Abstract
Surfactant lines the alveolar surface and prevents alveolar collapse. Derangements of surfactant cause respiratory failure and interstitial lung diseases. The collectins, surfactant proteins A and D, are also important in innate host defense. However, surfactant regulation in the postnatal lung is poorly understood. We found that the epithelial integrin, αvβ6, regulates surfactant homeostasis in vivo by activating latent transforming growth factor (TGF)-β. Adult mice lacking the β-subunit of αvβ6 (Itgb6−/−) developed increased bronchoalveolar lavage phospholipids and surfactant proteins A and D, and demonstrated abnormal-appearing alveolar macrophages, reminiscent of the human disease pulmonary alveolar proteinosis. Using lung-specific expression of constitutively active TGF-β1 in Itgb6−/− mice, we found that TGF-β1 was sufficient to normalize these abnormalities. Tgfβ1-deficient mice also demonstrated increased phospholipids and surfactant proteins A and D, but mice lacking the key TGF-β signaling molecule, SMAD3, did not. Therefore, integrin-mediated activation of latent TGF-β1 regulates surfactant constituents independent of intracellular SMAD3. In vivo increases in surfactant protein A and D were not associated with increases in mRNA for these proteins in alveolar tissue from Itgb6−/− mice. On the other hand, isolated alveolar macrophages from Itgb6−/− mice were defective in processing phospholipids in vitro, suggesting that reduced surfactant clearance contributes to altered surfactant homeostasis in these mice in vivo. These findings show that αvβ6 and TGF-β1 regulate homeostasis of phospholipids and collectins in adult mouse lungs and may have implications for anti-fibrotic therapeutics that inhibit active TGF-β in the lung.
Keywords: surfactant, macrophage, lung, integrin, transforming growth factor-β
CLINICAL RELEVANCE
Our findings may have significant implications for the treatment of fibrotic lung diseases, such as idiopathic pulmonary fibrosis, that target transforming growth factor-β in the lung. Our work also identifies mediators that may have relevance to human disorders of surfactant dysregulation.
Surfactant is the “surface acting agent” that lines the pulmonary alveolus and prevents alveolar collapse during breathing by reducing surface tension at the end of expiration. Surfactant is a spatially coordinated complex of proteins (10%) and phospholipids (90%) that covers the alveolar epithelium (1, 2). Surfactant proteins include the collectins, surfactant proteins A and D, and the hydrophobic proteins, surfactant proteins B and C. Surfactant is produced and secreted by specific transporters into the alveolar space by alveolar epithelial type II cells. It is cleared from the alveolar space by reuptake into type II epithelial cells and by catabolism within mature alveolar macrophages and type II cells (1, 3, 4). The inability to produce functional surfactant is a major cause of respiratory failure in newborns and interstitial lung diseases in children (5). Conversely, the presence of excessive surfactant in the disease pulmonary alveolar proteinosis is a cause of respiratory failure in adults (6). In addition, there is accumulating evidence that alterations in surfactant homeostasis occur in other adult pulmonary diseases, including sarcoidosis, idiopathic pulmonary fibrosis, acute respiratory distress syndrome (ARDS), and asthma (7–9). Thus, understanding the molecular pathways involved in surfactant homeostasis may lead to new ideas about the pathologic mechanisms involved in these varied lung diseases.
The lack of in vitro models that mimic the complex, multicellular interactions that occur in the normal alveolus has slowed progress in the understanding of the molecular mechanisms of surfactant regulation and made in vivo modeling indispensable. In this regard, the essential role of GM-CSF in alveolar macrophage differentiation and surfactant homeostasis was discovered via genetic manipulation of mice (2, 6, 10–13). However, whether there are additional mediators important for maintaining surfactant homeostasis in the postnatal adult lung remains an open question.
Integrins are heterodimeric transmembrane receptors composed of α and β subunits that mediate many cellular functions. In addition to their role in cell adhesion to extracellular matrix components, integrins are increasingly recognized as important initiators of biochemical signals that promote cellular survival, migration, and proliferation (reviewed in Refs. 14–16). The integrin subunit β6 is expressed on pulmonary epithelium and associates with the αv subunit. In previous studies, we discovered that integrin αvβ6 is a critical activator of latent transforming growth factor (TGF)-β in murine lungs (17). Mice carrying a deletion of the integrin β6 gene (Itgb6−/−) were protected from bleomycin-induced pulmonary fibrosis due to a lack of active TGF-β in the lung. We also found that Itgb6−/− mice develop spontaneous age-related pulmonary emphysema caused by activated alveolar macrophages (18). While studying this abnormal macrophage phenotype in Itgb6−/− mice, we serendipitously discovered that integrin αvβ6 plays a critical role in regulating surfactant homeostasis in adult mouse lungs. Based on these findings, we tested the hypothesis that the mechanism through which integrin αvβ6 mediates surfactant homeostasis is by regulating TGF-β activity.
MATERIALS AND METHODS
Mice
Mice deficient in the β6 subunit of the epithelial integrin αvβ6 (Itgb6−/−) were generated as described on a 129T2/SvEms genetic background (19). These mice were backcrossed five generations with wild-type C57BL6/J mice (The Jackson Laboratory, Bar Harbor, ME). In additional studies, we also analyzed Itgb6−/− mice backcrossed more than five generations with wild-type 129 mice. Deletion of Itgb6 was confirmed by Southern analysis. Double-transgenic mice expressing the reverse tetracycline transactivator (rtTA) under the spatial control of the Clara Cell 10 promoter and constitutively active TGF-β1 under the drug-inducible control of the tetracycline response element were generated as previously described (Tg[CCSP-rtTA],Tg[tetO-Tgfb1Cys-Ser223,225]) (18). Mice carrying both transgenes were verified by PCR genotyping and bred onto an FVB Itgb6−/− genetic background. Doxycycline-inducible expression of TGF-β1 was verified by enzyme-linked immunosorbent assay (ELISA) of fluid recovered by bronchoalveolar lavage (BAL) (BD Bioscience PharMingen, San Jose, CA) (18). Tgfb1-deleted mice were generated by breeding heterozygote mice (Tgfb1+/−), generated in an NIH/Olac genetic background (20) (generous gift from Rosemary Akhurst, University of California, San Francisco). Smad3-deleted mice (21) (generous gift of Anita Roberts, Center for Cancer Research, National Cancer Institute, National Institutes of Health) were generated by breeding 129 heterozygotes (Smad3+/−).
