Abstract
Amongst the various demyelinating diseases that affect the central nervous system, those induced by an inflammatory response stand out because of their epidemiological relevance. The best known inflammatory-induced demyelinating disease is multiple sclerosis, but the immune response is a common pathogenic mechanism in many other less common pathologies (e.g., acute disseminated encephalomyelitis and acute necrotizing haemorrhagic encephalomyelitis). In all such cases, modulation of the immune response seems to be a logical therapeutic approach. Cannabinoids are well known immunomodulatory molecules that act through CB1 and CB2 receptors. While activation of CB1 receptors has a psychotropic effect, activation of CB2 receptors alone does not. Therefore, to bypass the ethical problems that could result from the treatment of inflammation with psychotropic molecules, considerable effort is being made to study the potential therapeutic value of activating CB2 receptors. In this review we examine the current knowledge and understanding of the utility of cannabinoids as therapeutic molecules for inflammatory-mediated demyelinating pathologies. Moreover, we discuss how CB2 receptor activation is related to the modulation of immunopathogenic states.
Keywords: endocannabinoid system, neuroinflammation, oligodendrocyte, multiple sclerosis, neural stem cells
Introduction
Demyelination is the loss of the myelin sheath that surrounds axons and it may affect both the central nervous system (CNS) and peripheral nervous system (PNS). Demyelinating pathologies may have a primary genetic aetiology (leukodystrophies) or may be the secondary effect of infections, vascular alterations, toxic insults or inflammatory reactions. Regardless of the cause, the result will be the total or partial loss of function in the demyelinated area, depending on the region of the nervous system affected. In this review we shall focus on the CNS demyelinating diseases in which inflammation plays a role in their pathogenesis, like multiple sclerosis (MS). However, we will also discuss other effects mediated by CB2 that are fundamental in demyelinating diseases, including neuronal and glial protection, the regulation of stem/precursor cells and cell replacement. MS is the most frequent neurological disease in young adults. In addition to myelin loss, there is also neuronal damage in MS that further contributes to the symptomatology (Ferguson et al., 1997; Mews et al., 1998; Trapp et al., 1998, 1999). Even though several hypotheses have been proposed to explain its aetiopathology, this issue remains unresolved. Epidemiological studies suggest that MS occurs in a genetically susceptible population that has been in contact with an environmental factor, most probably a viral infection. This triggers an immune response that, by diverse mechanisms is finally directed towards myelin self-peptides (Kurtzke, 1993; Johnson, 1994; Coraddu et al., 1998; Haines et al., 1998). Accordingly, the CSF of MS patients contains antibodies against myelin basic protein (MBP) and myelin oligodendroglial glycoprotein (MOG; Olsson and Nilsson, 1979; Miller et al., 1983). This autoimmune response against myelin peptides has been reproduced in animal models of MS, such as experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD; Rauch et al., 1987; Rodriguez et al., 1988; Fujinami, 1989; Yamada et al., 1990). In both MS- and EAE-, activated lymphocytes are detected that recognize several epitopes of myelin proteins and these self-reactive clones have been reported to be involved in the pathogenesis of EAE and TMEV-IDD (Gerety et al., 1994; Steinman, 1996; Schmidt et al., 1997; Pope et al., 1998; Hellings et al., 2002). The main reason for inflammatory-mediated damage is thought to be the release of reactive oxygen and nitrogen species by immune cells, as well as that of proteases, which directly mediate cell damage (Correa et al., 2005a, 2005b). In addition, immune cells release cytotoxic/cytostatic cytokines, that not only cause damage but that may also enhance the release of more reactive species and glutamate by the cells in the surrounding tissue (the pathogenic mechanisms of demyelinating diseases are summarized in Figure 1). Modulation of the immune response could therefore be an important therapeutic approach to treat demyelinating conditions.
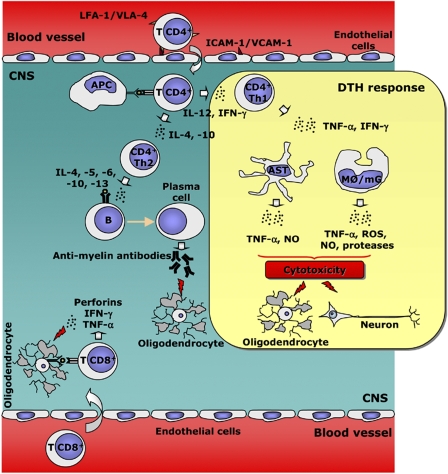
Figure 1.
Hypothetic immune-mediated demyelinating disease pathogenesis. Primed CD4+ T lymphocytes migrate to the CNS through blood vessels enriched in adhesion molecules. Once in the CNS, DCs or macrophages/microglia acting as APC present the antigen to antigen-specific CD4+ lymphocytes. Depending on the environmental signals that these cells receive, the CD4+ lymphocytes acquire different T helper (Th) phenotypes. Th1 cells will establish an antigen-specific DTH response that will lead to the liberation of several cytotoxic molecules by the Th1 themselves, astrocytes and microglia/macrophages. In addition, the generation of the Th2 phenotype will result in the activation of infiltrated B cells into plasma cells that will secrete antibodies against myelin antigens. This response has been related both with oligodendroglial damage and recovery. Even though their pathogenic contribution to MS and EAE is not clear, CD8+ T cells recognize specific antigens and they may elaborate a cytotoxic response against the cells that presented the antigen. CNS, central nervous system; DCs, dendritic cells; DTH, delayed-type hypersensitivity; EAE, experimental allergic encephalomyelitis; LFA-1, lymphocyte function-associated antigen-1; MS, multiple sclerosis; VLA-4, very late activation antigen-4.
