Abstract
Most cognitive neuroscientific research exploring the nature of age-associated compensatory mechanisms has compared old adults (high vs. average performers) to young adults (not split by performance), leaving ambiguous whether findings are truly age-related or reflect differences between high and average performers throughout the lifespan. Here, we examined differences in neural activity (as measured by ERPs) that were generated by high vs. average performing old, middle-age, and young adults while processing novel and target events to investigate the following three questions: 1) Are differences between cognitively high and average performing subjects in the allocation of processing resources (as indexed by P3 amplitude) specific to old subjects, or found throughout the adult lifespan? 2) Are differences between cognitively high and average performing subjects in speed of processing (as indexed by target P3 latency) of similar magnitude throughout the adult lifespan? 3) Where along the information processing stream does the compensatory neural activity attributed to cognitively high performing old subjects begin to take place? Our results suggest that high performing old adults successfully manage the task by a compensatory neural mechanism associated with the modulation of controlled processing and the allocation of more resources, whereas high performing younger subjects execute the task more efficiently with fewer resources. Differences between cognitively high and average performers in processing speed increase with age. Middle-age seems to be a critical stage in which substantial differences in neural activity between high and average performers emerge. These findings provide strong evidence for different patterns of age-related changes in the processing of salient environmental stimuli, with cognitive status serving as a key mediating variable.
Keywords: compensatory neural activity, cognitive aging, novelty, attention, ERPs, P3
Introduction
In recent years, there has been an increasing interest in not only making overall distinctions between ‘normal’ and diseased aging, but also in understanding the sources of individual differences within the older population (Daffner et al., 2006a;Fabiani et al., 1998;Friedman, 2003). Of particular interest has been elucidating the neural mechanisms that allow some older individuals to perform cognitive tasks at a level comparable to their younger counterparts (Cabeza et al., 2002;Daffner et al., 2006b;Duarte et al., 2006;Stern, 2002). In this context, we have published a series of related reports on differences between cognitively average and cognitively high performing adults across the lifespan in their P3 event-related potential and behavioral responses to novel and target events (Daffner et al., 2005;Daffner et al., 2006b;Daffner et al., 2006a;Daffner et al., 2007). Our emphasis has been on novelty processing because of the notion that increased responsiveness to novelty may not only be a reflection of successful cognitive aging, but also helps to promote it (Daffner et al., 2006a;Wilson et al., 2002). We have focused on the P3 component because its amplitude is likely to reflect (or be very sensitive to) the amount of processing resources allocated to reorienting attention, updating working memory, or categorizing an event (Daffner et al., 1998;Donchin and Coles, 1988;Escera et al., 1998;Isreal et al., 1980;Kok, 2001;Sirevaag et al., 1989). Additionally, P3 latency can serve as an index of information processing speed (time taken for stimulus evaluation or memory updating) and not motor response preparation (McCarthy and Donchin, 1981;Walhovd and Fjell, 2002).
To obtain a measure of behavioral response to both novel and target events, we have developed a subject-controlled variant of the novelty oddball task in which subjects control how long they look at standard, rare target, and infrequent unique novel images. In several previous studies, we have employed this paradigm with cognitively high and average performing young, middle-age and old adults (determined by dividing each age group into those scoring in the top (cognitively high) and middle third (cognitively average) on neuropsychological tests based on age-appropriate norms). These studies have revealed several major findings: 1) Behaviorally, the viewing duration of novel stimuli (an index of visual attention and exploratory behavior (Berlyne, 1960)) of cognitively high performing old individuals was comparable to that of cognitively high performing middle-age and young subjects, and longer than matched cognitively average performing old subjects (Daffner et al., 2007). 2) Cognitively high performing old individuals had faster, more accurate responses to targets than cognitively average performing old individuals (Daffner et al., 2006a) and performed comparably to cognitively high performing middle-age and young individuals (Daffner et al., 2007). 3) The P3 latency to targets of cognitively high performing old subjects was shorter than that of cognitively average performing old subjects (Daffner et al., 2006a), and comparable to that of middle-age and young cognitively high performing subjects (Daffner et al., 2006b). 4) Cognitively high performing old subjects generated a larger P3 amplitude to novel stimuli than both cognitively average performing old subjects (Daffner et al., 2006a) and cognitively high performing middle-age and young subjects (Daffner et al., 2006b).
Our research has left several outstanding issues. In the current study, we address 3 specific questions: 1) Are differences between cognitively high and average performing subjects in the allocation of processing resources (as measured by P3 amplitude) specific to old subjects, or found throughout the adult lifespan? 2) Are differences between cognitively high and average performing subjects in speed of processing (as measured by target P3 latency) of similar magnitude throughout the adult lifespan? 3) Where along the information processing stream does the compensatory neural activity attributed to cognitively high performing old subjects begin to take place?
The first issue, addressing differences in resource allocation between cognitively high and average performing individuals across the lifespan, is particularly relevant. Although recently there has been a number of studies comparing the neural activity of higher and lower performing old subjects to that of a group of young subjects (Cabeza et al., 2002;Duarte et al., 2006;Rosano et al., 2005;Rosen et al., 2002), only a few reports have taken into account differences in neural activity between groups of young subjects that differed in performance (e.g., Fjell and Walhovd, 2005;Madden et al., 2004;Stern et al., 2004). Without such a comparison group, it is difficult to be sure whether the differences between high and average performing old subjects are related to age, or reflect different levels of performance that would be observed for any age group. This issue was investigated by including cognitively high and cognitively average performing middle-age and young subjects in our sample. We hypothesized that the relationship between cognitive status and age would be a complex one. Old subjects with greater cognitive competence may compensate for age-related physiological changes by allocating more resources, which would be indexed by a larger P3 response, whereas younger subjects with greater cognitive competence may be able to manage the task more efficiently, which would be indexed by a smaller P3 response. Thus, we anticipated that analysis of the P3 response to novel events would be associated with a significant interaction between age and cognitive status.
The second issue, concerning whether differences between cognitively high and average performing subjects in speed of processing (as measured by target P3 latency) are similar across the lifespan, is also important to pursue. Prior studies have suggested that an increase in target P3 latency is associated with aging (O'Donnell et al., 1992;Polich, 1996;Walhovd and Fjell, 2002) and cognitive decline (Olichney and Hillert, 2004). Several groups also have reported a relationship between P3 latency and normal cognitive abilities, especially measures of attention, processing speed, and fluid intelligence (O'Donnell et al., 1992;Polich et al., 1983;Walhovd and Fjell, 2002). For example, Polich et al. (1983) and O’Donnell et al. (1992) found an inverse correlation between target P3 latency and performance on Digit Span, which remained after controlling for age. Walhovd and Fjell (2002) also reported an inverse correlation between target P3 latency and Block Design, Matricies, and Digit Span, of which only Digit Span remained significant after controlling for age. Prior studies have not explicitly evaluated whether the magnitude of the differences between cognitively high and average performing individuals varies across the lifespan. Moreover, we are aware of only one report, by Fjell and Walhovd (2005), that has examined whether age-related increases in P3 latency are modulated by differences in cognitive status. The results from this report suggested that they were not. In the current study, these issues were addressed by investigating P3 latency across subjects who differed in terms of cognitive status (high vs. average performers) and age (old vs. middle-age vs. young). We hypothesized that the magnitude of the difference in P3 latency between cognitively high and cognitively average performing adults would be largest among old subjects (as indicated by an interaction between cognitive status and age). Moreover, we anticipated that, in contrast to cognitively high performers, cognitively average performers would exhibit the commonly reported age-related increase in P3 latency.
The final issue, concerning where along the information processing stream compensatory neural activity begins, is difficult to address using functional imaging techniques, but is very well-suited to investigation by ERPs, which have excellent temporal resolution. To sort out this issue, we extended our previous analyses to include ERP components earlier than the P3. Earlier ERP components are believed to reflect perceptual encoding and sensory gating mechanisms (P1 (Luck et al., 1990;Mangun and Hillyard, 1988), N1 (Hillyard et al., 1973;Luck et al., 2000)), stimulus feature detection (P2 (Luck and Hillyard, 1994)), and preliminary stimulus categorization or detection of stimulus unfamiliarity (N2 (Daffner et al., 2000a;Pritchard et al., 1991)). We hypothesized, based on the available literature (e.g., Beck et al., 1980;Celesia and Daly, 1977;Curran et al., 2001;Ford and Pfefferbaum, 1991;Kenemans et al., 1995;Muller and Knight, 2002;Snyder and Hillyard, 1976;Yamaguchi et al., 1995), that the most salient difference between cognitively high and average performers in electrophysiologic response to novel and target events would involve controlled processing linked to directing attention to salient stimuli and updating the contents of working memory, which would be indexed by differences in the P3 amplitude and latency.