BAL Cell Analysis
For all mice over the age of 6 weeks, the lungs were lavaged five times with 0.8-ml aliquots of sterile tris-buffered saline. An aliquot of 400 μl was removed and processed for cell counts and cytocentrifugation. The remainder of the lavage fluid was centrifuged, and the cell free supernatant was stored at −70°C until further processing. The volume for each BAL sample was measured and for all samples ranged from 3.25 to 3.55 ml. We found no significant change in the outcomes of our results when the surfactant constituents were analyzed in concentration units. For 12-day- old mice (Tgfβ1-deficient), the lungs were lavaged five times with 0.2-ml aliquots of sterile tris-buffered saline. An aliquot of 400 μl was removed and processed for cell counts and cytocentrifugation. The remainder of the lavage fluid was centrifuged, and the cell free supernatant was stored at −70°C until further processing. Cytospin slides were stained with Diff Quik (Dade International, Miami, FL), and 300 total cells were counted for cell differentials.
Phospholipid Quantification
The phospholipid content of BAL fluid was determined after removing cells by centrifugation at 800 revolutions per minute for 5 minutes at 4°C. BAL phospholipid was extracted into methanol/chloroform, and the total phospholipid content was derived from the phosphorus concentration as previously described (22).
Surfactant Protein Measurement
Serial dilutions of cell-free BAL fluid from mice of each genotype were analyzed for surfactant protein (SP)-A and SP-D content using a dot blot assay with monospecific polyclonal antibodies against recombinant mouse SP-A and SP-D, respectively, as described previously (23). Standard curves using recombinant mouse SP-A and SP-D expressed in Chinese hamster ovary cells were used to determine the linear range of these assays and calculate absolute SPA and SPD bronchoalveolar lavage (BAL) fluid pool sizes.
Histologic Staining
For histologic staining, mouse lungs were inflated to 20 cm water pressure with 10% neutral buffered formalin (VWR Scientific Products, West Chester, PA) or OCT in 10% sucrose solution. Formalin-fixed lungs were embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin and eosin, or periodic acid Schiff (PAS) by the Pathology Department of San Francisco General Hospital using standard protocols. OCT inflated lungs were immediately frozen on dry ice, and frozen 5-μm sections were stained for oil red O using standard protocols. For high-power microscopic images of alveolar macrophages (Figure 1b) and type II epithelial cells (Figures E4 and E5 in the online supplement) from ultrathin lung sections, the lungs of Itgb6−/− mice and wild-type littermates were fixed and processed as previously described (24).
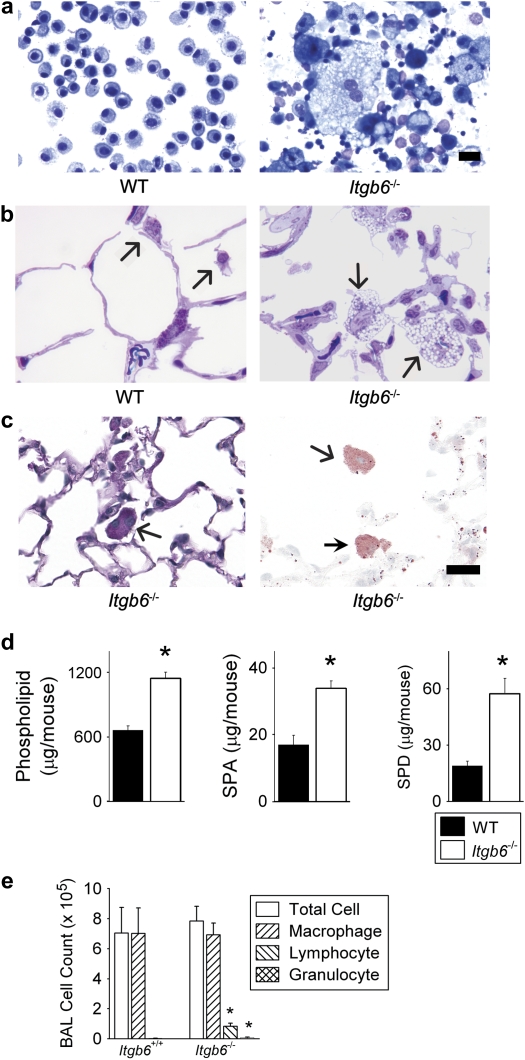
Figure 1.
Integrin β6 subunit-deleted (Itgb6−/−) mice spontaneously develop elevated bronchoalveolar lavage (BAL) phospholipid and collectin levels associated with large foamy alveolar macrophages and inflammation. (a) Representative BAL fluid cytospins from Itgb6−/− mice revealed enlarged alveolar macrophages with cytoplasmic vacuolization and extracellular debris. (b) Representative high-power microscopic images of lung tissue showed enlarged size and numerous vacuoles in macrophages from Itgb6−/− mice; this was not observed in any of the sections from littermate controls. Arrows indicate alveolar macrophages. (c) PAS (left) and Oil red O (right) staining of inflated lung sections from Itgb6−/− mice demonstrate cytoplasmic staining (PAS stains glycoprotein pink; Oil red O stains lipid red). Arrows indicate alveolar macrophages. (d) Total phospholipids, SP-A, and SP-D in BAL fluid from wild-type (WT) littermates and Itgb6−/− mice expressed as mean ± SEM (n = 6 per group). Results are representative of three separate experiments; *P < 0.005. (e) BAL fluid cell counts and differentials in wild-type littermates and Itgb6−/− mice; * P < 0.008. Mice studied in b were 6 months old, while all other data were collected from 2-month-old mice. Scale bars indicate 20 μm.
