Abstract
Cannabinoids suppress behavioural responses to noxious stimulation and suppress nociceptive transmission through activation of CB1 and CB2 receptor subtypes. CB1 receptors are expressed at high levels in the central nervous system (CNS), whereas CB2 receptors are found predominantly, but not exclusively, outside the CNS. CB2 receptors are also upregulated in the CNS and dorsal root ganglia by pathological pain states. Here, we review behavioural, neurochemical and electrophysiological data, which identify cannabinoid CB2 receptors as a therapeutic target for treating pathological pain states with limited centrally, mediated side effects. The development of CB2-selective agonists (with minimal affinity for CB1) as well as mutant mice lacking CB2 receptors has provided pharmacological and genetic tools required to evaluate the effectiveness of CB2 agonists in suppressing persistent pain states. This review will examine the efficacy of cannabinoid CB2-selective agonists in suppressing acute, inflammatory and neuropathic nociception following systemic and local routes of administration. Data derived from behavioural, neurochemical and neurophysiological approaches are discussed to better understand the relationship between antinociceptive effects induced by CB2-selective agonists in behavioural studies and neural mechanisms of pain suppression. Finally, the therapeutic potential and possible limitations of CB2-based pharmacotherapies for pathological pain states induced by tissue and nerve injury are discussed.
Keywords: allodynia, hyperalgesia, endocannabinoid, central sensitization, nerve injury
Introduction
The management of chronic and severe pain is the burden of clinicians. Multiple pharmacological agents have been employed to treat diverse pathological pain states including opiates, nonsteroidal anti-inflammatory drugs, anticonvulsants, antidepressants, ketamine and others (Guindon et al., 2007). However, adverse side effects constrain therapeutic dosing and limit therapeutic efficacy. Despite improvements in our understanding of pathophysiological mechanisms underlying chronic pain states and the identification of multiple analgesic mechanisms, the clinical need for pharmacotherapies for chronic pain that are effective, nontoxic and devoid of unwanted central side effects remains predominant.
Terminology
Animal models have been developed to experimentally assess pathophysiological mechanisms underlying distinct clinical pain states induced by tissue injury, inflammation, nerve trauma, chemotherapeutic agents and metabolic challenges. These models also permit preclinical evaluation and validation of the therapeutic efficacy of putative analgesics (for review see Dubner and Ren, 1999). Although the mechanisms underlying distinct pathological pain states differ and remain incompletely understood, persistent pain states may share common features. These features include the development of hyperalgesia and/or allodynia and the presence of spontaneous pain. Hyperalgesia is described as an increase in pain evoked by noxious stimuli and also a lowered threshold for pain. Allodynia is defined as an increase in sensitivity to previously non-noxious levels of stimulation. The term hyperalgesia, however, has also been used in the literature to collectively refer to both hyperalgesia and allodynia. This review will describe empirical studies from the literature, which evaluate the utility of exploiting cannabinoid CB2 receptor mechanisms for suppressing acute, inflammatory and neuropathic pain states.
Historical perspective
Indirect evidence first implicated a role for CB2 receptor mechanisms in the modulation of persistent pain states. Systemic and intraplantar (Calignano et al., 1998, 2001) administration of palmitoylethanolamide (PEA), an endogenous fatty acid amide, produces antinociception in the formalin test that is blocked by SR144528, a CB2 receptor-selective antagonist (Calignano et al., 1998). Orally administered PEA also reduced inflammatory hyperalgesia and oedema by inhibiting mast cell degranulation (Mazzari et al., 1996) and subsequent release of inflammatory mediators that excite nociceptors. However, PEA does not bind to CB2 receptors, demonstrating that PEA is not a direct CB2 receptor agonist (Showalter et al., 1996; Griffin et al., 2000; De Petrocellis et al., 2002; Lo Verme et al., 2005).
The subsequent development and evaluation of CB2-selective agonists such as HU308, AM1241, JWH-133 and GW405833 (L768242) have provided direct support for the hypothesis that activation of CB2 produces antinociceptive effects in persistent pain states. Importantly, CB2-selective agonists such as HU308 and AM1241 lack centrally mediated side effects associated with activation of CB1 receptors, including hypoactivity, hypothermia and catalepsy (Hanus et al., 1999; Malan et al., 2001). Such observations have led support to the view that CB2 agonists would be unlikely to be psychoactive or addictive. The absence of central nervous system (CNS) side effects is consistent with the relative paucity of CB2 receptors in brain of naive animals (Munro et al., 1993; Galiègue et al., 1995; Zimmer et al., 1999; Buckley et al., 2000). CB2 receptors are expressed predominantly, but not exclusively outside the CNS (Van Sickle et al., 2005; Beltramo et al., 2006), where they are localized extensively to cells of the immune system. These immune cells include mast cells, B cells, T4 and T8 cells, microglial cells, macrophages, natural killer cells and to a lesser extent monocytes and polymorphonuclear neutrophils (Facci et al., 1995; Howlett et al., 2004; Maresz et al., 2005). CB2 receptors have been identified in microglial cultures (Walter et al., 2003; Beltramo et al., 2006) and occur in immune tissues at levels 10–100 times greater than the CB1 receptor (Facci et al., 1995; Galiègue et al., 1995). An emerging literature implicates a role for neuroimmune interactions in contributing to the development or maintenance of pathological pain states (for review see DeLeo and Yezierski, 2001). However, the mechanism by which CB2 receptor activation may modulate these interactions remains poorly understood.
Cannabinoid receptor pharmacology
Activation of CB2 receptors inhibits adenylyl cyclase (Slipetz et al., 1995; Di Marzo and De Petrocellis, 2006) and activates mitogen-activated protein kinase (Bouaboula et al., 1996; Di Marzo and De Petrocellis, 2006) through binding of the α-subunit of the Gi/o protein. In contrast to CB1 receptors, CB2 receptors do not couple to calcium-Q or inward-rectifying potassium channels (Felder et al., 1995). Agonist binding to CB1 receptors, by contrast, suppresses calcium and activates inward-rectifying potassium conductances—effects associated with depression of neuronal excitability and transmitter release. Thus, differences in receptor distribution and signal transduction mechanisms are likely to account for the relative absence of the CNS side effects induced by CB2 agonists. These considerations suggest that novel pharmacotherapies targeting CB2 receptors may have considerable therapeutic potential.
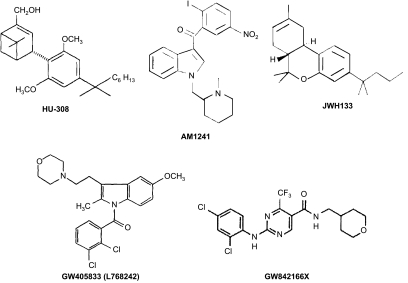
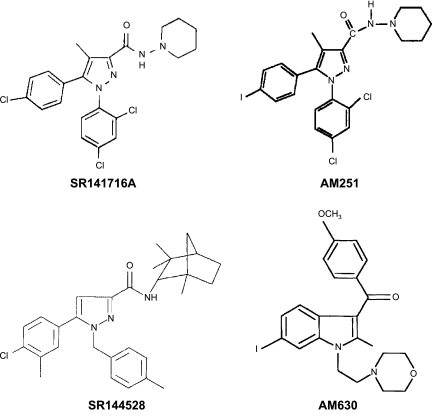
Significant drug discovery efforts have been directed towards developing and characterizing CB2-selective agonists (see Figure 1) both in vitro (see Table 1) and in vivo (see Tables 2, 3, 4 and 5). These efforts have sought to evaluate and validate the CB2 receptor as an analgesic target. HU308 (4-[4-(1,1-diemethylheptyl)-2,6-dimethoxyphenyl]-6,6-dimethyl-bicyclo[3.1.1]hept-2-ene-2-methanol) was the first CB2-selective agonist exhibiting low affinity for CB1 to be synthesized (Hanus et al., 1999). HU308 exhibits anti-inflammatory and peripheral antihyperalgesic properties, which are reversed by the CB2 antagonist SR144528 but not by the CB1 antagonist SR141716A (Hanus et al., 1999). HU308 fails to show CNS activity in a tetrad of behavioural tests, which assess cardinal signs of CB1 receptor activation associated with Δ9-tetrahydrocannabinol (Gaoni and Mechoulam, 1971), the psychoactive ingredient in cannabis.