The current study followed the schema proposed by Friedman and colleagues (2001). The novelty P3 (or P3 to novels) was defined as the P3 response elicited by infrequent events about which the subject received no instruction before the experiment (Friedman et al., 2001). The target P3 (or P3 to targets) was defined as the P3 response evoked by designated events about which the subject had been instructed prior to the experiment and to which the subject was required to generate a specific response (Friedman et al., 2001). We adopted the following strategy for interpreting our electrophysiologic results that is consistent with one suggested by several investigators (Cabeza et al., 2002;Friedman, 2003): if there were differences in neural activity between cognitively high and average performers within a designated age group, the pattern associated with the high performers was interpreted as representing the more adaptive response.
Materials and methods
Participants
After completing informed consent, participants underwent an evaluation that included a medical, neurological, and psychiatric history, a formal neurological examination, neuropsychological testing, and completion of questionnaires surveying mood and socioeconomic status. To be included in the study, participants had to be in one of three age groups (18–28 years old (young subjects), 45–55 years old (middle-age subjects), or 65–85 years old (old subjects)), English-speaking, and have ≥ 12 years of education, a Mini Mental State Exam (MMSE) (Folstein et al., 1975) score ≥ 26, and an estimated IQ on the American Modification of the National Adult Reading Test (AMNART) (Ryan and Paolo, 1992) ≥ 100. Subjects were excluded if they had a history of CNS diseases or major psychiatric disorders based on DSM-IV criteria (American Psychiatric Association, 1994), a history of clinically significant medical diseases, corrected visual acuity worse than 20–40, a history of clinically significant audiological disease, a Geriatric Depression Scale (Yesavage et al., 1981) score of ≥ 10 for the old subjects or a Beck Depression Inventory (Beck and Steer, 1987) score of ≥ 10 for middle-age or young subjects, or focal abnormalities on neurological examination consistent with a lesion in the CNS.
For all age groups, cognitive status was operationally defined based on performance on the following six neuropsychological tests: 1) Digit Span subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III) (Wechsler, 1997a); 2) Controlled Oral Word Association Test (COWAT) (Ivnik et al., 1996); 3) Logical Memory II subtest of the Wechsler Memory Scale-III (WMS-III) (Wechsler, 1997b); 4) Visual Retention Test (Youngjohn et al., 1993); 5) Boston Naming Test (Tombaugh and Hubley, 1997); 6) Category (animal) fluency (Spreen and Strauss, 1998). Each of the tests has available norms across a range of ages. To meet criteria for a cognitively high performer, subjects had to score in the top 3rd (≥ 67th percentile) of published age-matched norms on ≥ 4 of the 6 cognitive tests. To meet criteria for a cognitively average performer, subjects had to score in the middle 3rd (33rd to 66th percentile) of published age-matched norms on ≥ 3 of the 6 cognitive tests. A composite score was computed for each subject by averaging performance (percentile score) on each of the neuropsychological tests. Since performance on our experimental task was particularly dependent upon attention/executive functions, the average percentile score on Digit Span and COWAT was also computed. Subjects also completed the AMNART and the Raven’s Progressive Matrices Test (Raven et al., 1995) to determine estimated IQ scores, and the MMSE to obtain a gross measure of current mental state.
Experimental methods
The experimental procedures used were analogous to the ones described in prior reports (Daffner et al., 2000b;Daffner et al., 2006b). Two hundred and fifty line drawings, white on black background, were presented in 5 blocks of 50, each at the center of a high-resolution computer monitor. All stimuli subtended a visual angle of approximately 2.75° along their longest dimension. There were three categories of visual stimuli: 1) a repetitive Standard Stimulus (a triangle)--70% frequency, 2) a Target Stimulus (upside down triangle)--approximately 15% frequency, and 3) Novel Stimuli, randomly drawn from a set of unusual/unfamiliar line drawings (e.g., impossible or fragmented objects) shown only one time each--approximately 15% frequency, many of which came from the collection of drawings that have been used by Kroll and Potter (1984) and Kosslyn et al. (1994) (Figure 1). Stimuli appeared within a fixation box subtending a visual angle of approximately 3.5° x 3.5° that remained on the screen at all times.
Figure 1.
There were three categories of visual stimuli: a) a repetitive standard stimulus (a triangle— 70% frequency), b) a target stimulus (an upside down triangle— 15% frequency), and c) novel stimuli (drawn from a set of unusual/unfamiliar line drawings (e.g., impossible or fragmented objects) and shown only one time each— 15% frequency).
Participants were informed that the experiment involved the study of brain wave activity as they looked at different kinds of drawings. We emphasized that they could view each picture for however long or short they liked. They controlled the viewing duration by pressing a button that led to the erasure of the current stimulus and the onset of a blank screen that lasted between 800 and 1760 ms (mean ~1300 ms), followed by the presentation of the next stimulus. Also, participants were told to respond to the designated target stimulus by pressing a foot pedal (ipsilateral to the button press). Instructions indicated that accuracy was more important than speed. Left and right button press/foot pedal was counterbalanced for all subject groups. Although viewing durations were calculated by subtracting the stimulus onset time from the button press time, all stimuli were displayed for a minimum duration of 600 ms. Subjects also participated in two other conditions not reported here. The order of the conditions was counterbalanced for all subject groups.
ERP recordings
An electrode cap (Electro-Cap International, Eaton, OH, USA) was used to hold to the scalp 35 active tin electrodes whose locations were based on the International 10–20 system. Electrodes were arranged in 5 columns (midline, 2 inner lateral, 2 outer lateral), each with 7 antero-posterior sites (see Appendix A.1 for a montage of the electrode site locations). All sites were referenced to the left mastoid, and the impedance between each recording site and the reference was reduced to less than 5K ohms. An electrode was placed beneath the left eye (whose electrical activity was compared to an electrode placed above the left eye) to check for eye blinks and vertical eye movements. Another electrode was placed to the right of the subject’s right eye (referenced to an electrode to the left of the left eye) to check for lateral eye movements. A final electrode was placed over the right mastoid (referenced to the left one) to monitor asymmetrical mastoid activity. (None was identified.) The EEG was amplified by an SA Instrumentation (San Diego, CA, USA) system (model H & W 32BA), using a band filter with negative 3dB cutoffs of 0.01 and 40 Hz, and continuously digitized (200 Hz) by a computer yielding 1280 ms of data from each electrode site, beginning 100 ms before stimulus onset.
Data analysis
A continuous record of the raw EEG was stored on hard disk. Off-line, EEG epochs for the three stimulus types (novels, target hits, standards) were averaged separately. Trials with eye movements or amplifier blocking were excluded from data analysis. For subjects who had greater than 15% eye blinks in response to any of the stimulus types, a blink correction program (using principal component analysis) was employed (Dale AM, 1994) that computed the impact of the blink on the wave forms in each channel. The P3 in response to each stimulus type was defined as the peak positive amplitude between 350 and 850 ms after stimulus onset. We also examined other components, defined as follows: P1 (peak positive amplitude 0–125 ms), N1 (peak negative amplitude 50–125 ms), P2 (peak positive amplitude 150–250 ms), N2 (peak negative amplitude 200–350 ms). All components were measured with respect to the average of the 100 ms prestimulus baseline. The latency of a component was defined as the time from stimulus onset to the wave peak at midline sites.
In general, ERP data for each stimulus type were analyzed using analysis of variance (ANOVA), with age group (old, middle-age, and young subjects) and cognitive status (high performing, average performing) as the between-subjects variables and electrode site as the within-subjects variable. P3 data were analyzed separately along midline (ML), inner lateral (IL) column, and outer lateral (OL) column electrode sites (see Appendix A.1). P1, N1, P2, and N2 data were analyzed for midline sites only. Analyses of scalp distribution focused on evaluating antero-posterior differences across subject groups by determining whether there were electrode site (x 7) by group (e.g., x 3) interactions at midline, inner lateral and outer lateral locations. In looking at interactions between scalp electrode site and other variables (e.g., age group), the data were first normalized using a z score technique (Kounios and Holcomb, 1994) similar to the method recommended by McCarthy and Wood (1985) to avoid problems associated with interpreting site by factor interactions using ANOVA. Analyses that yielded significant interactions between subject group, stimulus type, or electrode site resulted in planned contrasts between the levels of the variable. The Geisser-Greenhouse correction was applied for all repeated measures with greater than 1 degree of freedom.