Real-Time Quantitative Polymerase Chain Reaction Gene Expression Measurement
For gene expression studies of alveolar macrophages, total cells were isolated from BAL fluid by centrifugation at 800 rpm for 5 minutes. Cells were resuspended in RPMI culture medium with 10% FBS and plated on tissue culture plastic to enrich for alveolar macrophages. After a 30-minute incubation, cells were washed twice using PBS, lysed, and total RNA was isolated using commercially available reagents according to the manufacturer's protocol (RNeasy Mini kit; Qiagen, Valencia, CA). Adherent cells were over 97% macrophages by Diff Quik staining based on our prior testing of this method (25).
For gene expression studies on alveolar cells, lungs from experimental and control littermates were harvested en bloc after canalization of the trachea and inflated with OCT in PBS at 20 cm pressure. The inflated lungs were then frozen in OCT-containing embedding molds on dry ice, and stored at −80°C until processed. Eight-micron-thick sections were cut on a cryostat at −20°C, with three sections placed on each PEN-membrane slide (Leica Microsystems, Inc., Bannockburn, IL). Samples were fixed in 70% EtOH at −20°C, then stained with a standard hematoxylin and eosin protocol, finishing with dehydration through stepwise ethanol concentrations. Slides were stored over dessicant until dissection occurred. Two slides of three sections each were dissected per sample, requiring 30 minutes of dissection per slide. Alveolar sections were isolated using a Leica AS LMD laser microdissection system at ×100 magnification, with care taken to avoid visible vascular structures and airways. RNA was purified and DNase treated using Qiagen Micro RNA Isolation kits as per manufacturer's instructions. RNA was assessed for quality and quantity using an Agilent BioAnalyzer Nano LabChip (Palo Alto, CA). An average of 14.5 ng of RNA was obtained per sample after purification, corresponding to approximately 1,200 to 1,600 cells.
Primer design and amplimer detection probe design were performed using Primer Express v 2.0 (Applied Biosystems, Foster City, CA) as we previously described (25). Primers and probe sequences are available in Table E1. The mean number of cycles to threshold (CT) of fluorescence detection was calculated for each sample and the results were normalized to the mean CT of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) for each sample tested. Results are expressed as a ratio of complementary DNA abundance compared with control mice.
For measurement of Gmcsf mRNA levels in homogenized lung tissue, total lung RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Total RNA was further purified with Qiagen RNeasy mini kit following DNase I digestion (Invitrogen). Two-step real-time reverse transcription PCR (RT-PCR) was used to determine the expression of mouse Gmcsf with Taqman Gold RT-PCR kit (Applied Biosystems). The primers and probes of Gmcsf and internal control Gapdh (or Beta-actin) were acquired from Applied Biosystems (Taqman Assay-on-Demand Gene Expression Assay).
Alveolar Epithelial Cell Isolation
Mouse lung vasculature was perfused with sterile PBS to remove visible red blood cells. The lungs were then inflated with dispase (5,000 Caseinolytic Units; BD Biosciences) followed by 1% low melting agarose, and then removed en bloc and incubated at room temperature with gentle agitation in dispase (5,000 Caseinolytic Units). After incubation the distal lung tissue was gently massaged with small curved forceps while resting in Dulbecco's modified Eagle's medium (DMEM) with 5% FBS. This procedure resulted in the dislodging of distal alveolar tissue while the large airways and associated connective tissue remained intact. This remaining lung “skeleton” was discarded. The tiny alveolar tissue fragments and cells were rocked in DMEM with 5% FBS for 10 minutes and then mixed by pipetting. Next the cells were filtered with successively smaller filters (70, 40, and 20 μm). The filtrate was centrifuged and the cells were counted. Next, alveolar epithelial cells were isolated by negative selection using biotinylated anti-CD16/CD32 and anti-CD45 (BD Biosciences) and streptavidin MagneSphere Paramagnetic particles (Promega, Madison, WI). Isolated epithelial cells from each mouse were counted and aliquoted in amounts of 100,000 and 400,000 cells, pelleted, and either frozen or lysed in 50 mM Tris HCl pH8/150 mM NaCl/1 mM EDTA/1% Triton X-100 and then frozen as required for subsequent analysis.
Macrophage 1,2-Dipalmitoyl-sn-Glycero-3-Phosphoethanolamine-N-Rhodamine Studies
A fluorescently labeled surfactant mixture was prepared by pooling Folch-extracted BAL fluid from C57BL6/J wild-type mice with 1,2-Dipalmitoyl-sn-Glycero-3-Phosphoethanolamine-N-Rhodamine (rhodamine-DPPE) (Avanti Polar Lipids, Inc., Alabaster, AL) in a 1:1 (wt:wt) ratio. This mixture was dried by rotary evaporation and resuspended in PBS without calcium in a final concentration of 500 μg/ml and sonicated before use. Alveolar macrophages were isolated from 6-week-old Itgb6−/− mice and wild-type littermate controls. Cells were incubated at 37°C with 5 or 50 μg/ml rhodamine-DPPE or unlabeled surfactant for 1 hour. The same set-up was performed on macrophages incubated at 4°C to serve as a control for nonspecific binding of labeled lipid to cells. Macrophages were then aggressively washed with PBS three to five times and a fraction of the macrophages were allowed to incubate in RPMI with 2% FBS for an additional 4 hours. At the end of the 1- and 5-hour time-points, macrophage fluorescence was analyzed by fluorescence-activated cell sorter (FACS). Macrophage fluorescence at 1 hour was regarded as cell-associated lipid, and any decay in fluorescence at the 5-hour time-point was considered to represent lipid catabolism as previously reported by Yoshida and coworkers (11).
α-Naphthyl Acetate Esterase Detection
Cytospins of BAL cells from five Itgb6−/− mice and five wild-type littermates were prepared and stained for fluoride-resistant α-naphthyl acetate esterase (α-NAE) activity using the commercial kit and recommended protocol (Sigma Diagnostics, St. Louis, MO). Black staining indicates the presence of fluoride-resistant α-NAE activity, which is an indicator of tissue macrophage differentiation (26–28). The number of alveolar macrophages with evidence of staining was determined for each mouse by counting 300 cells per cytospin.