Figure 1.
Chemical structures of cannabinoid CB2-selective agonists evaluated in Tables 1, 2, 3, 4 and 5.
Table 1.
In vitro binding profile of cannabinoid CB2 agonists and CB1 and CB1 antagonists
| Compound | CB1 | CB2 | Probe | Reference | |||
|---|---|---|---|---|---|---|---|
| HU-308 | CB2 agonist | Ki>10 μM | Rat brain | Ki=22.7±3.9 nM | Transfected cells | [3H]HU-243 | Devane et al., 1992; Mechoulam et al., 1995 |
| AM1241 | CB2 agonist | Ki=280±41 nM | Rat brain | Ki=3.4±0.5 nM | Mouse spleen | [3H]CP55,940 | Ibrahim et al., 2003 |
| JWH-133 | CB2 agonist | Ki=677±132 nM | Rat brain | Ki=3.4±1.0 nM | Human embryonic kidney 293 cells | [3H]CP55,940 | Huffman et al., 1999 |
| GW405833 | CB2 agonist | Ki=2043±183 nM | Cos-7 cells | Ki=14±6 nM | Cos-M6 cells | [3H]WIN55212-2 | Slipetz et al., 1995; Gallant et al., 1996 |
| (L768242) | Ki=273±42.6 nM | Rat brain | Ki=3.6±1.1 nM | Rat spleen | [3H]CP55,940 | Valenzano et al., 2005 | |
| GW842166 X | CB2 agonist | Not available | Not available | Not available | Giblin et al., 2007 | ||
| SR141716A | CB1 antagonist | Ki=2 nM | Rat brain | Ki>1000 nM | Mouse vas deferens | [3H]CP55,940 | Rinaldi-Carmona et al., 1995 |
| AM251 | CB1 antagonist | Ki=7.5 nM | Rat forebrain | Ki=2290 nM | Mouse spleen | [3H]CP55,940 | Gatley et al., 1997; Lan et al., 1999; Palmer et al., 2002 |
| SR144528 | CB2 antagonist | Ki=305±44 nM | Rat brain | Ki=0.30±0.38 nM | Rat spleen | [3H]CP55,940 | Rinaldi-Carmona et al., 1998 |
| AM630 | CB2 antagonist | Ki=5152±567 nM | CHO cells | Ki=31.2±12.4 nM | CHO cells | [3H]CP55,940 | Ross et al., 1999 |
Table 2.
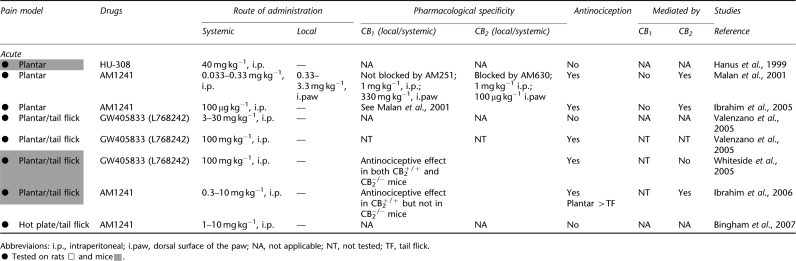
Antinociceptive effects of cannabinoid CB2 agonists in animal models of acute pain
Table 3.
Antinociceptive effects of cannabinoid CB2 agonists in the carrageenan model of inflammation
| Pain model | Drugs |
Route of administration |
Pharmacological specificity |
Antinociception |
Mediated by |
Studies | |||
|---|---|---|---|---|---|---|---|---|---|
| Systemic | Local | CB1 (local/systemic) | CB2 (local/systemic) | CB1 | CB2 | Reference | |||
| Inflammatory | |||||||||
| •Carrageenan i.pl. post | GW405833 (L768242) | 0.3–10 mg kg−1, i.p. | — | NT | Blocked by SR144528; 3 mg kg−1, i.p. | Yes WB |
NT | Yes | Clayton et al., 2002 |
| •Carrageenan i.pl. post | AM1241 | 33–330 μg kg−1, i.p. | 33 μg kg−1, i.pl. | Not blocked by SR141716A; 1 mg kg−1, i.p. | Blocked by SR144528; 1 mg kg−1, i.p. | Yes M and T Fos |
No | Yes | Nackley et al., 2003a |
| •Carrageenan i.paw pre | AM1241 | 0.1–1 mg kg−1, i.p. | 1–4 mg kg−1, i.paw | Not blocked by AM251; 300 μg kg−1, i.p.; 300 mg kg−1, i.paw | Blocked by AM630; 100 μg kg−1 i.p.; 100 μg kg−1, i.paw | Yes T |
No | Yes | Quartilho et al., 2003 |
| •Carrageenan i.pl. post | AM1241 | 330 μg kg−1, i.v. | 33 or 330 μg kg−1, i.pl. | Not blocked by SR141716A; 1 mg kg−1, i.v. | Blocked by SR144528; 1 mg kg−1, i.v. | Yes NP (E) Inf >Noninf |
No | Yes | Nackley et al., 2004 |
| •Carrageenan i.pl. pre | JWH-133 | — | 5–15 μg in 50 μl, i.pl. | Not blocked by SR141716A; 10 μg in 50 μl i.pl. | Blocked by SR144528; 10 μg in 50 μl i.pl. | Yes NP (M) Inf≈Noninf |
No | Yes | Elmes et al., 2004 |
| •Carrageenan i.pl. pre | JWH-133 | 0.3–10 mg kg−1, s.c. | — | Not blocked by SR141716A; 3 mg kg−1, s.c. | Blocked by SR144528; 3 mg kg−1, s.c. | Yes WB |
No | Yes | Elmes et al., 2005 |
| •Carrageenan i.pl. pre | AM1241 | — | 33 μg kg−1, i.pl. | Not blocked by SR141716A; 33 μg kg−1, i.pl. | Blocked by SR144528; 33 μg kg−1, i.pl. | Yes M and T M > T |
No | Yes | Gutierrez et al., 2007 |
| •Carrageenan i.pl. pre | AM1241 | 1–10 mg kg−1, i.p. | — | NT | Blocked by AM630; 1 mg kg−1, i.p. | Yes T |
NT | Yes | Bingham et al., 2007 |
Abbreviations: Fos, suppression of carrageenan-evoked spinal Fos protein in lamina I, II and V, VI; Inf, inflamed; i.p., intraperitoneal; i.paw, dorsal surface of the paw; i.pl., intraplantar; i.v., intravenous; E, transcutaneous electical stimulation; M, mechanical; Noninf, noninflamed; NP, neurophysiological evidence from extracellular recordings of spinal wide dynamic range neurons; NT, not tested; post, carrageenan injected after drugs; pre, carrageenan injected before drugs; s.c., subcutaneous; T, thermal; WB, weight bearing.