Since the late 1970s, it was clear that the psychoactive compounds of Cannabis sativa, such as delta-9-tetrahydrocannabinol (Δ9-THC), modulate the inflammatory response (Zimmerman et al., 1977; Smith et al., 1978). In the following years, numerous studies showed that cannabinoids increased the susceptibility of animals to experimentally induced infections, as these molecules acted as anti-inflammatory drugs. The discovery of this undesirable effect of cannabinoids within the context of infection led to the idea that they could be used against inflammation in diseases like MS. Indeed, in the 1980s, a cannabinoid agonist was first shown to be effective in a CNS demyelinating pathology (Lyman et al., 1989). In this study, animals pretreated with Δ9-THC displayed a delay in the onset of the symptoms and a reduction in severity when EAE was subsequently induced. In corresponding histological studies, a decrease in cell infiltration into the spinal cord was observed, which correlated with the symptomatology of the inflammatory process. The identification of the so-called peripheral cannabinoid receptor (Munro et al., 1993) and further studies on the function of this receptor identified the new CB2 receptor as the major participant in cannabinoid-mediated immune modulation. Indeed, the CB2 receptor was found to be expressed primarily in cells of the immune and haematopoietic systems (Munro et al., 1993) although more recently it was found in the brain (Van Sickle et al., 2005; Gong et al., 2006). CB2 receptors are G-protein-coupled receptors that primarily associate with the Gi/o subtypes of G proteins, and they activate signalling pathways that may involve adenylate cyclase inhibition or activation of mitogen-activated protein kinases (reviewed in Howlett and Mukhopadhyay, 2000; Pacher et al., 2006; Fernandez-Ruiz et al., 2007).
The effects of CB2 activation on demyelination through modulation of the inflammatory response
The first studies on the potential therapeutic role of cannabinoids in demyelinating diseases were aimed at evaluating its usefulness as a symptomatic treatment for spasticity and tremor in patients with MS (Clifford, 1983; Ungerleider et al., 1987). However, the involvement of CB1 or CB2 was not assessed since they were yet to be cloned at that time. While the administration of a CB2 agonist to mice with chronic relapsing experimental allergic encephalomyelitis (CREAE) ameliorated spasticity, the use of a CB2 antagonist worsened it (Baker et al., 2001). As well as using CB2 agonists for symptomatic treatment of demyelinating pathologies, cannabinoids may serve as therapeutic agents. In this scenario, CB2 is expressed in MS plaques by microglia, lymphocytes and astrocytes (Benito et al., 2007). Indeed, intraperitoneal injection of a selective CB2 agonist over 10 days to mice with established TMEV-IDD improves their motor function by modulating microglia and lymphocyte infiltration into the spinal cord (Arévalo-Martin et al., 2003). As with MS, there is an autoimmune response against myelin peptides in TMEV-IDD that contribute to the pathology (Gerety et al., 1994; Merrill and Benveniste, 1996; Steinman, 1996). In essence, myelin-specific CD4+ peripheral T cells are activated and enter the spinal cord where, after recognizing their specific epitope, they differentiate into a T-helper cell (Th1) phenotype and elaborate a delayed-type hypersensitivity (DTH) response (Figure 1). In DTH responses, not only the immune cells are involved but also, the surrounding tissue cells respond to the signals released by the immune system. CB2 receptors are expressed by all cells in the immune system both in humans and rodents (Galiegue et al., 1995; Lee et al., 2001; Matias et al., 2002), as well as by resident CNS cells. We shall analyse, here, how CB2 receptor activation can improve an immune-mediated demyelinating disorder through its effects on the following cell types: CD4+ T cells, B lymphocytes, dendritic cells (DCs), microglia/macrophages and astrocytes. However, it should be noted here that CB2 is highly inducible and its presence or absence in culture models may or may not reflect the native situation in vivo.
CD4+ T cells
Treatment of TMEV-IDD mice with a CB2 agonist reduces the infiltration of CD4+ T cells to the spinal cord (Arévalo-Martín et al., 2003). Among other effects, this could be the result of inhibiting the migration of these cells from the periphery to the CNS since the CB2 selective ligands JWH-133 and JWH-015 inhibit stromal cell-derived factor (CXCL12)-induced chemotaxis and transendothelial migration (Ghosh et al., 2006; Coopman et al., 2007). The reduced infiltration of CD4+ T cells into the spinal cord may also be explained through CB2 activation to induce apoptosis of these cells (Sanchez et al., 2006; Lombard et al., 2007). Remarkably, some of the effects induced by CB2 agonists are not completely blocked by CB2 antagonists and they seem to be independent of CB1 receptors (Sanchez et al., 2006). Thus, it should be borne in mind that there is increasing evidence of non-CB1- and non-CB2-mediated effects of many cannabinoids. Nevertheless, here we will focus on the studies where the effects are mediated partially or entirely by CB2 receptors.
Whether by inhibiting the migration of leukocytes or promoting the apoptosis of these cells, the effect of the reduced infiltration of these cells into the CNS is beneficial as there is a decrease in the release of Th1 cytokines (interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) or interleukin-12 (IL-12)) into the surrounding tissue, which is involved in tissue damage. It is known that cannabinoids alter the profile of cytokine expression from a Th1 to a Th2 phenotype in a CB2-dependent manner (Yuan et al., 2002). In addition, the treatment of TMEV-IDD mice with the non-selective CB1 and CB2 agonist WIN 55212-2 inhibits the myelin-specific DTH response and the release of IFN-γ from CD4+ T cells (Croxford and Miller, 2003). IFN-γ has been shown to exacerbate MS, reducing the survival of oligodendrocytes and affecting normal myelination (Panitch et al., 1987; Corbin et al., 1996; Horwitz et al., 1997; Molina-Holgado et al., 2001). In addition IFN-γ induces the expression of the vascular cellular adhesion molecule-1 (VCAM-1) by endothelial cells, which favours the access of activated lymphocytes to the CNS (Groves et al., 1993; Weiser et al., 2007). Therefore, reducing the release of IFN-γ by the Th1 cells could itself block the ‘calling' signal and therefore, decrease the migration of more primed T cells to the CNS that would amplify the damage.
B lymphocytes
As commented above, antibodies against myelin proteins are detected in both MS patients and in EAE and TMEV-IDD mice. Although their pathological contribution is not clear, since autoantibodies have been related both with damage and recovery (Rodriguez and Lennon, 1990; Brosnan and Raine, 1996), cannabinoids modulate the function of the cells that produce them through a mechanism that involves CB2. For instance, cannabinoids are involved in the maturation of B lymphocytes to antibody-secreting plasma cells, and they are known to induce the apoptosis of these cells (Carayon et al., 1998; Lombard et al., 2007).
