Abstract
Background and purpose:
Cannabinoids (CBs) are known to be vasoactive and to regulate tissue inflammation. The present study examined the in vivo vasomotor effects of the CB2 receptor agonists JWH015 and JWH133 in rat knee joints. The effect of acute and chronic joint inflammation on CB2 receptor-mediated responses was also tested.
Experimental approach:
Blood flow was assessed in rat knee joints by laser Doppler imaging both before and following topical administration of CB2 receptor agonists. Vasoactivity was measured in normal, acute kaolin/carrageenan inflamed and Freund's complete adjuvant chronically inflamed knees.
Key results:
In normal animals, JWH015 and JWH133 caused a concentration-dependent increase in synovial blood flow which in the case of JWH133 was blocked by the selective CB2 receptor antagonist AM630 as well as the transient receptor potential vanilloid-1 (TRPV1) antagonist SB366791. The vasodilator effect of JWH133 was significantly attenuated in both acute and chronically inflamed knees. Given alone, AM630 had no effect on joint blood flow.
Conclusion and implications:
In normal joints, the cannabinomimetic JWH133 causes hyperaemia via a CB2 and TRPV1 receptor mechanism. During acute and chronic inflammation, however, this vasodilatatory response is significantly attenuated.
Keywords: arthritis, blood flow, cardiovascular system, cannabinoids, CB2 receptor, endocannabinoids, knee joint, laser doppler imaging, transient potential vanilloid receptor
Introduction
Cannabis sativa is the source of at least 60 distinct alkaloids, which make up a group of compounds called cannabinoids. Cannabinoids can be categorized depending on whether they are synthetic, plant-derived (phytocannabinoids) or naturally produced in the body (endocannabinoids). The endocannabinoid system is believed to play an important role in health and disease where it can ameliorate the severity of disorders, such as pain, multiple sclerosis, schizophrenia and emesis (Di Marzo and Petrocellis, 2006; Pertwee, 2006a). Overproduction of endocannabinoids, however, could have a detrimental effect on the body, leading to problems such as obesity, infertility and inflammatory disorders. Thus, strategic control of the endocannabinoid system with selective agonists or antagonists may prove to have therapeutic value in the treatment of various diseases.
Two cannabinoid receptors have been cloned, viz. CB1 and CB2 (Matsuda et al., 1990; Munro et al., 1993). These Gi/o protein-coupled receptors are distributed throughout the body and are involved in the control of miscellaneous physiological processes, such as pain perception, appetite and vasoregulation. CB1 receptors are predominantly found on nerve terminals in the central and peripheral nervous systems (Tsou et al., 1998; Pertwee, 1999a; Farquhar-Smith et al., 2000), although they have also been localized in non-neuronal tissues, such as the pituitary gland, spleen and immunocytes (Bouaboula et al., 1993). The primary location of CB2 receptors is on immunocytes (Pertwee, 1999b, 2006a), but they have also been identified on peripheral nerves (Griffin et al., 1997) and in the central nervous system (Van Sickle et al., 2005). A plethora of cannabinoid ligands have been developed with fairly high selectivity for CB1 and CB2 receptors (Pertwee, 1999b, 2006b). Two selective CB2 receptor agonists are JWH015 ((2-methyl-1-propyl-1H-indol-3-yl)-1-napthalenylmethanone) and JWH133 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran). The Ki values for JWH015 are 383 and 13.8 nM for the CB1 and CB2 receptors, respectively (Chin et al., 1999), while JWH133 has Ki of 677 and 3.4 nM for the CB1 and CB2 receptors, respectively (Huffman et al., 1999). For antagonists, the aminoalkylindole AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl) methanone) has been shown to be an effective blocker of CB2 receptors (Hosohata et al., 1997).
Cannabinoids are known to exert potent cardiovascular effects in vivo. For example, systemic administration of the endocannabinoid anandamide, which acts on CB1 and CB2 receptors, causes hypotension and bradycardia (Varga et al., 1996; Högestatt and Zygmunt, 2002). Moreover, anandamide has been shown to produce vasodilatation in the brain (Ellis et al., 1995), liver (Garcia et al., 2001), kidney (Deutsch et al., 1997), heart (Wagner et al., 2001; Ford et al., 2002) and mesentery (Randall et al., 1996; White and Hiley, 1998; Jarai et al., 1999). A number of studies using more selective cannabinoid analogues have confirmed that CB1 receptor activation leads to relaxation of vascular smooth muscle and systemic hypotension (for review, see Randall et al., 2004). Indeed in the rat knee joint, local administration of the selective CB1 receptor agonist, arachidonyl-2-chloroethylamide causes an increase in synovial blood flow (Baker and McDougall, 2004). Less is known about the vasomotor effects of CB2 receptor agonists. Wagner et al. (2005) reported that in vitro perfusion of rat hearts with JWH133 caused an increase in coronary blood flow that was insensitive to AM630 antagonism. In vivo assessment of CB2 receptor agonists on tissue blood flow have so far not been reported.
Evidence has been emerging in recent years that suggests that cannabinoids are able to bind to and activate the transient receptor potential vanilloid-1 (TRPV1) channel. TRPV1 channels are non-selective cation channels expressed on the axon terminals of a subgroup of primary afferent neurons (Caterina et al., 1997; Szallasi, 2002). In joints, activation of TRPV1 channels by local capsaicin treatment leads to inflammatory changes, including increased synovial blood flow (Karimian et al., 1995; Varga et al., 2005). Interestingly, the CB1 receptor agonist arachidonyl-2-chloroethylamide has also been shown to act on TRPV1 channels to cause synovial hyperaemia in the rat knee (Baker and McDougall, 2004). Whether CB2 receptors are similarly coupled to TRPV1 channels has never been explored.
