Abstract
This review gives an overview of the CB2 receptor (CB2R) knockout (CB2R−/−) mice phenotype and the work that has been carried out using this mutant mouse. Using the CB2R−/− mice, investigators have discovered the involvement of CB2R on immune cell function and development, infection, embryonic development, bone loss, liver disorders, pain, autoimmune inflammation, allergic dermatitis, atherosclerosis, apoptosis and chemotaxis. Using the CB2R−/− mice, investigators have also found that this receptor is not involved in cannabinoid-induced hypotension. In addition, the CB2R−/− mice have been used to determine specific tissue CB2R expression. The specificity of synthetic cannabinoid agonists, antagonists and anti-CB2R antibodies has been screened using tissues from CB2R−/− mice. Thus, the use of this mouse model has greatly helped reveal the diverse events involving the CB2R, and has aided in drug and antibody screening.
Keywords: CB2R, CB2R−/− mice, antifibrogenic, pain, allergic dermatitis, atherosclerosis, chemotaxis, osteoporosis, multiple sclerosis
Introduction
The peripheral cannabinoid receptor (CB2R) was the second cannabinoid receptor discovered (Munro et al., 1993), after the central cannabinoid receptor (CB1R) (Matsuda et al., 1990). Both cannabinoid receptors are G-protein-coupled seven transmembrane (TM) receptors. Human CB1R and CB2R share 44% overall homology and 68% at the TM level (Munro et al., 1993). Human CB1R and mouse CB1R share 96% homology (Chakrabarti et al., 1995), while human CB2R and mouse CB2R share 82% homology (Shire et al., 1996b). Mouse CB1R and CB2R share 66% overall homology and 78% at the TM level (Shire et al., 1996a). CB1R is expressed at high levels in brain tissue and to a lesser extent in peripheral tissues such as the adrenal glands, reproductive organs and on immune cells (Matsuda et al., 1990; Bouaboula et al., 1993; Galiegue et al., 1995). In contrast, CB2R is mainly expressed in cells of haematopoietic origin. CB2R expression has been demonstrated in spleen and thymus (Schatz et al., 1997), lymph nodes, Peyer's patches (Lynn and Herkenham, 1994) and immune system-derived cell lines. Human blood cell populations were reported to have different degrees of CB2R expression with the following rank order: B cells>natural killer cells>monocytes>polymorphonuclear neutrophil cells>CD8+ T cells>CD4+ T cells (Galiegue et al., 1995; Carayon et al., 1998). The expression level of CB2R in lymphocytes and macrophages has been shown to vary in relation to cell differentiation and activation state (reviewed in Klein et al., 2003). Thus, Carayon et al. (1998) reported that CB2R expression varied depending on the stage of B-cell differentiation with virgin and memory B cells, expressing the highest levels of CB2R mRNA followed by germinal-centre B cells and centroblasts (Carayon et al., 1998). Carlisle et al. (2002) showed that while resident macrophages lack CB2R expression, thioglycollate-elicited and interferon-γ (IFN-γ)-primed macrophages have high-CB2R levels (Carlisle et al., 2002). The expression of CB2R gene in immune tissues has been reported to be 10–100 times that of CB1R (Galiegue et al., 1995). More recently, CB2R expression in osteoblasts, osteocytes and osteoclasts was observed (Ofeck et al., 2006). CB2R expression has also been found in preimplantation embryos (Paria et al., 1995), and more recently in the normal central nervous system (CNS) (Ross et al., 2001; Van Sickle et al., 2005; Ashton et al., 2006; Gong et al., 2006; Onaivi, 2006; Onaivi et al., 2006). The existence of a putative third cannabinoid receptor, GPR55, has been reported. GPR55 is an orphan G-protein-coupled receptor that has low-sequence homology (10–15%), compared to that of CB1R or CB2R, and is expressed in the testis at approximately a 15-fold higher level than in the brain (Baker et al., 2006). However, full characterization of this receptor is lacking, and it cannot be concluded that it is a true cannabinoid receptor (Petitet et al., 2006). Recently, Sugiura et al. (2007) were unable to stimulate GPR55 activation using three different cannabinoids (Sugiura et al., 2007). To investigate the role of CB1R and CB2R, mutant mice with deletions in these receptors have been developed. Thus far, there are three lines of CB1R knockout (CB1R−/−) mice (reviewed in Valverde et al., 2005), and two CB2R knockout (CB2R−/−) mice, one developed by Buckley et al. (2000) and the one recently developed by Deltagen (San Mateo, CA, USA) and commercially available through Jackson Laboratory (Bar Harbor, ME, USA) (http://jaxmice.jax.org/strain/005786.html#genes). The goal of the present review is to provide an update on the multiple findings investigators have achieved with the use of the CB2R−/− mice generated by Buckley et al. (2000).
CB2R−/− mice
The CB2R−/− mice were generated by homologous recombination. Upon homologous recombination, the last 341 bp of the CB2R-coding exon in the gene was replaced by the neomycin gene, effectively removing bp 1186 onwards from the coding sequence (see accession U21681 in GenBank). The CB2R deletion results in a gene lacking the coding region for part of the intracellular loop 3, TM regions 6 and 7 and the C terminus (Buckley et al., 2000). This deletion renders the CB2R nonfunctional as macrophages derived from CB2R−/− mice are unable to respond to THC (Buckley et al., 2000; Chuchawankul et al., 2004). However, these mice are responsive to the psychotropic effects of cannabinoids (Buckley et al., 2000). The CB2R−/− mice display no gross morphological differences from their wild-type counterparts, but at the cellular level, the CB2R−/− mice are deficient in splenic marginal zone B cells, peritoneal B1a (CD5+CD11b+CD23loB220lo) cells, splenic memory CD4+ T cells, and intestinal natural killer cells and natural killer T cells (Ziring et al., 2006). Furthermore, CB2R−/− mice have a decreased number of diaphyseal osteoblast precursors, but have an increased osteoclast number and increased activity of their trabecular osteoblasts. This phenotype causes CB2R−/− mice to undergo a greater age-related bone loss than their wild-type counterparts, a loss of bone mass that is apparent by 8 weeks of age (Ofeck et al., 2006).
