Abstract
The emerging potential for the cannabinoid (CB) system in modulating gastrointestinal inflammation has gained momentum over the last few years. Traditional and anecdotal use of marijuana for gastrointestinal disorders, such as diarrhoea and abdominal cramps is recognized, but the therapeutic benefit of cannabinoids in the 21st century is overshadowed by the psychoactive problems associated with CB1 receptor activation. However, the presence and function of the CB2 receptor in the GI tract, whilst not yet well characterized, holds great promise due to its immunomodulatory roles in inflammatory systems and its lack of psychotropic effects. This review of our current knowledge of CB2 receptors in the gastrointestinal tract highlights its role in regulating abnormal motility, modulating intestinal inflammation and limiting visceral sensitivity and pain. CB2 receptors represent a braking system and a pathophysiological mechanism for the resolution of inflammation and many of its symptoms. CB2 receptor activation therefore represents a very promising therapeutic target in gastrointestinal inflammatory states where there is immune activation and motility dysfunction.
Keywords: inflammatory bowel disease, colitis, cannabis, endocannabinoids, enteric nervous system, gastrointestinal motility, bowel cancer, visceral sensation
Introduction
It has been known for centuries that cannabis and its derivatives have beneficial effects on various disorders of the gastrointestinal (GI) tract (Di Carlo and Izzo, 2003). After the discovery of the two G-protein-coupled cannabinoid (CB) receptors, the majority of the actions of CB ligands in the gut were attributed to the activation of CB1 receptors. Notably, CB1 receptor activation leads to the inhibition of acetylcholine release through prejunctional receptors located on myenteric cholinergic neurones, which ultimately results in the inhibition of contractility in vitro or an attenuation of motility in vivo (Pertwee, 2001; Hornby and Prouty, 2004; Izzo and Coutts, 2005; Massa et al., 2005). Considerable effort has now established that these receptors are part of the endocannabinoid system in the GI tract. This consists of the natural ligands anandamide and 2-arachidonoyl glycerol (2-AG) and other receptors (Di Marzo et al., 2004), and the biosynthetic and degradative enzymes necessary for the formation and inactivation of the endocannabinoids (Piomelli, 2003; Di Marzo et al., 2004). Little evidence was found for either the presence or function of CB2 receptors in the normal gut, though as we shall describe, these receptors are indeed present and likely have roles to play in the regulation of GI motility.
The availability of animal models of intestinal inflammation has led to considerable progress in our understanding of these conditions, as well as for opportunities to examine the role of CB receptors. Here, it was found that CB1 receptor activation reduced GI inflammation in various animal models (Di Marzo and Izzo, 2006). Recently, a role for CB2 receptors has also emerged after important observations highlighting increased receptor expression in samples from patients with inflammatory bowel diseases (IBD; Wright et al., 2005).
Altered visceral perception and pain are commonly found in patients with IBD and may persist in those in remission from this condition (Farrokhyar et al., 2006). Pain is also one of the defining features of irritable bowel syndrome and other functional bowel disorders, which are conditions of altered bowel function and pain that are not associated with physical abnormalities of the gut wall (Azpiroz et al., 2007). Recent evidence has shed light on a role for CB2 receptors in pain states, particularly inflammatory pain models, including those originating from the gut. In this paper, we will focus on the regulatory role of CB2 receptors in the GI tract, highlighting their importance in pathophysiology.
Gastrointestinal endocannabinoid system
Cannabinoid receptors (CB1 and CB2), their endogenous ligands and enzymes for endocannabinoid biosynthesis and inactivation are the components of the endocannabinoid system and all have been detected in the GI system. Functions such as relaxation of the lower oesophageal sphincter and inhibition of gastric acid secretion, intestinal motility and fluid secretion have largely been attributed to the CB1 receptor and have been reviewed elsewhere (Hornby and Prouty, 2004; Duncan et al., 2005a; Izzo and Coutts, 2005; Massa et al., 2005). The concept that the endocannabinoid system in the small intestine and colon becomes overstimulated during inflammation in both animal models and human inflammatory disorders has recently been evaluated (Di Marzo and Izzo, 2006). There is good evidence that endocannabinoids and CB1 receptors are upregulated during intestinal inflammation (Izzo et al., 2001; D'Argenio et al., 2006) and that enhanced endocannabinoid production, acting mostly through CB1 receptors, can protect against both epithelial damage (Wright et al., 2005) and increased motility during intestinal inflammation (Izzo et al., 2003; Massa et al., 2004). In addition, increased endocannabinoids have also been reported in diverticular and coeliac disease (Guagnini et al., 2006; D'Argenio et al., 2007).