Microglia/macrophages
In both MS patients and animal models of the disease, activated microglia/macrophages have been related to tissue damage. The release of reactive oxygen and nitrogen species, seem to be two critical events involved in the pathogenesis of these cells, producing severe damage to myelin and toxicity in oligodendroglia and neurons (Chia et al., 1983; Konat and Wiggins, 1985; Rodriguez and Quddus, 1986; Liuzzi et al., 1995; Molina-Hogado et al., 2001). Treatment of activated microglia cultures with anandamide, UCM707 (an endocannabinoid uptake blocker) or WIN 55212-2, decreases the expression of the inducible NOS-2 and the production of nitrites in a CB1- and CB2-dependent manner (Ortega-Gutierrez et al., 2005; Eljaschewitsch et al., 2006). In addition, microglia and macrophages produce several cytokines considered to be pro-inflammatory, such as TNF-α, IL-1β or IL-12. Through CB2 receptors, cannabinoids inhibit the expression of TNF-α, IL-1β and the p40 subunit of IL-12 and IL-23 by microglia/macrophages (Klegeris et al., 2003; Correa et al., 2005a, 2005b). TNF-α causes neuronal death through an excitotoxic mechanism and recruits more lymphocytes to the CNS by upregulating the expression of VCAM-1 and intercellular adhesion molecule-1 (ICAM-1) on endothelial cells (Dobbie et al., 1999; Weiser et al., 2007). The benefits derived from inhibiting IL-1β release may be related to the increase in NMDA receptor-mediated intracellular calcium provoked by this cytokine, which in turn augments glutamate-mediated neurodegeneration (Viviani et al., 2003). On the other hand, cannabinoids may abrogate the beneficial effects of IL-1β, which promotes the survival of mature oligodendrocytes and therefore, impedes demyelination (Vela et al., 2002). IL-12 is necessary for the differentiation of the lymphocytes that enter into the CNS in the Th1 phenotype, explaining the therapeutic effect of CB2-mediated inhibition of the p40 subunit of IL-12 in monocytic cells (Shevach et al., 1999; Correa et al., 2005a, 2005b). In addition, the p40 subunit is a constituent of IL-23, which is directly involved in the generation of the Th17 lymphocyte phenotype. This has recently been uncovered as a major participant in tissue damage (Langrish et al., 2005; Kroenke and Segal, 2007).
Microglia/macrophages are also involved in antigen presentation, which is necessary for the activation of primed lymphocytes that enter the CNS. Treatment of TMEV-IDD mice with a CB2 agonist decreases microglial activation and major histocompatibility complex-II (MHC-II) expression in the spinal cord (Arévalo-Martin et al., 2003; Ortega-Gutierrez et al., 2005). Finally, specific stimulation of CB2 receptors by JWH-015 suppresses IFN-γ-induced CD40 expression, which is involved in phagocytosis, antigen presentation and the production of cytokines. CD40 is becoming considered as a new therapeutic target for experimental autoimmune encephalomyelitis (EAE), since blocking CD40–CD154 interactions with a neutralizing antibody diminishes murine EAE disease activity (Ehrhart et al., 2005 and references therein).
It should be noted that CB2 expression is highly inducible in macrophages or microglia, and its levels in vitro depend on the local environment and the combination of inflammatory molecules (Carlisle et al., 2002; Maresz et al., 2005). In addition, in vivo CB2 is not expressed equally in all microglial populations, but rather it is predominantly present in perivascular or activated microglia (Benito et al., 2003; Nunez et al., 2004). This variability in the expression of CB2 in vitro and in vivo must be taken into account when extrapolating the effects mediated by CB2 in culture models to the in vivo situation, not only for microglia but also for other cell types.
Dendritic cells
DCs, the major antigen-presenting cells (APCs), drive the cellular fate of lymphocytes and therefore, they are critical for the development of immune responses. These cells have been involved in the pathogenesis of MS and related animal models (Greter et al., 2005) even though there is no direct evidence that activation of CB2 receptors modulates their activity in MS or in animal models. However, CB2 receptor activation does induce apoptosis of these cells, as well as promoting their migration to lymph nodes (Maestroni, 2004).
Astrocytes
Astrocytes express CB1 and CB2 receptors and they respond to cytokines secreted by the immune cells by regulating the production of molecules involved both in the bystander injury and in the protection of CNS tissue (Molina-Holgado F et al., 2002; Sheng et al., 2005). Among these molecules, astrocytes express NOS-2 and they produce NO in response to several inflammatory signals. Cannabinoids inhibit the release of nitrites by astrocytes through a mechanism involving the CB2 receptor (Molina-Holgado et al., 1997; Molina-Holgado F et al., 2002; Sheng et al., 2005). In these cells, cannabinoids also inhibit the inflammation-induced expression of TNF-α, IL-1β and IL-6 through CB1 and CB2 receptors (Molina-Holgado et al., 1997, 1998; Ortega-Gutierrez et al., 2005). The implications of modulating TNF-α and IL-1β expression in inflammatory-demyelinating diseases have already been discussed. Regarding IL-6, this controversial cytokine is thought to be involved in disease development, in the protection of both neurons and oligodendroglial cells from excitotoxicity, and in remyelination (Rodriguez et al., 1994; Okuda et al., 1998; Pizzi et al., 2004). Regarding excitotoxic damage, it was recently shown that cannabinoids can prevent axonal damage in a viral model of MS, interfering with the excitotoxic component in the progression of this disease in a way that requires activation of CB2 receptors (Docagne et al., 2007).
