Abstract
Endocannabinoids are endogenous ligands of brain-type (CB1) and spleen-type (CB2) cannabinoid receptors. N-Arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) are prototype members of the fatty acid amides and the monoacylglycerols, two groups of endocannabinoids. Unlike CB1, CB2 receptors do not reside within ‘caveolae', specialized membrane microdomains that are well-known modulators of the activity of a number of G protein-coupled receptors. In this issue of the British Journal of Pharmacology, Rimmerman and coworkers demonstrate that 2-AG is entirely localized in the caveolae of dorsal root ganglion cells, where also part of AEA (∼30%) can be detected. However, most of AEA (∼70%) was detected in non-caveolae fractions, that is where CB2 receptors are localized. The different interaction of AEA and 2-AG with membrane microdomains might have significant implications for endocannabinoid-dependent autocrine and/or retrograde-paracrine signalling pathways. It also raises an important question about the structural determinants responsible for a different localization of two apparently similar endocannabinoids within lipid bilayers.
Keywords: cannabinoids, caveolae, cholesterol, endocannabinoids, lipid rafts, signal transduction
Endocannabinoids are lipid signalling molecules that modulate several physiological processes. They are endogenous ligands of brain-type cannabinoid receptors (CB1) and spleen-type cannabinoid receptors (CB2), two G protein-coupled receptors that also bind Δ9-tetrahydrocannabinol, the psychoactive component of Cannabis sativa (Howlett et al., 2002). N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) are prototype members of two groups of endocannabinoids, the fatty acid amides and the monoacylglycerols respectively (Piomelli, 2003; Di Marzo and Petrosino, 2007).
The dependence of endocannabinoid signalling on ‘lipid rafts' (LRs) is an emerging concept (Barnett-Norris et al., 2005; Dainese et al., 2007). LRs are specialized membrane microdomains that are enriched in cholesterol, sphingolipids and arachidonic acid and that have a tightly packed state (Hanzal-Bayer and Hancock, 2007). LRs are well-known modulators of the activity of a number of G protein-coupled receptors, for which a raft domain provides a more organized platform for the proper assembly of signalling complexes, also preventing cross-talks between different pathways. CB1 receptors have been shown to reside within LRs (Bari et al., 2005), and consistently they co-localize with LR markers (Sarnataro et al., 2006). More recently, CB1 receptors have been shown to co-localize with caveolin-1, a marker of ‘caveolae' (Bari et al., 2007). These are considered a subclass of LRs represented by non-clathrin-coated and flask-shaped invaginations (diameter of ∼60–80 nm) in the plasma membrane. Unlike CB1, CB2 receptors do not reside within LRs or caveolae (Bari et al., 2006), as also shown by Rimmerman et al. (2008) in this issue of the British Journal of Pharmacology. These authors provide further information that seems to add a new player in the arena of the modulation of endocannabinoid signalling. In fact, they demonstrate by liquid chromatography/tandem mass spectrometry that 2-AG is concentrated in the caveolae of dorsal root ganglion cells where it co-localizes with components of the diacylglycerol pathway responsible for 2-AG production. Instead, AEA was detected in LRs and non-LR fractions to comparable levels (Rimmerman et al., 2008). Therefore, much similar cannabinoid receptors (CB1 versus CB2), their endogenous ligands (AEA versus 2-AG) show a different interaction with LRs, an observation that might have significant implications for endocannabinoid-dependent autocrine and/or retrograde-paracrine signalling. On one hand, the findings presented by Rimmerman et al. (2008) demonstrate that the CB1 receptor agonists 2-AG and, to a lesser extent, AEA are present where they should be in order to activate their target: next to it, so that they can easily reach the binding site by lateral diffusion. On the other, these new data call for a reconsideration of the general concept that 2-AG is the only true agonist of CB2 receptors whereas AEA (a weak and partial ligand for this receptor) does not have any physiological relevance (Sugiura et al., 2000). In fact, AEA might be localized more likely than 2-AG next to CB2 receptors in dorsal root ganglion cells and possibly in other cell types.