CB2 receptor distribution in the GI tract
Messenger RNA for the CB2 receptor has been isolated from full-wall thickness preparations of rat oesophagus and stomach (Storr et al., 2002) and the guinea-pig ileum (Griffin et al., 1997). Storr et al. (2002) were also able to identify CB2 receptor mRNA expression in dissected preparations of the rat ileum containing only longitudinal muscle with the adherent myenteric plexus (LMMP). Using a similar preparation from the guinea-pig, Griffin et al. (1997) were unable to detect CB2 mRNA. This may reflect a species difference, but it may also be due to tissue preparation, since these authors reported only a faint band for CB1 receptor in their LMMP preparations, that are known to express high levels of this receptor. Interestingly, Storr et al. (2002) were unable to detect CB2 mRNA in mucosal samples of the rat ileum. Given the abundance of immune cells in the normal gut, and the well-described presence of CB2 receptor on these cells (Cabral and Staab, 2005; Klein, 2005; Lunn et al., 2006), this result again needs to be interpreted cautiously. Further studies are warranted to examine which cells in the normal gut express CB2 receptor mRNA.
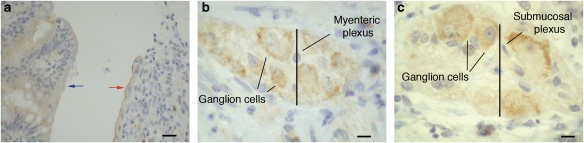
Antibodies to CB2 receptor have been used to examine the distribution of protein in the human GI tract. In humans, CB2 receptors are either absent or weakly expressed in intestinal epithelium (Figure 1), but are evident in the apical membranes at ulcerative margins in IBD (Wright et al., 2005; Figure 1). In addition, immune cells in the lamina propria that are CB2 immunoreactive include plasma cells and activated macrophages (Wright et al., 2005). Human colonic epithelial cell lines derived from colorectal tumours, such as HT29, Caco2 and DLD-1, all also express CB2 receptors (Ihenetu et al., 2003; Ligresti et al., 2003). The functional relevance of this observation is not known; suffice to say that patients with chronic intestinal inflammation have an increased risk of developing bowel cancers (Lu et al., 2006; see below). Recently, CB2 receptor expression has been observed in the enteric nervous system in rodents (Duncan et al., 2005b), and we show here that it is present on enteric neurons of the human ileum (Figure 1). Further studies exploring this in detail are underway.
Figure 1.
Immunohistochemical analysis of CB2 receptor protein in human ileum. Archival tissue sections were retrieved from files at the Royal United Hospital (Bath, UK) with the approval of the Bath Local Research Ethics Committee (RUH Bath NHS Trust, UK). Tissue blocks were fixed in 4% (w/v) formaldehyde and embedded in paraffin. Sections were stained with a primary rabbit polyclonal anti-human CB2 antibody (Cayman Chemicals Co., Boldon, Tyne and Wear, Newcastle UK) using standard immunohistochemical techniques (Wright et al., 2005). Brown staining indicates CB2 immunoreactivity. (a) Dense CB2 immunoreactivity was found in the luminal membrane at the ulcerative margin in active Crohn's disease (right-hand side, red arrow) with weak cytoplasmic staining in the non-involved epithelium (left-hand side, blue arrow). Bar=30 μm. Enteric ganglia in the (b) myenteric plexus and the (c) submucosal plexus in normal tissue were moderately CB2 positive. Results are representative of the staining pattern noted in the assessment of 21 separate patient samples. This is the first illustration of CB2 receptor immunoreactivity in the human enteric nervous system. Unpublished data, used with kind permission from Dr Leigh Biddlestone, Rachel Seymour and Dr Karen Wright (Bath, UK). Bar=10 μm. CB, cannabinoid.