The current study was undertaken to assess the vasomotor effects of the two CB2 receptor agonists, JWH015 and JWH133, on knee joint blood flow in vivo. The role of TRPV1 channels in these vasoregulatory processes was also investigated. Further studies were carried out to examine the effect of joint inflammation on CB2 receptor-mediated responses using animal models of acute and chronic arthritis.
Methods
All experimental procedures received prior approval from the University of Calgary Animal Care Committee, which is in accordance with the Canadian Council for Animal Care. Adult male Wistar rats (242–495 g) were obtained from Charles River Laboratories (Montreal, Quebec, Canada) and housed in pairs at room temperature under a 12 h light–dark cycle, with free access to water and standard rat chow. Animals were randomly assigned to one of three experimental groups: naïve control, acutely inflamed and chronically inflamed.
Induction of joint inflammation
Two distinct models of arthritis were used in the present investigation based on the different phases of inflammation. Thus, the kaolin/carrageenan arthritis model produces an acute inflammatory reaction within 24 h after induction, while adjuvant monoarthritis is more suitable to assess the chronic phase of joint inflammation. Prior to arthritis induction, knee joint diameters were measured using electronic digital callipers (Mitutoyo Instruments, Tokyo, Japan) oriented medio laterally across the joint line between the femoral condyle and the tibial plateau. Diameters were compared before and upon completion of inflammation development, which is at 24 h for the kaolin/carrageenan model and at 1 week for the adjuvant monoarthritis model.
In the acutely inflamed group of rats, inflammation was induced in the right knee joint by kaolin and carrageenan. Under isoflurane anaesthesia (2–5% isoflurane; 100% O2 at 1 l min−1), 0.2 ml of 2% kaolin was injected through a 27-gauge needle into the posterior and anterior synovial cavity, followed by repeated limb extensions and flexions for 10 min to ensure adequate dispersion of the suspension within the joint and to cause articular abrasion. Subsequently, 0.2 ml of 2% carrageenan was injected into the joint by the same procedure. These animals were allowed to recover for 24 h prior to blood flow assessment.
For chronic inflammation, rats were anaesthetized with 2–5% isoflurane (100% O2 at 1 l min−1), and a localized monoarthritis was induced as previously described (Donaldson et al., 1993; McDougall et al., 1995). The right knee joint was shaved and 0.2 ml of Freund's complete adjuvant (heat-killed Mycobacterium tuberculosis, 1 mg ml−1) was injected through a 27-gauge needle into the knee joint with 0.1 ml being introduced into the posterior compartment of the joint and 0.1 ml being injected anteriorly. Animals were allowed to recover for 1 week prior to the blood flow experiments. Sham-injected animals were not tested as an intra-articular injection of sterile saline has been shown to have no effect on synovial blood flow or vasoreactivity (Karimian et al., 1995).
Surgical preparation of animals
On the day of experimentation, animals were deeply anaesthetized with urethane (25% stock solution, 2 g kg−1, i.p.). Depth of anaesthesia was confirmed by a lack of a pinch withdrawal reflex applied to the hindpaw as well as an absence of a corneal blink reflex. A longitudinal incision was made in the neck and the trachea was isolated and cannulated to permit unobstructed breathing. The right carotid artery was then isolated and cannulated with heparinized saline (100 U ml−1)-filled polyethylene tubing (Portex Fine Bore Tubing, 0.5 mm ID, 1.00 mm OD; SIMS Portex Ltd., Kent, UK). The carotid cannula was attached to a pressure transducer (1050 Pressure Transducer; Stoelting Co., Wood Dale, IL, USA) and mean arterial pressure (MAP) was recorded continuously throughout the experiment via a blood pressure monitor (Pressure Monitor BP-1; World Precision Instruments, Sarasota, FL, USA). The animal was then placed in dorsal recumbency on an electric-heating blanket (TR-100; Fine Science Tools Inc., Vancouver, British Columbia, Canada) and the internal body temperature was maintained at 37 °C as measured by a rectally inserted electronic thermometer. An ellipse of skin covering the anteromedial region of the right knee joint was removed and the underlying tissue was kept moist by regular superfusion of 37 °C physiological saline (0.9% NaCl).
Measurement of knee joint blood flow
Knee joint blood flow was measured by laser Doppler perfusion imaging (LDI) as previously described (Karimian et al., 1995; McDougall et al., 1995). The technique involved placing a laser Doppler perfusion imager (MoorLDI V.2; Moor Instruments Ltd., Devon, UK) 30 cm above the exposed rat knee and angling the scanner head to avoid reflectance artefact. A low-power (2 mW) red He–Ne laser (λ=633 nm) was directed onto the surface of the knee joint and by means of a motor-controlled mirror the laser beam scanned in a raster pattern across the joint capsule. Careful placement of opaque black cloth at the margins of the knee ensured that only the joint capsule was scanned. The image resolution was set at 42 pixels × 55 pixels with a scan speed of 4 ms pixel−1. At each pixel point, photons in the incident laser beam underwent Doppler shift by virtue of erythrocytes flowing in the tissue. These Doppler-shifted photons were captured by a photodetector in the scanner head and a perfusion value was calculated based on the velocity and concentration of erythrocytes circulating in the microvasculature. A two-dimensional colour-coded image of tissue perfusion was then constructed and stored for later offline analysis.
