Abstract
Recently, it has been recognized that the cannabinoid receptor CB2 may play a functionally relevant role in the central nervous system (CNS). This role is mediated primarily through microglia, a resident population of cells in the CNS that is morphologically, phenotypically, and functionally related to macrophages. These cells also express the cannabinoid receptor CB1. The CB1 receptor (CB1R) is constitutively expressed at low levels while the CB2 receptor (CB2R) is expressed at higher levels and is modulated in relation to cell activation state. The relatively high levels of the CB2R correspond with microglia being in ‘responsive' and ‘primed' states, suggesting the existence of a ‘window' of functional relevance during which activation of the CB2R modulates microglial activities. Signature activities of ‘responsive' and ‘primed' microglia are chemotaxis and antigen processing, respectively. The endocannabinoid 2-arachidonylglycerol has been reported to stimulate a chemotactic response from these cells through the CB2R. In contrast, we have shown in vivo and in vitro that the exogenous cannabinoids delta-9-tetrahydrocannabinol and CP55940 inhibit the chemotactic response of microglia to Acanthamoeba culbertsoni, an opportunistic pathogen that is the causative agent of Granulomatous Amoebic Encephalitis, through activation of the CB2R. It is postulated that these exogenous cannabinoids superimpose an inhibitory effect on pro-chemotactic endocannabinoids that are elicited in response to Acanthamoeba. Furthermore, the collective results suggest that the CB2R plays a critical immune functional role in the CNS.
Keywords: Acanthamoeba, amoebic encephalitis, brain infection, cannabinoid receptors, CB2R, CB2 receptor, CP55940, delta-9-tetrahydrocannabinol, microglia, THC
Introduction
To date, two unique cannabinoid receptors, one located primarily in the brain (CB1 receptor (CB1R)) and the other in the immune system (CB2 receptor (CB2R)), have been identified. The CB1R appears to be responsible for most, if not all, of the centrally mediated effects of cannabinoids (Compton et al., 1993). This receptor has been well characterized using potent radiolabelled cannabinoid agonists that bind in the high picomolar to low nanomolar range. The CB1R is concentrated in areas of the brain that control movement, coordination, sensory perception, learning and memory, reward and emotions, hormonal function, and body temperature. This localization within the brain is consistent with the pharmacological profile of cannabinoids in that high densities are found in cerebellum, hippocampus and cerebral cortex, whereas low quantities are present in the brain stem (Herkenham et al., 1991; Thomas et al., 1992). The CB1R is G-protein coupled as evidenced by inhibition of adenylyl cyclase (Howlett et al., 1986), inhibition of N-type calcium channels (Mackie and Hille, 1992) and increased binding of non-hydrolyzable GTPγS in the presence of cannabinoids (Sim et al., 1996). In addition, the cloning of this cannabinoid receptor resulted from screening of a G-protein-coupled receptor library (Matsuda et al., 1990). It is not surprising that a cannabinoid receptor was found also in the immune system. Transcripts (that is, mRNAs) for the CB1R and CB2R have been found in spleen and tonsils (Munro et al., 1993; Galiègue et al., 1995) and other immune tissues (Munro et al., 1993; Bouaboula et al., 1996). However, in all cases reported to date, levels of message for the CB2R in immune cells exceed those for the CB1R. The distribution pattern of levels of CB2R mRNA displays major variation in human blood cell populations with a rank order of B lymphocytes>natural killer cells≫monocytes>polymorphonuclear neutrophils>CD8 lymphocytes>CD4 lymphocytes (Galiègue et al., 1995). A rank order for levels of CB2R transcripts similar to that for primary human cell types has been recorded for human cell lines belonging to the myeloid, monocytic and lymphoid lineages (Galiègue et al., 1995). A general similar pattern has been reported for mouse and rat (Daaka et al., 1995). In addition, cognate protein has been identified in rat lymph nodes, Peyer's Patches and spleen (Lynn and Herkenham, 1994). The presence of the CB2R primarily within immune cells suggests a role for this receptor in the activities attributed to these cells. However, CB1R also may be involved in cannabinoid-mediated modulation of select immune functions (Stefano et al., 1996; Sinha et al., 1998; Waksman et al., 1999). Recent studies support the presence of yet uncloned cannabinoid receptors, principally on the basis of pharmacological evidence of cannabinoid action in CB1R- and CB2R-deficient mice (Jaggar et al., 1998; Di Marzo et al., 2000; Breivogel et al., 2001). The first of these has been reported to couple to Gi/o proteins (Hajos and Freund, 2002; Offertaler et al., 2003), to be activated by micromolar concentrations of abnormal-cannabidiol (CBD), a synthetic analogue of CBD, and to be potentiated through cGMP and protein kinase G (Begg et al., 2003). A second putative non-CB1, non-CB2 receptor has been referred to as the ‘palmitoylethanolamide receptor' because palmitoylethanolamide, an analogue of anandamide that does not bind the CB2R, causes a reduction of pain associated with inflammatory response (Calignano et al., 1998; Jarai et al., 1999) that is blocked by the CB2R antagonist ((1S-endo)-5-(4-Chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)-N-(1,3,3-trimethylbicyclo(2.2.1)hept-2-yl)-1H-pyrazole-3-carboxamide (SR144528). The nuclear peroxisome proliferator-activated receptor-alpha (PPAR-α) has been reported as the mediator of the anti-inflammatory actions of this lipid amide (Lo Verme et al., 2005a, 2005b). Finally, Breivogel et al. (2001) reported the existence of a ‘WIN receptor' based on experiments of GTPγS binding in brain membranes from CB1R knockout mice. In their studies, anandamide and the aminoalkylindole WIN55212-2 stimulated GTPγS binding. However, the ‘novel' receptor was found to exhibit a pharmacology distinctive from that of the CB1R and the CB2R. It was not activated by delta-9-tetrahydrocannabinol (THC) and the classical cannabinoid agonists (−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP55940) and HU210 and was blocked weakly by the CB1R antagonist (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide hydrochloride (SR141716A).
