of L-plastin showed decreased expressin of CD25 and CD69 at the cell surface (Wabnitz et al., 2007), suggesting phosphorylation of L-plastin may control receptor transport to the cell surface.
All of these studies suggest that control of L-plastin phosphorylation may represent a unique mechanism independent of chemokine receptor desensitization by which triaryl bis-sulphones could control of immune cell mobility and immune modulation. This model suggests that the cannabinoid CB2 receptor-specific triaryl bis-sulphones decreases L-plastin phosphorylation, which decreases the ability to the L-plastin to organize the actin fibrils involved in cellular polarization, required for controlled cellular chemotaxis. We would argue that this biology is not the direct result of inhibition of the kinases involved in L-plastin phosphorylation. Both protein kinase A (Wang and Brown, 1999) and protein kinase C (Paclet et al., 2004) have been implicated in the phosphorylation of L-plastin. We have shown that Sch.414319 does not inhibit the biochemical activity of several protein kinases, including a Ca2+/calmodulin-dependent protein kinase II, epidermal growth factor receptor tyrosine kinase, extracellular signal-regulated kinase 1 serine/threonine kinase, the fyn, HER2 and lck tyrosine kinases, non-selective protein kinase A, a non-selective protein kinase C and selective assays for protein kinase C-, - (I and II) and - (Table 2). This suggests that the decreased phosphorylation of L-plastin following triaryl bis-sulphone treatment is not due to inhibition of these protein kinases independent of cannabinoid CB2 signalling.
The ability of cannabinoid CB2 receptor-specific triaryl bis-sulphones to modulate plastin phosphorylation may provide a rationale for linking this class of compounds with cancer cell motility (invasiveness). A number of neoplastic cells from solid tumours are shown to express L-plastin, including 68% from carcinoma cell lines and 53% from mesenchymal cell lines (Lin et al., 1993; Park et al., 1994). The L-plastin promoter has been used to specifically target gene expression to these cells (Chung et al., 1999; Peng et al., 2001; Akbulut et al., 2004), and in experiments seeking to control invasiveness using antisense RNA (Zheng et al., 1999). Klemke et al. (2007) showed that L-plastin expression per se did not correlate to the penetration depth or the stage of melanoma in human tissue biopsies. However, in the same paper, Klemke and co-workers did show a correlation between L-plastin phosphorylation and tumour cell invasiveness in vitro and in a mouse B16 melanoma model.
While these models are interesting, they are complicated by the biology of L-plastin knockout (LPL−/−) mice. Chen et al. (2003) showed that LPL−/− mice were unable to control Staphylococcus aureus infection in subcutaneous abscess model. This effect was not the result of altered immune cell migration—LPL−/− peripheral mononuclear cell (PMN) migration to infection site appeared normal. LPL−/− PMNs bound and ingested opsinized S. aureus normally. The authors concluded that the immune deficit in this model resulted from an attenuated respiratory burst in the LPL−/− PMNs. Further studies are needed for comparing this biology with that using small-molecule inhibitors to determine the ultimate mechanism by which cannabinoid inverse agonists function.
Cannabinoid CB2 receptor and disease: arthritis
The demonstrated ability of triaryl bis-sulphones to modulate immune cell motility provides a generalized, well-validated mechanism for generalized immunoregulation. With the identification of Sch.414319 as an optimized member of this compound class, we have confirmed the ability of this compound to modulate cell migration in a number of animal models described previously. Sch.414319 inhibits leukocyte migration into the pleural cavity of mice and rats sensitized to mBSA antigen, and blocks eosinophilia in fluid recovered from the lungs of mice and guinea pigs sensitized to ovalbumin antigen (data not shown). However, we were most interested in determining the ability of this compound to impact a more complex disease model—antigen-induced arthritis. Studies with a congener of Sch.414319, designated Sch.356036, showed that the compound can ameliorate bone damage in a rat model of relapsing–remitting arthritis, a particularly harmful property of this inflammatory joint disease. In leukocyte recruitment models, this CB2 receptor-selective compound shows efficacy when added in concert with suboptimal doses of selected anti-inflammatory agents, consistent with its unique function and indicative of its potential therapeutic utility (CA Lunn, et al., in press).