Statistical Analysis
The data are expressed as means ± SEM unless otherwise indicated. For two group comparisons we used Student's t test for normally distributed data or Wilcoxon rank sum test for nonnormally distributed data as appropriate (e.g., cell count data, for which some groups had values of zero). For three and four group comparisons and normally distributed data, we began with ANOVA and then performed pairwise comparisons using Bonferroni correction for greater than two pairwise comparisons. A P value of ⩽ 0.05 was considered statistically significant.
RESULTS
Adult Itgb6−/− Mice Have Abnormal-Appearing BAL Macrophages and Increased Levels of Phospholipids and Collectins
We previously found that alveolar macrophages from adult mice carrying a deletion for the integrin β6 gene (Itgb6−/−) were markedly activated producing high levels of matrix metalloproteinase-12 (MMP-12) (18). Genome-wide expression analysis of these alveolar macrophages further supported the idea that they possessed marked changes in baseline gene expression profiles compared with wild-type littermate macrophages (25). Morphologically, these macrophages were enlarged, vacuolated, and often contained more than one nucleus (Figures 1a and 1b). Histologic staining showed evidence of PAS and oil-red O staining in alveolar macrophages from Itgb6−/− mice, indicating intracellular glycoproteins and lipid (Figure 1c). In addition to the macrophage phenotype, we also observed that cell-free BAL fluid from adult Itgb6−/− mice was translucent but not transparent, with a statistically significant increase in fluid turbidity (mean ± SD visible light absorbance, 0.45 ± 0.07) compared with wild-type littermates (0.2 ± 0.1) (P = 0.002).
Given the similarities in the appearance of alveolar macrophages from Itgb6−/− mice to that from patients with pulmonary alveolar proteinosis, we measured constituents of surfactant in BAL fluid from these mice. Total phospholipid in cell-free BAL fluid was about 73% higher in Itgb6−/− mice than in wild-type mice (Figure 1d). Similarly, levels of the collectins SP-A and SP-D were increased by 100% and 200%, respectively, in BAL fluid from Itgb6−/− mice compared with that from wild-type littermates (Figure 1d). Inflammatory cells from Itgb6−/− mice were also increased compared with wild-type littermates (Figure 1e). These surfactant abnormalities could not be accounted for by a decrease in the numbers of BAL macrophages in Itgb6−/− mice compared with wild-type mice. These data indicate that the αvβ6 epithelial integrin regulates the homeostasis of pulmonary phospholipids and SP-A and -D. Furthermore, loss of αvβ6 integrin leads to abnormal macrophage morphology.
Reconstitution of Active TGF-β1 in Itgb6−/− Mice Normalized Levels of Surfactant Constituents and the Macrophage Phenotype
The αvβ6 integrin is an important activator of latent TGF-β in the lung, kidney, and skin (17). In the lung, deletion of the β6 subunit results in a local deficiency of active TGF-β, which prevents the development of bleomycin-induced pulmonary fibrosis (17). Since active TGF-β1 has been shown to directly inhibit macrophage function (29, 30) and surfactant protein expression in vitro (31–35), we hypothesized that the absence of active TGF-β1 in the lungs of Itgb6−/− mice leads to the observed macrophage activation and increased surfactant levels. To test whether TGF-β1 is sufficient to reverse the Itgb6−/− phenotype, we studied Itgb6−/− mice genetically engineered to contain a lung-specific tetracycline-inducible transgene for constitutively active TGF-β1. Treatment of these mice with doxycycline results in low but measurable levels of active TGF-β1 in BAL fluid (18). We then determined the in vivo effect of low level active TGF-β1 on the surfactant levels in Itgb6−/− mice by treating mice with 5 weeks of doxycycline, beginning at 3 weeks of age. We found that the levels of both phospholipid and SP-A and -D were restored to levels similar to those found in wild-type littermates (Figure 2). To determine whether low-level production of active TGF-β1 was sufficient to restore phospholipid and SP-A and -D levels in much older mice, we repeated the experiment, but this time started doxycycline treatment at about 3 months of age and found a similar response (data not shown). Analysis of the BAL fluid cells in Itgb6−/− mice after induced expression of active TGF-β1 also demonstrated near-normalization of the alveolar macrophage morphology (Figure E1). Neither TGF-β1 transgene expression nor doxycycline altered phospholipid or SP-A and -D levels in mice with normal αvβ6 expression (data not shown).
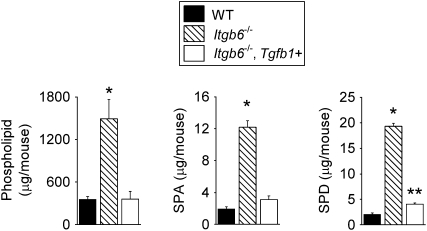
Figure 2.
Increases in surfactant constituents in Itgb6−/− mice are normalized by doxycycline-induced lung expression of active TGF-β1. Total phospholipid, SP-A, and SP-D in BAL fluid from 2-month-old wild-type (WT), Itgb6−/−, and Itgb6−/− mice carrying a transgene for active TGF-β1 (Itgb6−/−, Tgfb1+). Data are expressed as mean ± SEM (n = 4–6 per group). Doxycycline treatment was started in all groups at 3 weeks of age and continued until the mice were killed at 2 months of age. Results are representative of three separate experiments. *P < 0.008 compared with all other groups; **P = 0.02 compared with WT.
These in vivo data suggest that the mechanism by which integrin αvβ6 regulates phospholipid and SP-A and -D levels is through activation of latent TGF-β. Further, these data provide evidence that active TGF-β1 plays an important role in regulating levels of phospholipid and SP-A and -D in adult mouse lungs. Active TGF-β1 was also sufficient to restore the normal appearance of alveolar macrophages.