Microglia are CB2R-expressing resident macrophages in the CNS
There is increasing evidence that the CB2R, in addition to its linkage to immune cell activities at peripheral sites, plays a functionally relevant role in immunity in the CNS. This role appears to be exerted primarily through microglia, a resident population of cells in the brain, spinal cord and retina that is morphologically, phenotypically and functionally related to macrophages (Streit et al., 1988; Dickson et al., 1991; Ling and Wong, 1993; Gehrmann et al., 1995; Gehrmann, 1996; Aloisi et al., 1998; Stoll and Jander, 1999). The function of ‘quiescent' microglia in normal brain is not well understood, but in pathological conditions, they play an active role as immunoeffector/accessory cells. Microglia migrate and proliferate during and after injury and inflammation (Leong and Ling, 1992; Kreutzberg, 1995, 1996; Benveniste, 1997a). Once activated, they produce various cytokines including interleukin (IL)-1, IL-6 and tumour necrosis factor-α (Giulian et al., 1986; Reid et al., 1993; Benveniste, 1997b), and express major histocompatability complex (MHC) class I and II antigens and the complement receptor, CD11/CD18 complex. Microglia, also, are phagocytic and, upon activation, can process antigens and exert cytolytic functions. Paradoxically, these cells not only play a role in host defense and tissue repair in the CNS (Streit et al., 1988; Perry, 1990), but also have been implicated in nervous system disorders, such as Multiple Sclerosis (MS) (Matsumoto et al., 1992), Alzheimer's disease (Rogers et al., 1988), Parkinson's disease (McGeer et al., 1988) and acquired immune deficiency syndrome (AIDS) dementia (Dickson et al., 1991; Merrill and Chen, 1991; Spencer and Price, 1992). Neural histological features of AIDS dementia complex include diffuse leukoencephalopathy of the white matter, which is accompanied by severe loss of myelin and sparing of fibres (Kleihues et al., 1985). Discrete areas of demyelination with hypertrophied astrocytes, which also contain microglia, blood-derived macrophages and multinucleated giant cells, are observed. The multinucleated giant cells have been reported to constitute syncytia of macrophages or microglia, which are productively infected with the human immunodeficiency virus type 1 (HIV1) and are a histopathological hallmark of subacute encephalitis in HIV1-infected brains (Koenig et al., 1986; Michaels et al., 1988) and spinal cords (Eilbott et al., 1989; Maier et al., 1989). Thus, it has been proposed that in AIDS dementia, macrophages and microglia are the predominant cell types, which are infected and produce HIV1 (Koyanagi et al., 1987; Kure et al., 1990). Manifestations of AIDS dementia indicate that direct infection by the HIV probably does not account for CNS dysfunction. Tumours, such as extranodal primary malignant B cell lymphomas (Snider et al., 1983; Gray et al., 1988) and cerebral deposits of Kaposi's sarcoma resulting from metastases of lung foci (Gorin et al., 1985; Gray et al., 1988), have been shown to occur either before or at the time of patient seroconversion and before onset of immune suppression (Perry, 1990). Cerebrovascular complications, such as vasculitis and haemorrhages within cerebral tumour areas or in areas of demyelination, also have been shown to occur before onset of immune suppression. These observations suggest that inflammatory cells and their products are actively involved in these histopathological events.
The CB2R is differentially expressed by microglia
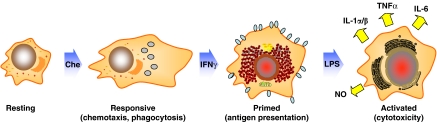
Microglia, consistent with other immune cells, undergo maturation, differentiation and activation, processes, which are characterized by differential gene expression and acquisition of correlative distinctive functional capabilities (Adams and Hamilton, 1984; Hamilton et al., 1986; Hamilton and Adams, 1987). Peritoneal macrophages, macrophage-like cells and microglia can be driven sequentially in response to multiple signals from ‘resting', to ‘responsive', ‘primed' and ‘fully' activated states, a process that mimics events in vivo (Figure 1). Using this in vitro model, it has been shown that levels of CB2R mRNA and protein are modulated differentially in relation to cell activation state (Carlisle and Cabral, 2002). The CB2R is not detected in ‘resting' cells, is present at high levels in ‘responsive' and ‘primed' cells, and is identified at greatly diminished levels in ‘fully' activated cells. In contrast, the CB1R is present in microglia at relatively low levels and is expressed constitutively in relation to cell activation state. These observations suggest that the CB2R is expressed ‘on demand' and that the modulation of CB2R levels is a feature common to cells of macrophage lineage as they participate in the inflammatory response. Furthermore, the relatively high levels of CB2R recorded for microglia when in ‘responsive' and ‘primed' states suggest that these cells exhibit a functionally relevant ‘window' during which they are most susceptible to the action of cannabinoids. Finally, since the kinetics of CB1R and CB2R expression by microglia are distinctive, activation of the two receptors by endogenous and/or exogenous cannabinoids may result in disparate functional outcomes.
Figure 1.
In vitro model of macrophage/microglial multi-step activation. Peritoneal macrophages, macrophage-like cells and microglia can be driven sequentially in response to multiple signals from ‘resting' to ‘responsive', ‘primed' and ‘fully' activated states, a process that mimics events in vivo. Each of these states is characterized by differential gene expression and acquisition of correlative distinctive functional capabilities (modified from: Adams and Hamilton, 1984; Hamilton et al., 1986; Hamilton and Adams, 1987).