•Tested on rats □ .
Table 4.
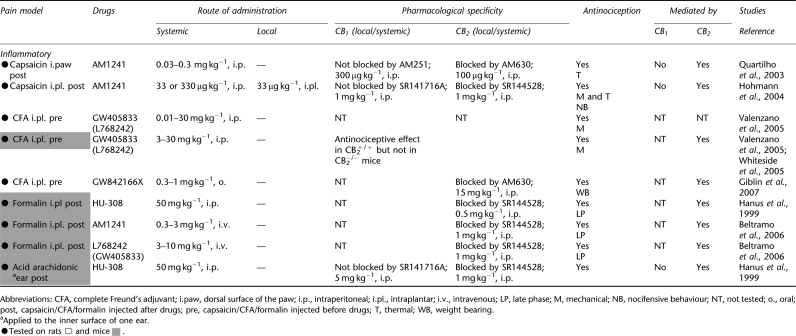
Antinociceptive effects of cannabinoid CB2 agonists in animal models of inflammatory pain
Table 5.
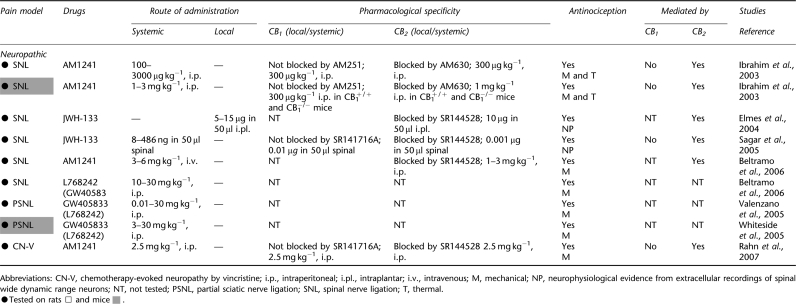
Antinociceptive effects of cannabinoid CB2 agonists in animal models of neuropathic pain
AM1241 (2-iodo-5-nitro-phenyl)-[1-(1-methyl-piperidin-2-ylmethyl)-1H-indol-3-yl]-methanone) (Ibrahim et al., 2003) was similarly shown to lack CNS side effects in the tetrad, but nonetheless produced peripheral-mediated antinociception in otherwise naive animals (see Table 1). AM1241 induces CB2-mediated antihyperalgesic effects in multiple models of persistent nociception, including those induced by tissue and nerve injury (see Tables 2, 3, 4 and 5). AM1241 stimulates the release of β-endorphin from skin keratinocytes (Ibrahim et al., 2005), suggesting that μ-opioid receptors contribute to antinociceptive effects of AM1241, but not necessarily other CB2 agonists, that are observed in otherwise naive animals. However, whether or not β-endorphin release contributes to AM1241-mediated antihyperalgesic efficacy in models of persistent nociception has not been evaluated.
AM1241 has recently been shown to behave as a protean agonist at the CB2 receptor in vitro, suggesting that functional efficacies displayed by AM1241 in vitro depend upon the level of receptor constitutive activities exhibited in the assay system (Yao et al., 2006). For example, AM1241 behaves as a neutral antagonist in FLIPR and cyclase assays and as a partial agonist in ERK (or mitogen-activated protein) kinase assays (Yao et al., 2006). However, at lower forskolin concentrations, AM1241 behaved as a partial agonist in the cyclase assay (Yao et al., 2006). Such factors may contribute to complexities (see Bingham et al., 2007) of in vivo actions of AM1241. More work is necessary to determine the signal transduction pathways implicated in the antihyperalgesic effects of AM1241. This review characterizes in vivo actions of AM1241 that are blocked by a CB2 antagonist. Therefore, AM1241 will be referred to in the present work as a CB2 agonist.
JWH-133 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran) is a well-characterized CB2 agonist (Huffman et al., 1999; Jonsson et al., 2006), which inhibits both inflammatory and neuropathic hyperalgesia (see Tables 3 and 5) through a CB2-selective mechanism. The CB2 agonist GW405833 (2,3-dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-morpholin-4-yl-ethyl)-indol-1-yl]-methanone) (Valenzano et al., 2005) is identical to the CB2 agonist referred to as L768242 (1-(2,3-dichlorobenzoyl)-2-methyl-3-(2-[1-morpholino]ethyl)-5-methoxyindole) (Huffman, 2000). Here, we will refer to this compound using the nomenclature employed in the original research article, with the other common name indicated in parentheses, to emphasize that these names refer to a single compound. GW405833 (L768242) exhibits anti-inflammatory and antihyperalgesic properties (Tables 2, 3, 4 and 5). The chemical structures of the CB2 agonists reviewed here are shown in Figure 1. The chemical structures of cannabinoid CB1 and CB2 antagonists are shown in Figure 2.
Figure 2.
Chemical structures of cannabinoid CB1 (SR141716A, AM251) and CB2 (SR144528, AM630) antagonists.
Nonselective cannabinoid agonists
CP55,940 and WIN55,212-2 are potent cannabinoid agonists that bind with high affinity to both CB1 and CB2 (Lan et al., 1999; Huffman, 2000; Palmer et al., 2002). These agonists suppress pain behaviour in different animal models of acute, tissue and nerve injury-induced nociception (for review see Walker and Hohmann, 2005). However, it is important to emphasize that the pharmacological profile exhibited by cannabinoid agonists in vivo may differ from the pharmacological profile demonstrated in vitro (for example, that suggested by their in vitro binding affinities). Despite possessing high affinity for CB2 in vitro, mixed CB1/CB2 agonists do not necessarily exhibit pharmacological properties in different pain models that are typical of other CB2-selective agonists in vivo. For example, antinociception induced by CP55,940, administered systemically, can be largely attributed to CB1 (Choong et al., 2007; Pryce and Baker, 2007). However, a role for CB2 in contributing to CP55,940-mediated antinociception has recently been described in both acute (tail flick assay) and neuropathic (spinal nerve ligation) pain models (Scott et al., 2004), whereas the antihyperalgesic effects of CP55,940 have solely been attributed to CB1 in an inflammatory pain model (Choong et al., 2007). Suppression of neuropathic nociception induced by systemically administered WIN55,212-2 has been shown to be mediated by CB1 (Herzberg et al., 1997; Bridges et al., 2001) and not by CB2 (Bridges et al., 2001). Studies employing intraplantar injections of WIN55,212-2 also confirm a role for CB1 receptors in suppressing neuropathic nociception following local administration; however, a role for CB2 mechanisms in contributing to the antihyperalgesic effects of WIN55,212-2 was not assessed (Fox et al., 2001). Thus, it is noteworthy that both CB1 and CB2 receptors have been implicated in the antihyperalgesic effects of locally (intraplantar) administered WIN55,212-2 in the carrageenan model of inflammatory nociception (Nackley et al., 2003b). Indeed, agonists that act on both CB1 and CB2 receptors in vitro can produce in vivo pharmacological effects, wherein activity at CB1 predominates (Dyson et al., 2005); these effects may differ with the route of agonist administration employed (systemic versus local) or nociceptive state (acute, tissue injury or nerve injury). Therefore, the present review will be restricted to evaluation of in vivo pharmacological effects of CB2-selective agonists that exhibit minimal affinity at CB1 (see Table 1). Here, we review preclinical studies that assess the role of CB2 receptor activation in suppressing pain in animal models of acute, inflammatory and neuropathic nociception using the best characterized CB2-selective agonists available to date. The antinociceptive effects of mixed cannabinoid agonists are reviewed elsewhere (Walker and Hohmann, 2005).