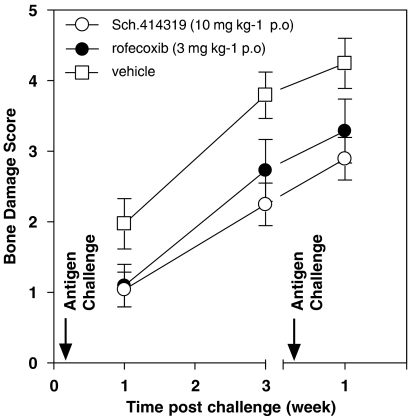
The recruitment of inflammatory cells into the joint space has been implicated in the initiation and maintenance of the pannus, a defining characteristic of rheumatoid arthritis. Both T cells (Kraan et al., 2004; Desmetz et al., 2007) and macrophages (Haringman et al., 2005; Szekanecz and Koch, 2007) have been suggested as playing a significant role in the pathology of this disease. The value of chemokine inhibition (Tak, 2006) as well as the ability of current therapeutics to modulate cellular infiltration into the joint (Taylor et al., 2000; Jenkins et al., 2002; Bukhari et al., 2003; Sesin and Bingham, 2005) add to the significance of this therapeutic approach. Based on these data, we sought to evaluate Sch.414319 in a rat model of relapsing–remitting arthritis. For studies using triaryl bis-sulphones, monoarticular arthritis was induced according to described procedures (Buchner et al., 1995). Male inbred Lewis rats were first sensitized with mBSA in complete Freund's adjuvant injected near the inguinal lymph node. Fourteen days later, arthritis was induced by injecting 500 μg of a sterile mBSA solution in a volume of 5 μl into the left talar–navicular joint space of animals anesthetized with isofurane vapors. The right paw was similarly injected with sterile saline to serve as an internal control. All animals are acclimated to treatment regime by dosing for two days before disease induction. Animals were handled in accordance with protocols and guidelines established by our institution's Animal Care and Use Committee, and showed no weight loss during progression of the monoarticular arthritis. Figure 4 shows that oral administration of 10 mg kg−1 Sch.414319 2 days before through 5 days after antigen challenge showed improved bone damage score when measured 2 weeks after the last drug dose. The effect was similar to that seen with 3 mg kg−1 rofecoxib. This result suggests that treatment with Sch.414319 slowed the development of the antigen-induced monoarticular arthritis, perhaps by slowing the movement of immune cells to the talar–navicular joint space. This fact and the ability of triaryl bis-sulphones to synergize with other anti-inflammatory agents including the cyclooxygenase inhibitor piroxicam, the PDE4 inhibitor rolipram and the steroid dexamethasone, provide a potential use for the class of compound (CA Lunn, et al., in press).
Figure 4.
Effect Sch.414319 on bone damage in antigen-induced mono-articular arthritis. Sensitized male inbred Lewis rats (nine per group) were challenged by injection of 5 μl (500 μg) mBSA antigen into the left talar–navicular joint space. The animals were treated 2 days before through 5 days after antigen challenges with vehicle, rofecoxib (3 mg kg−1 p.o.) or Sch.414319 (10 mg kg−1 p.o.)—animals received no treatment between first and second challenges. Radiologic evaluation was performed on the hind paws using Polaroid type 55 films and a microradiographic X-ray unit (Faxitron Hewlett Packard, McMinnville, OR, USA). Each paw was scored for soft tissue swelling (scale 0–3), erosions (scale 0–3), cartilage space changes (scale 0–3) and periostitis (scale 0–3), making for a total possible score of 12. Animals were used in accordance with protocols and guidelines established by our institution's Animal Care and Use Committee, and showed no weight loss during progression of the monoarticular arthritis. mBSA, methylated bovine serum albumin.
The ability to modulate the effects of antigen insult on bone structure using two cannabinoid CB2 receptor inverse agonists (Sch.414319 and Sch.356036) suggests a link between this receptor and bone physiology. However, mimicking the complex role of cannabinoid CB2 receptor pharmacology in cell migration, the role of the cannabinoid CB2 receptor in bone physiology is complex. Idris et al. (2005) have reported that CB2 (and CB1) receptor antagonists/inverse agonists AM630 and SR144528 inhibited osteoclast formation in C57BL/6 cell cultures stimulated with RANKL and M-CSF—agonists stimulated osteoclast formation. This result is consistent with the benefit of inverse agonists in maintaining bone density. On the other hand, a study of 388 postmenopausal women showed a significant association of polymorphisms and haplotypes containing the CB2 receptor gene CNR2 on human chromosome 1p36 with osteoporosis (Karsak et al., 2005). Assuming that the mutations detected in this study would decrease expression or activity of the CB2 receptor, Karsak suggested the data are consistent with the value of a cannabinoid CB2 receptor agonist as a valuable therapeutic. Ofek et al. (2006) also reported that, while young mice appeared normal, aged (1 year old), CB2 receptor-deficient mice exhibited an accelerated trabecular bone loss and cortical expansion. He also showed that the cannabinoid CB2 receptor-specific agonist HU308 stimulated proliferation of osteoblastogenic cultures and restricted osteoclastogenic cultures.