CB2 receptors and GI motility
Functional evidence in support of a role for CB2 receptors in the control of GI motility in normal animals is quite limited. In early studies, it was suggested that there may be a second (peripheral) CB receptor in the gut based on cross-tolerance studies (Fride, 1995). In support of this observation, Hanus et al. (1999) demonstrated that the CB2-selective receptor agonist HU-308 reduced defecation in mice under acute stress by being placed in a new environment. The effect of HU-308 was only partially (and not significantly) reversed by the CB2-selective receptor antagonist SR144528. While these data are consistent with an action on motility, stress plays a role in faecal pellet output, as does visceral sensation; hence, it is possible that this compound was not acting directly on the motor systems of the gut itself, but indirectly by interaction with other systems. In contrast to the action of HU-308, the CB2-selective receptor agonists JWH-133 had no effect on intestinal transit in normal rats (Mathison et al., 2004) and JWH-015 had no effect on colonic propulsion in mice (Pinto et al., 2002). Consistent with these data are the findings that SR144528 was unable to reverse the inhibitory action of the long-lasting anandamide analogue, methanandamide in mouse colon in vivo (Pinto et al., 2002) and likewise, the CB2-selective receptor antagonists AM630 or SR144528 were unable to reverse the effects of the non-selective CB agonist WIN 55,212-2 on intestinal transit in rats (Izzo et al., 1999). When administered alone neither CB2 receptor antagonist had effects on intestinal transit, which is in contrast to the actions of CB1 receptor antagonists where motility is enhanced (Izzo et al., 1999; Mathison et al., 2004; Duncan et al., 2005a, 2005b). Similarly, when assessed in vitro, several parameters of peristalsis elicited by fluid distension in the guinea-pig ileum were unaffected by SR144528 (Izzo et al., 2000). In contrast to the actions in the small and large intestine, AM630 appears to have an action in the rat stomach (Storr et al., 2002). Here, it was shown that AM630 alone potentiated the electrically stimulated, non-adrenergic, non-cholinergic relaxations of the rat fundus in vitro, as well as reversing the actions of anandamide, though curiously not WIN 55,212-2 (Storr et al., 2002). Coupled with the fact that these authors demonstrated CB2 receptor expression in these tissues, it points to a potential role for CB2 receptors in gastric motility that needs to be explored further. It should be pointed out that the selectivity of AM630 for CB2 versus CB1 receptors is not as great as that for SR144528 (Ross et al., 1999). Whether this has any bearing on the results presented by Storr et al. (2002) is not clear, but should be borne in mind when considering these data.
Intraperitoneal injection of bacterial cell wall lipopolysaccharide (LPS) at low doses causes an increased rate of GI transit in rats (Mathison et al., 2004). This increased transit was normalized by the CB2-selective receptor agonist JWH-133 but not by the CB1-selective receptor agonist arachidonyl-2-chloroethylamide (ACEA), which surprisingly had no effect in LPS-treated animals. The effect of JWH-133 was reversed by AM630, which alone had no effect, suggesting that there was not constitutive activation of the CB2 receptor in this model or that LPS does not elevate endocannabinoids that act at CB2 receptors. The mechanism of action of the CB2-selective agonist was evaluated in these studies. Here, it was shown to involve intact cyclooxygenase pathways, since the actions of JWH-133 were reversed by indomethacin (Mathison et al., 2004). These data are consistent with an action on immune cells that are activated by LPS, or potentially on the expression of CB2 receptors in the enteric nervous system after LPS treatment. Currently, these possibilities are under investigation. Preliminary data support the expression of CB2 receptors in the enteric nervous system (Duncan et al., 2005b; Figure 1).
In other inflammatory models of altered motility, the involvement of CB2 receptors has not been demonstrated. Administration of croton-oil causes a chronic ileitis associated with hypermotility (Izzo et al., 2001). Here, the CB2-selective receptor antagonist SR144528 alone had no effect on motility and failed to reverse the inhibitory effects of the CB agonist CP55,940. To date, the effects of CB2-selective agonists have not been examined in this model. Similarly, in acetic acid-induced ileus, SR144528 was inactive at reversing the delayed intestinal transit and JWH-015 was unable to counteract the actions of rimonabant (SR141716A), which increases transit in this model (Mascolo et al., 2002). Thus at this point, it seems that there may a role for CB2 receptors in the control of GI motility, but only in some regions of the gut or under some specific pathophysiological states. Further studies are required to explore these potentially interesting observations and to examine additional roles for the CB2 receptor in GI pathophysiology.
Intestinal immune system and IBD
The GI tract has the most extensive immune system of the body consisting of both lymphoid and granulocyte populations and numerous secreted factors that contribute to homeostasis and host defence. In patients with IBD (Crohn's disease and ulcerative colitis), the intestinal immune system becomes unbalanced and inappropriately responds to commensal bacteria and/or other luminal antigens. Initially, increased epithelial permeability, either through tight junction disruption or enteric neuronal or glial dysfunction, gives rise to a leaky intestinal barrier, which allows access to the underlying mucosal tissue (Baumgart and Carding, 2007). Functionally, this is represented by a change from immune tolerance to immune activation causing T and B lymphocytes to differentiate and proliferate. During active IBD, the proinflammatory cytokines secreted by activated effector T cells stimulate macrophages to secrete further proinflammatory cytokines. These events initiate a cascade of inflammatory events, which results in the arrival of numerous leukocytes from the mucosal and submucosal vasculature and further chemo- and cytokine release. This leads to further infiltration of inflammatory cells and a cycle of inflammation ensues that requires treatment to suppress the immune activation. Tissue damage results from the release of numerous noxious mediators.