To achieve a more accurate appreciation of the time course for cannabinoid activity, the imager was set to the single-point measurement mode to maximize the temporal resolution of the instrument. In these experiments, the laser beam remained stationary at a discrete locus on the surface of the joint capsule, and blood flow measurements were continuously acquired. The precise positioning of the laser beam was randomly chosen, but was usually placed in the vicinity of an observable microvascular network. A steady blood flow level was measured for about 1 min before a cannabinoid agonist was topically applied to the knee joint. Blood flow was then measured continually over the succeeding 5 min. In subsequent scanning experiments, the timeline for image acquisition was designed so as to coincide with the maximal vasomotor effect of the test agents.
Experimental protocol
All test reagents were placed in a water bath and gently warmed to 37 °C. A control LDI scan of the knee was taken and then the test drug was topically applied to the surface of the joint capsule as a 0.1 ml bolus. The CB2 receptor agonists tested in this study were JWH015 (10−14–10−11 mol) and JWH133 (10−14–10−12 mol). Blood flow scans of the joint were then taken at 0, 1.5, 2, 3 and 5 min following drug application. A cumulative concentration–response curve to each agonist was generated in separate groups of animals and sequential doses of agonist were applied to the joint following each 5-min scan. In other experiments, each dose of the CB2 receptor agonist was co-administered with the selective CB2 receptor antagonist AM630 (10−8 mol; 0.1 ml bolus topical). The concentration of AM630 was based on previous successful antagonism in the rat cardiovascular system (Ford et al., 2002). To determine whether the transient potential vanilloid receptor-1 (TRPV1) is involved in CB2 receptor-mediated vasoregulation, additional experiments were undertaken in which animals were pretreated with the TRPV1 receptor antagonist SB366791. At 20 min following an intraperitoneal injection of SB366791 (500 μg kg−1), concentration–response effects of JWH133 were assessed. This treatment regimen with SB366791 has previously been shown to successfully block TRPV1 receptors in the rat knee joint (Varga et al., 2005). It should be pointed out that JWH015 proved to be a weak, non-selective agonist for the CB2 receptor and as such was neither used in the SB366791 experiments nor tested in the inflammatory models.
At the conclusion of the experiment, the animal was killed by anaesthetic overdose (pentobarbital sodium, 240 mg intracardiac) and a final scan of the joint was performed. This dead scan constituted a ‘biological zero' measurement, which corresponded to tissue optical noise and residual Brownian motion. This ‘biological zero', which was typically about 5–10% of the basal perfusion level, was subtracted from all image perfusion values prior to data processing.
Image analysis and statistics
Knee joint LDI scans were analysed by proprietary perfusion image-processing software (Moor Instruments Ltd.). For each image, an average blood flow value for the entire anteromedial knee joint capsule was calculated and expressed in arbitrary perfusion units (PUs). Blood flow changes in response to drug administration were expressed as percentage change in perfusion between control and test scans. The maximal vasomotor effect (Emax) and the EC50 were calculated from a sigmoidal nonlinear regression curve fit. All data sets conformed to a Gaussian distribution and as such were tested with parametric statistical analyses using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). ANOVA was used to assess significant differences in blood flows within (one-way ANOVA) and between (two-way ANOVA) different animal groups. A Bonferroni post hoc test was used to determine whether groups of data were significantly different at a specific concentration of cannabinoid. A significance level of P<0.05 was used for each test and all reported values represent means±s.e.m. for ‘n' observations.
Drugs and reagents
JWH015, JWH133 and AM630 were all purchased from Tocris Bioscience, Ellisville, MO, USA; SB366791, Freund's complete adjuvant, λ-carrageenan, kaolin, urethane and heparin were all obtained from Sigma-Aldrich Ltd., Oakville, Ontario, Canada. SB366791 and all cannabinoid agonists and antagonists were dissolved in dimethyl sulphoxide and cremophor, diluted to their working concentrations, aliquoted and stored at either 4 °C or −20 °C as required. The final concentration of vehicle was maintained at 2% dimethyl sulphoxide and 1% cremophor for each concentration. This vehicle formulation has previously been found to have no significant effect on joint blood flow (Baker and McDougall, 2004).