TGF-β Is Directly Required for Normal Adult Surfactant Homeostasis, but SMAD3 Is Not
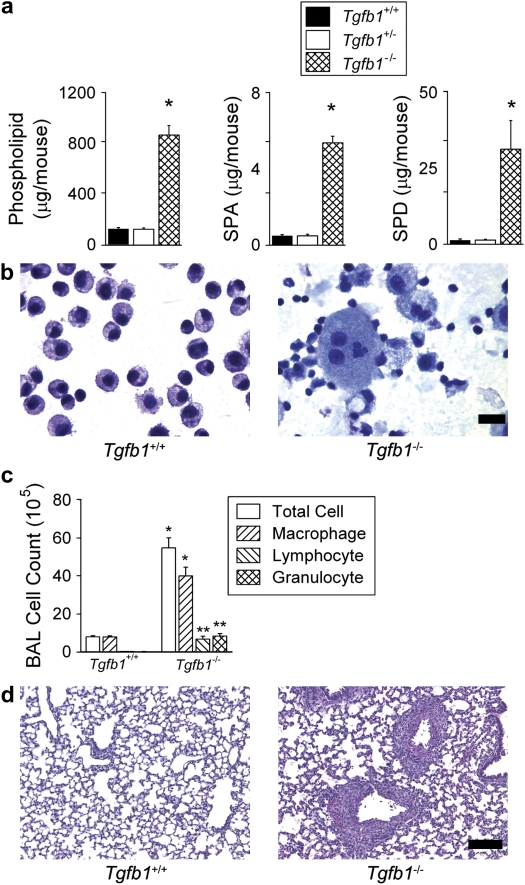
Since results from Itgb6−/− mice indicated that active TGF-β1 was the critical mediator of phospholipid and SP-A and -D homeostasis, we next examined the effect on these surfactant constituents caused by a direct genetic deletion of TGF-β1 itself. We therefore studied Tgfβ1-deficient mice (Tgfb1−/−) 12 days postpartum, shortly before they would be expected to die of a wasting syndrome (36, 37). Even at this early time-point, Tgfβ1-deficient mice had large increases in phospholipids (> 500%), SP-A (> 1,000%), and SP-D (> 2,000%) in BAL fluid (Figure 3a). Alveolar macrophages from Tgfβ1-deficient mice were markedly enlarged and vacuolated, and there was evidence of extracellular amorphous material (Figure 3b). Differential cell counts showed a significant increase of inflammatory cells in Tgfb1−/− mice compared with wild-type littermates (Figure 3c). Lung histology revealed that the inflammation was in a perivascular distribution (Figure 3d). The abnormalities in surfactant constituents found in Tgfβ1-deficient mice further support the requirement for TGF-β1 in surfactant homeostasis. Levels of surfactant constituents in Tgfβ1+/− mice were indistinguishable from wild-type littermates demonstrating that phospholipid and SP-A and -D homeostasis can be maintained with only one allele of TGF-β1.
Figure 3.
Tgfb1-deficient mice have elevated levels of surfactant constituents, enlarged, foamy alveolar macrophages and lung inflammation. (a) Total phospholipids, SP-A, and SP-D in BAL fluid from 12-day-old wild-type (Tgfb1+/+), heterozygote (Tgfb1+/−), and Tgfb1-deleted (Tgfb1−/−) littermate mice expressed as mean ± SEM (n = 6 per group). *P ⩽ 0.005 compared with all other groups. (b) BAL fluid cytospins from 12-day-old wild-type littermates and Tgfb1-deleted mice. Alveolar macrophages from heterozygote mice appeared similar to wild type (not shown) (scale bar, 20 μm). (c) BAL fluid cell counts and differentials in 12-day-old wild-type (Tgfb1+/+) and Tgfb1-deleted (Tgfb1−/−) littermate mice. (d) Representative hematoxylin and eosin–stained lung sections from wild-type littermates and Tgfb1-deleted mice, showing normal-appearing lung parenchyma with areas of peribronchovascular inflammation (scale bar, 100 μm).
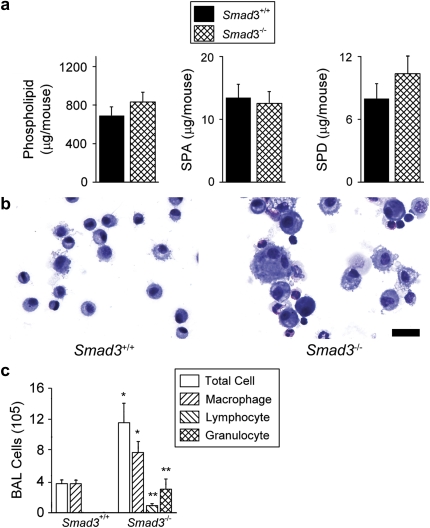
Since TGF-β1 was necessary and sufficient for homeostasis of phospholipid and SP-A and -D levels, we hypothesized that SMAD3, one of several critical intracellular signaling molecules for many effects of TGF-β1, would also be required. However, we found no difference in BAL fluid phospholipids or SP-A and -D levels in Smad3-deficient mice compared with levels found in wild-type littermates at either 4 or 2 months of age (Figures 4a and E2). Because the Smad3-deficient mice used in these analyses were generated in a 129 genetic strain, which was different than that of the previously studied Itgb6−/− mice (C57BL6/J and FVB) and Tgfb1−/− mice (NIH), we confirmed elevated phospholipid and SP-A and -D levels in Itgb6−/− mice on a 129 genetic mouse strain (Figure E3). This indicates that genetic background alone cannot explain the differences in the levels of phospholipids and SP-A and -D measured from the TGF-β–deficient mouse models and the Smad3-deficient mice.
Figure 4.
Smad3-deficient mice have normal levels of surfactant constituents in the setting of lung inflammation. (a) Total phospholipids, SP-A, and SP-D in BAL fluid from 4-month-old wild-type (Smad3+/+) and Smad3-deficient (Smad3−/−) littermate mice expressed as mean ± SEM (n = 10–11 per group). Differences in means were not statistically significant. (b) BAL cytospins from 4-month-old wild-type littermates and Smad3-deficient mice (Scale bar, 20 μm). (c) Two-month-old Smad3-deficient mice show increased numbers of BAL fluid macrophages, lymphocytes, and granulocytes expressed as mean ± SEM (n = 5–9 per group). *P ⩽ 0.05; **P ⩽ 0.007.
