Abstract
Cannabinoid type-1 (CB1) and type-2 (CB2) receptors belong to the family of G protein-coupled receptors and mediate biological effects of phyto-derived and endogenous cannabinoids. Whereas functions of CB1 receptor have been extensively studied, the CB2 receptor has emerged over the last few years as a critical player in regulation of inflammation, pain, atherosclerosis and osteoporosis. Therefore, although still at a preclinical stage, the development of selective CB2 molecules has gained of interest as new targets in drug discovery. Recent data have unravelled a key role of CB2 receptors during chronic and acute liver injury, including fibrogenesis associated to chronic liver diseases, ischaemia-reperfusion-induced liver injury, and hepatic encephalopathy associated to acute liver failure. This review summarizes the latest advances on the recently identified role of CB2 receptors in the pathophysiology of liver diseases.
Keywords: cannabinoid receptors, liver fibrosis, cirrhosis, ischaemia/reperfusion injury, liver diseases
Introduction
Although phytocannabinoids have long been used for recreational and therapeutic purposes, understanding the mechanisms involved came with the identification of an endocannabinoid system comprising at least two specific G-protein-coupled receptors (cannabinoid type-1 (CB1) and type-2 (CB2)), their endogenous ligands and a machinery dedicated to endocannabinoid synthesis and degradation (for reviews, see Mallat and Lotersztajn, 2006; Pacher et al., 2006; Mallat et al., 2007).
Over the last few years, the CB2 receptor has emerged as a critical target for regulation of inflammation, pain, bone loss and more recently in liver pathophysiology, including fibrogenesis associated with chronic liver diseases, ischaemia/reperfusion (I/R)-induced liver injury and hepatic encephalopathy-associated with acute liver failure. Therefore, although still at a preclinical stage, the development of selective CB2 molecules has gained interest as new targets in drug discovery.
The gene encoding the human CB2 receptor encodes a 360-amino-acid protein, which shares 44% homology to the CB1 receptor (Munro et al., 1993), and displays distinct but overlapping distribution. CB2 receptors show rather low levels of expression compared to CB1 receptors, predominating in cells of the immune system. In addition, expression of CB2 receptors is increasingly detected in a variety of peripheral tissues, particularly under pathological conditions. Intracellular CB2-dependent signalling pathways include Gi/o-dependent inhibition of adenylyl cyclase, stimulation of mitogen-activated protein kinase and phosphoinositide 3-kinase pathways, and activation of de novo ceramide production or cyclooxygenase-2 (COX-2) induction (Guzman et al., 2001).
Among the growing family of endocannabinoids identified (for reviews, see Piomelli et al., 2000; Di Marzo et al., 2004), 2-arachidonoylglycerol (2-AG) has been proposed to serve as the endogenous CB2 ligand, although it is also a full agonist for CB1 receptors. 2-AG is synthesized from diacylglycerol lipase and degraded intracellularly by monoacylglycerol lipase. CB2 receptors may also bind other endocannabinoid ligands; however, the signalling consequences of this binding is poorly known (Piomelli et al., 2000; Di Marzo et al., 2004).
Identification of functions associated with CB2 receptors has gained from the development of pharmacological agents specifically targeting CB2 receptors, including agonists (JWH-133 and analogues, HU-308 and AM 1241) and antagonists (SR 144528, AM 630) (Rinaldi-Carmona et al., 1998; Pertwee, 1999; Huffman, 2005), as well as from the availability of mice invalidated for CB2 (Buckley et al., 2000) However, hepatic effects of the CB1 and CB2 receptors have long remained unknown, probably owing to their low level of expression in the normal liver. Nonetheless, recent studies unravelled the major role of the endocannabinoid system in various liver pathological conditions (Mallat and Lotersztajn, 2006; Mallat et al., 2007).
Hepatic effects of CB2 receptors
The embryo liver shows a high expression of CB2 receptor that declines during development (Buckley et al., 1998). In contrast, adult normal liver shows a faint expression of CB2 receptors, although substantial amounts of 2-AG are produced (Kondo et al., 1998). Nevertheless, it has recently been shown that hepatic CB2 receptors are highly upregulated in several pathological conditions, including experimental and human liver fibrogenesis (Julien et al., 2005) and acute experimental I/R injury (Batkai et al., 2007). These observations stimulated investigations as to their pathophysiological significance.