Interaction of the immune and nervous system occurs through a network of communication between enteric neurons, enteric glia and intestinal epithelial cells and/or cytokine signalling with neurotransmitters. These interactions play a part in the induction and amplification of the inflammation, motility disturbances and pain (Baumgart and Carding, 2007). As noted above, CB1 receptors have been implicated as beneficial in experimental IBD and endocannabinoid levels in the gut are altered in IBD (Massa et al., 2004; D'Argenio et al., 2006). The role of CB2 receptors has not been fully explored in IBD, but observations of increased CB2 receptor expression on intestinal epithelium in IBD (Wright et al., 2005; Figure 1), as well as the presence of CB2 receptor on immune cells in the gut, points to a regulatory role in this system. The functional relevance of the increased CB2 receptor on epithelial cells is still unknown, but could be linked to barrier function. Importantly, CB2 receptor activation leads to reduced secretion of proinflammatory cytokines and a shift away from inflammation (Cabral and Staab, 2005; Klein, 2005; Lunn et al., 2006). This could have important roles in IBD and is now being explored experimentally.
Role of CB2 receptors in experimental animal models of IBD
There are over 20 experimental models of IBD, encompassing gene knockout, transgenic, spontaneous colitis, inducible colitis and adoptive transfer models (Elson et al., 2005). From these models, it has become clear that the luminal microbiota (the hundreds of species of commensal microbes found in the gut lumen) play important roles in initiating and sustaining inflammation and that other cellular elements including the intestinal epithelium, the enteric nervous system and the mucosal immune system of the host contribute to the initiation, maintenance and ultimately resolution of experimental inflammation (Elson et al., 2005; Vasina et al., 2006).
Chemically induced colitis using dextran sulphate sodium (DSS) to initiate inflammation is characterized by bloody diarrhoea, weight loss, shortening of the colon, neutrophil infiltration and epithelial changes including fibrosis, crypt loss, goblet cell hypoplasia and focal ulceration (reviewed by Elson et al., 2005). The CB2-selective receptor agonist JWH-133, improved microscopic and macroscopic scores of inflammation when administered prophylactically in DSS colitis, though it required relatively high doses of the compound (Kimball et al., 2006) and was somewhat less effective than treatment with the CB1-selective agonist ACEA. In this study, it has also been shown that there is an increase in CB2 receptor expression in the submucosal infiltrate, but not a dramatic increase in epithelial CB2 receptor expression, which reflects differences between the animal and human data (Wright et al., 2005).
Oil of mustard (OM)-induced colitis is an acute model of colitis that has an extensive neurogenic component that provides support for a neurogenic contribution to IBD (Kimball et al., 2006). OM-induced colitis is sensitive to prophylactic administration of a CB2 receptor agonist and, as with DSS colitis, JWH-133 was less effective than ACEA (Kimball et al., 2006). In this model, JWH-133 was also tested therapeutically, after OM-induced colitis was established. Interestingly, JWH-133 was far more effective at this time point than when given in advance of the development of colitis, and the levels of inhibition of the inflammatory parameters were similar to those obtained with the CB1 receptor agonist, which approached normalization of many indices. These data may be explained if the action of CB2 receptor agonists is primarily related to an inhibition of activation of infiltrating immune cells. The specificity of the action of these compounds was not tested by the use of antagonists in this study, which will be required in future work that extends this series of experiments.
Recent information regarding the impact of CB2 agonists in models of IBD was presented at the Digestive Disease Week 2007, (DDW, Washington, DC, May 2007). Three poster presentations utilized different animal models of colitis to assess the therapeutic potential of CB2 agonists. JWH-133-treated animals in a trinitrobenzene sulphonic acid-induced colitis model (TNBS, an acute and chronic model of colitis with extensive ulceration (Elson et al., 2005), showed significant, dose-dependent improvement in macroscopic score and myeloperoxidase (MPO) levels (Storr et al., 2007). CB2 receptor activation in the TNBS and DSS models limited immune cell recruitment, decreased cytokine and chemokine production and improved macroscopic and histological scores (Thuru et al., 2007). Both acute (DSS colitis) and an immune colitis model (Gαi2−/− T-cell transfer model of colitis) were compared for the ability of the CB2-selective agonist, AM1241, to protect against the development of colitis (Ziring and Braun, 2007). Gαi2-deficient mice develop a lethal colitis at 8–12 weeks of age, which is more severe in the distal colon, resembling human ulcerative colitis (Rudolph et al., 1995). In this study, it was found that AM1241 was unable to protect mice from acute DSS colitis, but was able to protect animals from the immune-mediated colitis. Ziring et al. (2006) found that mice deficient in CB2 receptor have profound deficiencies in splenic marginal zone, peritoneal B1a cells, splenic memory CD4+ T cells, and intestinal natural killer cells and natural killer T cells and suggest that these findings indicate that CB2-selective agonists may modulate the development and activity of immunoregulatory cell subsets and that the endocannabinoid system is required for the formation of T- and B-cell subsets involved in immune homeostasis. If this is the case, then the promising preliminary results presented recently may lead to important new therapeutics with value in the treatment of IBD.