Results
Participants
Ninety-six individuals participated in the study. Data from two subjects (one cognitively average performing old and one cognitively average performing middle-age subject) were not included in the analyses because their behavioral responses (viewing duration of novel stimuli) were more than 3 standard deviations from the mean of their respective groups. The characteristics of each group are summarized in Table 1 that includes the number of subjects, demographic information, the results on more global cognitive measures (e.g., estimated IQ), composite percentile scores on the neuropsychological tests, and the pertinent statistical analyses. Three cognitively high performing middle-age subjects and one cognitively average performing middle-age subject had a history of hypertension (p > 0.30, Mann-Whitney U test). Seven cognitively high performing old subjects and nine cognitively average performing old subjects reported cerebrovascular risk factors such as hypertension, high cholesterol, heart disease, or diabetes (p > 0.30).
Table 1.
Information about subjects
| Young | Middle-age | Old | Age Effects within each Cognitive Group | Overall Age Effect | Overall Cognitive Status Effect | Age by Cognitive Status Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-value | p-value | p-value | p-value | ||
| Number of Subjects | Ave. | 16 | 15 | 15 | |||||||
| High | 16 | 16 | 16 | ||||||||
| Gender (male/female) | Ave. | 6/10 | 4/11 | 7/8 | ns | ns | ns | ns | |||
| High | 10/6 | 9/7 | 7/9 | ns | |||||||
| Age | Ave. | 21.8 | 2.7 | 49.2 | 3.1 | 70.1 | 4.3 | <.01 | <.01 | ns | ns |
| High | 21.5 | 1.0 | 50.3 | 3.4 | 73.0 | 4.9 | <.01 | ||||
| Years of Education | Ave. | 14.9 | 1.7 | 17.7 | 2.8 | 16.1 | 4.0 | <.05a | <.01b,c | ns | ns |
| High | 15.4 | 0.6 | 18.9 | 4.3 | 16.5 | 4.2 | <.05a | ||||
| MMSE | Ave. | 28.9 | 1.2 | 28.4 | 1.4 | 28.5 | 1.6 | ns | ns | <.01 | ns |
| High | 29.1 | 0.7 | 29.4 | 1.0 | 29.2 | 0.8 | ns | ||||
| AMNART Estimated IQ | Ave. | 117.8 | 6.3 | 122.5 | 5.4 | 116.9 | 9.6 | <.10 | <.01a,b | <.05 | ns |
| High | 120.6 | 4.3 | 125.8 | 4.1 | 121.3 | 8.8 | <.05c | ||||
| Raven Estimated IQ | Ave. | 110.2 | 8.4 | 117.1 | 9.5 | 119.5 | 12.3 | <.05d | <.01a,d | <.05 | ns |
| High | 116.1 | 11.2 | 124.4 | 7.0 | 120.8 | 12.3 | ns | ||||
| Composite Percentile | Ave. | 47.1 | 9.7 | 48.4 | 11.6 | 50.5 | 9.2 | ns | ns | <.01 | ns |
| High | 71.5 | 7.6 | 76.0 | 10.3 | 72.3 | 9.8 | ns | ||||
| FAS/Digit Composite | Ave. | 57.6e | 16.7 | 47.6f | 19.5 | 43.1g | 20.5 | ns | ns | <.001 | <.07 |
| High | 72.1 | 19.8 | 75.4 | 16.1 | 79.2 | 17.7 | ns | ||||
Key: p<.05 Middle-aged vs. Young,
p<.05 Old vs. Middle-aged,
p<.01 Middle-aged vs. Young,
p<.05 Old vs. Young,
p<.05 Young Subjects: High vs. Average,
p<.001 Middle-aged Subjects: High vs. Average,
p<.0001 Old Subjects: High vs. Average.
Raven= Raven’s Progressive Matrices Test; AMNART= American Modification of the National Adult Reading Test; MMSE= Mini Mental State Exam; Composite Percentile Score for Digit Span WAIS-III, Controlled Oral Fluency (FAS), Logical Memory II, WMS-III, Visual Retention Test, Boston Naming Test, and Category Fluency (animals); FAS/Digit Composite= composite percentile score for Controlled Oral Fluency (FAS) and Digit Span WAIS-III.
Cognitively high performing subjects had a higher mean composite percentile score for the six neuropsychological tests than cognitively average performing subjects (73.2 %tile vs. 48.7 %tile, p < 0.001) (Table 1). There were no age-related differences in composite percentile score within either the cognitively high or cognitively average performing groups. Cognitively high performing subjects also had a higher mean percentile score than cognitively average performing subjects on the two neuropsychological tests that focus on attention and executive functions, Digit Span/COWAT (75.6%tile vs. 49.5%tile, p < 0.001). There was a marginally significant interaction between cognitive status and age on these tests of attention/executive function (F(2, 88) = 2.76, p = 0.07) due to the difference between cognitively high and average performing adults being smaller for young subjects (p < 0.05) than middle-age (p < 0.001) and old subjects (p < 0.001) (see Appendix A.2 for group scores and statistical analyses for each of the six neuropsychological tests administered).
Behavior
The behavioral results from this study were the focus of a previous report (Daffner et al., 2007). To summarize, cognitively high performing old subjects looked at novel stimuli as long as cognitively high performing middle-age and young subjects, and longer than cognitively average performing old subjects. As a group, cognitively high performing adults spent more time viewing novel stimuli than cognitively average performing adults. The size of the difference between cognitively high and cognitively average performers was largest among old subjects. Cognitively high performers also had faster and more accurate responses to targets than cognitively average performers (with no significant age-related effects).
P3 amplitude and scalp distribution
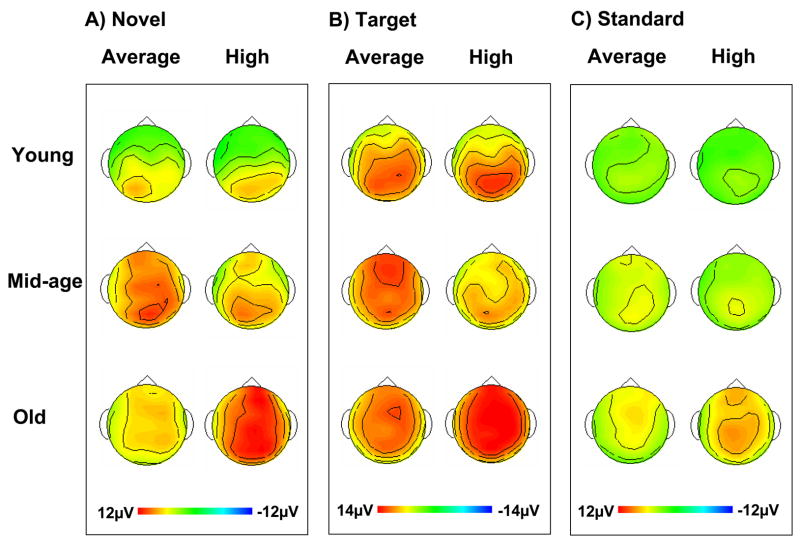
Figure 2 illustrates the grand average ERPs at midline sites for novel, target and standard stimuli for all groups. Figure 3 illustrates surface potential maps of the P3 response to each stimulus type for all groups. In the sections that follow, we will limit our discussion to the most salient findings.
Figure 2.
Midline grand average ERP plots in response to: A) novel, B) target, and C) standard stimuli across all subject groups. In (A), the green arrow marks the novelty P3 response at the Cz electrode site, illustrating that cognitively high performing old subjects generate a larger response than cognitively high performing middle-age and young subjects and than cognitively average performing old subjects. In (B), the yellow and purple arrows mark the target P3 response at Fz. The yellow arrow helps to illustrate that across cognitively average performers, middle-age and old subjects generated a similar target P3 response. The purple arrow helps to illustrate that across cognitively high performers, middle-age and young subjects generated a similar target P3 response.