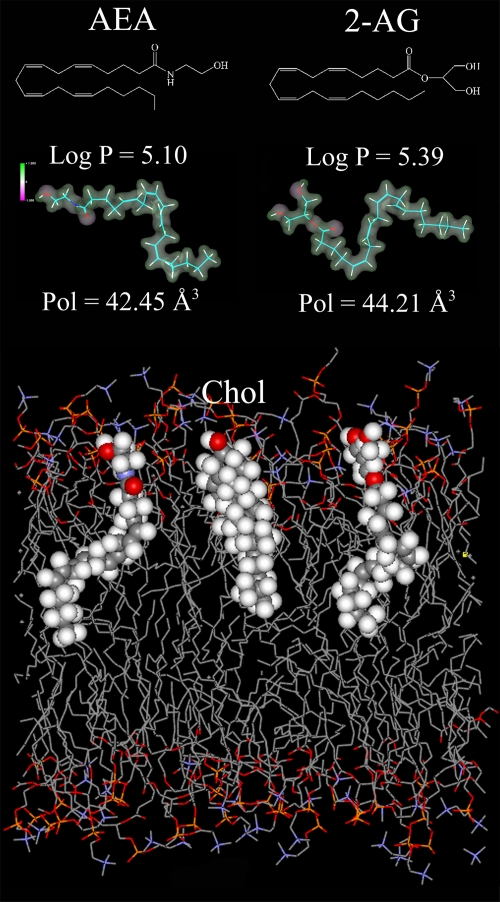
The paper by Rimmerman et al. (2008) raises another important question: ‘what are the structural determinants responsible for the different localization of AEA and 2-AG within lipid bilayers?' Like many other bioactive lipophilic molecules, endocannabinoids partition into membranes where they assume a thermodynamically favourable orientation and location. Quantitative structure–activity relationship (QSAR) studies have demonstrated that AEA and 2-AG share similar values of molecular descriptors like lipophilicity (expressed as logarithm of the octanol–water partition coefficient, log P), distribution of electrostatic potential and polarizability (Dainese et al., 2005). QSAR data were in accordance with the high flexibility of AEA observed by molecular dynamics simulations, which also showed that AEA embedded within the lipid bilayer tends to adopt a more extended conformation (Barnett-Norris et al., 2002). On the basis of the similarities of the molecular descriptors derived from QSAR analysis, it seems that 2-AG is embedded in the lipid bilayer in the same manner as AEA (Figure 1). Therefore, it is rather unexpected that these two endocannabinoids partition quite differently in raft and non-raft fractions. Maybe cholesterol, that is known to bind with AEA (Biswas et al., 2003), binds with 2-AG even better, presumably due to a better interaction with its acyl chain (Figure 1). As a consequence, cholesterol may favour the concentration of 2-AG within LRs. However, the study by Rimmerman et al. (2008) underlines the need for extensive investigations into the structural determinants that drive the interaction of apparently similar endocannabinoids with the surrounding lipid environment. Furthermore, the dependence of CB1 receptors on LRs integrity makes it challenging from the therapeutic point of view to selectively target CB1-dependent pathologies by means of LRs-oriented drugs. On a final note, the different interaction of AEA and 2-AG with raft and non-raft microdomains, along with the different localization of CB1 and CB2 receptors within these fractions, might represent a novel paradigm of ligand–receptor interactions whereby a third player comes into the game—the membrane lipids.
Figure 1.
AEA versus 2-AG. The chemical structures, lipophilicity (log P), electrostatic potential (violet −1; green +1) and polarizability (Pol) values of AEA and 2-AG are shown in the upper panel. Both log P and Pol values were calculated by means of the HyperChemTM 6.03 molecular modelling system (Hypercube, Inc., Gainesville, FL, USA), as reported (Dainese et al., 2005). A schematic representation of (from left to right) AEA, cholesterol (Chol) and 2-AG embedded within a dipalmitoylphosphatidilcholine bilayer is shown in the lower panel. Here, atoms in the space-filling models were coloured with the following codes: oxygen in red, nitrogen in violet, carbon in grey and hydrogen in light grey. The figure was kindly provided by Dr. Enrico Dainese (University of Teramo, Teramo, Italy).
Abbreviations
- AEA
N-arachidonoylethanolamine (anandamide)
- 2-AG
2-arachidonoylglycerol
- CB1
brain-type cannabinoid receptor
- CB2
spleen-type cannabinoid receptor
- LRs
lipid rafts
- QSAR
quantitative structure–activity relationship
References
- Bari M, Battista N, Fezza F, Finazzi-Agrò A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005;280:12212–12220. doi: 10.1074/jbc.M411642200. [DOI] [PubMed] [Google Scholar]
- Bari M, Oddi S, De Simone C, Spagnuolo P, Gasperi V, Battista N, et al. Type-1 cannabinoid receptors colocalize with caveolin-1 in neuronal cells Neuropharmacology 2007. in press [DOI] [PMC free article] [PubMed]
- Bari M, Spagnuolo P, Fezza F, Oddi S, Pasquariello N, Finazzi-Agrò A, et al. Effect of lipid rafts on CB2 receptor signaling and 2-arachidonoyl-glycerol metabolism in human immune cells. J Immunol. 2006;177:4971–4980. doi: 10.4049/jimmunol.177.8.4971. [DOI] [PubMed] [Google Scholar]
- Barnett-Norris J, Hurst DP, Lynch DL, Guarnieri F, Makriyannis A, Reggio PH. Conformational memories and the endocannabinoid binding site at the cannabinoid CB1 receptor. J Med Chem. 2002;45:3649–3659. doi: 10.1021/jm0200761. [DOI] [PubMed] [Google Scholar]
- Barnett-Norris J, Lynch D, Reggio PH. Lipids, lipid rafts and caveolae: their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci. 2005;77:1625–1639. doi: 10.1016/j.lfs.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Biswas KK, Sarker KP, Abeyama K, Kawahara K, Iino S, Otsubo Y, et al. Membrane cholesterol but not putative receptors mediates anandamide-induced hepatocyte apoptosis. Hepatology. 2003;38:1167–1177. doi: 10.1053/jhep.2003.50459. [DOI] [PubMed] [Google Scholar]
- Dainese E, Gasperi V, Maccarrone M. Partial QSAR analysis of some selected natural inhibitors of FAAH suggests a working hypothesis for the development of endocannabinoid-based drugs. Curr Drug Targets CNS Neurol Disord. 2005;4:709–714. doi: 10.2174/156800705774933096. [DOI] [PubMed] [Google Scholar]
- Dainese E, Oddi S, Bari M, Maccarrone M. Modulation of the endocannabinoid system by lipid rafts. Curr Med Chem. 2007;14:2202–2215. doi: 10.2174/092986707782023235. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–140. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Rimmerman N, Hughes HV, Bradshaw HB, Pazos MX, Mackie K, Prieto AL, et al. Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line Br J Pharmacol 2008153380–389.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnataro D, Pisanti S, Santoro A, Gazzerro P, Malfitano AM, Laezza C, et al. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid raft-mediated mechanism. Mol Pharmacol. 2006;70:1298–1306. doi: 10.1124/mol.106.025601. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, et al. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]