CB2R−/− mice and reproduction
The CB2R−/− mice are fertile, care for their young and have litter sizes comparable to their wild-type counterparts (Buckley et al., 2000). CB2R is present in the preimplantation embryo, but not in the oviduct or uterus (Das et al., 1995; Paria et al., 1995, 2001; Wang et al., 2004). CB2R is expressed from the one-cell through the blastocyts embryonic stage, restricted to blastocyts inner cell mass, but not in the trophectoderm-derived trophoblast stem cells, which are directly involved in implantation (Paria et al., 1995). On the other hand, CB1R expression is present in the trophectoderm of the preimplantation embryo (Paria et al., 1995; Yang et al., 1996). Congruent with this, was the finding that CB1R−/− and CB1R−/−/CB2R−/−, but not CB2R−/− embryos, were trapped in the oviduct (Wang et al., 2006). Furthermore, it has been shown that the number of uterine implantation sites and oviductal embryo transport in CB2R−/− pregnant female mice was comparable to that of wild-type pregnant female mice (Wang et al., 2006). Interestingly, embryonic development in CB2R−/− mice is retarded (Paria et al., 2001). It was found that on day 3 of pregnancy, only 41.5% of CB2R−/− embryos were at the eight-cell stage as compared to the 84% wild-type embryos. There were 24% CB1R−/− embryos at the eight-cell stage. On day 4 of pregnancy, 71.3% CB2R−/− embryos were blastocysts and 25.7% morulas compared to the 97.5% wild-type blastocysts and 2.5% morulas. There were 61.5% CB1R−/− embryos at the blastocyst stage and 37% at the morula stage (Paria et al., 2001). However, CB2R−/− mice breeding pairs have normal litter sizes (Buckley et al., 2000), suggesting that the asynchronous CB2R−/− embryonic development at days 3 and 4 of pregnancy does not hinder embryonic implantation at day 4 or 5. Taken together, these findings indicate that CB1R, and not CB2R, is mostly responsible for successful embryonic implantation, and that both receptors have a role in synchronizing embryonic development. The function of CB2R in the embryonic stem cells is unknown, but may implicate this receptor in specifying pluripotent inner cell mass cell lineage during blastocyst formation (Paria et al., 2001; Wang et al., 2006).
CB2R−/− mice and disease models
CB2R−/− mice and bone loss
Cannabinoid receptors have been implicated in bone mass, bone loss and osteoclast activity (Idris et al., 2005; Ofeck et al., 2006). Unregulated osteoclast (bone-resorbing cells) or osteoblast (bone-forming cells) activity can cause bone loss, resulting in diseases such as osteoporosis (Helfrich, 2003). The involvement of CB1R on bone mass has been reported previously (Idris et al., 2005). CB2R is expressed in osteoblasts, osteocytes and osteoclasts (Ofeck et al., 2006). Osteoclasts are derived from cells in the myeloid lineage (Boyle et al., 2003; Xing et al., 2005). Osteoblasts, the precursors of osteocytes, are derived from precursor cells in the stromal element of bone marrow (Rickard et al., 1996). CB2R−/− mice showed accelerated age-related trabecular bone loss and cortical expansion, although cortical thickness remained the same. CB2R−/− osteoclast number and trabecular osteoblast activity were increased. However, there was a significant decrease in the number of diaphyseal osteoblast precursors. In wild-type mice, the CB2R-specific agonist HU-308 enhanced endocortical osteoblast number and activity and restrained trabecular osteoclastogenesis, apparently by inhibiting proliferation of osteoclast precursors. These findings suggest that CB2R has a role in bone homeostasis and that this receptor is a potential drug target for the treatment of osteoporosis (Ofeck et al., 2006).
CB2R−/− mice and liver disorders
The CB2R has been implicated to have an antifibrogenic role in the liver (Julien et al., 2005). Liver fibrosis occurs due to chronic liver injury that can eventually lead to cirrhosis and its complications. Julien et al. (2005) found that while normal liver does not express CB2R, human cirrhotic liver does. In this study, it was found that CB2R activation had potent antifibrogenic effects, including hepatic myofibroblast growth inhibition and increased apoptosis. Furthermore, when liver fibrosis was induced in wild-type and CB2R−/− mice using carbon tetrachloride (CCl4), they found that CB2R−/− mice developed enhanced liver fibrosis compared to their wild-type counterparts. That is, they observed a higher collagen deposition as determined by histological examination and hydroxyproline measurement. In addition, smooth muscle α-actin mRNA (α-SMA) was higher for CCl4-treated CB2R−/− mice compared to CCl4-treated wild-type or vehicle-treated CB2R−/− mice (Julien et al., 2005). Hepatocyte proliferation was also impaired in CB2R−/− mice compared to wild-type mice (Deveaux et al., 2007). These findings provide evidence for a protective role of CB2R in liver injury.
The CB2R also seems to play a protective role in liver ischaemia/reperfusion (I/R) injury (Batkai et al., 2007). The I/R model of organ ischaemia followed by reperfusion mimics events that may develop in common disease such as myocardial infarction and stroke, coronary bypass surgery and organ transplantation. In the I/R model, liver ischaemia was induced for 60 min by clamping the hepatic artery and portal vein. Reperfusion was then allowed for 90 min or 24 h (Batkai et al., 2007). Liver damage to I/R was greater in CB2R−/− mice compared to wild-type mice as determined by increased serum transaminase AST/ALT and myeloperoxidase activities, malondialdehyde formation as an indicator or lipid peroxidation, and an increase in proinflammatory cytokines and chemokines. Histological examination also revealed much more extensive liver injury and neutrophil infiltration in the liver from CB2R−/− mice than that from wild-type mice. Interestingly, the hepatic levels of the endocannabinoids anandamide and 2-arachidonoylglycerol (2-AG) were also substantially elevated upon I/R (Batkai et al., 2007).