Figure 3.
Surface potential maps of the P3 response to A) novel, B) target and C) standard stimuli for all subject groups.
Novel stimuli
Cognitively high performing old subjects generated a larger novelty P3 component than cognitively high performing middle-age (F(1,30) = 5.15, p < 0.04 (ML); 3.54, p < 0.07 (IL); 3.99, p < 0.06 (OL)) and young subjects (F(1,30) = 7.90, p < 0.01 (ML); 6.02, p < 0.03 (IL); 3.16, p < 0.09 (OL)) (see Figures 2A, 3A). The difference in novelty P3 amplitude between cognitively high performing old and middle-age subjects was of similar magnitude across anterior and posterior locations (no age x site interaction), while the difference between cognitively high performing old and young subjects was largest anteriorly (age x site interaction, F(6,180) = 3.23, p < 0.02 (ML); 3.84, p < 0.02 (IL); 2.25, p = 0.10 (OL)). There were no differences between cognitively high performing young and middle-age subjects in the size or scalp distribution of the novelty P3. Cognitively high performing old subjects also generated a larger novelty P3 than cognitively average performing old adults at midline and inner lateral sites (F(1,29) = 5.87, p < 0.03 (ML); 4.32, p < 0.05 (IL); 1.62, p > 0.20 (OL)), which was of similar magnitude across anterior and posterior sites (no age x site interaction). The pattern of novelty P3 response that distinguished cognitively high from average performers was different for middle-age and young subjects than for old subjects (age x cognitive status interaction: middle-age vs. old, F(1,59) = 9.54, p < 0.01 (ML); 7.38, p < 0.01 (IL); 8.34, p < 0.01 (OL); young vs. old, F(1,59) = 5.18, p < 0.03 (ML); 4.91, p < 0.04 (IL); 1.30, p = 0.26 (OL)). (See Figure 4 for a scatter plot showing individual data points for the 6 groups, and Appendix A.3 which illustrates differences in the novelty P3 across 3 midline sites.) Among middle-age subjects, cognitively average performers had a larger novelty P3 response than cognitively high performers, especially at outer lateral sites (F(1,29) = 3.69, p < 0.07 (ML); 3.08, p < 0.09 (IL); 9.10, p < 0.01 (OL)), the magnitude of which was similar across anterior to posterior sites. Among young adults, cognitively average performing subjects also had a larger novelty P3 response than cognitively high performing subjects, but the difference was not significant. Also of note, the size of the novelty P3 response was larger for cognitively average performing middle-age subjects than both cognitively average performing old subjects (F(1,28) = 4.71, p < 0.04 (ML); 4.19, p = 0.05 (IL); 4.41, p < 0.05 (OL) (with similar magnitude across anterior to posterior electrode sites), and young subjects (F(1,29) = 4.86, p < 0.04 (ML); 3.16, p < 0.09 (IL); 5.45, p < 0.03 (OL). The largest difference between middle-age and young subjects was observed anteriorly at inner and outer lateral sites (age x site interaction, F(6, 174) = 1.39, p < 0.25 (ML); 3.57, p < 0.02 (IL); 3.48, p < 0.03 (OL)). There was no overall difference in the size of the novelty P3 response between the old and young cognitively average performers (no effect of age). However, young subjects had a larger response than old subjects posteriorly, whereas old subjects had a larger response than young subjects anteriorly (age x electrode site interaction F(6,174) = 3.19, p < 0.03 (ML); 4.38, p < 0.008 (IL); 3.83, p < 0.03 (OL)).
Figure 4.
Scatter plot illustrating individual mean midline P3 amplitude (in μV) to novel stimuli by subject group. Horizontal bars represent mean P3 amplitude for each group.
Target stimuli
What was most striking about the grand average waves in response to target stimuli was that for cognitively average performers, the ERPs of middle-age subjects looked very similar to those of old subjects (see Figures 2B and 3B). In contrast, for the cognitively high performers, the ERPs of middle-age subjects looked more similar to those of young subjects. To a large degree, statistical analyses validated this impression. Among cognitively average performers, there was no difference between old and middle-age subjects in either the amplitude or scalp distribution of the target P3 (no effect of age; no age x site interaction). Cognitively average performing young subjects had a larger P3 posteriorly and a smaller P3 anteriorly than both middle-age subjects (age x site interaction, F(6,174) = 7.49, p < 0.001 (ML); 9.41, p < 0.001 (IL); 7.93, p < 0.01 (OL)) and old subjects (age x site interaction, F(6,174) = 6.97, p < 0.001 (ML); 6.38, p < 0.01 (IL); 6.73, p < 0.01 (OL)). Among cognitively high performers, there was no difference between young and middle-age subjects in either the amplitude or scalp distribution of the target P3 (no effect of age; no age x site interaction). Cognitively high performing old subjects had a larger target P3 than cognitively high performing middle-age subjects (main effect of age, F(1,30) = 8.73, p < 0.01 (ML); 5.93, p < 0.03 (IL); 4.83, p < 0.04 (OL)) across the entire scalp (no age x site interaction). Cognitively high performing old subjects had a larger target P3 than cognitively high performing young subjects anteriorly, while cognitively high performing young subjects had a larger target P3 than cognitively high performing old subjects posteriorly, especially at midline sites (age x site interaction, F(6,180) = 3.98, p < 0.01 (ML); 2.62, p < 0.08 (IL); 2.43, p < 0.10 (OL)). Another way to characterize the results is to note that old and middle-age subjects exhibited opposite patterns. Among old subjects, cognitively high performing subjects tended to have a larger target P3 response than their cognitively average performing counterparts at midline sites (main effect of cognitive status, F(1,29) = 3.29, p = 0.08 (ML); 2.62, p = 0.12 (IL); 1.23, p = 0.28 (OL)), a finding that was uniform across the scalp (no age x electrode site interaction). In contrast, among middle-age subjects, cognitively average performers tended to have a larger target P3 than cognitively high performers (main effect of cognitive status, F(1,29) = 2.89, p = 0.10 (ML); 1.69, p = 0.20 (IL); 3.63, p = 0.07 (OL)). For middle-age subjects, there was a cognitive status by electrode site interaction (F(6,174) = 2.86, p < 0.05 (ML); 3.19, p = 0.05 (IL); 1.98, p = 0.16 (OL)) with high performers having a maximum amplitude at parietal sites (e.g., Pz) and average performers at frontal sites (e.g., Fz). Among young subjects, there was no difference in target P3 amplitude or distribution between high and average performers. Like cognitively high performing middle-age subjects, young subjects (across both cognitive groups) had a maximum amplitude at parietal sites (main effect of electrode site, F(6,180) = 11.36, p < 0.001 (ML); 16.05, p < 0.001 (IL); 4.06, p < 0.03 (OL)).
Standards
Among cognitively high performing adults, old subjects had a larger P3 response to standard stimuli than middle-age subjects (F(1,30) = 11.94, p < 0.002 (ML); 10.67, p < 0.003 (IL); 3.83, p < 0.06 (OL)), the magnitude of which was similar across anterior to posterior sites. Old cognitively high performers also had a larger P3 response compared to young cognitively high performers (F(1,30) = 12.79, p < 0.002 (ML); 12.11, p < 0.002 (IL); 6.73, p < 0.02 (OL)), especially at fronto-central midline and inner lateral sites (age x site interaction, F(6,180) = 5.05, p < 0.004 (ML); 4.66, p < 0.02 (IL); .58, p = 0.58 (OL)) (see Figures 2C, 3C). There were no differences between cognitively high performing middle-age and young subjects in the size or scalp distribution of the P3 to standards. Across old subjects, cognitively high performers had a larger P3 response to standard stimuli than cognitively average performers at midline and inner lateral sites (F(1,29) = 8.25, p < 0.008 (ML); 9.29, p < 0.005 (IL)), with no cognitive status by site interaction (see Appendix A.4 for a scatter plot illustrating this result). In contrast, across middle-age and young adults, there were no differences in the size or scalp distribution of the P3 response to standard stimuli between cognitively high and average performers. Among cognitively average performing adults, there were no age-related differences in the size of the P3 response to standard stimuli, which contrasts with the pattern of response to novel and target stimuli. There were no differences in the scalp distribution of the P3 to standards between cognitively average performing middle-age and young subjects, and between cognitively average performing middle-age and old subjects. However, compared to young subjects, old subjects generated a P3 to standards that was larger fronto-centrally, and smaller posteriorly (age x site interaction, F(6,174) = 3.54, p < 0.03 (ML); 2.15, p = 0.11 (IL); 2.6, p = 0.08 (OL)).