CB2 activation affects remyelination through modulation of the inflammatory response
In experimental models of demyelination there is, both neurological and histopathological, evidence of the therapeutic benefit of cannabinoids. Most data indicate that the activation of CB1 or CB2 receptors reduces deficits such as spasticity, tremor or neuropathic pain (Baker et al., 2000), whereas CB2 receptors also regulate inflammatory aspects related to disease progression (Maresz et al., 2007). Recent studies show that demyelination and axonal defects that initially occur after humoral and cellular immune-mediated attacks to elements of the CNS are affected by cannabinoid treatment. We previously reported extensive remyelination of the spinal cord in TMEV-infected mice, which paralleled the functional recovery of mice treated with WIN 55212-2, ACEA and JWH-015, CB1/CB2, CB1 and CB2 receptor agonists, respectively (Arèvalo-Martín et al., 2003). In this model, cannabinoid-induced attenuation of the inflammatory response was linked to axon remyelination. Oligodendrocyte death and the resulting destruction of myelin plays an important role in axonal degeneration, as demyelinated axons are highly vulnerable to oxidative stress and to cytokine and glutamate toxicity (Werner et al., 2001). Similarly, oligodendrocytes are sensitive to microglial-derived free-radicals and mediators of inflammation (Molina-Holgado et al., 2001; Back et al., 2002; Li et al., 2005). Therefore, strategies aimed at reducing or slowing down the demyelination and neurodegeneration process will certainly be beneficial in MS.
It is now well accepted that once oligodendrocytes have lost their myelin membranes, they are unable to remyelinate axons even if they survive the insult (Keirstead and Blakemore, 1997). Consequently, oligodendrocyte progenitors located in the brain parenchyma and as stem cells in the subventricular zone (SVZ) are recruited to demyelinated areas following experimental demyelination and in MS to remyelinate naked axons (Keirstead and Blakemore, 1999; Chang et al., 2000; Menn et al., 2006). The specific action of cannabinoids in neural stem cells is described in the next section. However, the failure in remyelination could either be due to the constant presence of lymphocytes invading the CNS, which release IFN-γ or to the activated microglia/astrocytes that produce IL-1β, TNF-α and IL-6, among other mediators. Cannabinoids may directly enhance myelin repair by acting on oligodendrocyte progenitors, or they may act indirectly by inhibiting the immune response that might be contributing to demyelination or hampering remyelination.
The synthetic cannabinoid agonists HU210 or WIN 55212-2 act on both CB1 and CB2 receptors to protect oligodendrocytes from apoptosis produced by deprivation of trophic support, a mechanism dependent on phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signalling (Molina-Holgado E et al., 2002). Moreover, cannabinoids suppress the production of inflammatory molecules by astrocytes and microglial cells including IL-1β, TNF-α and NO (Molina-Holgado et al., 1997; Molina-Holgado E et al., 2002; Puffenbarger et al., 2000; Cabral et al., 2001), as well as enhancing the release of the anti-inflammatory cytokines IL-4, IL-10, IL-6 and interleukin-1 receptor antagonist (IL-1ra) (Molina-Holgado et al., 1998, 2003; Klein et al., 2000).
Apoptotic death of oligodendrocytes occurs following treatment with IFN-γ or TNF-α (Vartanian et al., 1995; Baerwald and Popko, 1998; Ye and D'Ercole, 1999). Moreover, NO released by microglia or generated from exogenous NO donors is known to induce oligodendrocyte death (Merrill et al., 1993). In contrast, IL-1β is not toxic for oligodendrocytes in pure culture, but it does produce apoptosis of oligodendrocytes in mixed glial cultures containing astrocytes and microglia. However, this apoptosis can be blocked by antagonists of AMPA/kainate glutamate receptors (Takahashi et al., 2003). Interestingly, the cannabinoid HU-210 reduced neural cell death in response to S-AMPA or NMDA receptor activation, although neurons from IL-1ra knockout (KO) mice were not protected. These results suggest that the neuroprotective and anti-inflammatory effects of cannabinoids are mediated by the release of endogenous IL-1ra in primary neurons or glial cells in vitro. The specific CB1 and CB2 receptor antagonists (SR-141716A and SR-144528, respectively) abrogated lipopolysaccharide (LPS)-induced IL-1ra release, implicating both CB receptors in this effect (Molina-Holgado et al., 2003). However, in chronic relapsing EAE only cannabinoid agonists acting through CB1 receptors provide significant neuroprotection (Pryce et al., 2003).
The anti-inflammatory cytokines, IL-4 and IL-10 and their receptors are present in oligodendroglial cells (Molina-Holgado et al., 2001), and some of the effects attributed to these cytokines in models of MS (Kennedy et al., 1992) may have a direct influence on oligodendrocytes. Remarkably, IL-10 offered protection against oligodendroglial death evoked by LPS/IFN-γ. These data raise the question of whether IL-10 may play a protective role in demyelinating diseases, not only downregulating the function of inflammatory cells but also promoting the survival of progenitors and differentiated oligodendrocytes (Molina-Holgado et al., 2001).
Finally, the activation of cannabinoid receptors exerts protective effects on neurons and oligodendrocytes and suppresses chronic inflammatory responses through the attenuation of pro-inflammatory mediators. Therefore, cannabinoids might well modulate the progression of demyelinating diseases.
The effect of CB2 receptor activation on neural stem/precursor cells
CB2 receptors in the CNS
The past decade has seen a dramatic increase in our understanding of CB2 cannabinoid receptor expression in the CNS, where CB2 receptors display a more limited pattern of expression than CB1 receptors (Pazos et al., 2005; Van Sickle et al., 2005; Gong et al., 2006). CB2 receptors are thought to be mainly expressed in the immune system (Munro et al., 1993); however, recent findings have clearly demonstrated that CB2 receptors are also located in discrete neural cell populations (Van Sickle et al., 2005), oligodendrocyte progenitors and mature cells (Molina-Holgado E et al., 2002), cerebellar granular cells (Skaper et al., 1996), microglial cells (Benito et al., 2003; Walter and Stella, 2004) and endothelial cells (Golech et al., 2004). Moreover, cannabinoid CB2 receptors have been identified in neural stem cells (NSCs) both in vivo and in vitro, and CB2 selective cannabinoid agonists and antagonists modulate NSC formation and precursor cell proliferation through phosphoinositide-3 kinaseAkt and extracellular regulated kinase signalling (Palazuelos et al., 2006; Arevalo-Martin et al., 2007; Molina-Holgado et al., 2007). Indeed, this pathway was previously shown to mediate cannabinoid-induced survival of oligodendrocyte progenitor cells deprived of trophic support in vitro (Molina-Holgado E et al., 2002).