Acute pain
Cannabinoids induce antinociceptive effects through spinal, supraspinal and peripheral mechanisms (Martin et al., 1995; Pertwee, 2001; Hohmann, 2002; Walker and Hohmann, 2005). Recent studies suggest that some, but not all, CB2-selective agonists induce antinociception in tests of acute pain in otherwise naive animals. The magnitude of the observed antinociception may differ with the assay for acute nociception and agonist and dose employed (see Table 2). Systemic (intraperitoneal) and local (intraplantar) administration of AM1241 produces a thermal antinociceptive effect in the plantar test in otherwise naive animals (Malan et al., 2001; but see Bingham et al., 2007). This test measures the latency for animals to remove their paws from a radiant heat source that is focused onto the plantar surface of the paw through the floor of a glass platform. This antinociceptive effect was mediated by CB2 receptors because it was antagonized by the CB2-selective antagonist AM630, administered systemically or locally into the dorsal surface of the paw. By contrast, systemic or local administration of the CB1 antagonist AM251 did not alter AM1241-induced antinociception. AM1241 induces antinociception in the plantar test in rats (Malan et al., 2001) and mice (Ibrahim et al., 2006). The ability of AM1241 to inhibit acute nociception in the hot plate and tail flick tests is also lost in CB2−/− mice, confirming a role for CB2 receptors in these actions (Ibrahim et al., 2006) (see Table 2). These studies also reveal that AM1241 is less efficacious in producing antinociception in the spinally mediated tail flick test relative to the plantar test, which assesses latency to paw withdrawal. By contrast, systemic administration of HU308 and GW405833 (L768242) failed to induce antinociception in the hot plate (Hanus et al., 1999; Valenzano et al., 2005) and tail flick (Valenzano et al., 2005) tests.
Systemic administration of a high dose (100 mg kg−1) of GW405833 (L768242) elevated thermal paw withdrawal latencies in the hot plate and tail flick test in rats (Valenzano et al., 2005). However, these effects are unlikely to be attributed to activation of CB2 receptors; the same dose (100 mg kg−1) of GW405833 (L768242) induced antinociceptive effects in both CB2−/− and CB2+/+ mice and induced motor ataxia (Valenzano et al., 2005; Whiteside et al., 2005). Interestingly, antihyperalgesic doses of all three compounds—AM1241, HU308 and GW405833 (L768242)—failed to alter locomotor activity following systemic administration. These data suggest that CB2-selective agonists do not induce other centrally mediated effects associated with activation of CB1. The lack of CNS side effects observed with antihyperalgesic doses of CB2 agonists (that is, lower doses that can be specifically attributed to CB2-specific mechanisms) may also reflect limited CNS penetration of some but certainly not all CB2 agonists. For example, GW405833 (L768242) has been shown to penetrate the CNS (Valenzano et al., 2005). Complete pharmacokinetic profiles for new and existing CB2 agonists are needed to better address this issue.
More work is also necessary to verify that the antinociceptive effects of AM1241 (i.p.) in modulating acute nociception represent a class effect typical of other CB2 agonists. Electrophysiological studies employing transcutaneous electrical stimulation reveal that AM1241 preferentially suppresses the mechanism by which spinal neurons are sensitized; this suppression is more pronounced in the presence compared to the absence of inflammation (Nackley et al., 2004). Thus, it is noteworthy that three structurally distinct CB2 agonists (AM1241, GW405833 (L768242) and HU308) suppress acute responses to mechanical stimulation following tissue injury induced by hindpaw incision (LaBuda et al., 2005). Hindpaw incision induces microglial and astrocytic activation (Romero-Sandoval and Eisenach, 2007) as well as tactile allodynia (LaBuda et al., 2005). Hindpaw incision-induced tactile allodynia was suppressed by all three CB2 agonists. The antiallodynic effects of HU308 were also blocked by SR144528, consistent with mediation by CB2. Consequently, a better understanding of the mechanisms involved in CB2-mediated antinociceptive effects as well as the signal transduction mechanisms underlying these actions is required to understand how activation of CB2 modulates nociceptive responding in the presence versus absence of pathological pain states.
Persistent inflammatory nociception
Cannabinoids are antinociceptive in tissue injury models of persistent pain. Behavioural, electrophysiological and neurochemical studies all support a role for CB2 receptor activation in modulating inflammatory nociception. Effects of CB2-selective agonists in different inflammatory pain models (carrageenan, capsaicin, complete Freund's adjuvant, formalin and arachidonic acid) will be discussed separately (see Tables 3 and 4) because mechanisms underlying the development of hyperalgesia, allodynia and spontaneous pain in distinct models of tissue injury-induced nociception differ.
Carrageenan model
Intraplantar injection of carrageenan produces paw swelling (oedema) and hyperalgesia (Hargreaves et al., 1988) and induces expression of Fos, a nonspecific marker of neuronal activation (Honore et al., 1995). Systemic or local (intraplantar) administration of AM1241 suppresses the development of behavioural sensitization to both mechanical and thermal stimulation in the carrageenan model of inflammation (Nackley et al., 2003a). These antihyperaglesic effects were mediated by CB2 receptors because they were blocked by the CB2 antagonist SR144528, but not by the CB1 antagonist SR141716A (Nackley et al., 2003a). AM1241 also suppresses spinal Fos expression, a marker of neuronal activation, in the carrageenan model of inflammation; this suppression was similarly blocked by coadministration of AM1241 with SR144528 (Nackley et al., 2003a). AM1241 suppressed carrageenan-evoked Fos protein expression in a lamina-specific manner. CB2-mediated suppressions of carrageenan-evoked Fos protein expression were observed in the superficial (lamina I, II) and neck region (lamina V, VI) of the dorsal horn, spinal cord regions associated primarily with the termination of nociceptive primary afferents. By contrast, AM1241 did not alter Fos protein expression in the nucleus proprius (lamina III, IV) or ventral horn (Nackley et al., 2003a). These data are consistent with the hypothesis that antihyperalgesic effects of AM1241 in models of inflammatory nociception reflect a suppression of inflammation-evoked neuronal activation.
Local administration of AM1241 also attenuates the maintenance of thermal (Quartilho et al., 2003; Gutierrez et al., 2007) and mechanical (Gutierrez et al., 2007) hypersensitivity induced by hindpaw injection of carrageenan. These effects are blocked by CB2-selective antagonists such as AM630 or SR144528. Moreover, local injections of SR144528 but not SR141716A block the antihyperalgesic effects of locally administered AM1241 in a model of established (18 h post injection) carrageenan inflammation; these antihyperalgesic effects are observed with multiple modalities of stimulation (mechanical, thermal) (Gutierrez et al., 2007). The ability of intraplantar administration of SR144528 to block the antihyperalgesic effects of locally administered AM1241 cannot be attributed to nonspecific actions of the drug at CB1 receptors; under identical conditions, local administration of SR141716A, but not SR144528, blocked the antihyperalgesic effects of locally administered ACEA, a CB1-selective agonist (Gutierrez et al., 2007). This latter study also revealed more robust effects of AM1241 in suppressing responses to mechanical as opposed to thermal stimulation after the establishment of carrageenan inflammation.