Difference Waves
To help isolate endogenous components more specifically related to novelty and target processing, difference waves were computed for each subject by subtracting the ERP response to standard stimuli from the ERP response to novel and to target stimuli at midline sites. (Appendix A.5 illustrates the amplitude of the novelty P3 and target P3 difference waves at selected midline sites for each group.)
For the novelty P3 difference wave (Appendix A.5A), there was no overall effect of age or cognitive status. However, there was a marginally significant age by cognitive status interaction (F(2,88) = 2.92, p < 0.06). Across cognitively high performing adults, there were no age-related changes in novelty P3 difference wave. In contrast, across cognitively average performing adults, old subjects tended to have the smallest novelty P3 difference wave (F(2,43) = 2.82, p < 0.08). Among the cognitively high performing subjects, there was no evidence of age-related changes in the scalp distribution of the novelty P3 difference wave. In contrast, across cognitively average performing adults, old subjects had a more anteriorly distributed novelty P3 difference wave than young subjects (F(6,174) = 2.85, p < 0.04). The scalp distribution of the novelty P3 difference wave of cognitively average performing middle-age subjects was between that of old and young subjects (no age x site interactions between the middle-age subjects and either of the other age groups).
For the target P3 difference wave (Appendix A.5B) there was an overall effect of age (F(2,88) = 4.30, p < 0.02), no effect of cognitive status and no age by cognitive status interaction. The age effect was due to young subjects having the largest target P3 difference wave. There were no differences between old and middle-age subjects. Among cognitively high performing subjects, there were no age-related changes in the scalp distribution of the target P3 difference wave (no age x site interaction). In contrast, among cognitively average performing subjects, the scalp distribution of the target P3 difference wave varied across age groups (F(12,258) = 5.22, p < 0.00005). Young subjects had a less anteriorly distributed target P3 difference wave than old subjects (F(6,174) = 5.85, p < 0.001) and middle-age subjects (F(6,174) = 7.47, p < 0.0002), with no significant difference between the latter two groups.
P3 latency
Figure 5 illustrates the target P3 latency at midline sites for each of the groups. (Appendix A.6 is a scatter plot of the individual data points.) There was a marginal effect of age (F(2,88) = 2.82, p < 0.07) due to old subjects having a longer P3 latency than young subjects (p < 0.03). There was also a marginal effect of cognitive status (F(1,88) = 3.20, p < 0.08), with cognitively average performing subjects having a longer latency than cognitively high performing subjects. The most salient finding was an interaction between age and cognitive status (F(2,88) = 3.55, p < 0.04), which was of similar magnitude across anterior and posterior sites (no age x cognitive status x site interaction). The interaction between age and cognitive status reflected the fact that across cognitively high performing adults, there were no age-related differences in target P3 latency, whereas across cognitively average performing adults, age-related increases were observed (F(2,43) = 6.42, p < 0.01). Cognitively average performing young subjects had a shorter target P3 latency (459.33 (14.71), mean (SEM)) than cognitively average performing middle-age (522.52 (23.65)) (p < 0.03) and old subjects (535.81 (11.46)) (p < 0.001), with no significant difference between the latter two groups. Of note, across old adults, cognitively average performing subjects had a significantly longer target P3 latency than cognitively high performing subjects (F(1,29) = 8.77, p < 0.01). Across middle-age adults, cognitively average performing subjects had a marginally longer target P3 latency than cognitively high performing subjects (F(1,29) = 2.94, p = 0.10). However, across young subjects there were no differences in target P3 latency between cognitively high and average performing young subjects. Correlation analyses between P3 latency and performance on tests of attention/executive function were consistent with the results from the ANOVA (see Appendix A.7). The P3 latencies to novel and standard stimuli did not differ across age or cognitive status, and there were no interactions between age and cognitive status.
Figure 5.
Target P3 latency in ms (mean ± SEM ) at midline sites for all subject groups.
P1, N1, P2, N2 components
To address whether the relationship between age and cognitive status found for the P3 was specific to this ERP component, we examined the amplitude, scalp distribution, and latency of the P1, N1, P2 and N2 components at midline sites.
P1
The P1 in response to standard, target and novel stimuli did not differ in terms of amplitude, latency, or scalp distribution across age and cognitive groups, with no interaction between age and cognitive status. Across all subject groups the P1 was modulated by stimulus type, with target and novel stimuli evoking larger amplitudes (main effect of stimulus type, F(2,176) = 6.56, p < 0.003) and shorter latencies than standard stimuli (F(2, 176) = 5.68, p < 0.005).
N1
The N1 in response to standard, target and novel stimuli did not differ in terms of amplitude, latency, or scalp distribution across age and cognitive groups, with no interaction between age and cognitive status. The N1 was sensitive to stimulus type, with target and novel stimuli eliciting larger responses (main effect of stimulus type, F(2,176) = 11.71, p < 0.001), and longer latencies than those generated in response to standard stimuli (main effect of stimulus type, F(2,176) = 9.89, p < 0.001). This pattern was similar across all subject groups.
P2
The P2 in response to standard, target and novel stimuli did not differ in terms of amplitude or latency across cognitive groups, with no interaction between age and cognitive status. There were age-related effects on P2 amplitude seen only in response to novel stimuli, with old subjects generating a larger P2 than middle-age (main effect of age, F(1,58) = 5.07, p < 0.03) and young subjects (main effect of age, F(1,59) = 10.65, p < 0.01). The scalp distribution of the P2 varied across age groups and stimulus types. Old and middle-age subjects generated more anteriorly oriented P2 responses to novels and targets than young subjects (age by site interaction, novel stimuli: old vs. young, F(6,354) = 18.95, p < 0.001; middle-age vs. young, F(6,354) = 7.73, p < 0.001; target stimuli: old vs. young, F(6,354) = 3.03, p < 0.05; middle-age vs. young, F(6,354) = 3.35, p < 0.03).
N2
The N2 in response to standard, target and novel stimuli did not differ in terms of amplitude or latency across cognitively high and average performers, with no interaction between age and cognitive status. The effects of age on N2 amplitude and latency were only observed in response to novel stimuli, with young subjects generating a larger N2 than the middle-age (main effect of age, F(1,59) = 6.62, p < 0.02) and old subjects (main effect of age, F(1,59) = 13.97, p < 0.001), and old subjects having a longer N2 latency to novels compared to the middle-age (main effect of age, F(1,58) = 3.65, p = 0.06) and young subjects (main effect of age, F(1,59) = 4.26, p < 0.05). There were age-related differences in N2 scalp distribution, with young subjects consistently generating the most anteriorly-distributed N2 response to all types of stimuli, compared to both the middle-age subjects (age x site interaction, novel stimuli: F(6,354) = 4.13, p < 0.02; target stimuli: F(6,354) = 2.82, p < 0.06; standard stimuli: F(6,354) = 4.53 , p < 0.01) and old subjects (age x site interaction, novel stimuli: F(6,354) = 11.87, p < 0.001; target stimuli: F(6,354) = 4.47, p < 0.01; standard stimuli: F(6,354) = 16.24, p < 0.001). No differences were found between the old and middle-age subjects in the scalp distribution of the N2 to novel or target stimuli; however, in response to standard stimuli, middle-age subjects generated a more anterior N2 response than old subjects (age x site interaction, F(6,348) = 3.62, p < 0.02).
Estimated IQ as a covariate
To determine whether the major findings of this study could be explained by differences in IQ, pertinent statistical analyses (midline P3 analyses) were run again using estimated IQ (based on AMNART and Raven’s scores) as covariates. The statistical results had an almost identical pattern and magnitude as those reported earlier (see Appendix A.8 for details).
Discussion
The main goal of this study was to understand the nature of age-associated compensatory mechanisms by investigating differences in neural resources allocated to novel and target events among cognitively high and average performing old, middle-age, and young adults. The groups were relatively well matched for pertinent variables, including gender, education, mood, and socioeconomic status, with only minor differences in estimated IQ between cognitively high and average performing subjects. Of note, after controlling for the impact of differences in estimated IQ, the salient findings of the study remained.