Results
CB2 receptor effects in normal knee joints
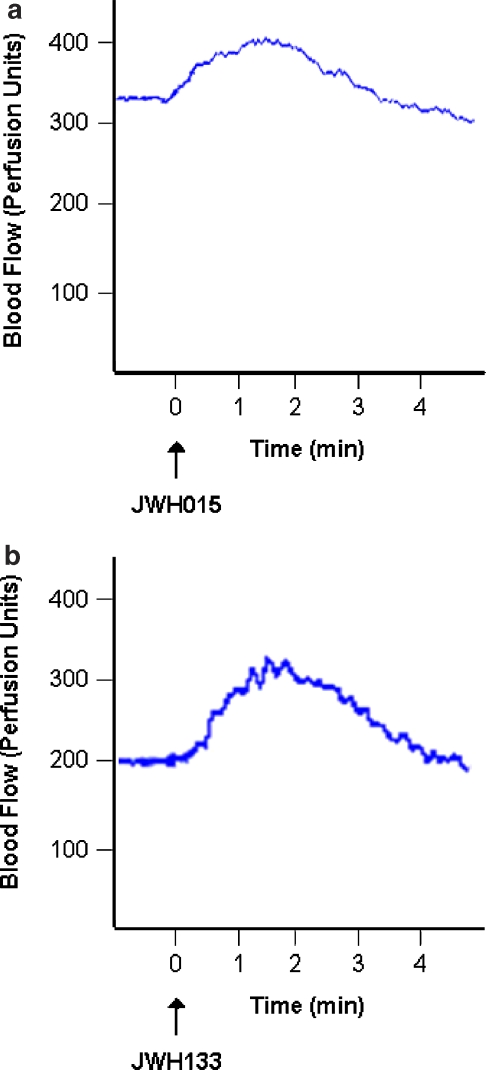
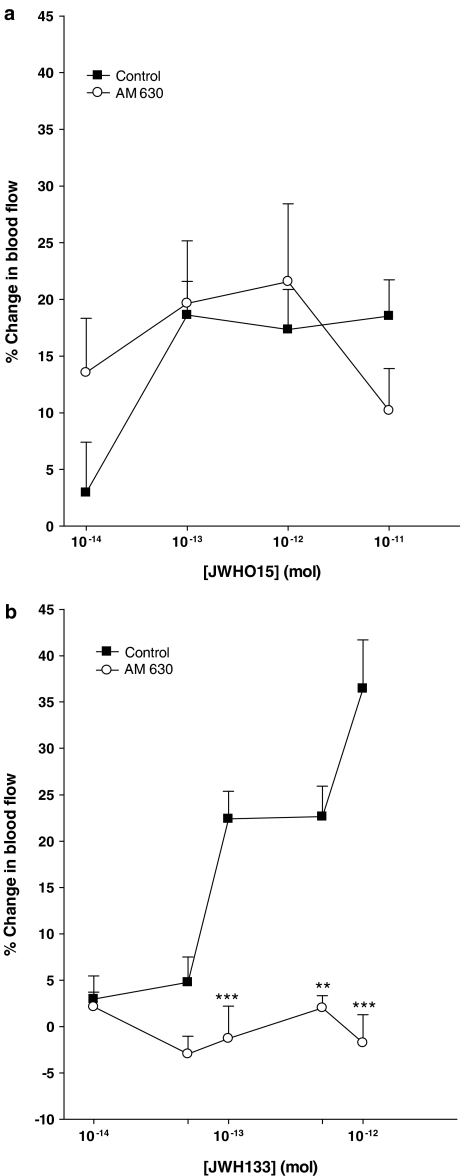
LDI-determined basal blood flow in normal rat knees was 421±26.8 PU (n=52). Application of the CB2 receptor agonists JWH015 and JWH133 onto the surface of normal rat knees caused a marked but transient increase in synovial blood flow. Continuous measurement of joint blood flow at a single discrete location indicated that vasodilatation was rapid in onset in response to CB2 receptor activation, with maximal blood flow being reached approximately 1.5 min after drug application (Figure 1). Synovial blood flow subsequently returned to control levels at around the 4-min time point. Concentration–response curves were constructed for JWH015 and JWH133 and both agonists showed a concentration-dependent effect (Figure 2). The calculated Emax for JWH015 was 18.5±3.2% with an EC50 of 1.9 × 10−14 mol. JWH133 on the other hand had an Emax of 36.4±5.3% and an EC50 of 7.9 × 10−14 mol. The selective CB2 receptor antagonist AM630 had no effect on JWH015-mediated vasodilatation (P=0.81, two-way ANOVA; n=9–10; Figure 2a). Conversely, co-administration of AM630 with JWH133 significantly inhibited the vasodilatatory effect of the cannabinoid (P<0.0001, two-way ANOVA; n=11–38; Figure 2b).
Figure 1.
Single-point measurements of knee joint blood flow at a discrete locus on the joint capsule showing typical responses to topical application of the CB2 receptor agonists JWH015 (a) and JWH133 (b). Blood flow gradually rose, with maximal vasodilatation occurring 1.5 min after drug administration. Joint blood flow subsequently returned to control levels by about 4 min.
Figure 2.
Concentration–response effects of JWH015 (a) and JWH133 (b) on synovial blood flow. The selective CB2 receptor antagonist AM630 (10−8 mol) was unable to block the vasodilator effect of JWH015, but significantly attenuated the hyperaemic response of JWH133 (***P<0.001, **P<0.01 vs control; two-way ANOVA followed by Bonferroni post hoc test; n=11–38). Data are presented as mean percent change in perfusion±s.e.m.
Administration of 10−12 mol JWH133 without previous application of the lower concentrations of the cannabinoid still elicited around a 40% increase in joint blood flow (data not shown), indicating that tachyphylaxis was not occurring in these experiments.
Since JWH015 only produced a weak, non-selective effect on synovial blood flow, the subsequent experiments were only carried out with the more efficacious JWH133.
Role of TRPV1 in CB2 receptor-mediated vasodilatation
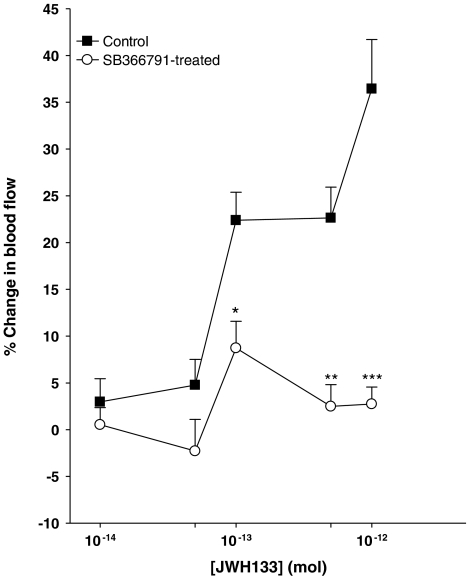
To determine if the TRPV1 ion channel is involved in the activity shown by JWH133, animals were treated with the TRPV1 antagonist SB366791. As shown in Figure 3, the vasodilator effect of JWH133 was blocked in SB366791-treated animals. A two-way ANOVA confirmed that the JWH133 concentration–response curves in SB366791-treated vs non-treated animals were statistically different from each other (P<0.0001; n=12–38).