Intravenous or local hindpaw administration of AM1241 also suppresses neuronal sensitization recorded in spinal nociceptive neurons during the development of carrageenan inflammation (Nackley et al., 2004). This observation suggests a neurophysiological mechanism capable of mediating the antihyperalgesic effects of AM1241. Spinal neuronal excitability was induced by applying trains of electrical stimulation to the peripheral receptive field in the ipsilateral hindpaw in the absence or presence of carrageenan inflammation. During the development of carrageenan inflammation, preemptive administration of AM1241 preferentially suppressed C fibre-mediated afterdischarge responses and windup—electrophysiological effects attributed to C fibre-mediated sensitization of wide dynamic range neurons (Nackley et al., 2004). The AM1241-induced suppression of electrically evoked responses was blocked by the CB2 antagonist SR144528, but not by the CB1 antagonist SR141716A (Nackley et al., 2004). Moreover, activity evoked in purely non-nociceptive neurons (that is, A-β fibre-mediated responses recorded in low threshold mechanosensitive cells) was unaffected. Thus, behavioural, electrophysiological and neurochemical studies suggest that AM1241 preferentially suppresses neuronal sensitization that is observed in the presence compared to the absence of an inflammatory pain state. These observations are also consistent with the ability of intraplantar injections of JWH-133 to suppress mechanically evoked responses of wide dynamic range neurons in carrageenan-treated rats through a CB2-specific mechanism; this electrophysiological response was blocked by local administration of the CB2 antagonist SR144528 but not by the CB1 antagonist SR141716A (Elmes et al., 2004).
Carrageenan inflammation also decreases weight bearing in the inflamed paw. Thus, it is noteworthy that both GW405833 (L768242) and JWH-133, administered systemically, reverse this effect. GW405833 (L768242) and JWH-133, cannabinoid CB2 agonists from different chemical classes, increase weight bearing in the carrageenan-inflamed paw through a mechanism that is dependent upon CB2 receptor activation (Clayton et al., 2002; Elmes et al., 2005). Like AM1241 (Quartilho et al., 2003; Nackley et al., 2004), both GW405833 (L768242) and JWH-133 also decrease carrageenan-evoked peripheral oedema (Clayton et al., 2002; Elmes et al., 2005). Thus, the available data suggest that multiple CB2-selective agonists suppress inflammatory nociception and peripheral oedema induced by hindpaw carrageenan administration; these effects are observed in behavioural, electrophysiological and neurochemical studies, involve multiple stimulus modalities (mechanical, thermal), are observed following systemic or local agonist administration and are blocked by CB2 but not CB1 antagonists (see Table 3). The ability of CB2 agonists to suppress persistent nociception in other tissue-injury models of persistent pain is summarized in Table 4.
Capsaicin model
Intradermal administration of capsaicin, the pungent ingredient in hot chilli peppers, induces hypersensitivity to mechanical and thermal stimulation as well as spontaneous pain (Gilchrist et al., 1996). Hyperalgesia evoked by capsaicin treatment refers to an increase in pain behaviour evoked by suprathreshold stimuli and/or lowered threshold for pain (Gilchrist et al., 1996). Primary hyperalgesia, especially that elicited by noxious thermal stimulation, is mediated in part by sensitization of C-fibre mechanoheat (polymodal) nociceptors (LaMotte et al., 1992; Torebjörk et al., 1992). Secondary (mechanical) hyperalgesia is observed in surrounding uninjured tissue and involves sensitization of the CNS (Baumann et al., 1991; LaMotte et al., 1992) as well as nociceptor sensitization (Serra et al., 2004).
AM1241, administered systemically, induced a dose-dependent suppression of capsaicin-evoked thermal hyperalgesia and spontaneous pain behaviour (Quartilho et al., 2003; Hohmann et al., 2004). These antihyperalgesic effects were mediated by CB2 receptors because they were antagonized by AM630 (Quartilho et al., 2003) and SR144528 (Hohmann et al., 2004). Both local (intraplantar) and systemic (intraperitoneal) administration of AM1241 suppresses mechanical hyperalgesia and allodynia as well as thermal hypersensitivity evoked by intradermal capsaicin injection (Hohmann et al., 2004). The suppressive effects of AM1241 were dose-dependent and antagonized by SR144528, but not SR141716A. Moreover, capsaicin-evoked nocifensive behaviour (licking, lifting and failure to bear weight on the injected paw) was also blocked by AM1241 through a CB2-specific mechanism (Hohmann et al., 2004). The antihyperalgesic effects of AM1241 were mediated, at least in part, by a local site of action; AM1241 injected into the capsaicin-injected paw suppressed capsaicin-evoked hypersensitivity to mechanical and thermal stimulation, whereas injection of the same dose into the contralateral (capsaicin-untreated) paw was inactive (Hohmann et al., 2004).
Complete Freund's adjuvant model
Intraplantar administration of complete Freund's adjuvant in rodents induces peripheral oedema as well as hypersensitivity to mechanical and thermal stimulation (Ren and Dubner, 1999). Inflammation appears approximately 2 h following injection of complete Freund's adjuvant, produces its maximal effect after 6−8 h and can persist for weeks following injection (Ren and Dubner, 1999; Walker et al., 2003). GW405833 (L768242), administered systemically, suppressed the development of adjuvant-induced tactile allodynia and mechanical hyperalgesia in a dose-dependent manner. This suppression was observed in both rats and mice (Valenzano et al., 2005; Whiteside et al., 2005). Although pharmacological specificity of GW405833 (L768242) was not assessed in rats, CB2 receptors are nonetheless likely to mediate the observed suppression of mechanical hypersensitivity (Valenzano et al., 2005; Whiteside et al., 2005). GW405833 (L768242) suppressed adjuvant-induced mechanical hypersensitivity in CB2+/+ mice, but these antihyperalgesic effects were absent in CB2−/− mice. Moreover, another CB2 agonist, GW842166X (2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide), administered orally, fully reversed complete Freund's adjuvant-induced hyperalgesia when weight bearing was used to assess behavioural sensitization. This effect was blocked by AM630, albeit at a high dose (15 mg kg−1, i.p.), and possible mediation by CB1 was not assessed (Giblin et al., 2007).
A better understanding of the mechanism of action of CB2-selective agonists has recently been obtained using GW405833 (L768242) and the complete Freund's adjuvant model of inflammatory pain (see Table 4). Whiteside et al. (2005) evaluated the ability of the opioid antagonist naltrexone to block the antihyperalgesic effects of GW405833 (L768242) in mice subjected to adjuvant-induced inflammation of the hindpaw. Naltrexone was ineffective in blocking the antihyperalgesic effects of GW405833 (L768242) (Whiteside et al., 2005). From this later study, it can be concluded that CB2-mediated antihyperalgesic effects of GW405833 (L768242) are not dependent upon the release of endogenous opioids (Whiteside et al., 2005). By contrast, AM1241 releases β-endorphin from skin keratinocytes following activation of CB2 receptors in otherwise naive animals (Ibrahim et al., 2005). It is noteworthy, therefore, that the antinociceptive efficacy of AM1241 (i.p.), the only CB2 agonist shown to date, to produce antinociception in an acute pain model (the plantar test) in otherwise naive animals is also lost in μ-opioid receptor knockout mice (Ibrahim et al., 2005). Thus, the available data suggest that multiple CB2-selective agonists suppress behavioural sensitization induced by complete Freund's adjuvant administration in both rats and mice through a CB2-specific mechanism. These effects are blocked by CB2 antagonists and are absent in CB2−/− mice. Moreover, antihyperalgesic efficacy of CB2-selective agonists in this model does not require opioid receptor activation or mobilization of β-endorphin. Importantly, the available data collectively suggest that β-endorphin release and μ-opioid receptor sensitivity are not a class effect associated with all CB2-selective agonists.