Antifibrogenic properties of CB2 receptors
Chronic liver injury is associated with liver fibrogenesis, ultimately leading to cirrhosis (Lotersztajn et al., 2005). The latter is a prominent cause of morbidity and mortality worldwide with about 800 000 deaths per year, owing to complications of portal hypertension and liver failure, and to a high incidence of hepatocellular carcinoma. In western countries, prevailing causes of cirrhosis include chronic alcohol consumption, chronic hepatitis C infection, and, increasingly, non-alcoholic steatohepatitis (Lotersztajn et al., 2005).
Liver fibrogenesis is driven by a heterogeneous population of non-parenchymal cells expressing smooth muscle α-actin that originate from hepatic stellate cells and hepatic myofibroblasts. In response to chronic liver injury, both cell types proliferate and accumulate in injured areas, synthesize fibrogenic cytokines (that is, transforming growth factor-β1, TGFβ1), growth factors, chemokines, fibrosis components and inhibitors of matrix degradation (Lotersztajn et al., 2005). The frequent inability to eradicate the cause of chronic liver disease warrants the development of liver-specific antifibrotic strategies. Strategies targeting fibrogenic cells have been extensively investigated and schematically aim at (i) reducing fibrogenic cell accumulation by growth inhibitory or proapoptotic compounds, and/or (ii) reducing fibrosis through inhibition of extracellular matrix synthesis or enhancement of its degradation. In addition, inhibition of parenchymal injury or reduction of liver inflammation has also shown some beneficial antifibrogenic effects (Lotersztajn et al., 2005). However, despite encouraging experimental results, proof of efficacy of potential antifibrogenic molecules in a clinical setting is currently lacking.
We recently showed that CB1 and CB2 receptors are marginally expressed in the normal liver and undergo marked upregulation in the cirrhotic human liver, predominating in hepatic myofibroblasts (Julien et al., 2005), an observation that was subsequently confirmed by others (Xu et al., 2006). These findings led us to investigate the function of CB2 receptors during liver fibrogenesis, owing to the use of an established liver fibrosis model induced by chronic carbon tetrachloride intoxication. Mice invalidated for CB2 receptors developed significantly enhanced liver fibrosis compared to wild-type mice, as assessed by morphometric analysis of Sirius red-stained slides, and by quantification of collagenous proteins in the liver. Enhanced fibrosis was associated with an increased density of hepatic myofibroblasts, as shown by enhanced expression of smooth muscle α-actin (Julien et al., 2005). The mechanisms involved were further investigated in cell cultures of liver fibrogenic cells. Activation of CB2 receptors in hepatic myofibroblasts and activated hepatic stellate cells reduced cell accumulation by triggering both growth inhibition and apoptosis (Julien et al., 2005). We further demonstrated that CB2-dependent growth inhibition is mediated by a COX-2 pathway, and that CB2-dependent apoptosis involves oxidative stress. Indeed, THC increased COX-2 protein expression and activity, and inhibition of COX-2 by a selective COX-2 inhibitor blunted the growth-inhibitory effect of THC (Julien et al., 2005). These observations corroborated our previous data demonstrating the central role of COX-2 in growth inhibition of hepatic myofibroblasts (Mallat et al., 1995, 1998; Gallois et al., 1998; Davaille et al., 2000). In contrast, apoptotic concentrations of THC stimulated production of reactive oxygen species, and, accordingly, CB-receptor-mediated apoptosis was blocked by antioxidants (Julien et al., 2005).
Endogenous ligands of hepatic CB2 receptors remain to be identified among the increasing number of endocannabinoids. In this respect, we showed that 2-AG exerts CB1- and CB2-independent apoptotic effects in liver fibrogenic cells (Julien et al., 2005), thereby suggesting that other endocannabinoids may be responsible for CB2 activation during liver fibrogenesis. Alternatively, CB2 receptors might be constitutively activated, independently of ligand occupation of the receptor (Portier et al., 1999).
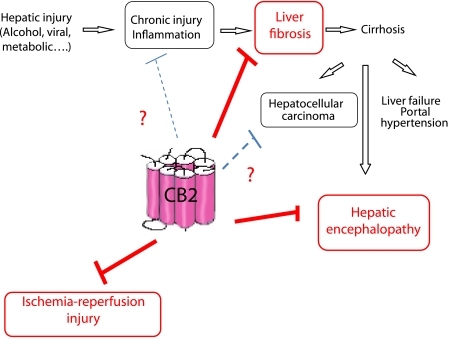
Taken together, our results indicate that during experimental liver fibrogenesis, endogenous activation of CB2 receptors limits progression of fibrosis by reducing accumulation of liver fibrogenic cells (Julien et al., 2005). These findings identify CB2 receptors as a potential novel therapeutic target for the control of fibrogenesis during chronic liver diseases (Figure 1). In a parallel study, we also demonstrated that CB1 receptors accelerate liver fibrogenesis (Hezode et al., 2005; Teixeira-Clerc et al., 2006), following enhanced accumulation of hepatic myofibroblasts. Overall, our data therefore suggest that combined therapy with selective CB1 antagonists and CB2 agonists might open new therapeutic avenues for the treatment of liver fibrosis.