Neural stem cells express CB2 receptors in vivo
Some discrete regions of active neurogenesis are maintained in the adult mammalian brain, with the capacity to generate functional neurons. These neurogenic areas include the SVZ and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus, and adult NSCs appear to be capable of proliferating and differentiating within these zones (for a review see Alvarez-Buylla and Lim, 2004). In both these areas, the SVZ and SGZ, functional CB2 receptors have been identified in neural progenitor cells and their activation has been associated with NSC proliferation (Palazuelos et al., 2006; Molina-Holgado et al., 2007). Mounting interest has focused on the dual function of the endocannabinoid system on neurogenesis in health and disease (Galve-Roperh et al., 2007). Hence, further studies will be necessary to determine how CB2 signalling pathways participate in the regulation of neurogenesis during CNS development, and whether CB2-associated signalling cascades are activated in neurodegenerative disorders.
The SGZ
Neural progenitor cells located in the SGZ of the hippocampus proliferate and give rise to immature neurons (Kempermann et al., 2003), which become functional neurons within 4–6 weeks (Van Praag et al., 2002). There is evidence that CB2 receptors play an active role in the modulation of hippocampal neurogenesis. Indeed, hippocampal progenitors produce endocannabinoids in a developmentally regulated pattern (Fernandez-Ruiz et al., 2000; Aguado et al., 2005). Moreover, the expression of CB2 receptors in the SGZ is restricted to neural progenitor cells (Palazuelos et al., 2006). The presence of functional CB2 receptors in SGZ progenitor cells was confirmed by assessing BrdU incorporation in response to HU-308 treatment in CB2 KO animals and their wild-type (WT) littermates. Pharmacological blockage with the CB2 antagonist SR-144528 blocked basal progenitor proliferation while HU-308 induced proliferation, suggesting that CB2 signalling modulates progenitor cell proliferation in the SGZ (Palazuelos et al., 2006).
The SVZ
In the SVZ, NSC proliferate and are a source of neurons and oligodendrocytes (Doetsch et al., 1999; Menn et al., 2006). CB2 cannabinoid receptors are present in the postnatal rat SVZ and exogenous administration of selective CB2 receptor agonists such as JWH-056 to newborn animals affects the development of this zone (Arevalo-Martin et al., 2007). Additional studies confirmed that CB2 receptors are expressed in the rat SVZ using PCR, western blotting and immunohistochemistry. This expression is somewhat complementary to that of the CB1 receptor since CB2 is predominantly expressed in polysialylated neural cell adhesion molecule (PSA-NCAM)-positive precursors, although there are a small number of stem cells that are immunoreactive for CB2 receptors. Furthermore, CB2 receptors affect SVZ function, since treatment with a selective agonist (JWH-056) increases the expression of PSA-NCAM. PSA-NCAM-expressing cells in the SVZ have been identified as migratory neuroblasts that enter the rostral migratory stream, as well as oligodendrocyte precursors that migrate towards the adjacent areas of the white matter (Doetsch et al., 1999; Menn et al., 2006). The signals controlling the physiology of postnatal SVZ development remain unknown, despite the fact that some candidates control these processes have been already identified (Lie et al., 2004). These findings indicate that cannabinoids may provide such signals, and they suggest that both CB1 and CB2 receptors may be implicated in postnatal oligodendrogenesis.
Neural stem cells express CB2 receptors in vitro
In vitro studies, using neurospheres (clonal cellular aggregates of neural stem cells/precursors cells), stem cell lines or primary cultures of NSC derived from embryonic and adult brain, have confirmed that cells proliferating in vitro express functional CB2 receptors (Palazuelos et al., 2006; Molina-Holgado et al., 2007). Indeed, CB2 receptors are expressed in PSA-NCAM-positive cells of the rat SVZ (Arevalo-Martin et al., 2007). Accordingly, NSCs express fully functional CB2 receptors and their activation promotes their proliferation leading to the generation of more neurospheres. Hence, CB2 receptor activation could be involved in maintaining the self-renewal capacity of stem cells. Moreover, the expression of the stem cell marker Sox-2 is maintained through several passages in cultures stimulated with CB2 agonists. Since the activation of CB2 receptors does not produce any psychoactive effects, these results open the possibility to study whether neural stem cell behaviour could be manipulated in vivo through the administration of exogenous CB2-selective agonists.
Collectively, these findings demonstrate that NSCs are targets for CB2 agonists, and in conjunction with recent synthesis of non-psychotropic CB2 agonists (Galve-Roperh et al., 2006; Zhang et al., 2007), this raises the possibility that CB2 agonists could have the potential to promote brain repair. In fact, brain or spinal cord lesions activate stem/precursor cells and recruit new cells to the injured areas (Hallbergson et al., 2003; Romanko et al., 2004). Thus, promoting remyelination by endogenous progenitors or transplantation of new exogenous progenitors are promising therapeutic strategies for demyelinating diseases (Imitola et al., 2003). Either way, CB2 agonists could be a powerful tool to aid future therapies.
Conclusions
Resident immune and CNS cells express functional CB2 receptors. The activation of CB2 receptors results in the modulation of the inflammatory response, restraining one of the agents responsible for the progress of demyelination and neuronal death, the ultimate causes of the symptoms in pathologies such as MS and EAE. The modulation of inflammatory molecules through CB2 receptors could also enhance remyelination, stimulating the survival of oligodendrocyte precursors and neural stem/precursor cells, and their development into mature oligodendrocytes.
However, the role of CB2 in controlling demyelination and enhancing remyelination is not limited to autoimmune diseases and it is not restricted to the control of the immune system (Figure 2). Both in MS, EAE and other non-immune-mediated demyelinating diseases, the protective effect of CB2 agonists on neural cells is a remarkable advantage. Moreover, CB2 receptor activation may be a relevant strategy in cellular replacement. However, before proposing the usefulness of CB2 agonists for myelin disorders, it is necessary to obtain a deeper understanding of what effects may be attributable to the activation of CB2 receptors alone, and which are also due to the participation of the CB1 receptor. Furthermore, we must be aware of what effects may be mediated by other receptors, since there is increasing evidence that cannabinoids can induce certain effects independently of CB1 or CB2. Nevertheless, given our current understanding of CB2 receptors and the pathogenesis of immune-mediated or other demyelinating disorders, these receptors seem to be of potential therapeutic interest.