Formalin model
The formalin test is a well-established model of persistent pain characterized by a transient, biphasic pattern of pain behaviour. The early phase is characterized by acute activation of C and Aδ fibres. The late phase involves an inflammatory reaction in peripheral tissue (Tjölsen et al., 1992), the development of CNS sensitization (Coderre and Melzack, 1992; Coderre and Katz, 1997) and additionally involves activation of primary afferent nociceptors (Puig and Sorkin, 1996). CB2 agonists are antinociceptive in the formalin test (see Table 4). The antinociceptive effect of HU308 was restricted to the late phase of the formalin test (Hanus et al., 1999), which is associated with CNS sensitization. Both AM1241 and the CB2-selective agonist L768242 (GW405833), administered intravenously, similarly reduced the late, but not the early phase, of formalin pain. The antinociceptive effect of each agonist was also dependent upon CB2 receptor activation (Beltramo et al., 2006). These observations are consistent with previous work demonstrating that intraplantar administration of PEA suppresses formalin-evoked pain behaviour through a mechanism that is blocked by the CB2 antagonist SR144528 (Calignano et al., 1998). Intraplantar administration of PEA also preferentially suppresses spinal neuronal sensitization evoked by hindpaw formalin administration; this suppression is observed under conditions in which acute responses to non-noxious mechanical stimulation are unaffected (LoVerme et al., 2006). Effects of CB2-selective agonists have not been characterized in the formalin model using electrophysiological methods, although they might be predicted to behave similarly to PEA.
Efficacy of multimodal therapies directed at CB2 receptors and other analgesic targets (for example, enzymes catalyzing endocannabinoid deactivation) is also supported in the literature. Endogenous anandamide and PEA can be detected in paw skin, where they may engage peripheral CB1 and CB2 receptor subtypes (Calignano et al., 1998). Thus, it is noteworthy that local coadministration of PEA with exogenous anandamide (an endocannabinoid acting at CB1/CB2 receptors) produces a synergistic analgesic effect in both phases of the formalin test through a mechanism that involves both CB1 and CB2 receptor subtypes (Calignano et al., 1998). The combination of anandamide with ibuprofen (a nonspecific cyclooxygenase inhibitor) produced a synergistic local antinociceptive effect in both phases of the formalin test that is similarly mediated by both CB1 and CB2 receptors (Guindon et al., 2006). Endocannabinoid levels are also enhanced by the combination of anandamide with ibuprofen/rofecoxib (Guindon et al., 2007b). Similarly, exogenous 2-arachidonoylglycerol, an endocannabinoid acting at CB1/CB2 receptors, in combination with the monoacylglycerol lipase inhibitor, URB602 (an inhibitor 2-arachidonoylglycerol deactivation), produces additive antinociceptive effects (Guindon et al., 2007a). The effects of 2-arachidonoylglycerol were mediated by CB2 receptors, whereas the effects of URB602 involved both CB1 and CB2 receptor subtypes. These studies raise the possibility that CB2 receptors may also be targeted indirectly by inhibiting endocannabinoid deactivation, thereby elevating levels of endocannabinoids at peripheral sites where they are produced on demand in a stimulation contingent fashion. More work is necessary to determine whether such adjunctive strategies may be exploited clinically to preferentially enhance the efficacy of local antihyperalgesic mechanisms. Such adjunctive therapies may exhibit a more beneficial and circumscribed spectrum of physiological effects compared to direct agonist administration.
Arachidonic acid-induced ear oedema model
Topical administration of arachidonic acid in the ear of the mouse induces a characteristic inflammatory response (Hanus et al., 1999). HU308, administered intraperitoneally prior to arachidonic acid application, significantly reduced ear tissue swelling (Hanus et al., 1999). This anti-inflammatory effect was reduced by SR144528, consistent with mediation by CB2 receptors (Hanus et al., 1999).
Nerve injury-induced nociception
Animal models of neuropathic pain have been developed to mimic symptoms associated with nerve injury observed clinically. Neuropathic pain may be induced by traumatic injury, metabolic challenges and chemotherapeutic agents (Seltzer et al., 1990; Polomano and Bennett, 2001; Cantón et al., 2004). Pharmacotherapies (for example, opioids, antidepressants and anticonvulsants) used to treat neuropathic pain produce inadequate pain relief and/or unwanted side effects. Thus, the identification of novel therapeutic approaches with limited side effect profiles remains an urgent medical need.
In behavioural studies, nonselective cannabinoid agonists reduce mechanical allodynia and thermal hyperalgesia (Herzberg et al., 1997; Bridges et al., 2001; Fox et al., 2001; Guindon and Beaulieu, 2006). However, the role of CB2 receptor activation in modulation of neuropathic pain remains poorly understood. Only a small number of studies have evaluated the efficacy of CB2-selective agonists for suppressing neuropathic nociception; these studies have employed models of neuropathic pain evoked by traumatic nerve injury (that is, partial sciatic nerve ligation and spinal nerve ligation models) and chemotherapeutic agents (that is, vincristine) (see Table 5). Below, we review the available data that uniformly supports a role for CB2 receptor activation in modulation of neuropathic nociception.
Spinal nerve ligation model
The efficacy of CB2 agonists in suppressing neuropathic nociception was first evaluated using a spinal nerve ligation model (Ibrahim et al., 2003). Neuropathic pain was induced by ligating the L5 and L6 spinal nerves according to the procedures described by Kim and Chung (1992). AM1241, administered systemically, produced a dose-dependent reversal of established mechanical and thermal hypersensitivity that was mediated by a CB2-specific mechanism (Ibrahim et al., 2003). The antihyperalgesic effects of AM1241 were reversed by the CB2 receptor antagonist AM630 (Ibrahim et al., 2003). Moreover, AM1241 blocked mechanical and thermal hypersensitivity in both CB1+/+ wild-type and CB1−/− mice, demonstrating that the antihyperalgesic efficacy of AM1241 does not require activity at CB1. Another group independently verified that AM1241, administered systemically, dose-dependently suppressed nerve injury-induced mechanical hypersensitivity on the ligated side compared with vehicle-treated controls; this antihyperalgesic effect was similarly mediated by a CB2-specific mechanism (Beltramo et al., 2006). In this study, L768242 (GW405833) also reduced allodynia elicited by spinal nerve ligation in a dose-dependent manner. However, pharmacological specificity of L768242 (GW405833)-induced actions was not verified using a CB2 antagonist (Beltramo et al., 2006). Additional support for CB2-mediated suppression of neuropathic nociception is derived from electrophysiological studies employing JWH-133. JWH-133, administered locally in the paw, reduced evoked responses to noxious mechanical stimulation in wide dynamic range neurons recorded in spinal nerve ligated rats; this effect was attenuated by SR144528 (Elmes et al., 2004). Moreover, spinal administration of JWH-133 also attenuated the mechanically evoked responses of neuropathic rats in a manner that was blocked by SR144528 (Sagar et al., 2005), suggesting that CB2 agonists may act at central sites to suppress pathological pain states. Responses in sham-operated animals were unaffected by JWH-133 (Sagar et al., 2005; but see Elmes et al., 2004). Thus, activation of CB2 receptors with multiple CB2-selective agonists—AM1241, JWH-133 and L768242 (GW405833)—alleviates neuropathic nociception in behavioural and electrophysiological studies.