Further investigations are required to rationalize the therapeutic benefit seen using our cannabinoid CB2 receptor-specific inverse agonists with that observed with cannabinoid CB2 receptor-specific agonists. We argue that the beneficial therapeutic effect of cannabinoid CB2 receptor inverse agonists on bone damage is mediated by limiting cell accumulation at the site of insult. Additional mechanisms could be involved in this complex disease, including precursor cell differentiation/proliferation (see above). Other laboratories have described effects of cannabinoid compounds on bone density based on other mechanisms (Sumariwalla et al., 2004; Burstein, 2005; Mbvundula et al., 2005; see Burstein and Zurier, 2004).
Cannabinoid CB2 receptor and disease: EAE
Another important therapeutic target for cannabinoid CB2 receptor-specific agents is inflammation of the central nervous system. Like arthritis, this disease involves migration of inflammatory cells to a restricted site, and then action of the cells at that site (Izikson et al., 2002). Early studies showed that the nonspecific cannabinoid agonist WIN55,212-2 could control spasticity and tremors in a chronic relapsing EAE (Baker et al., 2000), although this effect is believed to have been mediated by the cannabinoid CB1 receptor (Pryce and Baker, 2007). Although initially believed to be uniquely associated with immune cells, functional cannabinoid CB2 receptors have been found associated with central nervous system tissue (Van Sickle et al., 2005; Beltramo et al., 2006), and the detectible receptor mRNA increases in activated microglia cells with development of transgenic mice expressing a T-cell receptor transgene specific for the acetylated NH2-terminal peptide of myelin basic protein (Ac1-11) bound to I-Au (MBP-TCR) (CD4+ T-cell-induced EAE model (Maresz et al., 2005). This effect is believed due to the cannabinoid system within the central nervous system directly suppressing T-cell effector function via the CB2 receptor (Maresz et al., 2007). Ni et al. (2004) have shown that the increased leukocyte/endothelium interactions seen in mice following induction of EAE using myelin oligodendrocyte glycoprotein, can be alleviated with the cannabinoid agonist WIN55,212-2 via the CB2 receptor. Sipe et al. (2005) have extended these studies to human disease, showing the potential linkage of a cannabinoid CB2 receptor polymorphism associated with autoimmune disorders, including multiple sclerosis.
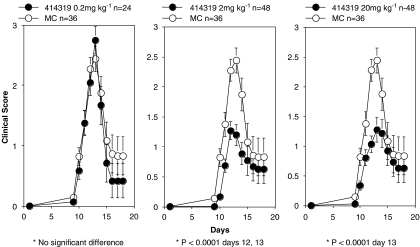
We have carried out preliminary investigations to investigate the role of the cannabinoid CB2 receptor in inflammatory diseases of the central nervous system, using the cannabinoid CB2 receptor-deficient mice. We have shown that the ability of a peptide derived from myelin oligodendrocyte glycoprotein (MOG35–55) to elicit EAE (Reich et al., 2005) is diminished in a cannabinoid CB2 receptor-deficient mouse strain (Lunn et al., 2006b). The CB2 receptor-deficient strain showed a significant delay in disease onset, attenuation in clinical disease score and improved survivability (with six of 15 mice dying in the control, but only one of 15 dying in the receptor-deficient group). We have now extended these initial studies to probe the ability of the cannabinoid CB2 receptor inverse agonist Sch.414319 to modulate EAE in the rat. For these experiments, male Lewis rats were injected with 30 mg of guinea pig spinal cord homogenate in complete Freund's adjuvant into the footpad (Smith et al., 1993). The animals were treated orally with Sch.414319 in 0.4% methylcellulose starting at day 0 then continuing throughout the 3-week disease course. Animals were evaluated daily for clinical disease score. Figure 5 shows a summation of the results of two independent experiments using at least 24 rats per treatment group. The figure shows that oral administration of 20 and 2 mg kg−1 Sch.414319 significantly modulates the clinical signs of EAE, converting complete hind-limb paralysis to a flaccid tail phenotype. Viewed in another way, we observed that roughly 30% of the vehicle-treated animals showed total limb paralysis, whereas no animals showed total limb paralysis at day 13, when treated with 2 mg kg−1 Sch.414319. The effect appears dose dependent—treatment with 0.2 mg kg−1 Sch.414319 is ineffective.