Role of CB2 receptors in the complications of chronic IBD
Fibrosis
Over one-third of patients with Crohn's disease will develop an intestinal stricture, and the great majority of these will require at least one surgical procedure. Crohn's disease-associated fibrosis results from chronic transmural inflammation and a complex interplay among intestinal mesenchymal cells, cytokines and local inflammatory cells (reviewed by Burke et al., 2007). The fibroblast is the key cell type mediating stricture formation. The cytoarchitecture of the bowel wall is altered with disruption of the muscularis mucosa, thickening of the muscularis propria and deposition of collagen throughout. The cytokine transforming growth factor (TGF)-β appears critical in this process, acting to increase growth factor and extracellular matrix (ECM) production and dysregulate ECM turnover. Potential therapeutic interventions are likely to concentrate on modulating downstream targets of TGF-β. It is interesting to note in this regard that TGF-β has been shown to downregulate CB2 receptors on lymphocytes (Gardner et al., 2002) and that CB2 receptor activity has an antifibrotic role in the liver (Julien et al., 2005). Whether this mechanism is relevant in intestinal fibrosis remains to be investigated.
Bowel cancer
Patients with chronic intestinal inflammation have an increased risk of developing bowel cancers (Itzkowitz and Yio, 2004; Lu et al., 2006). CB2 receptor mRNA is expressed in human adenomatous polyps and carcinomas (Ligresti et al., 2003) and CB2 protein is expressed in human colonic epithelial cell lines (Ihenetu et al., 2003; Ligresti et al., 2003; Joseph et al., 2004; Wright et al., 2005; Greenhough et al., 2007). Endogenous, synthetic and plant CBs have been shown to have an antiproliferative effect in human colonic epithelial cell lines through the CB1 receptor (Ligresti et al., 2003; Greenhough et al., 2007) and cyclooxygenase-2 (Patsos et al., 2005). Using DLD-1 colorectal cancer cells, Ligresti et al. (2003) showed that both CB1- and CB2-selective agonists inhibited proliferation to a similar extent. These interesting data suggest that CB2 receptor activation may be beneficial in cancer therapy. The functional relevance of CB2 receptor expression in cell lines with regard to human cancers in situ is not known, but it is interesting to note that normal intestinal epithelium expresses very little or no CB2 (Wright et al., 2005). This suggests that increased CB2 expression could be associated with the normal-inflamed-neoplasia-dysplasia-cancer sequence. This is not a new concept, in that CB2 receptor expression patterns change with respect to differentiation states and increased CB2 seems to promote de-differentiation and proliferation, which may favour increased malignancy (Fernández-Ruiz et al., 2007). Wound healing at the ulcerative margin in IBD would require epithelial dedifferentiation and proliferation to regain barrier integrity and the persistence of this feature in chronic inflammation might predispose this lineage to transformation and progression to cancer. Whether CB2 activity has the dual role of intestinal regeneration and antitumoral action remains to be investigated.
CB2 receptors and visceral sensations
Feelings of discomfort, nausea (that may result in emesis) and pain are commonly experienced sensory experiences that arise from the GI tract. Though few studies have explored the roles of the CB2 receptor in visceral sensation, there is increasing evidence of their involvement in states of inflammation or in the regulation of emesis. CB2 receptors have been implicated in pain processing in various neuropathic and inflammatory states of the skin and somatic structures. Recently, Sanson et al. (2006) investigated the involvement of CB receptors in inflammatory hypersensitivity of the colon. They showed that both CB1 and CB2 receptor agonists attenuated the degree of visceral sensitivity under baseline conditions and that the heightened sensitivity of colonic inflammation in TNBS colitis was also reversed by CB1 and CB2 receptor agonists. Interestingly, rimonabant, but not SR144528, further enhanced visceral sensitivity in colitis but not under baseline conditions. Though these results are clear, some caution needs to be exercised in their interpretation because of the observation that administration of synthetic THC to humans actually increased visceral sensitivity (Esfandyari et al., 2007). It must be noted that the human studies were performed only in normal subjects and used a low dose of the agonist, but the fact that the opposite findings were obtained indicates that this system may be more complex than we currently understand.