Partial sciatic nerve ligation model
Additional support for the hypothesis that CB2 agonists suppress neuropathic nociception is obtained from studies in which unilateral hindlimb neuropathy was induced by partial sciatic nerve ligation. Partial ligation of the sciatic nerve (Seltzer et al., 1990) resulted in the development of tactile allodynia and mechanical hyperalgesia within 2 weeks following surgery. Systemic administration of GW405833 (L768242) 3−5 weeks after the surgery reduced nerve injury-induced mechanical hyperalgesia in rats (Valenzano et al., 2005) and mice (Whiteside et al., 2005). Interpretation of these studies is somewhat limited by the fact that the pharmacological specificity of GW405833 (L768242) was not assessed in the partial sciatic nerve ligation model. However, the authors did demonstrate that antihyperalgesic effects of the same compound were blocked by a CB2 antagonist and were absent in CB2−/− mice following adjuvant inflammation of the hindpaw.
Chemotherapy-induced neuropathy
A single study has evaluated the possible role of CB2 receptors in suppressing neuropathic nociception evoked by treatment with chemotherapeutic agents (Rahn et al., 2007). Unlike neuropathy induced by traumatic nerve injury, neuropathy induced by chemotherapeutic agents may occur in the absence of peripheral nerve degeneration (Polomano and Bennett, 2001). A dysregulation of cellular calcium homeostasis, attributable to atypical mitochondrial function, has been implicated in chemotherapy-evoked neuropathy (Siau and Bennett, 2006). The vinca alkaloid vincristine is a chemotherapeutic agent commonly employed to treat leukaemia, lymphomas and solid tumours (Polomano and Bennett, 2001). Treatment with vincristine induces mechanical allodynia under conditions in which responses to thermal stimulation are preserved (Weng et al., 2003; Rahn et al., 2007). AM1241 partially reversed vincristine-induced mechanical allodynia in a manner that was blocked by a CB2 but not a CB1 antagonist (Rahn et al., 2007). By contrast, the mixed cannabinoid agonist WIN55,212-2 fully reversed vincristine-evoked mechanical allodynia. The anti-allodynic effects of WIN55,212-2 were mediated by both CB1 and CB2 receptors. Recent work also suggests that CB2 agonists are effective in suppressing peripheral neuropathy evoked by paclitaxel (taxol) administration in rats (Hohmann et al., 2007). More work is necessary to validate the effectiveness of CB2-selective agonists in suppressing the development of chemotherapy-induced neuropathic pain induced by diverse antitumour agents.
Mechanisms and implications
The complexity of the actions of CB2 agonists on neuronal and non-neuronal cells and their signalling properties are only beginning to be explored. CB2 receptors are present at or below the threshold for detection in normal CNS (Munro et al., 1993; Griffin et al., 1997; Zimmer et al., 1999). CB2 receptors and mRNA have, however, recently been reported within the CNS (Van Sickle et al., 2005), including the spinal cord (Beltramo et al., 2006), brainstem and cortex (Van Sickle et al., 2005). However, CB2 receptors localized within the CNS are not necessarily associated with neurons. In immunocytochemical studies, definitive evidence for the presence of CB2 protein within the CNS requires the demonstration that such staining is absent in CB2−/− mice.
CB2 receptors have been localized to peripheral nerve terminals (Pertwee et al., 1995; Griffin et al., 1997). CB2 receptors were first detected in cultured dorsal root ganglion cells derived from neonatal rats using fluorescence-activated cell sorting analyses (Ross et al., 2001). Two structurally distinct CB2-selective agonists (L768242 (GW405833) and AM1241) have recently been shown to suppress capsaicin-evoked release of calcitonin gene-related peptide in rat spinal cord in vitro (Beltramo et al., 2006), suggesting a neuronal mechanism of antihyperalgesic action. The presence of CB2 mRNA and protein has also been reported in rat and mouse paw tissues (Walczak et al., 2005, 2006). Finally, CB2 receptor protein has been identified in microglial cultures of neonatal rat spinal cord (Beltramo et al., 2006), suggesting that non-neuronal substrates contribute to the antihyperalgesic actions induced by CB2-selective agonists in vivo. Functional evidence in support of this hypothesis is derived from the ability of the CB2 agonist JWH-015, administered intrathecally, to reduce paw incision-induced microglial and astrocytic activation in the spinal cord; this reduction was reversed by the CB2 antagonist AM630 (Romero-Sandoval and Eisenach, 2007). Indeed, activation of CB2 receptors on non-neuronal cells has been postulated to suppress the release of inflammatory mediators that sensitize nociceptors (Mazzari et al., 1996). Thus, non-neuronal substrates as well as neuronal substrates may be responsible for the ability of CB2-selective agonists to suppress persistent pain states. These mechanisms may also contribute to the more pronounced effects of selective CB2 agonists in inflamed compared to noninflamed tissue (Nackley et al., 2004).
Electrophysiological studies demonstrate that CB2-selective agonists preferentially suppress activity in spinal nociceptive neurons under conditions in which these neurons are sensitized. For example, AM1241 suppresses C-fibre-mediated afterdischarge responses and windup in spinal wide dynamic range neurons through activation of CB2 receptors (Nackley et al., 2004). This suppression is more pronounced in the presence compared to the absence of persistent inflammation (Nackley et al., 2004). Selective activation of CB2 receptors by JWH-133 also suppresses mechanically evoked responses in neuropathic but not in sham-operated rats (Elmes et al., 2004; Sagar et al., 2005). JWH-133, administered locally in the paw, also inhibits carrageenan-evoked expansion of peripheral receptive field sizes in WDR neurons (Elmes et al., 2004). These studies collectively suggest that activation of CB2 receptor mechanisms preferentially suppresses neuronal sensitization. It is thus particularly noteworthy that pathological pain states and injury are associated with upregulation of CB2 receptor protein and mRNA. Expression of CB2 is markedly upregulated in dorsal root ganglia and spinal cord following sciatic nerve injury (Zhang et al., 2003; Walczak et al., 2005; Wotherspoon et al., 2005; Beltramo et al., 2006), whereas expression levels remain near the threshold for detection in naive animals. Understanding the functional consequence of upregulation of CB2 receptors along nociceptive pathways under conditions of pain and injury represents an important direction for future research.
Activation of CB2 receptors with AM1241 on skin keratinocytes stimulates the production of β-endorphin and induces antinociception in an acute pain model (the plantar test) in otherwise naive animals through activation of μ-opioid receptors (Ibrahim et al., 2005). The extent to which β-endorphin release may contribute to the antihyperalgesic effects of AM1241 in persistent pain state remains to be determined. Antihyperalgesic effects induced by GW405833 (L768242) in the complete Freund's adjuvant model are independent of μ-opioid receptors (Whiteside et al., 2005). This difference in μ-opioid sensitivity between these agonists may account for the ability of AM1241, but not other CB2 agonists described to date, to induce robust antinociception in the plantar test in otherwise naive animals (see Table 2; but see Bingham et al., 2007). Therefore, it is noteworthy that signalling changes downstream of initial CB2 receptor activation may differ depending upon the agonist employed and the presence or absence of injury. These factors must be considered in efforts to understand CB2 agonist actions as well as the antihyperalgesic/antinociceptive phenotype observed in a given nociceptive assay. Further work is required to identify the specific cellular elements that contain CB2 receptors and mechanism by which activation of these receptors suppresses neuronal sensitization.