Consistent with observations suggestive of a functionally relevant ‘window' for CB2R expression, a number of studies have indicated that activities attributed to ‘fully' activated microglia are not susceptible to cannabinoid-mediated action that is linked to the CB2R. Waksman et al. (1999) reported that the production of inducible nitric oxide (iNO), a potent inflammatory mediator that is released from microglia and macrophage-like cells upon their ‘full' activation, was inhibited by cannabinoids in a mode that was linked, at least in part, to the CB1R. The cannabinoid receptor high-affinity binding enantiomer CP55940 exerted a concentration-dependent (0.1–8 μM) inhibition of nitric oxide (NO) release from neonatal rat microglia subjected to activation with interferon-γ in concert with bacterial lipopolysaccharide, which far exceeds the binding and agonist activity at either of the CB receptors. In contrast, a minimal inhibitory effect on iNO production was exerted by the lower affinity binding paired enantiomer CP56667. These results implicated a cannabinoid receptor as linked functionally to the inhibition of iNO production, since the binding affinity of the paired enantiomers has been shown to correlate with bioactivity in vivo and in vitro (Compton et al., 1993; Felder et al., 1995), and enantiomeric selectivity is a characteristic feature of receptor-mediated cellular activity. To confirm the NO release data, the effect of the paired cannabinoid enantiomers on the activity of nicotinamide adenine dinuleotide phosphate-diaphorase was determined since its proportional intracellular activity correlates with that of NO synthase in neuronal cells (Hope et al., 1991). Consistent with the NO data, a differential inhibition of nicotinamide adenine dinuleotide phosphate-diaphorase activity in rat microglia was effected by CP55940 versus its paired enantiomer CP56667. Pretreatment of microglia with the Gαi/Gαo protein inactivator pertussis toxin, cyclic AMP reconstitution with the cell-permeable analogue dibutyryl-cAMP or treatment with the Gαs activator cholera toxin, resulted in reversal of the CP55940-mediated inhibition of NO release. Finally, functional studies performed with the CB1R-selective antagonist SR141716A (Rinaldi-Carmona et al., 1994) resulted in a reversal of the CP55940-mediated inhibition of iNO production. Collectively, these immune pharmacological results supported a functional linkage between the CB1R and cannabinoid-mediated inhibition of iNO production by neonatal rat microglia. Puffenbarger et al. (2000) extended these studies on the effects of cannabinoids on ‘fully' activated microglia and indicated that the inhibition of the inducible expression of pro-inflammatory cytokines was exerted through a non-CB1, non-CB2 receptor process. Exposure of neonatal rat cortical microglia to THC resulted in reduced amounts of lipopolysaccharide-induced mRNAs for IL-1α, IL-1β, IL-6 and tumour necrosis factor-α. Of these cytokine mRNAs, the response of that for IL-6 was exquisitely sensitive to THC treatment. Similarly, exposure of microglia cells to the putative endogenous cannabinoid (endocannabinoid) anandamide before lipopolysaccharide treatment resulted in a decrease in cytokine mRNA levels, but not to the same extent as that caused by THC; however, when methanandamide, the non-hydrolyzable analogue of anandamide, was tested, its ability to inhibit cytokine mRNA expression was comparable to that of THC. Exposure of microglia to either of the paired enantiomers CP55940 or CP56667 resulted in similar inhibition of lipopolysaccharide-induced cytokine mRNA expression. A comparable inhibitory outcome was obtained when the paired enantiomers levonantradol and dextronantradol were employed. Neither the CB1R-selective antagonist SR141716A nor the CB2R-selective antagonist SR144528 (Rinaldi-Carmona et al., 1998) was able to reverse the inhibition of cytokine mRNA expression by levonantradol. Collectively, the absence of stereoselectivity in the inhibition of cytokine mRNA expression and the inability of either the CB1R or CB2R antagonists to block the inhibitory effect of levonantradol demonstrated that while cannabinoids had the capacity to modulate levels of pro-inflammatory cytokine mRNAs in neonatal rat microglia, the inhibition of cytokine mRNA expression is apparently mediated neither through the CB1R nor through the CB2R.
Chemotaxis as a signature activity of ‘responsive' microglia
Signature activities of macrophage-like cells when in ‘responsive' and ‘primed' states of activation, states associated with the early inflammatory response, are chemotaxis and antigen presentation, respectively. Chemotaxis is the ability of cells to migrate in response to a stimulus and is distinctive from stimulus-independent random cellular motion (Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996). Chemotaxis differs from chemokinesis, a stimulus-dependent cellular motility whereby cells exhibit enhanced random motion that is dependent on a chemo-stimulant (Becker, 1977; Keller et al., 1978). Thus, chemotaxis is a process in which cell motility is directed towards a concentration gradient of chemo-stimulant (Harris, 1953, 1954; Jin and Hereld, 2006; Kehrl, 2006). In this chemotactic process, macrophage-like cell interaction with chemoattractants not only initiates a rapid and directed movement but also is associated with a complex array of cellular events that includes changes in ion fluxes, alterations in integrin avidity, production of superoxide anions and secretion of lysosomal enzymes (Murdoch and Finn, 2000). ‘Classical' chemoattractants include bacterial-derived N-formyl peptides, the complement fragment peptides C5a and C3a, and lipids such as leukotriene B4 and platelet-activating factor (Schiffmann et al., 1975; Goldman and Goetzl, 1982; Hanahan, 1986; Gerard and Gerard, 1994). Chemokines represent a second group of chemoattractants. These 8- to 17-kDa molecular mass range cytokines are selective for leucocytes in vitro and elicit accumulation of inflammatory cells in vivo (Baggiolini et al., 1994, 1997; Kim, 2004; Le et al., 2004). As in the case for cannabinoid receptors, the specific effects of chemokines on target cells are mediated by G-protein-coupled receptors (Murdoch and Finn, 2000; Charo and Ransohoff, 2006). Ligation of chemokines with their cognate receptors initiates a series of signal transductional events that results in regulation of leucocyte trafficking in inflammation, tissue injury, tumour development and host response to infection (Charo and Ransohoff, 2006).
Several studies have documented that cannabinoids affect the migratory activities of macrophages and macrophage-like cells. Stefano et al. (1998) reported that acute exposure to anandamide resulted in transformation of macrophages from an amoeboid and motile state to that of a rounded and non-motile conformation. These investigators proposed that the transforming events were linked to the CB1R since the CB1R-selective antagonist SR141716A blocked the transformation. Sacerdote et al. (2000) demonstrated that in vivo and in vitro treatment of rat peritoneal macrophages with CP55940, a full agonist at both CB1R and CB2R, resulted in decreased migration in vitro to the peptide formal-methionyl-leucine-phenylalanine. It was indicated, however, that while both the CB1R and CB2R were involved in this process, the cannabinoid-mediated effect was linked primarily to the CB2R. The chemotactic response of mouse macrophages to formal-methionyl-leucine-phenylalanine also has been shown to be decreased by CBD (Sacerdote et al., 2005), a cannabinoid that binds weakly to the CB2R. The CB2R antagonist SR144528 prevented this decrease, suggesting a functional linkage to the cognate receptor. Walter et al. (2003) found that the endocannabinoid 2-arachidonylglycerol (2-AG) triggered migration of microglia and that the CB2R was involved in this effect. Franklin and Stella (2003) demonstrated that arachidonylcyclopropylamide, an agonist selective for the CB1R, induced a dose-dependent increase in migration of mouse microglial cell line BV-2. However, while the arachidonylcyclopropylamide-induced response was blocked by pertussis toxin pretreatment consistent with the involvement of a Gi/o-protein-coupled receptor, the CB1R antagonist SR141716A did not prevent the arachidonylcyclopropylamide-mediated migration. In contrast, two antagonists of the CB2R (SR144528 and cannabinol) as well as two antagonists of ‘abnormal-CBD-sensitive' receptors (O-1918 and CBD) prevented the response. Based on these collective results, Franklin and Stella (2003) suggested that CB2Rs and ‘abnormal-CBD-sensitive' receptors regulated the migration of microglial-like cells. Stella and co-workers extended these studies and showed that P2X7 ionotropic receptors played a key role in controlling the production of 2-AG by microglia (Witting et al., 2004). Recently, Raborn et al. (in press) demonstrated that THC and CP55940 mediated inhibition of mouse peritoneal macrophage chemotaxis to the chemokine RANTES/CCL5 and that this event was linked to the CB2R. In these studies, the CB2R-selective ligand O-2137 (1-[4-(1,1-Dimethylheptyl)-2,6-dimethoxyphenyl]-3-methylcyclohexanol) exerted a robust inhibition of chemotaxis, while the CB1R-selective ligand (N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) had a minimal effect. The CP55940-mediated inhibition was reversed by the CB2R-selective antagonist SR144528 but not by the CB1R-selective antagonist SR141716A. In addition, THC treatment had a minimal effect on the chemotactic response of peritoneal macrophages from CB2R knockout mice. Collectively, the preponderance of data that has been obtained indicates that cannabinoids act through the CB2R to alter macrophage migration with exogenous cannabinoids, such as THC exerting inhibitory effects and endocannabinoids such as 2-AG eliciting an opposite stimulatory effect. Furthermore, the studies of Raborn et al. (in press) indicate that THC and CP55940 can transdeactivate migratory responsiveness to the chemokine RANTES/CCL5, suggesting that signaling through the CB2R leads to ‘cross-talk' with chemokine receptors. Thus, the CB2R may be a constituent element of a network of G-protein-coupled receptor signal transductional systems, inclusive of chemokine receptors, that act coordinately to modulate macrophage migration.