Figure 5.
Sch.414319 protects against development of EAE. Male Lewis rats challenged by injection of 50 μl (30 mg) of a guinea pig spinal cord homogenate in complete Freund's adjuvant into one footpad. The animals were treated starting at day 0 and oral dosing continued throughout the 3-week disease course, with varying amounts of Sch.414319 in 0.4% methylcellulose (MC) p.o. Animals were scored for disease severity: 0, no clinical signs; 1, flaccid tail; 2, hind limb weakness; 3, complete hind limb paralysis; 4, complete hind limb paralysis, forelimb weakness or paralysis; 5, death. Data presented represent the sum of two independent experiments. All animals were used in accordance with protocols and guidelines established by our institution's Animal Care and Use Committee. EAE, experimental autoimmune encephalomyelitis.
Conclusions
An ability to control the migration of inflammatory cells to the site of insult is a powerful strategy for the development of immunomodulators. Our work on triaryl bis-sulphones suggest that the cannabinoid CB2 receptor-specific inverse agonists may serve as such immune modulators. We have demonstrated that this class of compound behaves as inverse agonists in selected biochemical assays, including ligand-mediated GTPγS binding, forskolin-stimulated cAMP and in competition binding assays with known cannabinoid agonists/inverse agonists. Recent studies (Gonsiorek et al., 2006) have demonstrated that competition binding in the presence of a radiolabelled triaryl bis-sulphone significantly shifts the observed Ki relative to values obtained using an radiolabelled agonist ligand, consistent with the inverse agonist label. However, cell-based bioassays probing the effect of these compounds on the monocyte kinome (Table 3) suggest that the pharmacology of this class of compounds may be more complex. In this review, we have demonstrated that an optimized triaryl bis-sulphone, Sch.414319, modulates cell migration in vivo (Figure 1), can modulate bone damage in antigen-induced mono-articular arthritis (Figure 4) and can modulate the clinical signs of EAE in the Lewis rat strain (Figure 5). We have argued that these effects can all result from a control of inflammatory cell migration, and have presented preliminary evidence for a mechanism involving L-plastin phosphorylation could be involved.
We have not been the only investigators to identify immunomodulatory activities associated with cannabinoid CB2 receptor inverse agonists. Iwamura et al. (2001) showed that oral administration of the inverse agonists JTE-907 and SR144528 inhibited carrageenin-induced paw oedema in mice. Ueda et al. (2005) reported that orally administered JTE-907 (0.1–10 mg kg−1) and SR144528 (1 mg kg−1) significantly inhibited dinitrofluorobenzene-induced ear swelling, with increased cannabinoid CB2 receptor mRNA expression observed in the inflamed ear. Oka et al. (2005) showed that SR144528 treatment blocked 12-O-tetradecanoylphorbol-13-acetate-induced ear swelling, consistent with an observed decrease in leukotriene B4 production and decrease in neutrophil infiltration into the treated mouse ear. Oka et al. (2006) also observed that ear swelling was suppressed by administration of SR144528 immediately after sensitization (sensitization phase), or upon challenge (elicitation phase) of oxazolone-induced contact dermatitis. This result correlated with suppressed proinflammatory cytokine mRNA expression and attenuated eosinophil recruitment into the treated ear. Further studies, using these and other CB2 receptor-specific compounds, will be required to resolve the complex pharmacology of cannabinoids and the cannabinoid CB2 receptor, and to determine the most effective pharmacology to exploit this therapeutic target.
Acknowledgments
The author acknowledges the additional members of the Schering-Plough Research Institute, Department of Inflammation, including Long Cui, Alberto Rojas-Triana and James V Jackson, for their work on this project.
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- PMN
peripheral mononuclear cells
Conflict of interest
Work described has been carried out by members of the Schering-Plough Research Institute.