Conclusions and limitations
The available data suggest that CB2-selective agonists show promise for suppressing inflammatory and neuropathic pain states. In animal models of tissue and nerve injury-induced nociception, CB2-selective agonists suppress hyperalgesia and allodynia and normalize nociceptive thresholds without inducing analgesia. These behavioural observations are also consistent with electrophysiological data demonstrating that CB2-selective agonists such as AM1241 and JWH-133 suppress responses in nociceptive neurons preferentially under conditions in which these neurons are sensitized (that is, in the presence of pathological pain states). These agonists may also be more efficacious in suppressing hypersensitivity to mechanical as opposed to thermal stimulation for reasons that remain incompletely understood. A particularly beneficial aspect of the pharmacological profile of CB2 agonists is the failure of these compounds to induce adverse CNS side effects associated with activation of CB1 receptors. By contrast, unwanted CNS side effects (for example, psychoactivity, hypoactivity and hypothermia) limit the therapeutic potential of mixed cannabinoid agonists that exhibit high affinity for CB1 receptors. More work is necessary to demonstrate beyond doubt that CB2-selective agonists are unlikely to be psychoactive or addictive.
The available literature supports the efficacy of CB2 agonists in suppressing persistent pain states following acute administration. However, the impact of long-term treatment with CB2 agonists on antihyperalgesic efficacy and immune system function remains largely unknown. Individuals suffering from immunosuppressive diseases (for example, AIDS patients) could be poor candidates for CB2-mediated pharmacotherapies for pain because of the extensive distribution of CB2 receptors in immune tissue (for example, mast cells, B cells, microglial cells). More work is needed to identify the limitations associated with therapeutic strategies targeting CB2 receptors. Further research should also explore the therapeutic potential of multimodal analgesic strategies that combine CB2-mediated pharmacotherapies for pain with other agents directed at different analgesic targets. Such strategies offer the potential to produce synergistic antihyperalgesic effects with a more beneficial therapeutic ratio compared to conventional analgesics (for example, by combining a CB2-selective agonist with lower doses of opiates, CB1 agonists or nonsteroidal anti-inflammatory drugs that are below the threshold for inducing undesirable side effects). More work is necessary to determine whether activation of CB2 receptors can be employed effectively in chronic pain patients to suppress pathological pain states with limited side effect profiles.
Acknowledgments
We are grateful to Alexander Zvonok for helpful discussions. JG is supported by a Fonds de la recherche en santé du Québec (FRSQ) postdoctoral fellowship. AGH is supported by DA021644, DA022478 and DA022702.
Abbreviations
- CNS
central nervous system
- PEA
palmitoylethanolamide
Conflict of interest
The authors state no conflict of interest.
References
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, et al. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol. 2007;151:1061–1070. doi: 10.1038/sj.bjp.0707303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Piomelli D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur J Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]
- Cantón A, Fernández Castañer M, Conget I, Carreras G, Castell C, Tresserras R. Type 1 diabetes mellitus in Catalonia: chronic complications and metabolic control ten years after onset. Med Sci Monit. 2004;10:CR185–CR190. [PubMed] [Google Scholar]
- Choong KC, Su X, Urban MO. Effect of CP55,940 on mechanosensory spinal neurons following chronic inflammation. Neurosci Lett. 2007;414:105–109. doi: 10.1016/j.neulet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O'Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J.Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain Behav Brain Sci 199720404–419.discussion 435–513 [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Ligresti A, Bifulco M, Melck D, Di Marzo V. Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam Clin Pharmacol. 2002;16:297–302. doi: 10.1046/j.1472-8206.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Plant, synthetic, and endogenous cannabinoids in medicine. Annu Rev Med. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K.Assessing transient and persistent pain in animals Textbook of Pain 1999Churchill Livingstone: New York and Hong Kong; 359–369.In: Wall PD, Melzack R (eds).4th edn. [Google Scholar]
- Dyson A, Peacock M, Chen A, Courade JP, Yaqoob M, Groarke A, et al. Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat. Pain. 2005;116:129–137. doi: 10.1016/j.pain.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naïve rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, et al. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Gallant M, Dufresne C, Gareau Y, Guay D, Leblanc Y, Prasit P, et al. New class of potent ligands for human peripheral cannabinoid receptor. Bioorg Med Chem Lett. 1996;6:2263–2268. [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:PL 191–PL 197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Giblin GM, O'Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, et al. Discovery of 2-[(2,4-dichlorophenyl) amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem. 2007;50:2597–2600. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, et al. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB2 cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology. 2006;50:814–823. doi: 10.1016/j.neuropharm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Guindon J, De Léan A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007a;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Lo Verme J, De Léan A, Piomelli D, Beaulieu P. Synergistic antinociceptive effects of anandamide, an endocannabinoid, and nonsteroidal anti-inflammatory drugs in peripheral tissue: a role for endogenous fatty-acid ethanolamides? Eur J Pharmacol. 2007b;550:68–77. doi: 10.1016/j.ejphar.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Guindon J, Walczak JS, Beaulieu P. Recent advances in the pharmacological management of pain. Drugs. 2007;67:2121–2133. doi: 10.2165/00003495-200767150-00002. [DOI] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB2, a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Rahn EJ, Maxwell KW, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses chemotherapeutic neuropathy evoked by paclitaxel and vincristine administration. Eur J Pain. 2007;11 (Suppl 1):S121. [Google Scholar]
- Honore P, Chapman V, Buritova J, Besson JM. When is the maximal effect of pre-administered systemic morphine on carrageenin evoked spinal c-Fos expression in the rat? Brain Res. 1995;705:91–96. doi: 10.1016/0006-8993(95)01148-x. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Huffman JW. The search for selective ligands for the CB2 receptor. Curr Pharm Des. 2000;6:1323–1337. doi: 10.2174/1381612003399347. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Jonsson KO, Persson E, Fowler CJ. The cannabinoid CB2 receptor selective agonist JWH133 reduces mast cell oedema in response to compound 48/80 in vivo but not the release of β-hexosaminidase from skin slices in vitro. Life Sci. 2006;78:598–606. doi: 10.1016/j.lfs.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Koblish M, Little PJ. Cannabinoid CB2 receptor agonist activity in the hindpaw incision model of postoperative pain. Eur J Pharmacol. 2005;527:172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol (Lond) 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Patrick AL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur J Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003a;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Suplita RL, II, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003b;117:659–670. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Thakur GA, Makriyannis A. Cannabinergic ligands. Chem Phys Lipids. 2002;121:3–19. doi: 10.1016/s0009-3084(02)00143-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Pertwee R, Griffin G, Fernando S, Li X, Hill A, Makriyannis A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, et al. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007;152:765–777. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Inflammatory models of pain and hyperalgesia. ILAR J. 1999;40:111–118. doi: 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Alonso R, Shire D, Congy C, et al. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, et al. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Sagar DR, Kelly S, Millns PJ, O'Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 2005;22:371–379. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Evidence that CB1 and CB2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol. 2004;91:2770–2781. doi: 10.1152/jn.00565.2003. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor CB2: identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipetz DM, O'Neill GP, Favreau L, Dufresne C, Gallant M, Gareau Y, et al. Activation of the human peripheral cannabinoid receptor results in inhibtion of adenylyl cyclase. Mol Pharmacol. 1995;48:352–361. [PubMed] [Google Scholar]
- Tjölsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol (Lond) 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gootshall SL, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005;132:1093–1102. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Characterization of chronic constriction of the saphenous nerve, a model of neuropathic pain in mice showing rapid molecular and electrophysiological changes. J Neurosci Res. 2006;83:1310–1322. doi: 10.1002/jnr.20821. [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG.Cannabinoid mechanisms of pain suppression Cannabinoids- Handbook of Experimental Pharmacology 2005Springer-Verlag: Berlin; 509–554.In: Pertwee R (ed). [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, et al. The VR1 antagonist capsazepine reverses mechanical hyperalgesiain models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H-R, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/s0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, et al. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur J Pharmacol. 2005;528:65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, et al. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br J Pharmacol. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]