Figure 1.
CB2 receptors as novel targets for the treatment of liver diseases.
CB2 activation protects against liver I/R injury
I/R injury is a pathophysiologic process observed in several conditions, whereby hypoxic organ damage is accentuated following return of blood flow and oxygen delivery to the compromised tissue. Transient episodes of hepatic ischaemia occur during organ transplantation, trauma, hypovolaemic shock and selective liver resection, following inflow occlusion or total vascular exclusion. The pathophysiology of liver I/R injury includes both direct cellular damage as a result of the ischaemic insult, as well as delayed dysfunction and damage resulting from activation of inflammatory pathways. Histopathologic changes include cellular swelling, vacuolization, endothelial cell disruption, neutrophil infiltration and hepatocellular necrosis. Key events of the inflammatory reaction associated with liver damage include activation of Kupffer cells leading to oxidative stress, upregulation of the inducible nitric oxide synthase in hepatocytes, activation of c-jun terminal kinase, upregulation of proinflammatory cytokines and neutrophil accumulation.
Recent data have shown that CB2 receptors play a key role in the limitation of liver injury induced by I/R (Batkai et al., 2007). Indeed, the reperfusion phase is associated with an increase in hepatic synthesis of anandamide and 2-arachidonoyl glycerol. Parenchymal and non-parenchymal cells, including hepatocytes, Kupffer and endothelial cells contribute to enhanced endocannabinoid synthesis, as shown in isolated cell fraction from I/R-injured mice. Administration of the selective CB2 agonist, JWH-133, prior to the vascular occlusion protected against hepatic I/R injury, by a complex mechanism involving decreased inflammatory cell infiltration, reduced lipid peroxidation and expression of proinflammatory cytokines, chemokines and adhesion molecules. Moreover, mice invalidated for CB2 receptors displayed enhanced liver injury and inflammation following I/R (Batkai et al., 2007). Liver cells overexpressing CB2 receptors during I/R injury were not identified in this study. However, experiments in cultured sinusoidal endothelial cells indicated that CB2 activation reduced tumour necrosis factor alpha-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression and decreased adhesion of neutrophils to endothelial cells. Taken together, these data suggest that CB2 agonists may offer novel perspectives in prevention of hepatic I/R injury.
Hepatic encephalopathy
Hepatic encephalopathy is a major complication of chronic or acute liver failure. Pathogenesis remains partially understood, and involves multiple mechanisms, including altered production of neurotransmitters, astrocyte dysfunction and abnormalities in cerebral perfusion. A recent study showed that brain levels of CB2 receptors and of its ligand 2-AG are increased in the model of fulminant liver failure induced by thioacetamide (Avraham et al., 2005; Dagon et al., 2007). Moreover, neurological dysfunction was improved both by the non-selective CB1/CB2 agonist Δ9-THC (Dagon et al., 2007) and the CB2 agonists HU308 and 2AG, as well as by the CB1 antagonist rimonabant (Avraham et al., 2005). The neuroprotective effect of Δ9-THC was not associated with an improvement of liver functions, and was lost in CB2 KO mice, suggesting that neuroprotection results from a direct effect of THC on brain CB2 receptors. CB2-mediated improvement of cerebral dysfunction was related to stimulation of brain AMP-activated protein kinase (Dagon et al., 2007), a key regulator of energy balance that also controls cognitive function via regulation of neurogenesis and neuroapoptosis. These results identify CB2 as a novel protective target of encephalopathy. However, further studies are awaited to fully clarify the respective contribution of CB1 and CB2 receptors in this process.
In conclusion, recent studies have unravelled pleiotropic functions of CB2 receptors under physiological and pathological conditions, including acute and chronic liver diseases (Figure 1). Additional functions may soon arise, since recent studies have reported an upregulation of CB2 receptors in non-alcoholic fatty liver disease and in hepatocellular carcinoma (Xu et al., 2006). These findings may open novel therapeutic avenues, upon clinical development of CB2-specific molecules.