Taken together, the findings on the involvement of CB2R in the liver fibrosis model and in the I/R model implicate the CB2R in the liver repair mechanism.
CB2R−/− mice and pain
It is widely recognized that cannabinoids reduce pain in humans and animals. This effect by cannabinoids is thought to be largely mediated by the CB1R. More recently, however, the implication of CB2R in pain modulation is being recognized. CB2R has been shown to modulate acute pain, chronic inflammatory pain, post-surgical pain, cancer pain and pain associated with nerve injury (reviewed in Whiteside et al., 2007). The CB2R agonist, AM1241, has been shown to exert its antinociceptive effects locally without producing CNS effects (Malan et al., 2001). Furthermore, using three different assays of nociception, it was demonstrated that CB2R−/− mice have a lower threshold of pain compared to wild-type mice in the presence of cannabinoids. This was determined using the hot-plate, paw-withdrawal and tail-flick assays. While the hot-plate assay requires supraspinal responses, the paw-withdrawal and tail-flick assays require spinal reflexes. Thus, pain induced by these methods can be inhibited by drugs acting at these peripheral sites. In the absence of cannabinoids, using the Hargreaves' method (Hargreaves et al., 1988), the paw-withdrawal assay showed reduced latency in CB2R−/− mice compared their wild-type counterparts. Taken together, these findings provide evidence that CB2R is involved in acute nociception, and thus, is a likely target to treat acute pain (Ibrahim et al., 2006).
CB2R−/− mice and experimental autoimmune encephalomyelitis, a multiple sclerosis model
Cannabinoids have been shown to alleviate spasticity associated with experimental autoimmune encephalomyelitis (EAE) in rodents (Baker et al., 2000; Pryce and Baker, 2007). To determine whether CB2R is involved in the regulation of autoimmunity, EAE was induced in wild-type and CB2R−/− mice on a B10.PL background (Maresz et al., 2007). It was found that CB2R−/− mice exhibited a higher incidence of disease, a significantly increased clinical score and a reduced recovery rate than their wild-type counterparts. Furthermore, when EAE was induced in wild-type mice with CB2R−/− encephalitogenic T cells by adoptive transfer, there was a more severe clinical disease. The disease was characterized by a higher mortality rate and the presence of increased numbers of infiltrating mononuclear cells. Examination of the lesions revealed that there was an increase in the number of CB2R−/− T cells in and around the lesions when EAE was induced with CB2R−/− T cells as compared to that induced with wild-type T cells. This increase in T-cell number in the CNS was due to more proliferation and decreased apoptosis of the CB2R−/− T cells. Furthermore, the CNS CB2R−/− encephalitogenic T cells secreted more proinflammatory cytokines than the wild-type encephalitogenic T cells. This explains the more severe disease in mice receiving the CB2R−/− T cells (Maresz et al., 2007). It is known that endocannabinoids are synthesized in the CNS (Salzet et al., 2000) and that endocannabinoids have immune suppressive effects such as induction of apoptosis and inhibition of lymphocyte proliferation (Salzet et al., 2000; Klein et al., 2003), thus these findings provide evidence that the CNS actively suppresses T-cell function through the CB2R (Maresz et al., 2007).
CB2R−/− mice and allergic dermatitis
A model of cutaneous contact dermatitis was used to study the allergic response in mutant mice. It was found that, compared to wild-type mice, CB1R−/−, CB2R−/− and CB1R−/−/CB2R−/− mice exhibit enhanced allergic responses to 2,4-dinitrofluorobenzene (DNFB) (Karsak et al., 2007). In this model, mice were sensitized with DNFB on the shaved abdomen. On days 5, 13 and 21, the mice were challenged with DNFB on the right ear. Swelling was then measured on the right ear and compared to the left ear within the same animal. While swelling was increased in the right ear of wild-type mice, swelling was significantly higher in CB1R−/−, CB2R−/− and CB1R−/−/CB2R−/− ears. Furthermore, upon injection of the CB1R and CB2R antagonists, SR141716A and SR144528, respectively, the swelling was enhanced in wild-type animals. Swelling, however, was decreased by THC, but enhanced by HU-308. Moreover, the levels of the endogenous cannabinoids 2-AG and anandamide were elevated in CB1R−/−/CB2R−/− mice (Karsak et al., 2007). Interestingly, using a slightly different approach to induce a cutaneous reaction, another group reported a decrease, not an increase in the ear swelling of CB2R−/− mice. These investigators passively sensitized the mice by i.v. injection of 1 μg of monoclonal anti-dinitrophenol IgE followed by a topical challenge with DNFB (Ueda et al., 2007). Furthermore, this group reported that oral administration of SR144528 suppressed ear swelling (Ueda et al., 2005, 2007). Although both groups find that the CB2R is involved in the cutaneous allergic response, their findings, using the CB2R−/− mice as well as the CB2R antagonist, are opposite. While Karsak et al. (2007) implicate the endocannabinoid system in the attenuation of the inflammatory response, Ueda et al. (2005, 2007) propose that CB2R participate in the induction of the cutaneous reaction. The discrepancy in their findings, and hence conclusions, may be due to the different approaches used to induce the allergic response in wild-type and CB2R−/− mice, and in delivering the SR144528 compound.