The microflora of the gut contribute to homeostasis and to many aspects of digestive health including visceral sensation (Verdú et al., 2006; Azpiroz et al., 2007). Probiotic therapy improves digestive health in some conditions and may contribute to reductions in visceral sensitivity. Recently, it was shown that the probiotic agent Lactobacillus acidophilus, increased CB2 receptors in the epithelium of treated animals (rats and mice; Rousseaux et al., 2007). Furthermore, treatment with this agent was correlated with a reduction in colorectal distension-induced visceromotor responses (a surrogate of visceral pain) in rats treated with a butyrate enema to irritate the gut wall. The role of CB2 receptors was shown when this response was reversed by AM630, but not an opioid receptor antagonist (Rousseaux et al., 2007). These exciting new data suggest that CB2 receptors can be modulated in the gut and, somehow, these contribute to the restoration of normal visceral sensation in states of irritation, and as noted above, inflammation. How CB2 receptors on intestinal epithelial cells contribute to an anti-nociceptive response is not yet clear. One possibility is that inflammatory mediators released from the epithelium that activate primary afferent nerves are reduced by CB2 receptor activation. This is consistent with observations by Ihenetu et al. (2003), who showed that tumour-necrosis factor (TNF)α-induced release of interleukin-8 from cultured colonic epithelial cells was reduced by CB2 receptor agonists. Whether CB2 receptors in the nervous system also contribute to these responses is not yet known and remains to be determined.
Peripheral activation of primary afferent fibres by algesic chemicals released in the gut is frequently the cause of visceral pain (Blackshaw et al., 2007). In a pioneering study, Hillsley et al. (2007) recorded from mesenteric primary afferent fibres from normal and CB2 receptor-deficient mice. They showed that bradykinin excited mesenteric afferent nerves and that this response was reduced in a dose-dependent manner by the CB2-selective receptor agonist AM1241, and this effect was reversed by AM630. AM1241 had no significant effect in CB2 receptor-deficient mice. These studies do not reveal the site of CB2 receptor activation that leads to inhibition of neuronal activity or whether it is upregulated in visceral afferents in injury or inflammation, but they strongly suggest that the presence of CB2 receptors (on primary afferent nerves) in the gut may play a role in limiting the extent of neuronal activation, and hence pain, in states of inflammation.
Pain associated with pancreatitis is very severe and hard to treat. Biopsy samples from patients with pancreatitis reveal increases in the expression of both CB1 and CB2 receptors as well as increased levels of endocannabinoids (Michalski et al., 2007). In the same study, using an appropriate animal model of pancreatitis, pain responses were assessed in response to the CB agonist HU-210 at a dose that did not have central side effects associated with high doses of CB1 receptor agonists. Here, it was shown that either the CB1-selective receptor antagonist AM251 or the CB2-selective receptor antagonist AM630 reduced the effect of HU-210 and that the two antagonists together gave a similar response to either alone. It is not yet known if a CB2-selective receptor agonist would reduce pain in this model. These data suggest that both CB receptors are required for the anti-nociceptive effects of CBs in pancreatitis and they point out the potential benefit of CB2 receptor activation in visceral as well as somatic pain states, especially when they are of an inflammatory nature.
Though not painful, emesis is a condition commonly associated with GI dysfunction, though it is a frequent symptom of central nervous system disease. CBs have long been used for the treatment of emesis. There is very strong evidence for the involvement of the CB1 receptor in mediating the action of CBs in the brainstem (Izzo and Coutts, 2005; Storr and Sharkey, 2007). However, the expression of CB2 receptors in the dorsal vagal complex suggested that they may also be involved in the regulation of emesis. Van Sickle et al. (2005) showed that indeed CB2 receptor activation by endocannabinoids could reduce emesis evoked by a centrally acting emetic morphine-6-glucuronide. In this case, it seems that co-activation of CB1 and CB2 receptors were necessary for the anti-emetic action of endocannabinoids. CB2-selective receptor agonists alone did not reduce emesis, but AM630 was able to block the effects of both 2-AG and VDM11, a drug that raises the levels of 2-AG in the brainstem by blocking endocannabinoid reuptake. These studies also showed that activation of both CB receptors circumvented the unwanted central side effects of CB1 receptor activation alone. Whether CB2 receptors in the brainstem play a role in the sensation of nausea remains to be determined.