Figure 3.
Vasodilator effect of JWH133 is attenuated in animals pretreated with the TRPV1 antagonist SB366791 (500 μg kg−1). ***P<0.001, **P<0.01, *P<0.05 two-way ANOVA followed by Bonferroni post hoc test; n=12–38. Data are shown as means±s.e.m.
Joint oedema following inflammation
Comparison of joint diameters before and after inflammation induction confirmed oedema formation in the animal models of arthritis. In kaolin/carrageenan acutely inflamed knees, joint diameter increased from 9.0±0.07 to 15.1±0.44 mm, while chronically inflamed adjuvant monoarthritic knees showed an increase in knee diameter from 9.0±0.13 to 12.2±0.42 mm. Both increases were found to be statistically significant (P<0.0001, paired Student's t-test; n=10–13 knees).
Assessment of JWH133 in inflamed knee joints
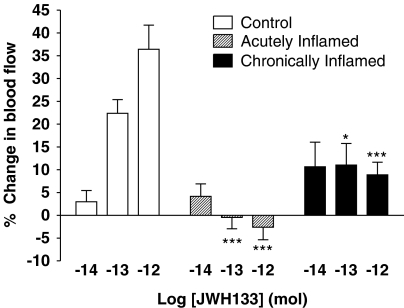
Basal blood flow to acutely inflamed knees was 566±65 PU (n=10). Administration of JWH133 to acutely inflamed rat knee joints failed to elicit any significant change in synovial blood flow (Figure 4). A two-factor ANOVA confirmed that the vasodilator effect of JWH133 seen in normal joints was abrogated in the acute inflammatory model (P<0.0001, two-way ANOVA; n=8–12).
Figure 4.
Effect of joint inflammation on JWH133-mediated vasodilatation. The hyperaemic effect of JWH133 was significantly reduced in both acute and chronically inflamed knees (***P<0.001, *P<0.05 two-way ANOVA followed by Bonferroni post hoc test; n=8–38). Data are presented as means±s.e.m.
In chronically inflamed joints, the hyperaemic effect of JWH133 was also significantly attenuated compared to normal control knees (P<0.0001, two-way ANOVA; Figure 4). Basal blood flow to adjuvant monoarthritic knees was 513±47.5 PU (n=10).
Effect of AM630 on normal, acutely and chronically inflamed knees
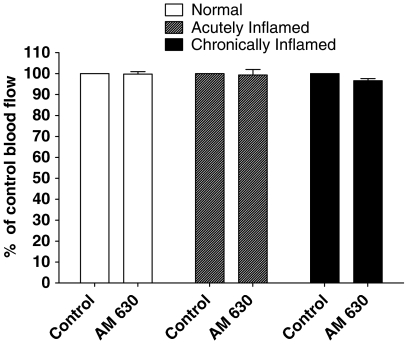
Topical administration of the selective CB2 receptor antagonist AM630 (10−8 mol) had no effect on synovial blood flow in normal (P=0.29), acutely inflamed knees (P=0.81) or chronically inflamed (P=0.05) rat knee joints (Figure 5). Thus, AM630 does not appear to be acting as an inverse or partial agonist to increase blood flow in this model system.
Figure 5.
Effect of topical application of the selective CB2 antagonist AM630 (10−8 mol) on knee joint blood flow. AM630 had no effect on joint blood flow in normal, acutely inflamed or chronically inflamed knees. Data are mean % of control values±s.e.m.
Blood pressure effects
As shown in Table 1, local administration of all of the test compounds had no significant effect on MAP. Thus, all reported vasomotor changes are a direct effect of the drugs on articular blood vessels and are not a secondary consequence of systemic changes in blood pressure.
Table 1.
MAP taken before (control) and at 1.5 min following topical application of the CB2 receptor agonists JWH015 and JWH133, and the CB2 receptor antagonist AM630
| Drugs | MAP (mm Hg) | n values |
|---|---|---|
| JWH015 (mol) | ||
| Control | 77±5 | 10 |
| 10−14 | 75±4 | 10 |
| Control | 75±5 | 10 |
| 10−13 | 73±4 | 10 |
| Control | 74±4 | 10 |
| 10−12 | 73±3 | 10 |
| Control | 71±3 | 10 |
| 10−11 | 69±2 | 10 |
| JWH133 (mol) | ||
| Control | 89±3 | 41 |
| 10−14 | 87±4 | 38 |
| Control | 90±4 | 38 |
| 5 × 10−14 | 88±5 | 28 |
| Control | 88±5 | 28 |
| 10−13 | 82±5 | 23 |
| Control | 82±5 | 24 |
| 5 × 10−13 | 75±6 | 11 |
| Control | 85±5 | 28 |
| 10−12 | 89±6 | 19 |
| AM630 (mol) | ||
| Control | 94±5 | 17 |
| 10−8 | 93±6 | 17 |
| SB366971 (μg kg−1) | ||
| Control | 94±9 | 8 |
| 500 | 93±9 | 10 |
The effect of the TRPV1 antagonist SB366791 on MAP is also shown (values are expressed as means±s.e.m.).