CB1R−/−/CB2R−/− and CB2R−/− mice in hypotension
Prolonged and profound hypotension is associated with diverse forms of shock, such as haemorrhagic (Wagner et al., 1997), endotoxaemic (Varga et al., 1998) and cardiogenic (Wagner et al., 2001) shock and the hypotension occurring in advanced liver cirrhosis (Batkai et al., 2001). THC, anandamide and 2-AG are known to cause long-lasting hypotension and bradycardia in most animal models (Benowitz and Jones, 1975; Varga et al., 1995; Jarai et al., 2000; Cohen et al., 2002). The cannabinoids anandamide and abnormal cannabidiol are known to induce vasodilation of mesenteric arteries in wild-type and CB1R−/−/CB2R−/− mice. This vasodilation is inhibited by SR141716A, but not by other CB1R antagonists (Jarai et al., 1999). A newly discovered endocannabinoid-like molecule, N-arachidonoyl-L-serine (ARA-S) has also been shown to produce endothelium-dependent vasodilation that is not reversed by SR141716 nor by SR144528. ARA-S binds very weakly to CB1R and does not bind to CB2R. ARA-S decreases LPS-induced plasma TNF-α levels in wild-type, CB1R−/−/CB2R−/− and CB2R−/− mice (Milman et al., 2006). This LPS-induced hypotension and cardiac contractility were prevented by SR141716. However, LPS-induced hypotension and its inhibition by SR141726 were similar in wild-type, CB1R−/− and CB1R−/−/CB2R−/− mice (Batkai et al., 2004). These findings prove that these cannabinoids cause hypotension via a non-CB1R/CB2R mechanism.
CB2R−/− mice and atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the large arteries, and is the primary cause of heart disease and stroke in Western countries (Libby, 2002). It has been found that oral administration of THC significantly reduced the progression of atherosclerosis in the apolipoprotein mouse model (ApoE−/−), an animal model for atherosclerosis. The CB2R antagonist SR144528 reversed the effect of THC, suggesting the involvement of the CB2R. Splenocytes derived from ApoE−/− mice treated with THC showed reduced concanavalin A (Con A)-induced proliferation and IFN-γ secretion. THC was also found to decrease ApoE−/− and wild-type thioglycollate-induced macrophage migration in response to the monocyte chemoattractant protein-1, but not the migration of CB2R−/− macrophages (Steffens et al., 2005). Apoptosis of macrophages is an important event in the pathophysiology of atherosclerosis (Liu et al., 2005). Oxidized low-density lipoproteins (OxLDL) are a major lipid component of atherosclerotic lesions and endocytosis of OxLDL is a potent inducer of apoptosis in cultured macrophages (Reid et al., 1993; Hardwick et al., 1996). Several macrophage processes associated with ongoing atherogenesis are regulated by CB2R (Zhu et al., 1998; Maccarrone and Finazzi-Agro, 2003; Steffens et al., 2005). It has been found that wild-type peritoneal macrophages treated with OxLDL and its cholesterol derivative 7-ketocholesterol readily undergo apoptosis. OxLDL- and 7-ketocholesterol-induced apoptosis in CB2R−/− peritoneal macrophages was significantly decreased compared to wild-type macrophages. Staurosporine-induced apoptosis was similar in wild-type and CB2R−/− peritoneal macrophages, indicating that apoptotic mechanism are intact in the CB2R−/− macrophages. These findings suggest that CB2R influences the development and progression of atherosclerotic lesions by mediating the apoptotic response of macrophages to OxLDL (Thewke et al., 2007). Taken together, these observations suggest that drugs targeting the CB2R may be valuable tools to treat atherosclerosis.
CB2R and macrophage chemotaxis
A crucial event occurring early in an inflammatory response is the migration of macrophages towards the chemostimulant. It has been found that peritoneal macrophage response to the chemokine RANTES/CCL5 is significantly inhibited by THC, CP55,940 and by the CB2R-specific agonist O-2137, but not by the CB1R agonist, ACEA. Moreover, the inhibition by THC was reversed by SR144528 but not by SR141716. THC treatment had a minimal effect on the chemotactic response of CB2R−/− peritoneal macrophages (Raborn et al., 2007). These findings implicate the CB2R in the modulation of macrophage migration in response to chemoattractants.
CB2R−/− mice and infectious models
CB2R−/− mice and bacterial infections
Δ-9-Tetrahydrocannabinol treatment of mice has been shown to suppress the immune response to Legionella pneumophila, an intracellular bacterium that causes Legionnaires' disease. In this model, THC suppresses Th1 immunity while enhancing Th2 response (Newton et al., 1994; Klein et al., 2000). The Th2-biasing effect of THC involves cannabinoid receptors, suppression of IL-12 and IL-12 receptor and IFN-γ along with an increase in the Th2-biasing transcription factor GATA 3 (Klein et al., 2004). Dendritic cells are potent antigen-presenting cells that upon maturation produce co-stimulatory molecules and cytokines such as IL-12 that bias helper T cells towards Th1 immunity (Kapsenberg, 2003). Since THC treatment significantly suppressed IL-12 serum levels in infected mice, dendritic cells were isolated from wild-type, CB1R−/− and CB2R−/− mouse bone marrow, they were infected in vitro with L. pneumophila and treated with THC. THC was able to suppress IL12p40 production in dendritic cells derived from all three mice genotypes. When SR141716 was used on CB2R−/− dendritic cells and SR144528 on CB1R−/− dendritic cells, the antagonists only partially attenuated the THC-induced suppression of IL-12 production by the cells. These findings suggest partial participation of CB1R and CB2R in the dendritic response to THC in this infectious model (Lu et al., 2006).
CB2R−/− mice and parasitic infections
Infection of wild-type mice with the Plasmodium berghei is a model for cerebral malaria. This infection leads to 100% mortality after 6–7 days of infection. It was found that CB2R−/− mice are resistant to cerebral disease after being infected with this parasite. Infection leads to comparable microglial migration in the CNS of wild-type and CB2R−/− mice. However, there is an increase in the number of CD11b+ cells in spleens of infected CB2R−/− mice compared to infected wild-type mice. The reason for the resistance of CB2R−/− mice to cerebral malaria is still unknown (Alferink et al., 2007).