References
- Akbulut H, Tang Y, Maynard J, Zhang L, Pizzorno G, Deisseroth AB. A vector targeting makes 5-fluorouracil chemotherapy less toxic and more effective in animal models of epithelial neoplasms. Clin Cancer Res. 2004;10:7738–7746. doi: 10.1158/1078-0432.CCR-04-0490. [DOI] [PubMed] [Google Scholar]
- Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- Babb SG, Matsudaira P, Sato M, Correia I, Lim SS. Fimbrin in podosomes of monocyte-derived osteoclasts. Cell Motil Cytoskeleton. 1997;37:308–325. doi: 10.1002/(SICI)1097-0169(1997)37:4<308::AID-CM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Benamar K, Yondorf M, Meissler JJ, Geller EB, Tallarida RJ, Eisenstein TK, et al. A novel role of cannabinoids: implication in the fever induced by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 2007;320:1127–1133. doi: 10.1124/jpet.106.113159. [DOI] [PubMed] [Google Scholar]
- Boldt K, Rist W, Weiss SM, Weith A, Lenter MC. FPRL-1 induces modifications of migration-associated proteins in human neutrophils. Proteomics. 2006;6:4790–4799. doi: 10.1002/pmic.200600121. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- Buchner E, Brauer R, Schmidt C, Emmrich F, Kinne RW. Induction of flare-up reactions in rat antigen-induced arthritis. J Autoimmun. 1995;8:61–74. [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum. 2003;48:46–53. doi: 10.1002/art.10727. [DOI] [PubMed] [Google Scholar]
- Burstein S. Ajulemic acid (IP-751): synthesis, proof of principle, toxicity studies, and clinical trials. AAPS J. 2005;7:E143–E148. doi: 10.1208/aapsj070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S, Zurier RB. Pain reduction and lack of psychotropic effects with ajulemic acid: comment on the article by Sumariwalla et al. Arthritis Rheum. 2004;50:4078–4079. doi: 10.1002/art.20805. [DOI] [PubMed] [Google Scholar]
- Chen H, Mocsai A, Zhang H, Ding RX, Morisaki JH, White M, et al. Role for plastin in host defense distinguishes integrin signaling from cell adhesion and spreading. Immunity. 2003;19:95–104. doi: 10.1016/s1074-7613(03)00172-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Oppenheim JJ, Howard OM. Chemokines and chemokine receptors as novel therapeutic targets in rheumatoid arthritis (RA): inhibitory effects of traditional Chinese medicinal components. Cell Mol Immunol. 2004;1:336–342. [PubMed] [Google Scholar]
- Chung I, Schwartz PE, Crystal RG, Pizzorno G, Leavitt J, Deisseroth AB. Use of L-plastin promoter to develop an adenoviral system that confers transgene expression in ovarian cancer cells but not in normal mesothelial cells. Cancer Gene Ther. 1999;6:99–106. doi: 10.1038/sj.cgt.7700017. [DOI] [PubMed] [Google Scholar]
- Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharmacol. 2007;7:360–371. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KA, Dunn-Meynell K, Korfmacher WA, Broske L, Nomeir AA, Lin CC, et al. Novel procedure for rapid pharmacokinetic screening of discovery compounds in rats. Drug Discov Today. 1999;4:232–237. doi: 10.1016/s1359-6446(98)01299-9. [DOI] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran NM, Griffin BD, O'Toole D, Brady KJ, Fitzgerald SN, Moynagh PN. The synthetic cannabinoid R(+)WIN 55,212-2 inhibits the interleukin-1 signaling pathway in human astrocytes in a cannabinoid receptor-independent manner. J Biol Chem. 2005;280:35797–35806. doi: 10.1074/jbc.M507959200. [DOI] [PubMed] [Google Scholar]
- De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho) physiological cellular processes. Acta Pharmacol Sin. 2005;26:769–779. doi: 10.1111/j.1745-7254.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Derocq JM, Jbilo O, Bouaboula M, Segui M, Clere C, Casellas P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60. Possible involvement of the CB2 receptor in cell differentiation. J Biol Chem. 2000;275:15621–15628. doi: 10.1074/jbc.275.21.15621. [DOI] [PubMed] [Google Scholar]
- Desmetz C, Lin YL, Mettling C, Portales P, Noel D, Clot J, et al. Cell surface CCR5 density determines the intensity of T cell migration towards rheumatoid arthritis synoviocytes. Clin Immunol. 2007;123:148–154. doi: 10.1016/j.clim.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Fine JS, Rojas-Triana A, Jackson JV, Engstrom LW, Deno GS, Lundell DJ, et al. Impairment of leukocyte trafficking in a murine pleuritis model by IL-4 and IL-10. Inflammation. 2003;27:161–174. doi: 10.1023/a:1025076111950. [DOI] [PubMed] [Google Scholar]