Summary and future directions
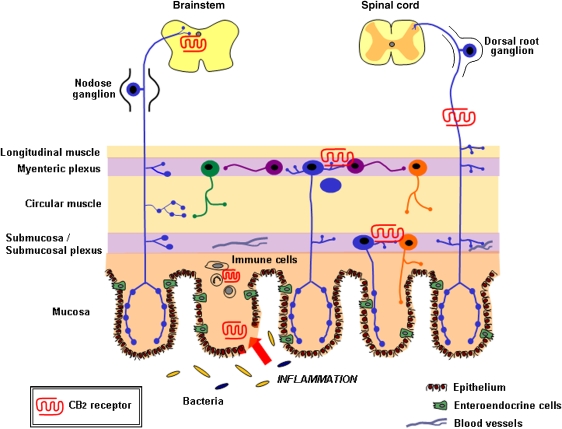
A model for the GI cannabinoid system in inflammation was put forward by Kunos and Pacher (2004). In this model, they outlined the sites within the gut wall and cell types expressing CB receptors that could be activated leading to a reduction in the development of intestinal inflammation. We propose an extension to this model incorporating the known and putative functions of CB2 receptors in the GI tract (Figure 2). Commensal bacteria (yellow) and pathogens (blue) are allowed access to the mucosal and submucosal regions through breaches in the epithelial barrier. CB2 receptor expression is upregulated at this site and may play a role in barrier integrity or regenerative processes. Immune cell activation, cytokine and chemokine production, proliferation and recruitment lead to infiltration of further immune cell types. The expression of CB2 receptors on a number of these cell types may serve to downregulate leukocyte infiltration and inflammation through inhibition of cytokine and chemokine production, inhibition of adhesion and migration, and apoptosis of activated immune cells (Cabral and Staab, 2005; Klein, 2005; Lunn et al., 2006). Endocannabinoid release during this inflammatory insult may then lead to activation of CB2 receptors on enteric neurons of the submucosal and myenteric plexuses, resulting in inhibition of gut motility that may be enhanced during inflammation. CB2 activation of primary afferent nerves reduces visceral sensitivity, relieving pain and central activation of CB2 receptors, together with CB1 receptors, reduces emesis.
Figure 2.
CB2 receptor expression in the gastrointestinal (GI) tract and its innervation. CB2 receptors are expressed in the enteric nervous system and on immune cells in the normal gut. During inflammation, there is enhanced expression on epithelial cells at the ulcer margins (red) and in infiltrating immune cells. CB2 receptor is also expressed on visceral afferent nerves and in the dorsal vagal complex in the brainstem. Commensal bacteria (yellow) and pathogens (blue) are allowed access to the mucosal and submucosal regions through breaches in the epithelial barrier during inflammation. The figure is extensively adapted from Furness et al. (1999). CB, cannabinoid.
As indicated throughout this review, there are substantial areas where we have little or no knowledge of the CB2 receptor in the GI tract. These include further knowledge of the sites of receptor expression in normal animals and in disease states, the regulation of the CB2 receptor in the gut and the full consequences of CB2 receptor activation. However, based on what we know so far, it is clear that CB2 receptors represent a braking system and a pathophysiological mechanism for the resolution of inflammation and its many symptoms. CB2 receptor activation therefore represents a very promising therapeutic target in GI inflammatory states where there is immune activation and motility dysfunction.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- ACEA
arachidonyl-2-chloroethylamide
- CB
cannabinoid
- DSS
dextran sulphate sodium
- ECM
extracellular matrix
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- LMMP
longitudinal muscle-myenteric plexus
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- OM
oil of mustard
- TGF
transforming growth factor
- TNBS
trinitrobenzene sulphonic acid
- TNF
tumour necrosis factor
Conflict of interest
The authors state no conflicts of interest.
References
- Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19 (Suppl 1):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Carding SR. Gastroenterology 1—inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJH, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19 (Suppl 1):1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Burke JP, Mulsow JJ, O'Keane C, Docherty NG, Watson RWG, O'Connell PR. Fibrogenesis in Crohn's disease. Am J Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Staab A. Effects on the immune system. Handb Exp Pharmacol. 2005;168:385–423. doi: 10.1007/3-540-26573-2_13. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Petrosino S, Gianfrani C, Valenti M, Scaglione G, Grandone I, et al. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J Mol Med. 2007;85:523–530. doi: 10.1007/s00109-007-0192-3. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- Di Carlo G, Izzo AA. Cannabinoids for gastrointestinal diseases: potential therapeutic applications. Expert Opin Investig Drugs. 2003;12:39–49. doi: 10.1517/13543784.12.1.39. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006;55:1373–1376. doi: 10.1136/gut.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005a;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Duncan M, Ho W, Shariat N, Pittman QJ, Mackie K, Patel KD, et al. Distribution of the CB2 receptor in enteric nerves of the rat ileum 2005 Symposium on the Cannabinoids 2005b. Burlington, VT, International Cannabinoid Research Society, p 159