As predicted, there was a significant interaction between age and cognitive status for P3 response to novel stimuli, with the pattern of differences between cognitively high and average subjects varying between old and younger age groups. Cognitively high performing old subjects allocated more attentional resources, as indexed by the novelty P3 response, than cognitively high performing middle-age and young subjects, and than cognitively average performing old subjects. These electrophysiological results occurred in the context of the behavioral findings that cognitively high performing old subjects were as engaged by novelty as their cognitively high performing younger counterparts and more engaged by novelty than cognitively average performing old adults. These results provide a strong basis for arguing that the increased allocation of resources to novel stimuli observed in cognitively high performing old adults does not simply represent less efficient processing, but a successful compensatory mechanism, perhaps in response to other age-related declines in neurophysiological functioning. Our findings are consistent with those results from the functional imaging literature that have shown that old subjects who perform comparably to young subjects on source or episodic memory tasks recruit more brain activity than young subjects, and than old subjects who perform worse (Cabeza et al., 2002;Reuter-Lorenz et al., 2000;Rosen et al., 2002) (but see Nielson et al. (2002) and Logan et al. (2002), whose data indicate that this pattern is not associated with all cognitive functions). Of note, the age-related anterior shift in scalp distribution of the novelty P3 was not different for the cognitively high and average performing old subjects, suggesting that these groups process novelty by relying on a similar underlying neural system, but differ mainly in terms of the amount of resources appropriated.
Middle-age and young subjects exhibited a pattern of P3 response to novelty opposite to that of old subjects: cognitively high performing subjects generated a smaller novelty P3 component than their cognitively average performing counterparts. This result suggests that for younger adults, cognitively high performing subjects may be able to process novelty more efficiently, thus appropriating fewer resources than cognitively average performing subjects (Haier et al., 1988;McGarry-Roberts et al., 1992). Consistent with this notion are studies that have shown that young subjects with greater intellectual capacity generate smaller target P3 amplitudes (Egan et al., 1994;McGarry-Roberts et al., 1992); however, this has not been an invariant finding (e.g., Jausovec and Jausovec, 2000). The literature on ‘cognitive reserve’ (Katzman, 1993;Satz, 1993;Stern, 2002) also is informative. According to current theory, older individuals who have greater cognitive reserve can utilize such resources to compensate for age-related physiological changes, which may manifest as increased neural activity, or the utilization of alternative cerebral networks or cognitive strategies. By contrast, younger individuals with greater cognitive reserve may, in general, have the capacity to manage assignments more efficiently and only call upon their reserve when a task becomes sufficiently demanding.
Of note, although the pattern of resource allocation, as measured by the novelty P3, was similar for middle-age and young subjects, the differences between cognitively high and average performing young subjects were not statistically significant. Several factors may help to explain the discrepancy between middle-age and young subjects. One possibility is that the group of young subjects, which consisted of college students and recent graduates, was much more homogeneous than the group of middle-age subjects. The difference between cognitively high and average performers on tests of attention and executive functioning was found to be marginally smaller for young subjects than middle-age subjects. There is evidence that a person’s frontal-executive system may play a central role in mediating the novelty P3 component (Daffner et al., 2000b). Furthermore, as suggested by theories about cognitive reserve, eliciting electrophysiological differences between cognitively high and average performing young subjects may require a much more demanding task than the one employed in the current study. An alternative explanation for why middle-age subjects have a different pattern from young subjects is that cognitively average performing adults may experience age-associated changes in neurophysiologic function years earlier than cognitively high performing adults. At middle-age, cognitively average performers may be able to compensate for such a decline by appropriating more processing resources (as measured by a larger P3). By the time they reach old age and have less capacity, this mechanism may no longer be viable, which is indexed by a decline in P3 amplitude. In contrast, cognitively high performing adults may not require such compensatory activity until they reach old age, at which time their P3 amplitude becomes larger.
Although additional research on subjects with a wider range of ages will be necessary to test this hypothesis, the pattern of P3 response to target stimuli also suggests that middle-age may be a critical period in the aging process. Among cognitively average performers, middle-age subjects generated a P3 response to targets that was most similar to old subjects with no differences between the two groups in amplitude or scalp distribution (both groups with an anterior maximum). The anterior shift in target P3 scalp distribution suggests that by middle-age, cognitively average performers are using a different set of neural processors to respond to targets than their younger counterparts. In contrast, among cognitively high performers, middle-age subjects generated a P3 response to targets that was most similar to young subjects in both amplitude and scalp distribution (with a posterior maximum). As discussed below, an analogous pattern was observed for the P3 latency: among cognitively average performers, there was a significant increase in target P3 latency between young and middle-age subjects, but not between middle-age and old subjects. Similarly, there were no significant differences between cognitively average performing middle-age and old subjects in terms of the peak amplitude and latency of their P1, N1, P2, and N2 response to target events. The divergence between cognitively average and cognitively high performing individuals at middle-age is particularly striking since these two groups did not differ in terms of socioeconomic background, years of education, or cerebrovascular risk factors. These results raise the possibility that the aging process either follows a faster pace for cognitively average performers, whose electrophysiological responses “look” old by middle-age, or that the aging process is slower for, or better countered by cognitively high performers who continue to “look” young during middle-age. Our findings emphasize the importance of including middle-age subjects in research aimed at understanding different patterns of cognitive aging. Recently, other studies also have suggested that there can be significant age-associated changes in brain structure and cognitive functioning that emerge by middle-age. For example, reports have suggested that significant age-related decreases in white matter (Brickman et al., 2006) and gray matter (Salat et al., 2004) volume are occurring by middle-age, especially in the frontal lobes. Another study performed a cluster analysis of neuropsychological performance of middle-age subjects and revealed three groups of individuals, including those who were cognitively intact, those with poor motor speed, and those with reduced executive function (Gunstad et al., 2006).
A review of the data regarding the P3 response to standard stimuli reveals that cognitively high performing old subjects generated a larger response than cognitively average performing old subjects and than cognitively high performing younger subjects (demonstrating a pattern similar to the one observed in response to novel stimuli and, to a lesser extent, in response to target stimuli). Thus, the increased allocation of resources by cognitively high performing old subjects was not limited to a specific stimulus type, but rather appeared to reflect the way in which they approached the task in general. These results are consistent with the notion that cognitively high performing old subjects differ from other groups in what has been labeled cognitive set or overall neural state (Rugg and Morcom, 2005) in response to the requirements of the task. This approach appears to be successful: in terms of visual exploration of novel stimuli and behavioral responses to target stimuli, cognitively high functioning old adults performed better than cognitively average functioning old subjects, and more like cognitively high functioning younger subjects. However, one would predict important limitations to this postulated age-related compensatory mechanism, with performance of the cognitively high functioning old group at risk for deteriorating under more challenging circumstances (such as dual task conditions) in which greater demands are placed on their limited resources.
Analysis of P3 difference waves, which addresses processing more specific to novel and target events, also reveals important age-related differences between cognitively high and average performing adults. Cognitively high performing older subjects were not different from young subjects in the size or scalp distribution of the novelty P3 or target P3 difference waves. This suggests a strong overlap among cognitively high performing adults in processing directed toward novel and target events. In contrast, cognitively average performing old subjects tended to have a smaller, more anteriorly distributed novelty P3 difference wave and a more anteriorly distributed target P3 difference wave compared to young subjects. Cognitively average performing middle-age subjects had a more anteriorly distributed target P3 difference wave than their younger counterparts and a novelty P3 wave whose scalp distribution was between that of their younger and older counterparts. One way to interpret this pattern of results is that for cognitively high performing old and middle-age subjects, the age-related anterior shift in P3 scalp distribution that occurs when processing novel and target stimuli was no greater than when processing standard stimuli. In contrast, for cognitively average performing old and middle-age subjects, the age-associated anterior shift in scalp distribution was larger in response to novel and target stimuli than in response to standard stimuli. This suggests that, in terms of information processing more specific to novel and target events, cognitively average performing middle-age and old individuals may rely more on frontal systems that are managing the task less efficiently (Fabiani et al., 1998). Additional research that varies the level of task demands may provide further insight into this issue.