- Giudice ED, Rinaldi L, Passarotto M, Facchinetti F, D'Arrigo A, Guiotto A, et al. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J Leukoc Biol. 2007;81:1512–1522. doi: 10.1189/jlb.1206738. [DOI] [PubMed] [Google Scholar]
- Gokoh M, Kishimoto S, Oka S, Mori M, Waku K, Ishima Y, et al. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces rapid actin polymerization in HL-60 cells differentiated into macrophage-like cells. Biochem J. 2005;386:583–589. doi: 10.1042/BJ20041163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsiorek W, Hesk D, Chen SC, Kinsley D, Fine JS, Jackson JV, et al. Characterization of peripheral human cannabinoid receptor (hCB2) expression and pharmacology using a novel radioligand [35S] Sch225336. J Biol Chem. 2006;281:28143–28151. doi: 10.1074/jbc.M602364200. [DOI] [PubMed] [Google Scholar]
- Greasley PJ, Clapham JC. Inverse agonism or neutral antagonism at G-protein coupled receptors: a medicinal chemistry challenge worth pursuing? Eur J Pharmacol. 2006;553:1–9. doi: 10.1016/j.ejphar.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834–838. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- Horuk R. Development and evaluation of pharmacological agents targeting chemokine receptors. Methods. 2003;29:369–375. doi: 10.1016/s1046-2023(02)00361-4. [DOI] [PubMed] [Google Scholar]
- Idris AI, van't Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- Izikson L, Klein RS, Luster AD, Weiner HL. Targeting monocyte recruitment in CNS autoimmune disease. Clin Immunol. 2002;103:125–131. doi: 10.1006/clim.2001.5167. [DOI] [PubMed] [Google Scholar]
- Janji B, Giganti A, De Corte V, Catillon M, Bruyneel E, Lentz D, et al. Phosphorylation on Ser5 increases the F-actin-binding activity of L-plastin and promotes its targeting to sites of actin assembly in cells. J Cell Sci. 2006;119 (Part 9):1947–1960. doi: 10.1242/jcs.02874. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Derocq JM, Segui M, Le Fur G, Casellas P. Stimulation of peripheral cannabinoid receptor CB2 induces MCP-1 and IL-8 gene expression in human promyelocytic cell line HL60. FEBS Lett. 1999;448:273–277. doi: 10.1016/s0014-5793(99)00380-4. [DOI] [PubMed] [Google Scholar]
- Jenkins JK, Hardy KJ, McMurray RW. The pathogenesis of rheumatoid arthritis: a guide to therapy. Am J Med Sci. 2002;323:171–180. doi: 10.1097/00000441-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Jones SL, Brown EJ. FcgammaRII-mediated adhesion and phagocytosis induce L-plastin phosphorylation in human neutrophils. J Biol Chem. 1996;271:14623–14630. doi: 10.1074/jbc.271.24.14623. [DOI] [PubMed] [Google Scholar]
- Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, et al. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Ouyang Y, Rockwell CE, Rao GK, Kaminski NE. 2-Arachidonoyl-glycerol suppresses interferon-gamma production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2. J Leukoc Biol. 2005;77:966–974. doi: 10.1189/jlb.1104652. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306:1077–1085. doi: 10.1124/jpet.103.051961. [DOI] [PubMed] [Google Scholar]
- Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005;14:3389–3396. doi: 10.1093/hmg/ddi370. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Oka S, Gokoh M, Sugiura T. Chemotaxis of human peripheral blood eosinophils to 2-arachidonoylglycerol: comparison with other eosinophil chemoattractants. Int Arch Allergy Immunol. 2006;140 (Suppl 1):3–7. doi: 10.1159/000092704. [DOI] [PubMed] [Google Scholar]
- Klemke M, Rafael MT, Wabnitz GH, Weschenfelder T, Konstandin MH, Garbi N, et al. Phosphorylation of ectopically expressed L-plastin enhances invasiveness of human melanoma cells. Int J Cancer. 2007;120:2590–2599. doi: 10.1002/ijc.22589. [DOI] [PubMed] [Google Scholar]