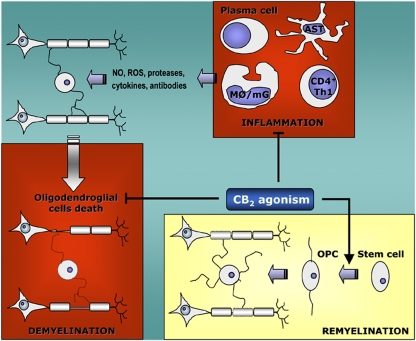
Figure 2.
Points of interaction of CB2 receptors with the pathogenesis of demyelinating diseases. The activation of CB2 receptors decreases the deleterious inflammatory response that results in the death of both oligodendrocyte progenitors and mature oligodendrocytes in immune-mediated demyelinating pathologies. Also, CB2 stimulation in oligodendroglial cells promotes their survival and therefore, CB2 agonists may be useful in other non-immune-mediated demyelinating pathologies. In addition, the activation of CB2 receptors may not only have an effect in protecting from demyelination but also, in promoting repair. In this sense, CB2 agonists promote the proliferation of neural stem/precursor cells and increase the expression of migration-related molecules such as PSA-NCAM in the SVZ. Therefore, CB2 receptor agonists could be therapeutic molecules that prevent the loss of myelin and promote the activation of the neural stem/precursor cells involved in the recovery of the myelin sheath. PSA-NCAM, polysialylated neural cell adhesion molecule; SVZ, subventricular zone.
Acknowledgments
EM-H is funded by grants from the Spanish Fondo de Investigaciones Sanitarias (04/2120) and from the Consejería de Sanidad de la Junta de Comunidades de Castilla-La Mancha (04061-00). FM-H is funded by The Wellcome Trust (UK). Personal support to AR-A was from the Consejería de Educación y Ciencia de la JCCM, and to B.N-G from the Ministerio de Educación y Ciencia (Programa Juan de la Cierva). CG is supported by Grants (SAF2004/0416) from Ministerio de Educación y Ciencia (Spain). We thank Dr Mark Sefton for critical reading of the manuscript and grammatical assistance.
Abbreviations
- APCs
antigen-presenting cells
- CNS
central nervous system
- CREAE
chronic relapsing experimental allergic encephalomyelitis
- CXCL12
stromal cell-derived factor
- DCs
dendritic cells
- DHT
delayed-type hypersensitivity
- EAE
experimental allergic encephalomyelitis
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-γ
- IL
interleukin
- IL-1ra
interleukin-1 receptor antagonist
- KO
knockout
- LPS
lipopolysaccharide
- MBP
myelin basic protein
- MHC
major histocompatibility complex
- MOG
myelin oligodendroglial glycoprotein
- MS
multiple sclerosis
- NSCs
neural stem cells
- PI3K/Akt
phosphoinositide 3-kinase/protein kinase B
- PNS
peripheral nervous system
- PSA-NCAM
polysialylated neural cell adhesion molecule
- SGZ
subgranular zone
- SVZ
subventricular zone
- Ths
T-helper cells
- TMEV-IDD
Theiler's murine encephalomyelitis virus-induced demyelinating disease
- TNF-α
tumour necrosis factor alpha
- VCAM-1
vascular cellular adhesion molecule-1
- WT
wild type
- Δ9-THC
delta-9-tetrahydrocannabinol
Conflict of interest
The authors state no conflict of interest.
References
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arévalo-Martín A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. Neuroscience. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Martin A, Garcia-Ovejero D, Rubio-Araiz A, Gomez O, Molina-Holgado F, Molina-Holgado E.Cannabinoids modulate OLIG2 and PSA-NCAM expression in the subventricular zone of postnatal rats through CB1 and CB2 receptors Eur J Neurosci 2007. e-pub ahead of print: doi:10.1111/j.460-9568.2007.05782 [DOI] [PubMed]
- Back SA, Han BH, Lou NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia–ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald KD, Popko B. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J Neurosci Res. 1998;52:230–239. doi: 10.1002/(SICI)1097-4547(19980415)52:2<230::AID-JNR11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively over expressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Romero JP, Tolon RM, Clemente D, Docagne F, Hillard CJ, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Raine CS. Mechanisms of immune injury in multiple sclerosis. Brain Pathol. 1996;6:243–257. doi: 10.1111/j.1750-3639.1996.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Harmon KN, Carlisle SJ. Cannabinoid-mediated inhibition of inducible nitric oxide production by rat microglial cells: evidence for CB1 receptor participation. Adv Exp Med Biol. 2001;493:207–214. doi: 10.1007/0-306-47611-8_24. [DOI] [PubMed] [Google Scholar]
- Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–3615. [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia LS, Thompson JE, Moscarello MA. Disorder in human myelin induced by superoxide radical: an in vitro investigation. Biochem Biophys Res Commun. 1983;117:141–146. doi: 10.1016/0006-291x(83)91552-8. [DOI] [PubMed] [Google Scholar]
- Clifford DB. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann Neurol. 1983;13:669–671. doi: 10.1002/ana.410130616. [DOI] [PubMed] [Google Scholar]
- Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharmacol. 2007;7:360–371. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Coraddu F, Sawcer S, Feakes R, Chataway J, Broadley S, Jones HB, et al. HLA typing in the United Kingdom multiple sclerosis genome screen. Neurogenetics. 1998;2:24–33. doi: 10.1007/s100480050048. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Kelly D, Rath EM, Baerwals KD, Suzuki K, Popko B. Targeted CNS expression of interferon-gamma in transgenic mice leads to hypomyelination, reactive gliosis and abnormal cerebellar development. Mol Cell Neurosci. 1996;7:354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- Correa F, Mestre L, Docagne F, Guaza C. Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: role of IL-10 and ERK1/2 kinase signaling. Br J Pharmacol. 2005a;145:441–448. doi: 10.1038/sj.bjp.0706215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Mestre L, Molina-Holgado E, Arevalo-Martin A, Docagne F, Romero E, et al. The role of cannabinoid system on immune modulation: therapeutic implications on CNS inflammation. Mini Rev Med Chem. 2005b;5:671–675. doi: 10.2174/1389557054368790. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Miller SD. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55, 212. J Clin Invest. 2003;111:1231–1240. doi: 10.1172/JCI17652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbie MS, Hurst RD, Klein NJ, Surtees RA. Upregulation of intercellular adhesion molecule-1 expression on human endothelial cells by tumour necrosis factor-alpha in an in vitro model of the blood-brain barrier. Brain Res. 1999;830:330–336. doi: 10.1016/s0006-8993(99)01436-5. [DOI] [PubMed] [Google Scholar]