It has been shown that the CB2R also is involved in cannabinoid-mediated inhibition of processing of select antigens by macrophages. Antigen processing and presentation constitute a complex set of events. The activation of helper/inducer CD4+ T cells requires their physical contact with another cell type called an antigen-presenting cell, which is partially due to the specificity of the T-cell antigen receptor, a plasma membrane protein. Unlike antibodies, the T-cell receptor does not bind to antigen alone, but rather the receptor recognizes a complex composed of the antigen and MHC class II molecules that are expressed at the surface of antigen-presenting cells (Schwartz, 1985). CD4+ T cells are usually specific for protein antigens, not carbohydrates or lipids. The form of the antigen in the complex with MHC class II molecules is a peptide fragment, not the native antigen (Guillet et al., 1987). In addition, these antigens usually are not synthesized by the antigen-presenting cells but are exogenous proteins (Germain, 1986). This process is distinctive from that which occurs for cytotoxic CD8-positive T cells, which recognize peptide antigens that can be derived from endogenous proteins in the context of MHC class I molecules on antigen-presenting or infected target cells. Important functions of antigen-presenting cells are internalization of antigen, proteolytic cleavage of antigen into peptides, formation of the peptide-class molecule complex and expression of the complex at the cell surface (Germain, 1986). These series of events comprise antigen processing. Once the complex is expressed at the cell surface, the process is referred to as antigen presentation. Several steps in antigen processing have been characterized. Soluble protein antigens are internalized by antigen-presenting cells through endocytosis, which is either nonspecific or receptor mediated (Unanue and Allen, 1987). Of the various types of antigen-presenting cells, only macrophages internalize particulate antigens by phagocytosis (Unanue and Allen, 1987). Other phagocytic cells lack MHC class II molecules and do not function as antigen-presenting cells. Regardless of the mode of antigen uptake, intracellular antigen enters an acidic organelle, where antigen processing probably occurs. Treatment of antigen-presenting cells with acidotropic agents, such as chloroquine, ammonium chloride and monensin (McCoy and Schwartz, 1988), that neutralize intracellular acidic pH eliminate antigen processing (Seglen, 1983). Cathepsins, acid proteases, within the acidic organelles are thought to cleave the antigens. Various protease inhibitors prevent antigen processing by antigen-presenting cells, depending on the antigen (Van der Drift et al., 1990). Thus, the interference by cannabinoids such as THC with any one of these steps could result in impaired antigen processing.
McCoy et al. (1999) examined the effect of THC on the processing of intact lysozyme by macrophages. It was demonstrated that THC impaired the ability of a macrophage hybridoma to function as an antigen-presenting cell based on its ability to secrete IL-2 upon stimulation of a soluble protein antigen-specific helper T-cell hybridoma. THC exposure significantly reduced the T-cell response to the native form of the antigen after pretreatment of the macrophages with nanomolar drug concentrations. However, THC did not affect IL-2 production when the macrophages presented a synthetic peptide of the antigen to the T cells, suggesting that the drug interfered with antigen processing, not peptide presentation. The cannabinoid inhibition of the T-cell response to native lysozyme was stereoselective consistent with the involvement of a cannabinoid receptor. That is, the bioactive CP55940 diminished T-cell activation, whereas the inactive stereoisomer CP56667 did not. The macrophage hybridoma expressed mRNA for the CB2R but not for the CB1R, whereas the T cells expressed an extremely low level of mRNA for the CB2R. The CB1R-selective antagonist SR141716A did not reverse the suppression caused by THC, demonstrating that the CB1R was not responsible for the drug's inhibitory effect. In contrast, the CB2R-selective antagonist SR144528 completely blocked the THC suppression of the T-cell response. These collective results implicated the macrophages as the target of cannabinoid inhibition of antigen processing in a mode that was linked functionally to the CB2R.