It is worth discussing several of the study’s findings regarding the novelty P3 that may seem puzzling because of their apparent inconsistency with other reports in the literature. For example, in contrast to many previous studies (e.g., Comerchero and Polich, 1998;Courchesne et al., 1975), young subjects generated a novelty P3 component that had a posteriorly-oriented scalp distribution, similar to that of their target P3 component. We strongly suspect that this result reflects the use of a subject-controlled variant of the novelty oddball task, which was employed to obtain a behavioral measure of a subject’s interest in novelty. In this variant, novel stimuli become more ‘task-relevant’ (and thus have a more ‘target-like’ quality), as an active decision must be made about each event (i.e., how long to look) and all events require a button press (to advance to the next visual stimulus). It is very likely that in this context, there may be a more prominent posteriorly-oriented P3b in response to novel events, as this component is believed to be a reflection of voluntary attention (Chong et al., in press). Another potential concern is that even among the cognitively average performing group, old subjects did not have the commonly reported age-related decline in novelty P3 amplitude (e.g., Fabiani and Friedman, 1995;Fjell and Walhovd, 2001;Friedman et al., 1998;Walhovd and Fjell, 2001). This outcome is likely to be a reflection of the kinds of novel stimuli that were presented. Most studies of aging using novelty oddball paradigms have not been carried out in the visual modality. In one well-controlled study (Fjell and Walhovd, 2004) that reported an age-related reduction in novelty P3 amplitude to visual stimuli, deviant stimuli consisted of a repeating geometric figure. In contrast, our novel visual events were non-repeating, highly unusual/unfamiliar stimuli. Two prior studies of aging that have employed similar kinds of novel stimuli to the ones used in the current investigation also have reported no age-related changes in the novelty P3 amplitude (Beck et al., 1980;Snyder and Hillyard, 1976).
In addressing our study’s second major question, we found that the target P3 latency (a measure of information processing speed) was shorter for cognitively high performing adults than cognitively average performing adults, a result consistent with the findings of several previous investigations (Egan et al., 1994;O'Donnell et al., 1992;Polich et al., 1983;Walhovd and Fjell, 2002). As predicted, the largest difference between the cognitively high and average groups was observed for old subjects. Our study also demonstrated that cognitively average performing individuals had the commonly reported age-related increase in target P3 latency. In striking contrast, among cognitively high performing individuals there were no age-associated changes in target P3 latency. These results provide additional support for the notion that there are different patterns of normal cognitive aging. Of note, our findings differ from the report by Fjell and Walhovd (2005), which found that the correlation between age and the latency of the P3a to deviant stimuli was similar for cognitively high and average performing adults who participated in a 3-stimulus visual oddball task. Additional research is needed to determine the source of this disparity. Possibilities include differences in the particular cognitively high or average samples used, differences between the novelty oddball and 3-stimulus oddball paradigm (as discussed above), or differences between the subject-controlled and traditional variants of the oddball task.
If we assume that relative to cognitively average performing old individuals, cognitively high performing old individuals are better able to manage age-related declines in neurophysiological function by compensatory neural activity, at what stages along the information processing stream does this take place? In contrast to the P3 component, we found no evidence for an interaction between age and cognitive status for the amplitude or latency of the P1, N1, P2, or N2 components in response to any stimulus type. In addition, the age-related slowing of the P3 response to targets among cognitively average performers cannot be attributed to delays in earlier processing stages, as none was found. Taken as a whole, these results suggest that cognitively high performers do not process novel (and other visual stimuli) differently from cognitively average performers by modulating early perceptual encoding, selective attention, or preliminary categorization. Rather, cognitively high and average performers are distinguished by differences in the modulation of controlled processing involved in directing attention to salient stimuli and updating internal models about a changing environment.
Several limitations of this study should be considered. Although we refer to different patterns of normal aging, we fully acknowledge the limits of cross-sectional studies. In contrast to longitudinal studies, cross-sectional data sets do not allow direct inferences regarding changes over time within individuals. We do not know that cognitively high performing old subjects were necessarily cognitively high performing middle-age subjects (although this seems likely). Certainly, some cognitively average performing old subjects were cognitively high performers when middle-aged. For our old subjects, generational cohort effects, selective mortality, and pathological aging processes are likely to have contributed to the outcome. Also, in future studies, it would be informative to expand the number of subjects to include a wider range of ages that would represent the whole adult lifespan. The current study intentionally limited its sample to individuals with estimated IQs of at least 100 to help ensure comparability among different age groups (especially since we were recruiting young adults from university campuses). In the future, it would be of interest to expand the pool of subjects to include individuals with below-average estimated IQs to determine the extent to which our findings apply to adults with a broader range of intellectual capacity. Also, because some of the major conclusions of the current study were derived from the findings of the neural activity of the cognitively high performing old subjects, it will be important to confirm that these 16 subjects are typical of this population. Support for the idea that they are a representative sample comes from a comparison that was made between these subjects and another group of 20 older adults with a very similar neuropsychological profile (i.e., cognitively high performing) who had participated in an analogous experimental paradigm. The two groups of cognitively high performing old subjects did not differ in terms their P3 response to novel, target, and standard stimuli (Daffner et al., 2006b).
In summary, the current study strongly suggests that there are different patterns of normal aging for cognitively high and average performing adults. Cognitively high performing old subjects are as engaged by novelty as their younger cognitively high performing counterparts and more engaged than cognitively average performing old subjects. It appears that cognitively high performing old adults accomplish this by a compensatory neural mechanism associated with the modulation of controlled processing and the appropriation of more resources (as indexed by the P3) and not the modulation of earlier perceptual processing or preliminary categorization (as indexed by the P1, N1, P2, or N2). Cognitively high performing young and middle-age adults may be able to process novelty more efficiently and appropriate fewer resources, as indexed by a smaller P3 amplitude, than their cognitively average performing counterparts. By middle-age there is a clear divergence between cognitively high and cognitively average performers in the way in which they process target stimuli. Cognitively average performing middle-age adults exhibit electrophysiological responses to targets (P1, N1, P2, N2, P3 amplitude and latency) that are very similar to those of their older counterparts. In contrast, cognitively high performing middle-age adults exhibit electrophysiological responses to targets that are closer to those of their younger counterparts. Additional studies that investigate a wider range of ages and that utilize longitudinal designs are necessary to confirm these interesting cross-sectional findings.
Supplementary Material
Acknowledgments
This research was supported in part by NIA grant 1R01 AGO17935 and by the S. Muss Foundation. The authors thank Danielle Williams for her assistance with data collection and analysis.
Appendix Legends
A.1. Montage illustrating the location of electrode sites, based on the International 10–20 system. Electrodes were arranged in 5 columns (midline (ML), 2 inner lateral (IL), 2 outer lateral (OL)) each with 7 antero-posterior sites.
A.2. Table with mean group scores and statistics for each of the 6 neuropsychological tests.
A.3. Bar graph illustrating P3 amplitude (in μV) (mean ± SEM) in response to novel stimuli at Fz, Cz, and Pz for all subject groups.
A.4. Scatter plot illustrating individual mean midline P3 amplitude (in μV) to standard stimuli for the cognitively high and cognitively average performing old subjects. Horizontal bars represent the mean P3 amplitude of each group.
A.5. Bar graph illustrating amplitude (in μV) (mean +/− SEM) of the A) novelty P3 difference wave and B) target P3 difference wave at Fz, Cz, and Pz for all subject groups.
A.6. Scatter plot illustrating individual mean midline P3 latency (in ms) to target stimuli for all subject groups. Horizontal bars represent group mean P3 latency.
A.7. Description of correlation analyses between target P3 latency and performance on tests of attention/executive function.