- Kraan MC, Haringman JJ, Weedon H, Barg EC, Smith MD, Ahern MJ, et al. T cells, fibroblast-like synoviocytes, and granzyme B+ cytotoxic cells are associated with joint damage in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63:483–488. doi: 10.1136/ard.2003.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Wintersberger W, Kress HG. Cannabinoid receptor-independent suppression of the superoxide generation of human neutrophils (PMN) by CP55 940, but not by anandamide. Life Sci. 2004;75:969–977. doi: 10.1016/j.lfs.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kurihara R, Tohyama Y, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, et al. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–12918. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- La Rana G, Russo R, Campolongo P, Bortolato M, Mangieri RA, Cuomo V, et al. Modulation of neuropathic and inflammatory pain by the endocannabinoid transport inhibitor AM404 (N-(4-hydroxyphenyl)-eicosa-5,8,11,14-tetraenamide) J Pharmacol Exp Ther. 2006;317:1365–1371. doi: 10.1124/jpet.105.100792. [DOI] [PubMed] [Google Scholar]
- Lavey BJ, Kozlowski JA, Hipkin RW, Gonsiorek W, Lundell DJ, Piwinski JJ, et al. Triaryl bissulfones as a new class of cannabinoid CB2 receptor inhibitors: identification of a lead and initial SAR studies. Bioorg Med Chem Lett. 2005;15:783–786. doi: 10.1016/j.bmcl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lavey BJ, Kozlowski JA, Shankar BB, Spitler JM, Zhou G, Yang DY, et al. Optimization of triaryl bissulfones as cannabinoid-2 receptor ligands. Bioorg Med Chem Lett. 2007;17:3760–3764. doi: 10.1016/j.bmcl.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Lin CS, Park T, Chen ZP, Leavitt J. Human plastin genes. Comparative gene structure, chromosome location, and differential expression in normal and neoplastic cells. J Biol Chem. 1993;268:2781–2792. [PubMed] [Google Scholar]
- Lopez-Cepero M, Friedman M, Klein T, Friedman H. Tetrahydrocannabinol-induced suppression of macrophage spreading and phagocytic activity in vitro. J Leukoc Biol. 1986;39:679–686. doi: 10.1002/jlb.39.6.679. [DOI] [PubMed] [Google Scholar]
- Lunn CA, Fine JS, Rojas-Triana A, Jackson JV, Fan X, Kung TT, et al. A novel cannabinoid peripheral cannabinoid receptor-selective inverse agonist blocks leukocyte recruitment in vivo. J Pharmacol Exp Ther. 2006a;316:780–788. doi: 10.1124/jpet.105.093500. [DOI] [PubMed] [Google Scholar]
- Lunn CA, Reich EP, Bober L. Targeting the CB2 receptor for immune modulation. Expert Opin Ther Targets. 2006b;10:653–663. doi: 10.1517/14728222.10.5.653. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2001;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Mbvundula EC, Bunning RA, Rainsford KD. Effects of cannabinoids on nitric oxide production by chondrocytes and proteoglycan degradation in cartilage. Biochem Pharmacol. 2005;69:635–640. doi: 10.1016/j.bcp.2004.11.018. [DOI] [PubMed] [Google Scholar]
- McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor-independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321:930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Ni X, Geller EB, Eppihimer MJ, Eisenstein TK, Adler MW, Tuma RF. Win 55212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult Scler. 2004;10:158–164. doi: 10.1191/1352458504ms1009oa. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, et al. 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. 2004;76:1002–1009. doi: 10.1189/jlb.0404252. [DOI] [PubMed] [Google Scholar]
- Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006;177:8796–8805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, et al. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–18497. doi: 10.1074/jbc.M413260200. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Paclet MH, Davis C, Kotsonis P, Godovac-Zimmermann J, Segal AW, Dekker LV. N-formyl peptide receptor subtypes in human neutrophils activate L-plastin phosphorylation through different signal transduction intermediates. Biochem J. 2004;377 (Part 2):469–477. doi: 10.1042/BJ20031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T, Chen ZP, Leavitt J. Activation of the leukocyte plastin gene occurs in most human cancer cells. Cancer Res. 1994;54:1775–1781. [PubMed] [Google Scholar]
- Peng XY, Won JH, Rutherford T, Fujii T, Zelterman D, Pizzorno G, et al. The use of the L-plastin promoter for adenoviral-mediated, tumor-specific gene expression in ovarian and bladder cancer cell lines. Cancer Res. 2001;61:4405–4413. [PubMed] [Google Scholar]
- Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich EP, Cui L, Yang L, Pugliese-Sivo C, Golovko A, Petro M, et al. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 2005;35:1027–1036. doi: 10.1002/eji.200425954. [DOI] [PubMed] [Google Scholar]