Cannabinoids modulate chemotaxis of microglia
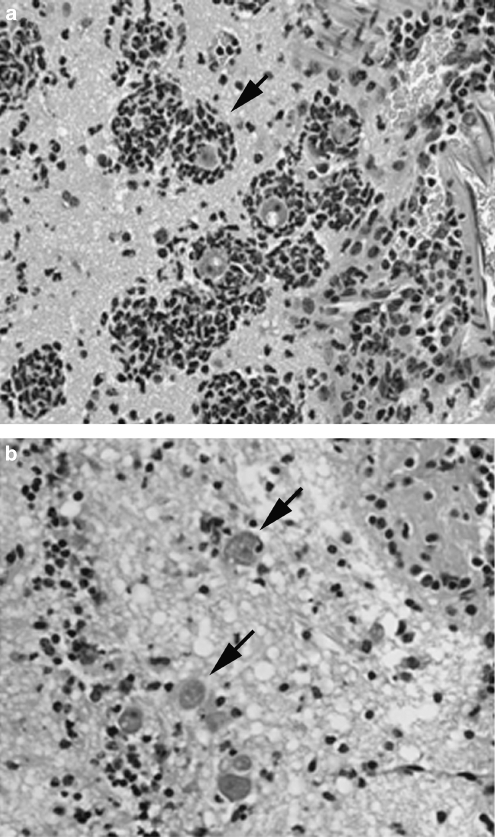
The cumulative results of immune pharmacological studies implicate the CB2R as playing a relevant functional role in the early inflammatory process by macrophages and macrophage-like cells, namely chemotaxis and antigen-processing functional attributes of these cell types when in ‘responsive' and ‘primed' states. Since microglia constitute a resident population of macrophages in the brain, exhibit phenotypic and functional properties of macrophages, and express the CB2R at maximal levels when in ‘responsive' and ‘primed' states, a ‘window' of functional relevance for the CB2R comparable to that for macrophages at peripheral sites may be operative. That is, antigen processing and/or chemotaxis by these cells may be particularly susceptible to cannabinoids in a mode linked to activation of the CB2R. To address this possibility, we have employed in vivo and in vitro rodent models of Granulomatous Amoebic Encephalitis, a chronic progressive infection of the CNS that is caused by Acanthamoeba culbertsoni (A. culbertsoni). A. culbertsoni is a free-living amoeba that can infect both immune-competent and immune-suppressed individuals (Martinez, 1993; Marciano-Cabral and Cabral, 2003) and has two morphologic forms as part of its life cycle, a trophozoite and a dormant cyst. The trophozoite is the invasive form of this protozoan. The portal of entry of A. culbertsoni may be the nasal passages, the lower respiratory tract, open wounds or ulcers in the skin, or any mucosal or serosal surface (Martinez, 1993). For brain infections, trophozoites are thought to enter either by the olfactory neuroepithelial route following the nerve pathway from the nasal mucosa to the olfactory bulb or by haematogenous spread from a primary site of infection, such as a cutaneous lesion (Martinez, 1993; Marciano-Cabral and Cabral, 2003). Once in the brain, amoebae may be destroyed by immune effector cells, such as microglia. Alternatively, amoebae may cause a subacute infection that is characterized by encystment and establishment of a chronic state associated with granuloma formation. The formation of granulomas around amoebae is thought to play a role in limiting dissemination. Although the incubation period for Acanthamoeba spp. infections is unknown, several weeks may be necessary to establish clinical signs. The relatively prolonged course of neuropathological events associated with Granulomatous Amoebic Encephalitis in rodent animal models affords the opportunity to investigate the outcome of infection with sublethal levels of Acanthamoeba as well as the characterization of the cellular elements within the brain whose functional activities against this protozoan may be affected by cannabinoids. Utilizing a (B6C3)F1 mouse model of Granulomatous Amoebic Encephalitis in which trophozoites were introduced through the intranasal route to mimic a natural route of infection in humans, we demonstrated that THC exacerbated Acanthamoeba-induced neuropathogenesis. Mice treated with THC exhibited higher mortalities from infection with Acanthamoeba as compared to similarly infected vehicle control mice (Marciano-Cabral et al., 2001). Serial frozen sections of brain from vehicle-treated infected mice processed for immunofluorescence for colocalization of amoebae and macrophage-like cells utilizing hyperimmune rabbit polyclonal anti-Acanthamoeba (Marciano-Cabral et al., 2000) and rat monoclonal anti-Mac-1 (anti-CD11b/CD18) antibody were found to contain few amoebae (Cabral and Marciano-Cabral, 2004). In contrast, numerous Acanthamoeba were detected in brain sections from infected animals treated with THC. Staining of paired serial sections with anti-Mac-1 antibody demonstrated that Mac-1+ cells in vehicle-treated animals were abundant in focal areas of infected brain tissue. However, these focal areas contained few amoebae. In contrast, foci in brain tissue from infected, THC-treated mice were replete with amoebae but contained few Mac-1+ cells. Comparable results were obtained when paraffinized brain sections were subjected to haematoxylin and eosin staining (Figure 2, unpublished data). For vehicle-treated mice, numerous foci of individual amoebic trophozoites surrounded by clusters of cells that resembled microglia morphologically were observed. Assessment of replicate sections using isolectin B4, a marker for microglia, indicated that cells clustering around amoebae were predominantly microglia. In contrast, for THC-treated mice, individual amoebic trophozoites were dispersed in the olfactory lobe and frontal areas of the brain in the absence of immune cell aggregates. The paucity of Mac-1+ cells at focal sites of Acanthamoeba infection in the brain of mice treated with THC suggests that these immune cells either do not migrate to infected areas or are selectively targeted by the Acanthamoeba and destroyed.
Figure 2.
THC downregulates accumulation of macrophage-like cells at focal sites of Acanthamoeba in mouse brain. (B6C3)F1 mice were treated once intraperitoneally with THC (25 mg kg−1) or vehicle (ethanol:emulphor:saline, 1:1:18), inoculated intranasally with 3 LD50 of A. culbertsoni, killed and the brains were removed. Paraffin sections were stained with haematoxylin and eosin. (a) Section from vehicle-treated mouse depicting accumulation of macrophage-like cells around Acanthamoeba (arrow). (b) Section from THC-treated mouse depicting Acanthamoeba in the brain in the absence of macrophage-like cell accumulation (arrows). THC, delta-9-tetrahydrocannabinol.
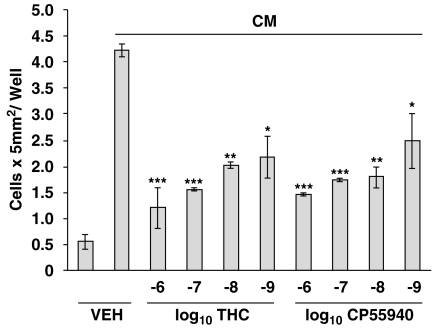
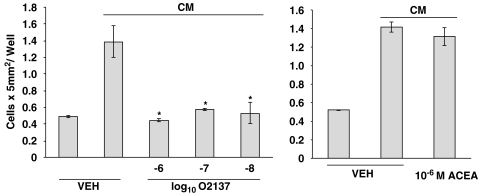
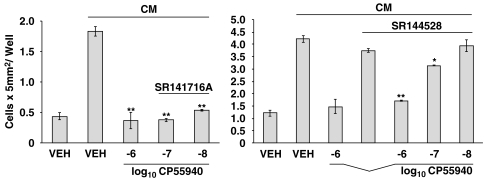
To determine whether THC exerted a direct effect on microglia, and to assess for a role of the CB2R in this process, in vivo studies were complemented with those in vitro. Acanthamoeba were maintained in culture for 24 h to generate an Acanthamoeba-conditioned medium (CM) that harbours proteases and other factors released from amoebae that serve as chemotactic stimuli for attracting microglia. THC treatment of neonatal rat cerebral cortex microglia in vitro resulted in a significant inhibition of the migratory response to CM (Figure 3, unpublished data). Experiments performed with THC were replicated using CP55940, the full agonist at the CB1R and CB2R. Again, treatment of microglia with CP55940 resulted in a significant concentration-related decrease in migration in response to CM. The concentration-related inhibitory effect of THC and CP55940 on the migratory response of neonatal rat cerebral cortex microglia to CM implicated a role for a cannabinoid receptor. Thus, to obtain insight as to the cannabinoid receptor linked to the inhibitory effect, microglia were treated with compounds exhibiting selective high affinity binding to the CB1R or the CB2R antecedent to assessment of the migratory response (Figure 4, unpublished data). Treatment of microglia with the highly selective CB2R ligand O-2137 resulted in a profound and significant inhibition in the migratory response to CM. In contrast, the CB1R-specific ligand ACEA exerted a minimal inhibitory effect on the microglial migratory response to CM. To confirm the data indicating that activation of the CB2R with a cannabinoid receptor selective ligand exerted a major inhibitory effect on the migratory response to CM, cannabinoid receptor agonist–antagonist experiments were performed (Figure 5, unpublished data). Treatment of microglia with the CB1R antagonist SR141716A did not block the inhibitory effect of CP55940. In contrast, treatment of microglia with the CB2R-specific antagonist SR144528 resulted in a reversal of the inhibitory effect of CP55940 indicating that the cannabinoid-mediated inhibition of the CM-stimulated microglial response was linked, at least in part, to the CB2R.