Abbreviation: MAP, mean arterial pressure.
Discussion
Cannabinoids acting via the CB2 receptor are known to heavily influence the activity and function of the nervous system; however, the effect of selective CB2 receptor agonists on the cardiovascular system are less well understood. The present study found that the synthetic CB2 receptor agonists JWH015 and JWH133 produced a significant increase in synovial blood flow when applied locally to the joint. These hyperaemic responses were a direct effect of the drugs on the joint microenvironment and not a secondary systemic reaction since the agonists were restricted to the periphery and had no influence on mean arterial blood pressure (see Table 1). Both agonists exhibited similar potencies; however, JWH133 was found to be more efficacious and showed greater selectivity for the CB2 receptor than JWH015, whose mild vasodilator effect was not blocked by the selective CB2 receptor antagonist AM630. The difference in responsiveness of these two drugs is likely related to the comparatively low affinity of JWH015 for the CB2 receptor (Chin et al., 1999; Huffman et al., 1999). Thus, JWH133 is the pharmacological tool of choice for in vivo vasomotor assessment and, as such, follow-up experiments investigating the mechanism of action of cannabinoids and the effect of joint inflammation on CB2 receptor responses was confined to this particular agonist.
It should be noted that the pharmacological tools currently available for cannabinoid research all possess some limits to selectivity. For example, JWH015, JWH133 and AM630 all show a mild affinity for the CB1 receptor. Thus, it is entirely feasible that the vasodilator responses observed in this study are being mediated by CB1 receptors and not CB2 receptors as reported. Attempts to block CB1 receptors with an antagonist such as AM251 were not carried out in this study, as AM251 also has a mild affinity for CB2 receptors. A further consideration is that the JWH compounds may be acting via the putative GPR55 cannabinoid receptor (Johns et al., 2007; Ryberg et al., 2007) or indeed via other, so far undefined, orphan receptors. Until robust, highly selective drugs become available and all potential cannabinoid receptor subtypes have been identified, the customary caveat with respect to the precise cannabinoid receptors involved in JWH-mediated vasomotor control should be considered.
The site of action of the tested cannabinoids is not readily identifiable in this in vivo model. The general viewpoint that CB2 receptors are localized primarily on immunocytes would indicate that these cells could be involved in the vasomotor effects of JWH015 and JWH133. Indeed, cannabinoids have been shown to activate human leukocytes, leading to the release of vasodilating agents, such as nitric oxide and pro-inflammatory chemokines (Stefano et al., 1996; Sugiura et al., 2004). This, however, would not account for the increased blood flow observed in normal joints that do not contain an appreciable number of immunocytes. Alternatively, the cannabinoids could be acting directly on the vascular smooth muscle to cause vasorelaxation. The endocannabinoid anandamide produces vasodilatation by opening large-conductance calcium-activated potassium channels (Grainger and Boachie-Ansah, 2001; White et al., 2001; Wagner et al., 2005), which causes hyperpolarization of smooth muscle cells and vascular relaxation. The vascular endothelium has also been implicated in the vasodilatatory effect of cannabinoids. A number of in vitro studies have shown that cannabinoids can act either as an endothelially derived hyperpolarizing factor or by stimulating the endothelium to cause the secondary release of vasodilator chemicals, such as nitric oxide (Randall et al., 1996; Deutsch et al., 1997; Stefano et al., 2000). A final putative mechanism by which cannabinoids alter tissue blood flow is via their modulatory effects on sensory and sympathetic neurotransmission. Cannabinoids are able to inhibit noradrenaline release from postganglionic sympathetic efferents (Ishac et al., 1996; Varga et al., 1996; Niederhoffer and Szabo, 1999), thereby attenuating sympathetic vascular tone and leading to hyperaemia. Articular blood vessels are also innervated by sensory nerves containing vasoactive neurotransmitters, such as substance P, vasoactive intestinal peptide and calcitonin gene-related peptide (Bjurholm et al., 1990; Abramovici et al., 1991; McDougall et al., 1997), all of which have been shown to increase synovial blood flow (Lam and Ferrell, 1993; McDougall et al., 1995, 1999; McDougall and Barin, 2005). These neuropeptides are released from vasosensory nerves in response to activation of the capsaicin-sensitive TRPV1 channel. Interestingly, anandamide and its synthetic analogues are thought to act on TRPV1 channels to cause the secondary release of pro-inflammatory neurotransmitters, which in turn cause vasodilatation of neighbouring blood vessels (Zygmunt et al., 1999; Ralevic et al., 2000; Smith and McQueen, 2001; Baker and McDougall, 2004). In the present study, blockade of TRPV1 channels with SB366791 significantly attenuated the vasodilator effect of JWH133. This is the first reported evidence showing that TRPV1 channels are essential for CB2 receptor functional activity. Similar observations have been described for CB1 receptor-mediated vasomotor control where the hyperaemic effect of the CB1 receptor agonist arachidonyl-2-chloroethylamide was attenuated following TRPV1 channel blockade (Baker and McDougall, 2004). It appears, therefore, that both CB1 and CB2 receptors are somehow functionally coupled to TRPV1 channels to orchestrate vasoregulation in the knee joint. Whether JWH133 binds directly to TRPV1 receptors to cause secondary release of pro-inflammatory neuropeptides or whether some intracellular pathway exists to allow cross-talk between TRPV1 and CB2 receptors remains to be resolved. While any combination of the mechanisms discussed above may be responsible for the vasodilator effect of the JWH compounds described here, other pathways cannot be discounted. Indeed, such is the complex nature of cannabinoid biology that several studies report contradictory evidence as to the mechanism of action of these eicosanoids on the vasculature (for reviews, see Mendizabal and Adler-Graschinsky, 2003; Randall et al., 2004). Future studies using highly selective cannabinoid agonists in different vascular beds will hopefully reveal the precise mechanism by which CB2 receptor activation leads to vascular smooth muscle relaxation.