A.8. Information on the impact of using estimated IQ as a covariate in analyses of P3 response at midline sites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory: Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Beck EC, Swanson C, Dustman RE. Long latency components of the visually evoked potential in man: effects of aging. Experimental Aging Research. 1980;6:523–545. doi: 10.1080/03610738008258385. [DOI] [PubMed] [Google Scholar]
- Berlyne D. Conflict, Arousal and Curiosity. McGraw-Hill; New York, NY: 1960. [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Daly RF. Effects of aging on visual evoked responses. Arch Neurol. 1977;34:403–407. doi: 10.1001/archneur.1977.00500190037005. [DOI] [PubMed] [Google Scholar]
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb P, Daffner K. To Ignore or Explore: Top Down Modulation of Novelty Processing. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.20003. in press. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Cognitive Brain Research. 1998;7:41–48. doi: 10.1016/s0926-6410(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Riis J, Rentz DM, Wolk DA, Budson AE, Holcomb PJ. Cognitive status impacts age-related changes in attention to novel and target events in normal adults. Neuropsychology. 2007;21:291–300. doi: 10.1037/0894-4105.21.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Calvo V, Faust R, Scinto LFM, Holcomb PJ. An electrophysiological index of stimulus unfamiliarity. Psychophysiology. 2000a;37:737–747. [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000b;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Cohen LG, Kennedy BP, West WC, Holcomb PJ. Regulation of attention to novel stimuli by frontal lobes: an event-related potential study. NeuroReport. 1998;9:787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Increased Responsiveness to Novelty is Associated with Successful Cognitive Aging. Journal of Cognitive Neuroscience. 2006a;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Scinto LFM, Holcomb PJ. Age-related differences in novelty and target processing among cognitively high performing adults. Neurobiology of Aging. 2005;26:1123–1295. doi: 10.1016/j.neurobiolaging.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Age-related differences in attention to novelty among cognitively high performing adults. Biological Psychology. 2006b;72:67–77. doi: 10.1016/j.biopsycho.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Dale AM. Source localization and spatial discriminant analysis of event-related potentials: linear approaches (brain cortical surface) Dissertation Abstracts International . 1994;55-07B:2559. [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neuroscience. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Egan V, Chiswick A, Santosh C, Naidu K, Remmington J, Best J. Size isn't everything: A study of brain volume, intelligence and auditory evoked potentials. Person Individ Diff. 1994;17:357–367. [Google Scholar]
- Escera C, Alho K, Winkler I, Naatanen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topography. 2001;14:25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Life-span changes in P3a. Psychophysiology. 2004;41:575–583. doi: 10.1111/j.1469-8986.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. High versus average cognitive function: Implications for the age-sensitivity of P3. Neurobiology of Aging. 2005;26:1305–1306. doi: 10.1016/j.neurobiolaging.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ford JM, Pfefferbaum A. Event-related potentials and eyeblink responses in automatic and controlled processing: effects of age. Electroencephalography and Clinical Neurophysiology. 1991;78:361–377. doi: 10.1016/0013-4694(91)90098-o. [DOI] [PubMed] [Google Scholar]
- Friedman D. Cognition and aging: a highly selective overview of event-related potential (ERP) data. Journal of Clinical and Experimental Neuropsychology. 2003;25:702–720. doi: 10.1076/jcen.25.5.702.14578. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski VA, Cycowicz YM. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35:508–520. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Brickman AM, Cohen RA, Arns M, Roe D, Lawrence JJ, Gordon E. Patterns of cognitive performance in middle-aged and older adults: A cluster analytic examination. J Geriatr Psychiatry Neurol. 2006;19:59–64. doi: 10.1177/0891988705284738. [DOI] [PubMed] [Google Scholar]
- Haier R, Siegel B, Nuechterlein K, Hazlett E, Wu J, Paek J, Browning H, Buchsbaum M. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Isreal JB, Wickens CD, Chesney GL, Donchin E. The event-related brain potential as an index of display-monitoring workload. Human Factors. 1980;22:211–224. doi: 10.1177/001872088002200210. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- Jausovec N, Jausovec K. Correlations between ERP parameters and intelligence: a reconsideration. Biological Psychology. 2000;55:137–154. doi: 10.1016/s0301-0511(00)00076-4. [DOI] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Smulders FT, Kok A. Selective processing of two-dimensional visual stimuli in young and old subjects: electrophysiological analysis. Psychophysiology. 1995;32:108–120. doi: 10.1111/j.1469-8986.1995.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints: a PET investigation. Brain. 1994;117:1055–1071. doi: 10.1093/brain/117.5.1055. [DOI] [PubMed] [Google Scholar]
- Kounios JH, Holcomb PJ. Concreteness effects in semantic processing: event-related potential evidence supporting dual-coding theory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: a comparison of lexical, object and reality decisions. Journal of Verbal Learning and Verbal Behavior. 1984;23:39–66. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. II Functional dissociation of P1 and N1 components. Electroencephalography and Clinical Neurophysiology. 1990;75:528–542. doi: 10.1016/0013-4694(90)90139-b. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends Cogn Sci. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Luck S, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA. Age-related changes in neural activity during visual target detection measured by fMRI. Cerebral Cortex. 2004;14:143–155. doi: 10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Spatial gradients of visual attention: behavioral and electrophysiological evidence. Electroencephalography and Clinical Neurophysiology. 1988;70:417–428. doi: 10.1016/0013-4694(88)90019-3. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–79. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- McGarry-Roberts P, Stelmack R, Campbell K. Intelligence, Reaction Time, and Event-Related Potentials. Intelligence. 1992;16:289–313. [Google Scholar]
- Muller NG, Knight RT. Age-related changes in fronto-parietal networks during spatial memory: an ERP study. Brain Res Cogn Brain Res. 2002;13:221–234. doi: 10.1016/s0926-6410(01)00119-7. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Friedman S, Swearer JM, Drachman DA. Active and passive P3 latency and psychometric performance: influence of age and individual differences. Int J Psychophysiol. 1992;12:187–195. doi: 10.1016/0167-8760(92)90010-9. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Hillert DG. Clinical applications of cognitive event-related potentials in Alzheimer's disease. Phys Med Rehabil Clin N Am. 2004;15:205–233. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Howard L, Starr A. P300 latency correlates with digit span. Psychophysiology. 1983;20:665–669. doi: 10.1111/j.1469-8986.1983.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Shappell SA, Brandt ME. Psychophysiology of N200/N400: a review and classification scheme. In: Jennings JR, Ackles PK, Coles MG, editors. Advances in Psychophysiology. Vol. 4. Jessica Kingsley Publishers Ltd. (UK); London : 1991. pp. 43–106. [Google Scholar]
- Raven JC, Court JH, Raven J. Coloured Progressive Matrices, section 2., Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford Psychologists Press; Oxford, UK: 1995. pp. 1–73. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, Kuller LH, Lopez OL, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old. Neuroimage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Morcom AM. The Relationship Between Brain Activity, Cognitive Performance, and Aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging:Linking Cognitive and Cerebral Aging. Oxford University Press; 2005. pp. 132–154. [Google Scholar]
- Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist. 1992;6:53–62. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the Cerebral Cortex in Aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- Sirevaag EJ, Kramer AF, Coles MG, Donchin E. Resource reciprocity: an event-related brain potentials analysis. Acta Psychologica. 1989;70:77–97. doi: 10.1016/0001-6918(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Snyder E, Hillyard SA. Long-latency evoked potentials to irrelevant, deviant stimuli. Behavioral Biology. 1976;16:319–331. doi: 10.1016/s0091-6773(76)91447-4. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York: 1998. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R. Brain Networks Associated with Cognitive Reserve in Healthy Young and Old Adults. Cerebral Cortex. 2004 doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Hubley AM. The 60-item Boston Naming test: norms for cognitively intact adults aged 25 to 88 years. Journal of Clinical and Experimental Neuropsychology. 1997;19:922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. The relationship between P3 and neuropsychological function in an adult life span sample. Biological Psychology. 2002;62:65–87. doi: 10.1016/s0301-0511(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Walhovd K, Fjell A. Two- and three-stimuli auditory oddball ERP tasks and neuropsychological measures in aging. NeuroReport. 2001;12:3149–3153. doi: 10.1097/00001756-200110080-00033. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. WAIS-III. Administration and Scoring Manual. The Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. WMS-III. Administration and Scoring Manual. The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Tsuchiya H, Kobayashi S. Electrophysiologic correlates of age effects on visuospatial attention shift. Brain Res Cogn Brain Res. 1995;3:41–49. doi: 10.1016/0926-6410(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Rose TL, Lapp D. Validity of the Geriatric Depression Scale in Subjects with Senile Dementia. Veterans Administration Medical Clinic; Palo Alto, CA: 1981. [Google Scholar]
- Youngjohn JR, Larrabee GJ, Crook TH. New adult- and education-correction norms for the Benton Visual Retention Test. The Clinical Neuropsychologist. 1993;7:155–160. doi: 10.1080/13854049308401517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.