Figure 3.
THC and CP55940 inhibit chemotaxis of microglia. Microglia were isolated from neonatal Sprague–Dawley rats and purified as described (Waksman et al., 1999), treated (3 h) with cannabinoid or vehicle (0.01% ethanol) and assessed (2 h) for migration against CM. The CB1R/CB2R partial agonist THC has a Ki=46 nM at the CB2R, while the potent full agonist CP55940 has a Ki=0.9 nM at the CB2R. *P<0.05, **P<0.01, ***P<0.001. n=3/group. CB1R, CB1 receptor; CB2R, CB2 receptor; CM, Acanthamoeba-conditioned medium; THC, delta-9-tetrahydrocannabinol.
Figure 4.
The CB2R, but not the CB1R, agonist inhibits chemotaxis of microglia. Microglia were treated (3 h) with cannabinoid or vehicle (0.01% ethanol) and assessed (2 h) for migration against CM. O2137: CB1R Ki=2700 nM, CB2R Ki=11 nM; ACEA CB1R Ki=1.4 nM, >1400-fold selectivity over the CB2R. *P<0.05. n=3 per group. ACEA, (N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide; CB1R, CB1 receptor; CB2R, CB2 receptor; CM, amoeba-conditioned medium.
Figure 5.
The CB2R antagonist reverses CP55940-mediated inhibition of chemotaxis of microglia. Microglia were treated (1 h) with antagonist (10−6 M) or vehicle (VEH), treated (30 min) with CP55940 or vehicle and assessed (2 h) for migration against CM. *P<0.05, **P<0.01. n=3 per group. CB2R, CB2 receptor; CM, amoeba-conditioned medium.
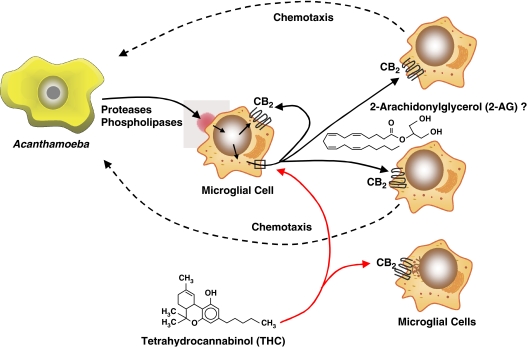
The mode by which THC and other exogenous cannabinoids such as CP55940 inhibit the chemotactic response of microglia to Acanthamoeba remains to be defined. However, it is known that Acanthamoeba produce proteases, phospholipases and other factors (Marciano-Cabral and Cabral, 2003) that may act on phospholipids in microglial membranes, generating cleavage products (Cabral, 2005). It is postulated that bioactive lipid mediators thus generated include the endocannabinoid 2-AG that serves to drive chemotaxis by autocrine and/or paracrine activation of the CB2R. The exogenous cannabinoid THC may inhibit this chemotactic response by superimposing a signal transductional activation of the CB2R. That is, THC could inhibit the synthesis and/or release of 2-AG or, alternatively, by virtue of its relative long half-life as compared to that of 2-AG, preclude this endocannabinoid from ligating to the CB2R. A proposed model of the role of the CB2R in modulation of the microglial chemotactic response to Acanthamoeba is shown in Figure 6.
Figure 6.
Model of role of CB2R in modulation of microglial chemotactic response to Acanthamoeba. Acanthamoeba elicit proteases, phospholipases and other factors that serve to generate cleavage products of phospholipids in microglial membranes through the action of phospholipases. It is postulated that bioactive lipid mediators thus generated include the endocannabinoid 2-AG that serves to drive chemotaxis of microglia by autocrine and/or paracrine activation of the CB2R. The exogenous cannabinoid THC may inhibit this chemotactic response by superimposing its effect on 2-AG by inhibiting its synthesis and/or release or by exerting a relatively long-lasting ligation to the CB2R. 2-AG, 2-arachidonylglycerol; CB2R, CB2 receptor; THC, delta-9-tetrahydrocannabinol.
Conclusion
The CNS is a complex arena that consists of a diverse group of cell types, including neurons, oligodendrocytes, microglia and astrocytes. While astrocytes are the predominant cell type of the CNS, microglia are the resident macrophages of the brain and provide the first line of defense against injury, assault and/or infection. These myeloid lineage cells also play an important role in remodelling and regeneration of the CNS. The combined cellular functions of both astrocytes and microglia form the innate immune system of the CNS. Although the CNS is a highly sophisticated network of checks and balances, it possesses a vulnerability to a multitude of neurodegenerative and neuroinflammatory processes. Some of these include Alzheimer's disease, Parkinson's Disease, MS, amyotrophic lateral sclerosis and HIV-associated dementia. The pathological hallmark of these diseases is chronic inflammation induced by persistent cell activation and elicitation of proinflammatory mediators (that is, NO, cytokines and chemokines). Studies have shown that microglia and microglia-derived cells are the major cell types responsible for this neuroinflammation. For example, during the first stages of brain inflammation, microglia-derived macrophages play a key role, and their presence is the consequential step in the neurodegeneration that follows (Ashton et al., 2007). Studies performed using immunohistochemistry have demonstrated that activated microglia are detected in senile plaques of Alzheimer's disease patients (Ramirez et al., 2005). In addition, activated microglia, depicted by a change from a ramified morphology to an amoeboid morphology, have been detected in the spinal cords of MS and amyotrophic lateral sclerosis patients immediately following death (Yiangou et al., 2006).