Changes in JWH133-mediated vasodilatation in acute and chronically inflamed knees
When applied topically to acutely inflamed knee joints, JWH133 failed to elicit any significant change in articular blood flow across the concentration range tested. Similarly in chronically inflamed knees, the vasodilator effect of the cannabinoid was conspicuously attenuated compared to normal control joints. One possible explanation for this inability of JWH133 to elicit a hyperaemic response in arthritic knees could be that the synovial microvasculature is already maximally vasodilated such that supplementary smooth muscle relaxation is unattainable in these models. Studies testing other vasodilators in these same inflammatory models, however, have successfully demonstrated that arthritic joint blood vessels possess a vasodilator reserve, indicating that vascular smooth muscle relaxation is still possible in these joints (McDougall and Barin, 2005; Zhang and McDougall, 2006). An alternative reason for the lack of a vasodilatatory response to JWH133 in arthritic knees is that there could be an alteration in articular CB2 receptor expression and/or sensitivity in inflamed joints; however, this possibility requires further experimental assessment. CB2 receptor expression on macrophages, microglia, dendritic cells and splenocytes is known to be downregulated following exposure to an inflammatory stimulus (Lee et al., 2001; Carlisle et al., 2002; Matias et al., 2002). Inflammation can cause overactivity of the endocannabinoid system, leading to increased release of anandamide and/or 2-arachidonoylglycerol (Varga et al., 1998; D'Argenio et al., 2006; Oka et al., 2006), and prolonged exposure of cannabinoid receptors to endogenous and synthetic cannabinoid ligands can cause internalization of these Gi/o protein-coupled receptors (Fan et al., 1996; Rubino et al., 2000; Shoemaker et al., 2005). In other organ systems, such as the gut, however, there is evidence of cannabinoid receptor upregulation despite elevated endocannabinoid levels (Izzo et al., 2001; Massa et al., 2004; Kimball et al., 2006). It appears, therefore, that the regulation of cannabinoid receptor expression during inflammation depends upon the tissue involved as well as the nature of the inflammatory stimulus. Interestingly, fatty acid amide hydrolase, the enzyme responsible for the degradation of endocannabinoids, is more active in inflamed gut compared to control animals (Izzo et al., 2001). This continuous turnover of endocannabinoids during intestinal inflammation implies that endocannabinoids cannot accumulate in the tissue and therefore cannabinoid receptor downregulation is averted. Future examination of fatty acid amide hydrolase activity, cannabinoid receptor expression and endocannabinoid levels in inflamed joints is required to help to explain the altered vasomotor responses to JWH133 demonstrated in the present investigation.
In conclusion, the present investigation clearly demonstrated the functional presence of CB2 receptors in rat knee joints, whose activation leads to a vasodilator response. Vanilloid TRPV1 channels are essential for the hyperaemic action of JWH133 and further studies are required to determine the molecular and biochemical pathways that link TRPV1 and CB2 receptors. The attenuation of CB2 receptor-mediated vasodilatation in acute and chronically inflamed joints suggests an alteration in CB2 receptor expression or sensitivity following an arthritic insult.
Acknowledgments
The technical assistance of Dr Chunfen Zhang is gratefully acknowledged. This work was supported by the Alberta Heritage Foundation for Medical Research (AHFMR), the Arthritis Society of Canada and the Canadian Institutes of Health Research. JJMcD is an AHFMR Senior Scholar and an Arthritis Society Investigator.
Abbreviations
- CB
cannabinoid
- LDI
laser Doppler perfusion imaging
- MAP
mean arterial pressure
- PU
perfusion unit
- TRPV1
transient receptor potential vanilloid-1
Conflict of interest
The authors state no conflict of interest.