- Docagne F, Muñetón V, Clemente D, Ali C, Loría F, Correa F, et al. Excitotoxicity in a chronic model of multiple sclerosis: neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fujinami RS. Immune responses against myelin basic protein and/or galactocerebroside cross-react with viruses: implications for demyelinating disease. Curr Top Microbiol Immunol. 1989;145:93–100. doi: 10.1007/978-3-642-74594-2_8. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon S, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Aguado T, Palazuelos J, Guzman M. The endocannabinoid system and neurogenesis in health and disease. Neuroscientist. 2007;13:109–114. doi: 10.1177/1073858406296407. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Aguado T, Rueda D, Velasco G, Guzman M. Endocannabinoids: a new family of lipid mediators involved in the regulation of neural cell development. Curr Pharm Des. 2006;12:2319–2325. doi: 10.2174/138161206777585139. [DOI] [PubMed] [Google Scholar]
- Gerety SJ, Rundell MK, Dal Canto MC, Miller SD. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Groves RW, Ross EL, Barker JN, MacDonald DM. Vascular cell adhesion molecule-1: expression in normal and diseased skin and regulation in vivo by interferon gamma. J Am Acad Dermatol. 1993;129:67–72. doi: 10.1016/0190-9622(93)70154-l. [DOI] [PubMed] [Google Scholar]
- Haines JL, Terwedow HA, Burgess K, Pericak-Vance MA, Rimmler JB, Martin ER, et al. Linkage of the MHC to familial multiple sclerosis suggest genetic heterogeneity. Hum Mol Genet. 1998;7:1229–1234. doi: 10.1093/hmg/7.8.1229. [DOI] [PubMed] [Google Scholar]
- Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellings N, Gelin G, Medaer R, Bruckers L, Palmers Y, Raus J, et al. Longitudinal study of antimyelin T-cell reactivity in relapsing-remitting multiple sclerosis: association with clinical and MRI activity. J Neuroimmunol. 2002;126:143–160. doi: 10.1016/s0165-5728(02)00052-8. [DOI] [PubMed] [Google Scholar]
- Horwitz MS, Evans CF, McGavern DB, Rodriguez M, Oldstone MB. Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- Imitola J, Snyder EY, Khoury SJ. Genetic programs and responses of neural stem/progenitor cells during demyelination: potential insights into repair mechanisms in multiple sclerosis. Physiol Genomics. 2003;14:171–197. doi: 10.1152/physiolgenomics.00021.2002. [DOI] [PubMed] [Google Scholar]
- Johnson RT. The virology of demyelinating diseases. Ann Neurol. 1994;36:54–60. doi: 10.1002/ana.410360715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of postmitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. Neuropathol Exp Neuro. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol. 1999;468:183–197. doi: 10.1007/978-1-4615-4685-6_15. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokines mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- Konat GW, Wiggins RC. Effect of reactive oxygen species on myelin membrane proteins. J Neurochem. 1985;45:1113–1118. doi: 10.1111/j.1471-4159.1985.tb05530.x. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Segal BM. Th17 and Th1 responses directed against the immunizing epitope, as opposed to secondary epitopes, dominate the autoimmune repertoire during relapses of experimental autoimmune encephalomyelitis. J Neurosci Res. 2007;85:1685–1693. doi: 10.1002/jnr.21291. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:422–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur J Pharmacol. 2001;423:235–241. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Riccio P, dal Canto MC. Release of myelin basic protein-degrading proteolytic activity from microglia and macrophages after infection with Theiler's murine encephalomyelitis virus: comparision between susceptible and resistant mice. J Neuroimmunol. 1995;62:91–102. doi: 10.1016/0165-5728(95)00110-n. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman WD, Sonett JR, Brosnan CF, Elkin R, Bornstein MB. Δ9-Tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol. 1989;23:73–81. doi: 10.1016/0165-5728(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004;18:1914–1916. doi: 10.1096/fj.04-2190fje. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur J Biochem. 2002;269:3771–3778. doi: 10.1046/j.1432-1033.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Mews I, Bergmann M, Bunkowski S, Gullotta F, Bruck W. Oligodendrocyte and axon pathology in clinically silent multiple sclerosis lesions. Mult Scler. 1998;4:55–62. doi: 10.1177/135245859800400203. [DOI] [PubMed] [Google Scholar]
- Miller JR, Burke A, Bever CT. Occurrence of oligoclonal bands in multiple sclerosis and other CNS diseases. Ann Neurol. 1983;13:53–58. doi: 10.1002/ana.410130112. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arévalo-Martín A, Guaza C. LPS/IFN-gamma cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. Eur J Neurosci. 2001;13:493–502. doi: 10.1046/j.0953-816x.2000.01412.x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Molina-Holgado F, Almazan G, Borrell J, et al. Expression of cannabinoid CB1 receptors in oligodendroglial cells: activation of PI-3KAkt signalling pathway promotes cell survival. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Lledo A, Guaza C. Anandamide suppresses nitric oxide and TNF-alpha responses to Theiler's virus or endotoxin in astrocytes. NeuroReport. 1997;8:1929–1933. doi: 10.1097/00001756-199705260-00027. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Molina-Holgado E, Guaza C. The endogenous cannabinoid anandamide potentiates interleukin-6 production by astrocytes infected with Theiler's murine encephalomyelitis virus by a receptor-mediated pathway. FEBS Lett. 1998;433:139–142. doi: 10.1016/s0014-5793(98)00851-5. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide induced nitric oxide release in astrocyte cultures. J Neurosci Res. 2002;67:829–836. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Pinteaux E, Moore J, Molina-Holgado E, Gibson RM, Guaza C, et al. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurones and glia. J Neurosci. 2003;23:6470–6474. doi: 10.1523/JNEUROSCI.23-16-06470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Rubio-Araiz A, Williams RJ, Moore JD, Arévalo-Martín A, García-Ovejero D, et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2007;25:629–634. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, et al. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- Olsson JE, Nilsson K. Gamma globulins of CSF and serum in multiple sclerosis: isoelectric focusing on polyacrylamide gel and agar gel electrophoresis. Neurology. 1979;29:1381–1391. doi: 10.1212/wnl.29.10.1383. [DOI] [PubMed] [Google Scholar]