During activation, microglia upregulate an array of cell-surface receptors that may be critical in microglial regeneration and/or degeneration of the CNS. Included among these are immunoglobulin (Ig) superfamily receptors, complement receptors, toll-like receptors, cytokine/chemokine receptors, opioid receptors and cannabinoid receptors. Microglia have been found to express both the CB1R and CB2R in vitro (Carlisle and Cabral, 2002; Carrier et al., 2005) and to produce the endocannabinoids 2-AG as well as anandamide in lesser quantities (Carrier et al., 2004). Thus, these cells appear to harbour a fully constituted system of endogenous cannabinoid ligands and cognate receptors. Activation of the CB2R on these cells appears to promote migration and proliferation. Walter et al. (2003) demonstrated that 2-AG induced migration of microglia and that this occurred through the CB2R and abnormal-CBD-sensitive receptors, with subsequent activation of the extracellular signal-regulated kinase 1/2 signal transduction pathway. These investigators also demonstrated that microglia expressed the CB2R at the leading edge of lamellipodia, consistent with their involvement in cell migration.
There is accumulating evidence that the CB2R is also expressed in the CNS. Van Sickle et al. (2005) reported the presence of CB2R mRNA and protein in brainstem neurons. Furthermore, the CB2Rs were found to be activated by the cognate agonist 2-AG and by elevated endogenous levels of endocannabinoids that also signal through the CB1R. In addition, Fernandez-Ruiz et al. (2007), using a variety of neurodegenerative disease models, reported the expression of the CB2R in microglia, astrocytes and neuron sub-populations. This expression of the CB2R in vivo apparently is attributed, in large measure, to microglia. In several neurodegenerative diseases, upregulation of microglial CB2R has been observed (Zhang et al., 2003; Benito et al., 2005, 2007; Maresz et al., 2005; Yiangou et al., 2006; Ashton et al., 2007). In addition, CB2R-positive microglia have been identified dispersed within active MS plaques and localized in the periphery of chronic active plaques (Benito et al., 2007).
The collective findings refute the concept that the only cannabinoid receptor that has a functionally relevant role in the CNS is the CB1R. The current data indicate that the CB2R can also be present in the CNS and that its expression is associated with a variety of inflammatory processes. This expression is manifest primarily when microglia are in ‘responsive' and primed' states of activation, signature activities of which include cell migration and antigen processing. It has been proposed that the role of the CB2R in immunity in the CNS is primarily that of anti-inflammatory (Carrier et al., 2005). In this context, this receptor has the potential to serve as a therapeutic target for appropriately designed CB2R-specific ligands that could act as anti-inflammatory agents in MS and other neuropathological processes. For example, in Theiler's virus infection of mouse CNS, an animal model for human MS, it was demonstrated that the synthetic cannabinoids WIN55,212-2, ACEA and JWH-015 improved neurological deficits, and reduced microglial activation, MHC class II expression and T-lymphocyte infiltration (Arevalo-Martin et al., 2003). Thus, selective targeting of the CB2R could lead to ablation of neuropathological processes while minimizing psychotropic effects that could be exerted by activation of the CB1R.
Acknowledgments
These studies were supported in part by NIH/NIDA awards DA05832 and DA05724. We thank Dr Billy Martin of the Department of Pharmacology and Toxicology, Virginia Commonwealth University for providing cannabinoids used in this study.
Abbreviations
- ACEA
(N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide
- 2-AG
2-arachidonylglycerol
- AIDS
acquired immune deficiency syndrome
- CM
A. culbertsoni-conditioned medium
- CP55940
(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- HIV1
human immunodeficiency virus type 1
- IL
interleukin
- MS
multiple sclerosis
- NO
nitric oxide
- O-2137
1-[4-(1,1-Dimethylheptyl)-2,6-dimethoxyphenyl]-3-methylcyclohexanol
- SR141716A
(5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide hydrochloride
- SR144528
((1S-endo)-5-(4-Chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)-N-(1,3,3-trimethylbicyclo(2.2.1)hept-2-yl)-1H-pyrazole-3-carboxamide
- THC
delta-9-tetrahydrocannabinol
Conflict of interest
The authors state no conflict of interest.
References
- Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Ria F, Penna G, Adorini L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J Immunol. 1998;160:4671–4680. [PubMed] [Google Scholar]
- Arevalo-Martin A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Becker EL. Stimulated neutrophil locomotion: chemokinesis and chemotaxis. Arch Pathol Lab Med. 1977;101:509–513. [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, et al. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–2536. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Romero JP, Tolon RM, Clemente D, Docagne F, Hillard CJ, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997a;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Cytokines: influence on glial cell gene expression and function. Chem Immunol. 1997b;69:31–75. doi: 10.1159/000058653. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;37:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Cabral GA. Lipids as bioeffectors in the immune system. Life Sci. 2005;77:1699–1710. doi: 10.1016/j.lfs.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Marciano-Cabral F. Cannabinoid-mediated exacerbation of brain infection by opportunistic amebae. J Neuroimmunol. 2004;147:127–130. doi: 10.1016/j.jneuroim.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Carlisle SJ, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. International Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Patel S, Hillard CJ. Endocannabinoids in neuroimmunology and stress. Curr Drug Targets CNS Neurol Disord. 2005;4:657–665. doi: 10.2174/156800705774933023. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. Mechanisms of disease: the many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Daaka Y, Klein TW, Friedman H. Expression of cannabinoid receptor mRNA in murine and human leukocytes. Adv Exp Med Biol. 1995;373:91–96. doi: 10.1007/978-1-4615-1951-5_13. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, et al. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Mattiace LA, Kure K, Hutchins K, Lyman WD, Brosnan CF. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991;64:135–156. [PubMed] [Google Scholar]
- Eilbott DJ, Peress N, Burger H, La Neve D, Orensteins J, Gendelman HE, et al. Human immunodeficiency virus type I in spinal cords of acquired immune deficiency syndrome patients with myelopathy: expression and replication in macrophages. Proc Natl Acad Sci USA. 1989;86:3337–3341. doi: 10.1073/pnas.86.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur J Pharmacol. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gehrmann J. Microglia: a sensor to threats in the nervous system? Res Virol. 1996;147:79–88. doi: 10.1016/0923-2516(96)80220-2. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immune effector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- Germain RN. The ins and outs of antigen processing and presentation. Nature. 1986;322:687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LN, Lachman LB. IL-1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DW, Goetzl EJ. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982;129:1600–1604. [PubMed] [Google Scholar]
- Gorin FA, Bale JF, Halks-Miller M, Schwartz RA. Kaposi's sarcoma metastatic to the CNS. Arch Neurol. 1985;42:162–165. doi: 10.1001/archneur.1985.04060020076020. [DOI] [PubMed] [Google Scholar]
- Gray F, Gherardi R, Scaravilli F. The neuropathology of the acquired immune deficiency syndrome (AIDS) Brain. 1988;111:245–266. doi: 10.1093/brain/111.2.245. [DOI] [PubMed] [Google Scholar]