With regard to other features of the pulmonary phenotype in Smad3-deficient mice, we found that there was more heterogeneity in the alveolar macrophage size compared with alveolar macrophages from wild-type littermates (Figure 4b). BAL fluid from Smad3-deficient mice revealed increased inflammatory cells in a pattern similar to that observed in Tgfβ1-deficient mice (Figure 4c). Overall, these data suggest that there may be alternative or compensatory TGF-β signaling pathways for surfactant regulation in Smad3-deficient mice. However, TGF-β signaling through SMAD3 does appear to be important for regulating other aspects of pulmonary homeostasis, such as inflammatory cell recruitment and activation of these cells.
Analysis of Isolated Alveolar Epithelial Cells from Itgb6−/− Mice
Our data indicate that a deficiency of active TGF-β is responsible for the elevated levels of surfactant constituents measured in Itgb6−/− and Tgfb1−/− mouse lungs. However, how TGF-β mediates this regulation in vivo is not known. Previously, it was shown that exogenous TGF-β treatment decreased SP-A mRNA and protein levels in cultured fetal lung tissue (32). Therefore, we analyzed isolated alveolar epithelial cells and lung tissue from Itgb6−/− mice to determine whether loss of integrin αvβ6-mediated TGF-β activation led to increased SP-A mRNA levels or had other effects that might contribute to the surfactant and collectin abnormalities we identified in vivo.
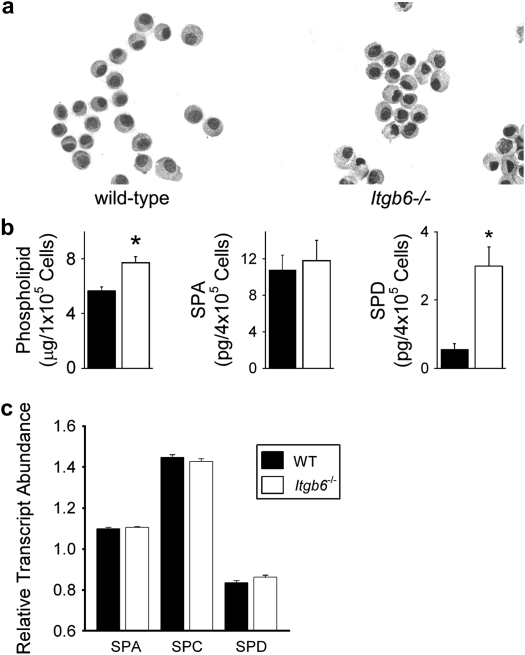
There were no obvious differences in the appearance of wild-type and Itgb6−/− epithelial cells in the alveoli (Figures E4 and E5) or after isolation (Figure 5a). Protein levels of SP-A were similar in isolated wild-type and Itgb6−/− epithelial cells (Figure 5b), and SP-A mRNA levels were similar in alveolar tissue obtained from wild-type and Itgb6−/− mice by laser capture dissection (Figure 5c). There was a 36% increase in phospholipid content of alveolar epithelial cells from Itgb6−/− mice as well as a larger increase in SP-D content (Figure 5b). Since the increase in SP-D protein was not accompanied by an increase in SP-D mRNA levels (Figure 5c), it is not clear whether increases in SP-D content in isolated epithelial cells are a result of increased reuptake and/or production or decreased catabolism by these cells. Immunohistochemical staining for SP-A and -D revealed a similar distribution of these proteins within cells lining the alveoli (Figures E6 and E7).
Figure 5.
Alveolar epithelial cells from Itgb6−/− mice contained increased phospholipids and SP-D with no detectable change in surfactant protein transcript levels. (a) Representative cytospins of alveolar epithelial cells from 4-month-old Itgb6−/− mice and littermate controls. (b) Total phospholipids and SP-A and SP-D in alveolar epithelial cells isolated from Itgb6−/− mice and littermate controls. Data are expressed as average μg or pg per designated cell number with n = 5 per group. (c) RNA was isolated from laser captured alveolar tissue from Itgb6−/− mice and littermate controls. Messenger RNA levels of SP-A, -C, and -D were measured using real-time PCR (n = 4 per group). Values for wild-type littermates are represented by solid bars and for Itgb6−/− mice by open bars. Data are expressed as means ± SEM.
Analysis of Isolated Alveolar Macrophages from Itgb6−/− Mice
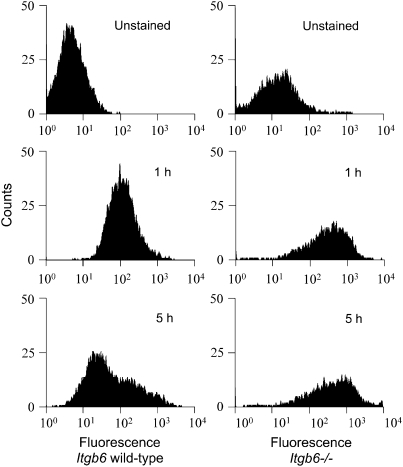
Since alveolar macrophages from Itgb6−/− mice appear abnormal, we hypothesized that a deficiency of TGF-β may alter alveolar macrophage catabolic function. Therefore we performed a functional assay to assess whether alveolar macrophages from Itgb6−/− mice demonstrate abnormalities of lipid processing in vitro. Using FACS analysis (Figure 6), we found that alveolar macrophages from both wild-type and Itgb6−/− mice were able to associate with the fluorescently labeled phospholipid, rhodamine-DPPE. Macrophages from wild-type littermates were able to degrade most of the labeled DPPE over 4 hours (median fluorescence intensity 104 units at 1 h versus 41 units at 5 h). In contrast, macrophages from Itgb6−/− mice were unable to degrade the labeled DPPE (86 units at 1 h versus 107 units at 5 h). This finding suggests that at least part of the abnormality in surfactant homeostasis in Itgb6−/− mice may be due to defective catabolism by alveolar macrophages.
Figure 6.