- Ortega-Gutierrez S, Molina-Holgado E, Arevalo-Martin A, Correa F, Viso A, Lopez-Rodriguez ML, et al. Activation of the endocannabinoid system as therapeutic approach in a murine model of multiple sclerosis. FASEB J. 2005;19:1338–1340. doi: 10.1096/fj.04-2464fje. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exarcebation associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Pazos MR, Núñez E, Benito C, Tolón RM, Romero J. Functional neuroanatomy of the endocannabinoid system. Pharmacol Biochem Behav. 2005;81:239–247. doi: 10.1016/j.pbb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Sarnico I, Boroni F, Benarese M, Dreano M, Garotta G, et al. Prevention of neuron and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6 receptor/IL-6 fusion protein in organotypic hippocampal slices. Mol Cell Neurosci. 2004;25:301–311. doi: 10.1016/j.mcn.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Pope JG, Vanderlugt CL, Rahbe SM, Lipton HL, Miller SD. Characterization of and functional antigen presentation by central nervous system mononuclear cells from mice infected with Theiler's murine encephalomyelitis virus. J Virol. 1998;72:7762–7771. doi: 10.1128/jvi.72.10.7762-7771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce G, Ahmed Z, Hankey DJR, Jackson SJ, Croxford JL, Pocock JM, et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Rauch HC, Montgomery IN, Hinman CL, Harb W, Benjamins JA. Chronic Theiler's virus infection in mice: appearance of myelin basic protein in the cerebrospinal fluid and serum antibody directed against MBP. J Neuroimmunol. 1987;14:35–48. doi: 10.1016/0165-5728(87)90099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Lennon VA. Immunoglobulins promote remyelination in the central nervous system. Ann Neurol. 1990;27:12–17. doi: 10.1002/ana.410270104. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Lucchinetti C, Clark RJ, Yaksh TL, Markowitz H, Lennon VA. Immunoglobulins and complement in demyelination induced in mice by Theiler's virus. J Immunol. 1988;140:800–806. [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, McKinney CW, Leibowitz JL. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J Immunol. 1994;153:3811–3821. [PubMed] [Google Scholar]
- Rodriguez M, Quddus J. Effect of cyclosporin A, silica quartz dust, and protease inhibitors on virus-induced demyelination. J Neuroimmunol. 1986;13:159–174. doi: 10.1016/0165-5728(86)90062-7. [DOI] [PubMed] [Google Scholar]
- Romanko MJ, Rola R, Fike JR, Szele FG, Dizon M, Felling RJ, et al. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog Neurobiol. 2004;74:77–99. doi: 10.1016/j.pneurobio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Sanchez AJ, Gonzalez-Perez P, Galve-Roperh I, Garcia-Merino A. R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)-pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphtalenylmethanone (WIN-2) ameliorates experimental autoimmune encephalomyelitis and induces encephalitogenic T cell apoptosis: partial involvement of the CB(2) receptor. Biochem Pharmacol. 2006;72:1697–1706. doi: 10.1016/j.bcp.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55, 212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;15:211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- Shevach EM, Chang JT, Segal BM. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin Immunopathol. 1999;21:249–262. doi: 10.1007/BF00812256. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Linigton C, Zipp F, Sotgiu S, de Waal MR, Wekerle H, et al. Multiple sclerosis: comparison of the human T-cell response to S100b and myelin basic protein reveals parallels to rat experimental autoimmune panencephalitis. Brain. 1997;120:1437–1445. doi: 10.1093/brain/120.8.1437. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, et al. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc Natl Acad Sci USA. 1996;93:3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SH, Harris LS, Uwaydah IM, Munson AE. Structure-activity relationships of natural and synthetic cannabinoids in suppression of humoral and cell-mediated immunity. J Pharmacol Exp Ther. 1978;207:165–170. [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong V. Interleukin-1β promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003;53:588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransahoff R, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Ransahoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol. 1999;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Ungerleider JT, Andyrsiak T, Faibanks L, Ellison GW, Myers LW. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse. 1987;7:39–50. doi: 10.1300/j251v07n01_04. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Li Y, Zhao M, Stefansson K. Interferonγ-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–743. [PMC free article] [PubMed] [Google Scholar]
- Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20:489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser S, Miu J, Ball HJ, Hunt NH. Interferon-gamma synergises with tumour necrosis factor and lymphotoxin-alpha to enhance the mRNA and protein expression of adhesion molecules in mouse brain endothelial cells. Cytokine. 2007;37:84–91. doi: 10.1016/j.cyto.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Yamada M, Zurbriggen A, Fujinami RS. The relationship between viral RNA, myelin-specific mRNAs and demyelination in central nervous system disease during Theiler's virus infection. Am J Pathol. 1990;137:1467–1479. [PMC free article] [PubMed] [Google Scholar]
- Ye P, D'Ercole AJ. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology. 1999;140:3063–3072. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. 2002;133:124–131. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S, Zimmerman AM, Cameron IL, Laurence HL. Delta1-tetrahydrocannabinol, cannabidiol and cannabinol effects on the immune response of mice. Pharmacology. 1977;15:10–23. doi: 10.1159/000136658. [DOI] [PubMed] [Google Scholar]