References
- Abramovici A, Daizade I, Yosipovitch Z, Gibson SJ, Polak JM. The distribution of peptide-containing nerves in the synovia of the cat knee joint. Histol Histopathol. 1991;6:469–476. [PubMed] [Google Scholar]
- Baker CL, McDougall JJ. The cannabinomimetic arachidonyl-2-chloroethylamide (ACEA) acts on capsaicin-sensitive TRPV1 receptors but not cannabinoid receptors in rat joints. Br J Pharmacol. 2004;142:1361–1367. doi: 10.1038/sj.bjp.0705902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjurholm A, Kreicbergs A, Ahmed M, Schultzberg M. Noradrenergic and peptidergic nerves in the synovial membrane of the Sprague–Dawley rat. Arthritis Rheum. 1990;33:859–865. doi: 10.1002/art.1780330613. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, et al. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther. 1999;291:837–844. [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Petrocellis LD. Plant, synthetic, and endogenous cannabinoids in medicine. Annu Rev Med. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- Donaldson LF, Seckl JR, McQueen DS. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J Neurosci Methods. 1993;49:5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Moore SF, Willoughby KA. Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. Am J Physiol Heart Circ Physiol. 1995;269:H1859–H1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- Fan F, Tao Q, Abood M, Martin BR. Cannabinoid receptor down-regulation without alteration of the inhibitory effect of CP 55940 on adenylyl cyclase in the cerebellum of CP 55940-tolerant mice. Brain Res. 1996;706:13–20. doi: 10.1016/0006-8993(95)01113-7. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N, Jr, Jarai Z, Mirshahi F, Kunos G, Sanyal AJ. Systemic and portal hemodynamic effects of anandamide. Am J Physiol Gastrointest Liver Physiol. 2001;280:G14–G20. doi: 10.1152/ajpgi.2001.280.1.G14. [DOI] [PubMed] [Google Scholar]
- Grainger J, Boachie-Ansah G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol. 2001;134:1003–1012. doi: 10.1038/sj.bjp.0704340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, et al. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- Högestatt ED, Zygmunt PM. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot Essent Fatty Acids. 2002;66:343–351. doi: 10.1054/plef.2001.0346. [DOI] [PubMed] [Google Scholar]
- Hosohata K, Quock RM, Hosohata Y, Burkey TH, Makriyannis A, Consroe P, et al. AM630 is a competitive cannabinoid receptor antagonist in the guinea pig brain. Life Sci. 1997;61:PL115–PL118. doi: 10.1016/s0024-3205(97)00596-1. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1',1'-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects Br J Pharmacol 2007 10.1038/sj.bjp.0707419[e-pub ahead of print: 20 August 2007; doi: [DOI] [PMC free article] [PubMed]
- Karimian SM, McDougall JJ, Ferrell WR. Neuropeptidergic and autonomic control of the vasculature of the rat knee joint revealed by laser Doppler perfusion imaging. Exp Physiol. 1995;80:341–348. doi: 10.1113/expphysiol.1995.sp003851. [DOI] [PubMed] [Google Scholar]
- Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006;291:G364–G371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- Lam FY, Ferrell WR. Effects of interactions of naturally-occurring neuropeptides on blood flow in the rat knee joint. Br J Pharmacol. 1993;108:694–699. doi: 10.1111/j.1476-5381.1993.tb12863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid CB(2) receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur J Pharmacol. 2001;423:235–241. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur J Biochem. 2002;269:3771–3778. doi: 10.1046/j.1432-1033.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Barin AK. The role of joint nerves and mast cells in the alteration of vasoactive intestinal peptide (VIP) sensitivity during inflammation progression in rats. Br J Pharmacol. 2005;145:104–113. doi: 10.1038/sj.bjp.0706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JJ, Bray RC, Sharkey KA. A morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit and human knee joints. Anat Rec. 1997;248:29–39. doi: 10.1002/(SICI)1097-0185(199705)248:1<29::AID-AR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Ferrell WR, Bray RC. Neurogenic origin of articular hyperemia in early degenerative joint disease. Am J Physiol. 1999;276:R345–R352. doi: 10.1152/ajpregu.1999.276.3.R745. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Karimian SM, Ferrell WR. Prolonged alteration of sympathetic vasoconstrictor and peptidergic vasodilator responses in rat knee joints by adjuvant-induced arthritis. Exp Physiol. 1995;80:349–357. doi: 10.1113/expphysiol.1995.sp003852. [DOI] [PubMed] [Google Scholar]
- Mendizabal VE, Adler-Graschinsky E. Cannabinoid system as a potential target for drug development in the treatment of cardiovascular disease. Curr Vasc Pharmacol. 2003;1:301–313. doi: 10.2174/1570161033476583. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br J Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006;177:8796–8805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Evidence for the presence of CB1 cannabinoid receptors on peripheral neurones and for the existence of neuronal non-CB1 cannabinoid receptors. Life Sci. 1999a;65:597–605. doi: 10.1016/s0024-3205(99)00282-9. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999b;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006a;147 (Suppl 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. 2006b;30 (Suppl 1):S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, et al. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55940. J Neurochem. 2000;75:2080–2086. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor Br J Pharmacol 2007 10.1038/sj.bjp.0707460[e-pub ahead of print: 17 September 2007; doi: [DOI] [PMC free article] [PubMed]
- Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314:868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- Smith PJ, McQueen DS. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br J Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Bilfinger TV, Rialas CM, Deutsch DG. 2-Arachidonyl-glycerol stimulates nitric oxide release from human immune and vascular tissues and invertebrate immunocytes by cannabinoid receptor 1. Pharmacol Res. 2000;42:317–322. doi: 10.1006/phrs.2000.0702. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Liu Y, Goligorsky MS. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem. 1996;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Oka S, Gokoh M, Kishimoto S, Waku K. New perspectives in the studies on endocannabinoid and cannabis: 2-arachidonoylglycerol as a possible novel mediator of inflammation. J Pharmacol Sci. 2004;96:367–375. doi: 10.1254/jphs.fmj04003x3. [DOI] [PubMed] [Google Scholar]
- Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol. 2002;118:110–121. doi: 10.1309/7AYY-VVH1-GQT5-J4R2. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varga A, Nemeth J, Szabo A, McDougall JJ, Zhang C, Elekes K, et al. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci Lett. 2005;385:137–142. doi: 10.1016/j.neulet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Abesser M, Karcher J, Laser M, Kunos G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J Cardiovasc Pharmacol. 2005;46:348–355. doi: 10.1097/01.fjc.0000175437.87283.f2. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Jarai Z, Batkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur J Pharmacol. 2001;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McDougall JJ. Stimulation of sensory neuropeptide release by nociceptin/orphanin FQ leads to hyperaemia in acutely inflamed rat knees. Br J Pharmacol. 2006;148:938–946. doi: 10.1038/sj.bjp.0706804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]