Itgb6−/− mice demonstrated abnormal handling of lipid by alveolar macrophages. FACS histogram plot of alveolar macrophages from wild-type (left column) and Itgb6−/− mice (right column) after incubation with a mixture of natural surfactant and rhodamine-DPPE as described in Materials and Methods. Macrophages were exposed to 50 μg/ml rhodamine-DPPE for 1 hour, then washed, and a fraction of the cells were then allowed to incubate for an additional 4 hours. Wild-type macrophages demonstrate a decay in fluorescence intensity indicating a loss of rhodamine-DPPE at 5 total hours of incubation that was not observed with Itgb6−/− macrophages. Similar results were obtained using different concentrations of rhodamine-DPPE. Minimal nonspecific binding of rhodamine-DPPE was seen in macrophages incubated at 4°C (data not shown). Ten thousand cell counts were collected for each sample and counts shown are gated to exclude lymphocytes.
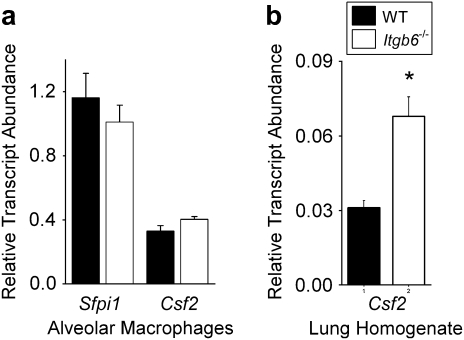
Given the finding that alveolar macrophages from Itgb6−/− mice may have defects in surfactant catabolism, we next measured the expression of several macrophage differentiation and maturation factors necessary to induce normal surfactant catabolic activity. The cytokine granulocyte macrophage colony-stimulating factor (GM-CSF) plays a critical role in inducing alveolar macrophage differentiation and maturation, and deficiencies in GM-CSF are associated with abnormalities in surfactant metabolism (10–12). Alveolar macrophages from Itgb6−/− mice expressed GM-CSF (Csf2) mRNA at levels comparable with those of controls (Figure 7a), whereas total lung Csf2 was actually increased in whole lung homogenates from Itgb6−/− mice (Figure 7b). We attempted to measure GM-CSF protein in concentrated BAL fluid from wild-type and Itgb6−/− mice, but as previously reported by other investigators (38, 39), levels were below the detection limit for the ELISA (sensitivity 0.5 pg/ml; data not shown). In addition, we analyzed the serum of Itgb6−/− mice for auto-antibodies against GM-CSF that have been shown to inhibit normal macrophage differentiation in many patients with pulmonary alveolar proteinosis, but did not find evidence for anti–GM-CSF (data not shown). As an alternative approach to assess whether alveolar macrophages from Itgb6−/− mice are in an immature state of differentiation, akin to that identified in GM-CSF–deficient mice, we analyzed expression of mRNA for the key transcription factor PU.1 (Sfpi1) and stained alveolar macrophages for fluoride-resistant α-naphthyl acetate esterase activity, which is an indicator of tissue macrophage differentiation (26–28). Alveolar macrophages from wild-type and Itgb6−/− mice did not differ significantly in either PU.1 mRNA expression (Figure 7a) or fluoride-resistant α-naphthyl acetate esterase activity (94% ± 4.5 versus 96% ± 2.7 positively staining cells, P = 0.45). Taken together, these data suggest that the abnormal macrophage phenotype observed in Itgb6−/− mice is not due to a global disruption of macrophage maturation.
Figure 7.
Alveolar macrophages from Itgb6−/− mice and control littermates had similar mRNA levels of GM-CSF and PU.1 while Itgb6−/− lung homogenate showed increased expression of GM-CSF compared with controls. (a) Total RNA was isolated from purified BAL macrophages, and expression of Sfpi1 (PU.1) and Csf2 (GM-CSF) were measured using real-time PCR (n = 5 per group). (b) Lung homogenate total RNA was analyzed for Csf2 (GM-CSF). Data are expressed as relative transcript abundance normalized to GAPDH ± SEM. Values for wild-type littermates are represented by solid bars and for Itgb6−/− mice by open bars.
DISCUSSION
Disorders of surfactant homeostasis play prominent roles in many lung diseases. Surfactant deficiency is central to the pathogenesis of newborn respiratory distress syndrome and is also important in adult respiratory distress syndrome (40). At the other extreme, excessive surfactant accumulation is found in patients with pulmonary alveolar proteinosis (6). In addition, the collectin family members, SP-A and SP-D, are increasingly recognized to play important roles in the pulmonary innate immune response (41) by opsonizing pathogens and facilitating cellular bacteriocidal activity. However, the factors that regulate levels of surfactant in the alveolar space are incompletely understood. In this study, we identified a novel mediator involved in homeostasis of the collectins and phospholipids in the adult mouse lung: the epithelial integrin αvβ6. We also determined that the mechanism by which integrin αvβ6 mediates this homeostasis is through regulation of TGF-β activation.
We found that active TGF-β was both necessary and sufficient to regulate surfactant phospholipid and collectin homeostasis in adult mouse lungs. This was evidenced by an increase in phospholipids and collectins in the absence of active TGF-β and alternatively an almost complete normalization of these surfactant constituents after induced expression of the active form of TGF-β1 in the lungs of Itgb6−/− mice. This regulatory effect of active TGF-β on surfactant components in Itgb6−/− mice was observed even in mice several months old, well after postnatal lung development was complete. Thus, our findings extend the observations of others by demonstrating the in vivo role of active TGF-β on the regulation of these surfactant components. In addition, these findings illustrate how critical TGF-β is to the homeostasis of surfactant in adult mouse lungs. This may have significant implications for treatment approaches that inhibit active TGF-β in fibrotic lung diseases, such as idiopathic pulmonary fibrosis. Furthermore, the identification of a role for TGF-β in regulating surfactant in adult lungs may aid the understanding of certain aspects of phospholipid derangements noted in patients with idiopathic pulmonary fibrosis (42), a condition found to be associated with increased levels of TGF-β (43).