CB2R−/− mice, lymphocyte proliferation and cytokine production
It is known that cannabinoids and cannabinoid receptors modulate lymphocyte proliferation and cytokine production (reviewed by Klein et al., 2003; Massi et al., 2006). To investigate whether the endogenous cannabinoid 2-AG modulates cytokine production via CB1R and CB2R, Ouyang, Kaplan and colleagues used the CB1R−/−/CB2R−/− double knockout mice. They found that 2-AG suppressed interleukin-2 (IL-2) and IFN-γ production in phorbol myristate 13-acetate/ionomycin (PMA/Io)-treated wild-type mouse splenocytes (Ouyang et al., 1998; Kaplan et al., 2005). In addition, 2-AG suppressed IFN-γ production in splenocytes derived from CB1R−/−/CB2R−/− mice (Kaplan et al., 2005). 2-AG ether, a nonhydrolysable analogue of 2-AG, also suppressed IL-2 expression in wild-type and CB1R−/−/CB2R−/− splenocytes (Rockwell et al., 2006). Our findings are consistent with these. We isolated splenocytes and CD4+ T cells from wild-type and CB2R−/− mice, and treated them in vitro with the T-cell mitogen Con A or with anti-CD3 and anti-CD28 antibodies. The cells were cultured in the presence or absence of 2-AG or WIN 55,212-2. We found that 2-AG and WIN 55,212-2 inhibited the secretion of IL-2 and IFN-γ in wild-type and CB2R−/− splenocytes stimulated with Con A (Figures 1 and 2). Furthermore, WIN 55,212-2 inhibited the secretion of IL-2 and IFN-γ in wild-type and CB2R−/− and CD4+ T cells stimulated with anti-CD3 and anti-CD28 (Table 1). However, WIN 55,212-2 did not alter the secretion of TGF-β in wild-type or CB2R−/− splenocytes and CD4+ T cells (data not shown). In addition, 2-AG and WIN 55,212-2 did not alter proliferation in wild-type or CB2R−/− splenocytes stimulated with Con A. However, WIN 55,212-2 inhibited proliferation in wild-type and CB2R−/− CD4+ T cells stimulated with anti-CD3 and anti-CD28 antibodies. This suggests that the effect of WIN 55,212-2 on cell proliferation may be responsible for decrease in cytokine production seen with this cannabinoid (Buranapramest, 2006). Taken together, these findings suggest that CB1R and CB2R are not involved in the inhibition of splenocyte proliferation and IL-2 and IFN-γ secretion by 2-AG and WIN 55,212-2, respectively.
Figure 1.
Cannabinoids inhibited the secretion of interleukin-2 (IL-2) in CB2R+/+ and CB2R−/− splenocytes stimulated with concanavalin A (Con A). CB2R+/+ or CB2R−/− splenocytes (1 × 106 cells per ml per well) were stimulated with Con A (2.5 μg ml−1) and treated with the indicated concentrations of 2-arachidonoylglycerol (2-AG) (a) or WIN 55,212-2 (b). IL-2 secretion levels were determined from 72-h cell culture supernatants by ELISA. Data are expressed as the mean of triplicate samples ±s.d. and are representative of three independent experiments. *Significantly different from untreated control, P<0.05.
Figure 2.
Cannabinoid inhibited the secretion of interferon-γ (IFN-γ) in CB2R+/+ and CB2R−/− splenocytes stimulated with concanavalin A (Con A). CB2R+/+ or CB2R−/− splenocytes (1 × 106 cells per ml per well) were stimulated with Con A (2.5 μg ml−1) and treated with the indicated concentrations of 2-arachidonoylglycerol (2-AG) (a) or WIN 55,212-2 (b). IFN-γ secretion levels were determined from 72-h cell culture supernatants by ELISA. Data are expressed as the mean of triplicate samples ±s.d. and are representative of three independent experiments. *Significantly different from untreated control, P<0.05.
Table 1.
IL-2 and IFN-γ secretion in anti-CD3 and anti-CD28 stimulated CD4+ cells treated with WIN 55,212-2
| WIN 55,212-2 (nM) | CB2R+/+ cells, IL-2 (pg ml−1±s.d.) | CB2R−/− cells, IL-2 (pg ml−1±s.d.) | CB2R+/+ cells, IFN-γ (pg ml−1±s.d.) | CB2R−/− cells, IFN-γ (pg ml−1±s.d.) |
|---|---|---|---|---|
| 0 | 49.17±9.42 | 158.20±52.84 | 134.50±17.05 | 139.00±27.02 |
| 3 | 47.64±5.20 | 115.60±90.35 | 133.50±32.23 | 124.40±42.46 |
| 10 | 22.37±15.19 | 77.04±21.67 | 87.54±6.35* | 80.20±2.86* |
| 31 | 14.22±2.42* | 30.72±9.90* | 110.40±23.10 | 101.50±13.88 |
| 100 | 38.98±27.10 | 64.98±11.11 | 122.20±5.86 | 123.80±24.58 |
| 316 | 48.92±45.70 | 72.78±53.71 | 93.67±18.04 | 96.37±13.11 |
| 1000 | 7.65±1.87* | 45.38±0.60* | 96.85±12.90 | 88.89±15.90 |
| 3162 | 13.10±8.50* | 18.75±5.36* | 91.21±5.21* | 82.98±10.50* |
Abbreviations: IFN-γ, interferon-γ; IL-2, interleukin-2.
Purified total T cells or CD4+ T cells were isolated by negative selection using the Pan T Cell Isolation Kit or CD4+ T cell Isolation Kit, respectively (Miltenyi Biotech, Auburn, CA, USA) from spleens. CB2R+/+ or CB2R−/− CD4+ T cells (1 × 105 cells per 0.1 ml per well) were stimulated with anti-CD3 (5 μg ml−1) and anti-CD28 (0.5 μg ml−1) antibodies and treated with the indicated concentrations of WIN 55,212-2. IL-2 and IFN-γ secretion levels were determined from 72-h cell culture supernatants by ELISA. Data are expressed as the mean of triplicate samples ±s.d. and are representative of three independent experiments. *Significantly different from untreated control, P<0.05.