- Rodriguez LG, Guan JL. 14-3-3 regulation of cell spreading and migration requires a functional amphipathic groove. J Cell Physiol. 2005;202:285–294. doi: 10.1002/jcp.20122. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Samstag Y, Eibert SM, Klemke M, Wabnitz GH. Actin cytoskeletal dynamics in T lymphocyte activation and migration. J Leukoc Biol. 2003;73:30–48. doi: 10.1189/jlb.0602272. [DOI] [PubMed] [Google Scholar]
- Sesin CA, Bingham CO., III Remission in rheumatoid arthritis: wishful thinking or clinical reality? Semin Arthritis Rheum. 2005;35:185–196. doi: 10.1016/j.semarthrit.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Shankar BB, Lavey BJ, Zhou G, Spitler JA, Tong L, Rizvi R, et al. Triaryl bissulfones as cannabinoid-2 receptor ligands: SAR studies. Bioorg Med Chem Lett. 2005;15:4417–4420. doi: 10.1016/j.bmcl.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol. 2005;78:231–238. doi: 10.1189/jlb.0205111. [DOI] [PubMed] [Google Scholar]
- Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- Smith SR, Terminelli C, Denhardt G. Modulation of cytokine responses in Corynebacterium parvum-primed endotoxemic mice by centrally administered cannabinoid ligands. Eur J Pharmacol. 2001;425:73–83. doi: 10.1016/s0014-2999(01)01142-6. [DOI] [PubMed] [Google Scholar]
- Smith SR, Watnick AS, Kung T, Siegel MI. In vitro and in vivo immunopharmacological profile of Sch 40120. Immunopharmacol Immunotoxicol. 1993;15:13–44. doi: 10.3109/08923979309066931. [DOI] [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13:209–222. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Sumariwalla PF, Gallily R, Tchilibon S, Fride E, Mechoulam R, Feldmann M. A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. Arthritis Rheum. 2004;50:985–998. doi: 10.1002/art.20050. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- Tak PP. Chemokine inhibition in inflammatory arthritis. Best Pract Res Clin Rheumatol. 2006;20:929–939. doi: 10.1016/j.berh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor α blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Miyagawa N, Matsui T, Kaya T, Iwamura H. Involvement of cannabinoid CB2 receptor-mediated response and efficacy of cannabinoid CB2 receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur J Pharmacol. 2005;520:164–171. doi: 10.1016/j.ejphar.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varnes JG, Gardner DS, Santella JB, III, Duncia JV, Estrella M, Watson PS, et al. Discovery of N-propylurea 3-benzylpiperidines as selective CC chemokine receptor-3 (CCR3) antagonists. Bioorg Med Chem Lett. 2004;14:1645–1649. doi: 10.1016/j.bmcl.2004.01.059. [DOI] [PubMed] [Google Scholar]
- Wabnitz GH, Kocher T, Lohneis P, Stober C, Konstandin MH, Funk B, et al. Costimulation induced phosphorylation of L-plastin facilitates surface transport of the T cell activation molecules CD69 and CD25. Eur J Immunol. 2007;37:649–662. doi: 10.1002/eji.200636320. [DOI] [PubMed] [Google Scholar]
- Wang J, Brown EJ. Immune complex-induced integrin activation and L-plastin phosphorylation require protein kinase A. J Biol Chem. 1999;274:24349–24356. doi: 10.1074/jbc.274.34.24349. [DOI] [PubMed] [Google Scholar]
- Widdowson KL, Elliott JD, Veber DF, Nie H, Rutledge MC, McCleland BW, et al. Evaluation of potent and selective small-molecule antagonists for the CXCR2 chemokine receptor. J Med Chem. 2004;47:1319–1321. doi: 10.1021/jm034248l. [DOI] [PubMed] [Google Scholar]
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. J Biol Chem. 2003;278:12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Oppenheim JJ. Crosstalk between chemokines and neuronal receptors bridges immune and nervous systems. J Leukoc Biol. 2005;78:1210–1214. doi: 10.1189/jlb.0405224. [DOI] [PubMed] [Google Scholar]
- Zhang N, Yang D, Dong H, Chen Q, Dimitrova DI, Rogers TJ, et al. Adenosine A2a receptors induce heterologous desensitization of chemokine receptors. Blood. 2006;108:38–44. doi: 10.1182/blood-2005-06-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Rudra-Ganguly N, Powell WC, Roy-Burman P. Suppression of prostate carcinoma cell invasion by expression of antisense L-plastin gene. Am J Pathol. 1999;155:115–122. doi: 10.1016/S0002-9440(10)65106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]