Acknowledgments
This work was supported by the INSERM, the Université Paris-Val-de-Marne, and by grants (to SL) of the Agence Nationale de la Recherche, Fondation pour la Recherche Médicale and Sanofi-Aventis.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- COX-2
cyclooxygenase-2
- I/R injury
ischaemia/reperfusion injury
Conflicts of interest
Part of the work described in this review was supported by a grant from Sanofi-Aventis (to SL).
References
- Avraham Y, Israeli E, Gabbay E, Okun A, Zolotarev O, Silberman I, et al. Endocannabinoids affect neurological and cognitive function in thioacetamide induced hepatic encephalopathy in mice. Neurobiol Dis. 2005;12:12. doi: 10.1016/j.nbd.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, Hansson S, Harta G, Mezey E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience. 1998;82:1131–1149. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- Buckley NE, Mccoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Ilan Y, Mechoulam R, Berry EM.Cannabinoids ameliorate cerebral dysfunction following liver failure via AMP-activated protein kinase FASEB J 2007(in press) [DOI] [PubMed]
- Davaille J, Gallois C, Habib A, Li L, Mallat A, Tao J, et al. Antiproliferative properties of sphingosine 1-phosphate in human hepatic myofibroblasts. A cyclooxygenase-2 mediated pathway. J Biol Chem. 2000;275:34628–34633. doi: 10.1074/jbc.M006393200. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Gallois C, Habib A, Tao J, Moulin S, Maclouf J, Mallat A, et al. Role of NF-kappaB in the antiproliferative effect of endothelin-1 and tumor necrosis factor-alpha in human hepatic stellate Cells. Involvement of cyclooxygenase-2. J Biol Chem. 1998;273:23183–23190. doi: 10.1074/jbc.273.36.23183. [DOI] [PubMed] [Google Scholar]
- Guzman M, Galve-Roperh I, Sanchez C. Ceramide: a new second messenger of cannabinoid action. Trends Pharmacol Sci. 2001;22:19–22. doi: 10.1016/s0165-6147(00)01586-8. [DOI] [PubMed] [Google Scholar]
- Hezode C, Roudot-Thoraval F, Nguyen S, Grenard P, Julien B, Zafrani ES, et al. Daily cannabis smoking as a risk factor for fibrosis progression in chronic hepatitis C. Hepatology. 2005;42:63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–649. doi: 10.2174/1389557054368844. [DOI] [PubMed] [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerc F, Tran-Van-Nhieu J, Li L, Karzak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, et al. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through Ca2+-dependent and -independent mechanisms. FEBS Lett. 1998;429:152–156. doi: 10.1016/s0014-5793(98)00581-x. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Ann Rev Pharmacol Toxicol. 2005;45:605–628. doi: 10.1146/annurev.pharmtox.45.120403.095906. [DOI] [PubMed] [Google Scholar]
- Mallat A, Fouassier L, Preaux AM, Gal CS, Raufaste D, Rosenbaum J, et al. Growth inhibitory properties of endothelin-1 in human hepatic myofibroblastic Ito cells. An endothelin B receptor mediated pathway. J Clin Invest. 1995;96:42–49. doi: 10.1172/JCI118052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat A, Gallois C, Tao J, Habib A, Maclouf J, Mavier P, et al. Platelet-derived growth factor-BB and thrombin generate positive and negative signals for human hepatic stellate cell proliferation. Role of a prostaglandin/cyclic AMP pathway and cross-talk with endothelin receptors. J Biol Chem. 1998;273:27300–27305. doi: 10.1074/jbc.273.42.27300. [DOI] [PubMed] [Google Scholar]
- Mallat A, Lotersztajn S. Endocannabinoids as novel mediators of liver diseases. J Endocrinol Invest. 2006;29:58–65. [PubMed] [Google Scholar]
- Mallat A, Teixeira-Clerc F, Deveaux V, Lotersztajn S. Cannabinoid receptors as new targets of antifibrosing strategies during chronic liver diseases. Expert Opin Ther Targets. 2007;11:403–409. doi: 10.1517/14728222.11.3.403. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Piomelli D, Giuffrida A, Calignano A, Rodriguez De Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- Portier M, Rinaldi-Carmona M, Pecceu F, Combes T, Poinot-Chazel C, Calandra B, et al. SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J Pharmacol Exp Ther. 1999;288:582–589. [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, Tran-Van-Nhieu J, Deveaux V, Serriere-Lanneau V, et al. CB1 cannabinoid receptor antagonism: a novel strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J, et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006;171:31–38. doi: 10.1016/j.cancergencyto.2006.06.014. [DOI] [PubMed] [Google Scholar]