CB2R−/− mouse cells and intracellular Ca2+ levels
The CB1R−/−/CB2R−/− double knockout mice have also been used to show that the effects of cannabinoids on intracellular calcium increase is not dependent on CB1R and CB2R. Rao and Kaminski (2006) found that THC (12.5 μM), cannabinol (20 μM) and HU-210 (20 μM) induced a rise in intracellular calcium in wild-type and CB1R−/−/CB2R−/− splenocytes. Interestingly, SR141716A and SR144528 (1 μM) attenuated the rise in calcium elicited by the cannabinoids. The partial attenuation of the cannabinoid-induced calcium increase in wild-type and CB1R−/−/CB2R−/− splenocytes, suggest that the antagonists used may be acting via mechanism distinct from those involving CB1R and CB2R.
CB2R−/− mice and drug specificity
Recently, the CB2R−/− mouse has been used to test drug specificity. The specificity of GW405833 for CB2R has been tested using this mutant mouse. Inflammatory hyperalgesia was induced in wild-type or CB2R−/− mice by an intraplanar injection of Freund's complete adjuvant and 24 h later GW405833 was given intraperitoneally. Tactile allodynia was developed by wild-type and CB2R−/− mice in response to the Freund's complete adjuvant injection. While GW405833 was able to reverse this effect in wild-type mice, it did not produce a reduction in tactile allodynia in the CB2R−/− mice (Valenzano et al., 2005; Whiteside et al., 2005). Furthermore, it was shown that the central effects of high dose of GW405833 were not mediated by CB2R as determined by carrying out the hot-plate, tail-flick and rotarod tests in CB2R−/− mice and finding similar responses to those found in wild-type mice (Whiteside et al., 2005). These findings show that GW405833 is an effective drug that, at low doses, targets the CB2R.
The specificity of AM1241 and HU-308 in in vivo LPS-induced TNF-α and IL-10 production has also been tested using the CB2R−/− mouse. AM 1241 (50 mg kg−1, i.p.) and HU-308 (30 mg kg−1, i.p.) inhibited LPS-induced TNF-α production. To test if this effect was mediated by the CB2R, AM1241 and HU-308 were given to wild-type and CB2R−/− mice. An hour later, mice were given LPS (0.15 mg kg−1, i.p.). It was found that plasma TNF-α was significantly inhibited and IL-10 significantly elevated in wild-type and CB2R−/− mice in response to these agonists. These findings indicate that in vivo administration of AM1241 and HU-308 alter LPS-induced TNF-α and IL-10 production independent of the presence of CB2R (Huang et al., 2007). However, another study revealed that some LPS-induced physiological events are mediated by the CB2R. Recently, Duncan et al. (2007) have found that while wild-type animals are able to mount a febrile response to an in vivo LPS (100 μg kg−1, i.p.) challenge, CB2R−/− mice do not (Duncan et al., 2007).
Thus, it can be concluded that the CB2R−/− mouse model is a very useful tool in drug discovery.
CB2R−/− mice and antibody specificity
Tissues from the CB2R−/− mice have been used to test the specificity of antibodies raised against the CB2R. The expression of CB2R protein in wild-type and CB2R−/− mice has been studied by immunohistochemistry in diverse tissues.
Although several laboratories failed to detect CB2R in the brain (Derocq et al., 1995; Galiegue et al., 1995; Schatz et al., 1997; Carlisle et al., 2002; Ibrahim et al., 2003, 2006), recent studies have revealed the presence of this receptor in diverse brain regions. Using polyclonal antibodies generated against the C terminus of CB2R from Alpha Diagnostics (San Antonio, TX, USA), Van Sickle et al. (2005) reported the expression of CB2R protein in neurons of the brainstem from wild-type animals. To test the specificity of their antibody, they carried out immunostaining on the dorsal motor nucleus of the vagus from wild-type and CB2R−/− mice. While they found CB2R expression in the wild-type mice, there was no immunostaining in CB2R−/− mice (Van Sickle et al., 2005). Using rabbit anti-human CB2R polyclonal antibody (Cayman Chemicals, Ann Arbor, MI, USA and Sigma, St Louis, MO, USA), Onaivi, Gong and colleagues have found CB2R widely expressed in the brain. Unfortunately, the specificity of the Cayman anti-CB2R antibody was not tested using the CB2R−/− brain. Instead, using a different antibody, one raised against the C terminus of CB2R (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), they showed that while CB2R expression was found in the interpolar part of spinal 5th nucleus of the wild-type mouse brain, CB2R expression was not found in the same brain region of CB2R−/− mice (Gong et al., 2006; Onaivi, 2006; Onaivi et al., 2006). Coincident with these data is the observation that CB2R is present in neural progenitors from late embryonic stages to adult brain. Moreover, activation of the CB2R in vitro promoted neural progenitor cell proliferation, this was not seen in CB2R−/− cells. In addition, in vivo treatment with HU-308 increased hippocampal progenitor proliferation in wild-type but not in CB2R−/− mice (Palazuelos et al., 2006). On the other hand, Wotherspoon et al. (2005) have not been able to find CB2R expression in sections of naive mouse dorsal root ganglia or spinal cord using the anti-CB2R antibody from Cayman. Although CB2R immunoreactivity was seen following unilateral nerve damage, and was localized to the superficial laminae of the dorsal horn of the spinal cord, ipsilateral to the nerve damage. Antibody specificity was confirmed in CB2R−/− spinal cord and spleen sections.
Using the anti-CB2R antibody from Cayman, expression of CB2R was also found in mouse atherosclerotic plaques (Steffens et al., 2005) and in osteoblasts, osteocytes and osteoclasts (Ofeck et al., 2006) of wild-type mice. CB2R antibody specificity was determined by using spleens and bone from CB2R−/− mice.
When obtaining data using the currently available polyclonal anti-CB2R antibodies, one should be cautious. At the moment, there are no CB2R-specific monoclonal antibodies available. It must therefore be recognized that the fine specificity of such commercial polyclonal antisera may change over time, depending on the bleed and individual animals being immunized during antibody production. Thus, tissues derived from CB2R−/− mice will continue to provide a very useful tool to elucidate the specificity of current and future anti-CB2R antibodies.