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol. 2007;293:G137–G145. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12:38–46. doi: 10.1097/01.mib.0000195391.49762.89. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fride E. Anandamides: tolerance and cross-tolerance to delta 9-tetrahydrocannabinol. Brain Res. 1995;697:83–90. doi: 10.1016/0006-8993(95)00790-w. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol Gastrointest Liver Physiol. 1999;277:G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- Gardner B, Zu LX, Sharma S, Liu Q, Makriyannis A, Tashkin DP, et al. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem Biophys Res Commun. 2002;290:91–96. doi: 10.1006/bbrc.2001.6179. [DOI] [PubMed] [Google Scholar]
- Greenhough A, Patsos HA, Williams AC, Paraskeva C.The cannabinoid delta(9)-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells Int J Cancer 2007. e-pub ahead of print 21 June 2007 [DOI] [PubMed]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford MLJ, Shire D, et al. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- Guagnini F, Valenti M, Mukenge S, Matias I, Bianchetti A, Di Palo S, et al. Neural contractions in colonic strips from patients with diverticular disease: role of endocannabinoids and substance P. Gut. 2006;55:946–953. doi: 10.1136/gut.2005.076372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. Hu-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, McCaul C, Aerssens J, Peeters PJ, Gijsen H, Moechars G, et al. Activation of the cannabinoid 2 (CB2) receptor inhibits murine mesenteric afferent nerve activity Neurogastroenterol Motil 2007. e-pub ahead of print 21 May 2007 [DOI] [PubMed]
- Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol. 2004;141:1335–1345. doi: 10.1038/sj.bjp.0705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihenetu K, Molleman A, Parsons ME, Whelan CJ. Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur J Pharmacol. 2003;458:207–215. doi: 10.1016/s0014-2999(02)02698-5. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso F, Costagliola A, Bisogno T, Marsicano G, Ligresti A, et al. An endogenous cannabinoid tone attenuates cholera toxin-induced fluid accumulation in mice. Gastroenterology. 2003;125:765–774. doi: 10.1016/s0016-5085(03)00892-8. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005;168:573–598. doi: 10.1007/3-540-26573-2_19. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Tonini M, Capasso F. Modulation of peristalsis by cannabinoid CB(1) ligands in the isolated guinea-pig ileum. Br J Pharmacol. 2000;129:984–990. doi: 10.1038/sj.bjp.0703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Niggemann B, Zaenker KS, Entschladen F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother. 2004;53:723–728. doi: 10.1007/s00262-004-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006;291:G364–G371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Kunos G, Pacher P. Cannabinoids cool the intestine. Nat Med. 2004;10:678–679. doi: 10.1038/nm0704-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V, et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–687. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- Lunn CA, Reich EP, Bober L. Targeting the CB2 receptor for immune modulation. Expert Opin Ther Targets. 2006;10:653–663. doi: 10.1517/14728222.10.5.653. [DOI] [PubMed] [Google Scholar]
- Mascolo N, Izzo AA, Ligresti A, Costagliola A, Pinto L, Cascio MG, et al. The endocannabinoid system and the molecular basis of paralytic ileus in mice. FASEB J. 2002;16:1973–1975. doi: 10.1096/fj.02-0338fje. [DOI] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski CW, Laukert T, Sauliunaite D, Pacher P, Bergmann F, Agarwal N, et al. Cannabinoids ameliorate pain and reduce disease pathology in cerulein-induced acute pancreatitis. Gastroenterology. 2007;132:1968–1978. doi: 10.1053/j.gastro.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsos HA, Hicks DJ, Dobson RR, Greenhough A, Woodman N, Lane JD, et al. The endogenous cannabinoid, anandamide, induces cell death in colorectal carcinoma cells: a possible role for cyclooxygenase 2. Gut. 2005;54:1741–1750. doi: 10.1136/gut.2005.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, et al. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil. 2006;18:949–956. doi: 10.1111/j.1365-2982.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- Storr M, Gaffal E, Saur D, Schusdziarra V, Allescher HD. Effect of cannabinoids on neural transmission in rat gastric fundus. Can J Physiol Pharmacol. 2002;80:67–76. doi: 10.1139/y02-005. [DOI] [PubMed] [Google Scholar]
- Storr M, Keenan CM, Patel KD, Sharkey KA. The cannabinoid (CB2) receptor mediates protection against TNBS colitis in mice. Gastroenterology. 2007;132 (Suppl 2):A231. [Google Scholar]
- Storr MA, Sharkey KA.The endocannabinoid system and gut-brain signalling Curr Opin Pharmacol 2007(in press) [DOI] [PubMed]
- Thuru X, Chamaillard M, Karsak M, Gantier E, Erdual E, Dubuquoy C, et al. Cannabinoid receptor 2 is required for homeostatic control of intestinal inflammation. Gastroenterology. 2007;132 (Suppl 2):A228. [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, et al. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006;126–127:264–272. doi: 10.1016/j.autneu.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Verdú EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:185–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Ziring D, Wei B, Velazquez P, Schrage M, Buckley NE, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- Ziring DA, Braun J. AM1241, a CB2-specific agonist protects against immune but not acute colitis. Gastroenterology. 2007;132 (Suppl 2):A232. [Google Scholar]