- Guillet JG, Lai MZ, Briner TJ, Buus S, Sette A, Grey HM, et al. Immunological self, nonself discrimination. Science. 1987;235:865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Hamilton TA, Adams DO. Molecular mechanisms of signal transduction in macrophages. Immunol Today. 1987;8:151–158. doi: 10.1016/0167-5699(87)90145-9. [DOI] [PubMed] [Google Scholar]
- Hamilton TA, Jansen MM, Somers SD, Adams DO. Effects of bacterial lipopolysaccharide on protein synthesis in murine peritoneal macrophages: Relationship to activation for macrophage tumoricidal function. J Cell Physiol. 1986;128:9–17. doi: 10.1002/jcp.1041280103. [DOI] [PubMed] [Google Scholar]
- Hanahan DJ. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Harris H. Chemotaxis of monocytes. Br J Exp Pathol. 1953;34:276–279. [PMC free article] [PubMed] [Google Scholar]
- Harris H. Role of chemotaxis in inflammation. Physiol Rev. 1954;34:529–562. doi: 10.1152/physrev.1954.34.3.529. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Hereld D. Moving toward understanding eukaryotic chemotaxis. Eur J Cell Biol. 2006;85:905–913. doi: 10.1016/j.ejcb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res. 2006;34:211–227. doi: 10.1385/IR:34:3:211. [DOI] [PubMed] [Google Scholar]
- Keller HU, Wissler JH, Hess MW, Cottier H. Distinct chemokinetic and chemotactic responses in neutrophil granulocytes. Eur J Immunol. 1978;8:1–7. doi: 10.1002/eji.1830080102. [DOI] [PubMed] [Google Scholar]
- Kim CH. Chemokine-chemokine receptor network in immune cell trafficking. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:343–361. doi: 10.2174/1568008043339712. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Lang W, Burger PC, Budka HJ, Vogt M, Maurier R, et al. Progressive diffuse leukoencephalopathy in patients with acquired immune deficiency syndrome (AIDS) Acta Neuropathol (Berlin) 1985;68:333–339. doi: 10.1007/BF00690837. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein TM, dal Canto MC, Pezeshkpour GH, Yungbluth M, et al. Detection of AIDS virus in macrophages brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen ISY. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia, the first line of defense in the brain pathologies. Drug Res. 1995;45:357–360. [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kure K, Lyman WD, Weidenheim KM, Dickson DW. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990;136:1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- Leong S, Ling E. Ameboid and ramified microglia: their interrelationship and response to brain injury. Glia. 1992;6:39–47. doi: 10.1002/glia.440060106. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005a;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005b;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Lynn AB, Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier H, Budka H, Lassmann H, Pohl P. Vacuolar myelopathy with multinucleated giant cells in the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1989;78:497–503. doi: 10.1007/BF00687711. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F, Cabral GA. Acanthamoeba spp. As agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F, Ferguson T, Bradley SG, Cabral G. Delta-9-tetrahydrocannabinol (THC), the major psychoactive component of marijuana, exacerbates brain infection by Acanthamoeba. J Eukaryot Microbiol. 2001;48:4S–5S. doi: 10.1111/j.1550-7408.2001.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F, Puffenbarger R, Cabral G. The increasing importance of Acanthamoeba infections. J Eukaryot Microbiol. 2000;47:29–36. doi: 10.1111/j.1550-7408.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Martinez AJ. Free living amebas: infection of the central nervous system. The Mount Sinai J Med. 1993;60:271–278. [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Ohmori K, Fujiwara M. Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system. J Neuroimmunol. 1992;37:23–33. doi: 10.1016/0165-5728(92)90152-b. [DOI] [PubMed] [Google Scholar]
- McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J Pharmacol Exptl Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- McCoy KL, Schwartz RH. The role of intracellular acidification in antigen processing. Immunol Rev. 1988;106:129–147. doi: 10.1111/j.1600-065x.1988.tb00777.x. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Chen ISY. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Michaels J, Price RW, Rosenblum MK. Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: proliferation, infection, and fusion. Acta Neuropathol. 1988;76:373–379. doi: 10.1007/BF00686974. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious disease. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Perry SW. Organic mental disorders caused by HIV: update on early diagnosis and treatment. Am J Psychiatry. 1990;147:696–710. doi: 10.1176/ajp.147.6.696. [DOI] [PubMed] [Google Scholar]
- Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Raborn ES, Marciano-Cabral F, Buckley NE, Martin BR, Cabral GA.The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor J NeuroImmun Pharmacol 2007(in press) [DOI] [PMC free article] [PubMed]
- Ramirez BG, Blazquez C, Gomez DP, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DM, Perry V, Andersson P, Gordon S. Mitosis and apoptosis of microglia in vivo induced by an anti-CR3 antibody which crosses the blood-brain barrier. Neuroscience. 1993;56:529–533. doi: 10.1016/0306-4522(93)90353-h. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letters. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq J, Casellas P, Congy C, et al. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Rogers J, Luber-Nardo J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, et al. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159:97–105. doi: 10.1016/j.jneuroim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Schiffmann E, Corcoran BA, Wahl SM. N-formyl-methionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Ann Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Inhibitors of lysosomal function. Meth Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with Δ9-tetrahydrocannabinol on cannabinoid-stimulated [35]GTPγS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Bonner TI, Bhat NR, Matsuda LA. Expression of the CB1 cannabinoid receptor in macrophage-like cells from brain tissue: Immunocytochemical characterization of fusion protein antibodies. J Neuroimmunol. 1998;82:13–21. doi: 10.1016/S0165-5728(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14:403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Spencer DC, Price RW. Human immunodeficiency virus and the central immune system. Ann Rev Microbiol. 1992;46:655–693. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Liu Y, Goligorsky MS. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem. 1996;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Salzet M, Rialas CM, Mattocks D, Fimiani C, Bilfinger TV. Macrophage behavior associated with acute and chronic exposure to HIV GP120, morphine and anandamide: endothelial implications. Int J Cardiol. 1998;64 (Suppl 1):S3–S13. doi: 10.1016/s0167-5273(98)00030-8. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Wei X, Martin BR. Characterization and autoradiographic localization of the cannabinoid binding site in rat brain using [3H]11-OH-Δ9-THC-DMH. J Pharmacol Exp Ther. 1992;263:1383–1390. [PubMed] [Google Scholar]
- Unanue ER, Allen PM. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987;236:551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Van der Drift AC, van Noort JM, Kruse J. Catheptic processing of protein antigens: enzymic and molecular aspects. Seminars Immunol. 1990;2:255–271. [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A, Walter L, Wacker J, Moller T, Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci USA. 2004;101:3214–3219. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]