Conclusion
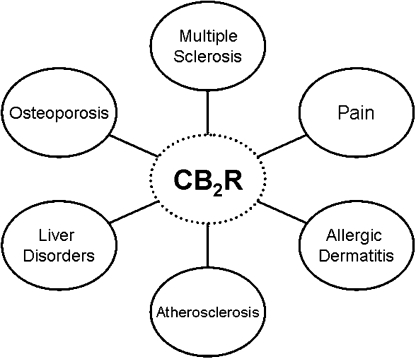
The reports reviewed here emphasize the usefulness of the CB2R−/− mouse. Using this mutant mouse, investigators have discovered, or confirmed, CB2R tissue and/or cellular expression under normal or abnormal conditions. Because the CB2R was originally cloned and reported to be present in immune cells (Munro et al., 1993), it is not surprising that the CB2R is found in cells of the haematopoietic lineage. The presence of this receptor in preimplantation embryonic stem cells (Paria et al., 1995) is very interesting and may implicate this receptor in the early development of haematopoietic cells and perhaps other cells. We found that in the embryonic rat, CB2R was expressed in the liver in cells that appeared to be Kuffer cells (Buckley et al., 1998). The CB2R has also been shown to be expressed in other normal adult cells. These include cells within the CNS (Van Sickle et al., 2005; Gong et al., 2006; Onaivi, 2006; Onaivi et al., 2006) and bone (Ofeck et al., 2006). In other instances, however, CB2R expression is only evident under certain disease conditions. Thus, CB2R expression is upregulated in pain models (Wotherspoon et al., 2005), atherosclerosis (Steffens et al., 2005), liver disorders (Julien et al., 2005) and during inflammation (Maresz et al., 2007) (Figure 3). On the basis of the findings using the CB2R−/− mouse, investigators have speculated as to the function of this receptor. CB2R activation is thought to be antinociceptive and anti-inflammatory. It is also thought to be involved in bone homeostasis and in protective mechanisms during atherosclerosis and liver injury. On the other hand, using this mutant mouse, investigators have found that the CB2R is not involved in hypotension. The implication of CB2R in so many events lends validity to the notion that CB2R is biologically functionally relevant. Nevertheless, living organisms are complex and one can reason that CB2R and its endocannabinoid ligands do not work alone and other important molecular players are equally necessary. Finally, the CB2R−/− mouse has been a very useful tool to help determine drug and antibody specificity and promises to continue to be very useful in drug and antibody discovery.
Figure 3.
Using the CB2R−/− mice, the CB2R has been implicated in diverse diseases.
Acknowledgments
The data presented in the figures and tables were possible through funding by NIH GM53933.
Abbreviations
- 2-AG
2-arachidonoylglycerol, endogenous cannabinoid
- AM1241
synthetic cannabinoid, CB2R selective agonist
- SR141716
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride, synthetic cannabinoid, CB1R selective antagonist
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicycle [2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide, synthetic cannabinoid, CB2R selective antagonist
- THC
Δ-9-Tetrahydrocannabinol
- WIN 55,212-2
(R)-[2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl](1-naphthalenyl)methanone, synthetic cannabinoid agonist
Conflict of interest
The author states no conflict of interest.
References
- Alferink J, Specht S, Arends H, Cron M, Poppensieker K, Maier W, et al. Resistance to cerebral malaria in CB2−/− mice 2007. Paper presented at CB2 Cannabinoid Receptors, Banff, Alberta, Canada
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Jarai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol. 2004;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther. 1975;18:287–297. doi: 10.1002/cpt1975183287. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, et al. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Buckley NE, Hanson S, Harta G, Mezey E. Expression of the CB1 and CB2 receptor mRNAs during embryonic development in the rat. Neuroscience. 1998;82:1131–1149. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Buranapramest M.The role of the peripheral cannabinoid receptor in modulating CD4+ T-cell function 2006. Master's thesis, California State University, Pomona
- Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–3615. [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral A, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Onaivi ES, Chaudhuri G. Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq. 1995;5:385–388. doi: 10.3109/10425179509020870. [DOI] [PubMed] [Google Scholar]
- Chuchawankul S, Shima M, Buckley NE, Hartman CB, McCoy KL. Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int Immunopharmacol. 2004;4:265–278. doi: 10.1016/j.intimp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Das SK, Paria BC, Chakraborty L, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci USA. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derocq J-M, Segui M, Marchand J, Le Fur G, Casellas P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995;369:177–182. doi: 10.1016/0014-5793(95)00746-v. [DOI] [PubMed] [Google Scholar]
- Deveaux V, Teixeira-Clerk F, Ichigotani Y, Manin S, Tran Van Nhiew J, Karsak M, et al. Role of CB2 receptors in hepatic wound healing 2007. Paper presented at CB2 Cannabinoid Receptors: New Vistas, Banff, Alberta, Canada
- Duncan M, Ho W, Buckley NE, Sharkey KA, Pittman QJ.CB2 receptors may play a role in fever induced by the endotoxin lipopolysaccharide 2007. Paper presented at CB2 Cannabinoid Receptors: New Vistas, Banff, Alberta, Canada
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Hardwick SJ, Hegyi L, Clare K, Law NS, Carpenter KL, Mitchinson MJ, et al. Apoptosis in human monocyte–macrophages exposed to oxidized low density lipoprotein. J Pathol. 1996;179:294–302. doi: 10.1002/(SICI)1096-9896(199607)179:3<294::AID-PATH590>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown R, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Helfrich MH. Osteoclast diseases. Micosc Res Tech. 2003;61:514–532. doi: 10.1002/jemt.10375. [DOI] [PubMed] [Google Scholar]
- Huang M, Buckley NE, Huang L, Emkey R, Hoffman BJ, Fremeau RT.CB2 agonists regulate LPS-stimulated cytokine production in mice via a non-CB2 receptor mechanism 2007. Paper presented at CB2 Cannabinoid Receptors: New Vistas, Banff, Alberta, Canada
- Ibrahim MM, Deng H, Zvonok K, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the brain. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Idris AI, Van't Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Goparaju SK, Wang L, Razdan RK, Sugiura T, et al. Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension. 2000;35:679–684. doi: 10.1161/01.hyp.35.2.679. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerk F, Tran Van Nhiew J, Li L, Karsak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Kaplan BLF, Ouyang Y, Rockwell CE, Rao GK, Kaminski NE. 2-Arachidonoyl-glycerol suppresses interferon-γ production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2. J Leukoc Biol. 2005;77:966–974. doi: 10.1189/jlb.1104652. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Karsak M, Gaffal E, Date R, Wang-Eckardt L, Rehnelt J, Petrosino S, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Larsen K, Chou J, Perkins I, Lu L, et al. Cannabinoid receptors and T helper cells. J Neuroimmunol. 2004;147:91–94. doi: 10.1016/j.jneuroim.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Nakachi N, Friedman H. Delta-9-tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12 and IL-12 receptor beta 2 responses to Legionella pneumophila infection. J Immunol. 2000;16:6461–6466. doi: 10.4049/jimmunol.164.12.6461. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Liu J, Thewke D, Su YR, Linton MF, Fazio S, Sinenski MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Newton C, Perkins I, Friedman H, Klein T. Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow derived dendritic cells infected with Legionella pneumophila. Eur J Pharmacol. 2006;532:170–177. doi: 10.1016/j.ejphar.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Lynn AB, Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. The endocannabinoid system, anadamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003;10:946–955. doi: 10.1038/sj.cdd.4401284. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah TW. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford L, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Parolaro D. Cannabinoids, immune system and cytokine network. Curr Pharm Des. 2006;12:3135–3146. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- Matsuda L, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun. 1994;62:4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofeck O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinod receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann NY Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53:676–683. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonist stimulate neuroal progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand–receptor signaling. Proc Natl Acad Sci USA. 1995;92:9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HHO, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276:20523–20528. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- Petitet F, Donlan M, Michel A. GPR55 as a new cannabinoid receptor: still a long way to prove it. Chem Biol Drug Des. 2006;67:252–253. doi: 10.1111/j.1747-0285.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raborn ES, Marciano-Cabral A, Buckley NE, Martin BR, Cabral GA.The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor J NeuroImmune Pharmacol 2007. online version available at [DOI] [PMC free article] [PubMed]
- Rao GK, Kaminski NE. Cannabinoid-mediated elevation of intracellular calcium: a structure–activity relationship. J Pharmacol Exp Ther. 2006;317:820–829. doi: 10.1124/jpet.105.100503. [DOI] [PubMed] [Google Scholar]
- Reid VC, Mitchinson MJ, Skepper JN. Cytotoxicity of oxidized low-density lipoprotein to mouse peritoneal macrophages: an ultrastructural study. J Pathol. 1993;171:321–328. doi: 10.1002/path.1711710413. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, et al. Actions of cannabinoid receptor ligands on rat culture sensory neurons: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Salzet M, Breton C, Bisogno T, Di Marzo V. Comparative biology of the endocannabinod system and possible role in the immune response. Eur J Pharmacol. 2000;267:4917–4927. doi: 10.1046/j.1432-1327.2000.01550.x. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Shire D, Calandra B, Delpech M, Dumont X, Kaghad M, Le Fur G, et al. Structural features of the central cannabinoid CB1 receptor involved in the binding of the specific CB1 antagonist SR141716A. J Biol Chem. 1996a;271:6941–6946. doi: 10.1074/jbc.271.12.6941. [DOI] [PubMed] [Google Scholar]
- Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessegue B, Bonnin-Cabanne O, et al. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim Biophys Acta. 1996b;1307:132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Aenaud C, Pelli G, Burger F, Staub C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Nakajima K, Kishimoto S, Oka S.An endogenous ligand for GPR55, a G-protein-coupled receptor 2007. Paper presented at 17th Annual Symposium on the Cannabinoids, Saint-Sauveur, Quebec, International Cannabinoid Research Society, Burlington, Vermont
- Thewke D, Freeman-Anderson N, Buckley NE.Cannabinoid receptor (CB2) deficiency inhibits oxidized LDL/Oxysterol-induced apoptosis in macrophages 2007. Paper presented at Arteriosclerosis Thrombosis and Vascular Biology meeting
- Ueda Y, Miyagawa N, Matsui T, Kaya T, Iwamura H. Involvement of cannabinoid CB2 receptor-mediated response and efficacy of cannabinoid CB2 receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur J Pharmacol. 2005;520:164–171. doi: 10.1016/j.ejphar.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Miyagawa N, Wakitani K. Involvement of cannabinoid CB2 receptors in the IgE-mediated triphasic cutaneous reaction in mice. Life Sci. 2007;80:414–419. doi: 10.1016/j.lfs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee GP, Harrison JE, Boulet JM, Gottshall SL, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Valverde O, Karsak M, Zimmer A. Analysis of the endocannabinoid system by using CB1 cannabinoid receptor knockout mice. Handb Exp Pharmacol. 2005;168:117–145. doi: 10.1007/3-540-26573-2_4. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:270–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, et al. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol. 2001;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- Wang H, Huirong X, Dey SK. Endocannabinoid signaling directs periimplantation events. AAPS J. 2006;8:E425–E432. doi: 10.1007/BF02854916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, et al. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur J Pharmacol. 2005;528:65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 2007;14:917–936. doi: 10.2174/092986707780363023. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Paria B, Dey S. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biol Reprod. 1996;55:756–761. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]
- Zhu W, Friedman H, Klein TW. Delta-9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. J Pharmacol Exp Ther. 1998;286:1103–1109. [PubMed] [Google Scholar]
- Ziring D, Wei B, Velazquez P, Schrage M, Buckley NE, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]