While active TGF-β was required for homeostasis of lung phospholipids and collectins, SMAD3, a key intracellular signaling molecule for TGF-β, was not. We speculate there are several possible explanations for this. First, this finding is not unprecedented in light of the accumulating evidence that regulatory SMAD2 and 3 can mediate different cellular functions in vivo and in vitro (44–46). Second, there is the possibility that compensatory pathways are activated in Smad3-deficient mice, and thus are able to bypass the deficiency of SMAD3. While this explanation may account for the normal levels of surfactant components in Smad3-deficient mice, it is clear that other aspects of pulmonary homeostasis, such as control over inflammatory cell recruitment, requires signaling by SMAD3. We excluded the possibility that our findings might be the result of genetic strain differences between the TGF-β–deficient mouse models and Smad3-deficient mice by analyzing the alveolar content of phospholipids and collectins in Itgb6−/− mice on the same genetic background as Smad3-deficient mice. We speculate that SMAD3-independent TGF-β signaling pathways allow for normal surfactant homeostasis in Smad3-deficient mice, and that failure to activate these SMAD3-independent TGF-β pathways accounts for the surfactant abnormalities in Itgb6−/− and Tgfb1−/− mice.
Additional evidence to support the idea that active TGF-β can regulate pulmonary homeostasis through signaling pathways independent of SMAD3 comes from alveolar macrophages from Smad3-deficient mice. Compared with alveolar macrophages from Itgb6−/− or Tgfb1−/− mice, Smad3−/− macrophages show less dramatic morphologic changes (Figures 1a, 2b, and 4b). In addition, we previously found that alveolar macrophages from Itgb6−/− mice expressed mRNA transcripts for matrix metalloproteinase-12 at levels approximately 100- to 200-fold higher than that from control mice, which was regulated by active TGF-β (18). In contrast, alveolar macrophages from Smad3−/− mice have barely detectable increases of matrix metalloproteinase-12 mRNA (Figure E8), indicating that there are additional signaling pathways that can respond to active TGF-β despite a total deficiency of SMAD3. Overall, these differences in alveolar macrophage homeostasis between Smad3-deficient mice and Itgb6−/− mice identify an important area for further research to understand the various signaling pathways used by active TGF-β to regulate macrophage function.
Our results suggest that the mechanism of TGF-β–mediated surfactant regulation is not through GM-CSF. Mice lacking GM-CSF (Csf2−/−) also demonstrate elevated levels of surfactant constituents (12). In these GM-CSF–deficient mice, the primary cause for surfactant dysregulation was found to be a defect in catabolism of surfactant (47). Specifically, the majority of alveolar macrophages from these mice were unable to effectively catabolize surfactant components due to an immature state of differentiation (11, 27, 48). Reconstitution of GM-CSF–deficient mice with GM-CSF protein was able to restore surfactant homeostasis (38, 49). Additional studies identified that GM-CSF stimulates induction of the PU.1 transcription factor, which causes alveolar macrophage differentiation and enables surfactant clearance (11, 48). In support of these data, the majority of alveolar macrophages from GM-CSF–deficient mice lacked staining for fluoride-resistant α-naphthyl acetate esterase activity, a marker of fully differentiated alveolar macrophages (27). While our findings from Itgb6−/− mice also suggest a defect in lipid processing by alveolar macrophages, the majority of macrophages from these mice were positive for fluoride-resistant α-naphthyl acetate esterase activity, suggesting that they are fully differentiated. Furthermore, we found increased, not decreased, levels of GM-CSF mRNA in lung homogenate from Itgb6−/− mice compared with wild-type littermates, as well as similar mRNA levels of PU.1 in alveolar macrophages from wild-type and Itgb6−/− mice. Taken together, these data suggest that TGF-β may directly or indirectly influence alternative catabolic pathways in macrophages that are not dependent on GM-CSF.
We also considered the possibility that a deficiency of active TGF-β in the lung leads to macrophage apoptosis, since alveolar macrophages from Itgb6−/− mice appear to contain pycnotic nuclei (data not shown). We did find that alveolar macrophages from Itgb6−/− mice were highly fluorescent without staining, and with annexin V and acridine orange staining these macrophages showed a staining pattern that suggested apoptosis (data not shown). However, we were unable to measure additional markers of apoptosis or senescence using multiple methods (Western blots for caspase 3, p21, tunnel staining, immunocytochemistry for caspase 3, propidium iodine staining, and DNA laddering). Thus, we cannot convincingly conclude that macrophage apoptosis contributes to the surfactant abnormalities that we measured.
Despite differences in macrophage maturation states between GM-CSF–deficient mice and Itgb6−/− mice, there appear to be several lung phenotypic features shared between these two models that we speculate may represent a common phenotype observed when clearance of surfactant is defective. Both models have features consistent with an elevation of surfactant in the airspace, notably the accumulation of eosinophilic material in the alveolar spaces and foamy lipid–laden macrophages. These two mouse models also share the feature of spontaneous development of increased inflammation in the lung—specifically, the presence of peribronchovascular aggregates of lymphoid cells, which include B and CD4+ T cells (10, 19). Another feature shared by these two models is the fact that the deletion of GM-CSF or integrin β6 subunit had no detectable influence on mRNA levels for surfactant proteins even though alveolar surfactant protein and lipid levels were elevated. This supports the idea that impaired clearance rather than increased surfactant synthesis is likely responsible for the accumulation of phospholipids and collectins in Itgb6−/− mice as it is in GM-CSF–deficient mice.
Although addition of exogenous TGF-β1 has been shown to reduce surfactant protein A mRNA and protein in ex vivo human fetal lung tissue (32), our in vivo data show that a depletion of TGF-β results in elevated BAL levels of surfactant protein A without influencing protein or transcript levels within epithelial cells. This apparent discrepancy may be attributable to important differences in experimental design. The in vivo models that we studied represent the consequences of interfering with normal levels of active TGF-β in the lung, whereas the fetal lung tissue model represents the effects of exogenous TGF-β1, which may or may not represent normal levels that are realized in vivo. In any case, our results suggest that in vivo, an increase in transcript levels is not the mechanism responsible for the elevation of SP-A in mice lacking active TGF-β. Rather, our findings suggest that a depletion of active TGF-β leads to a decrease in clearance of SP-A in the lung.
In contrast to SP-A, we found that depletion of TGF-β led to increased SP-D levels in both BAL fluid and alveolar epithelial cells, without an increase in transcript levels. The disproportionate increase of protein levels for SP-D compared with SP-A in Itgb6−/− alveolar epithelial cells was also observed in the BAL fluid, where we measured a 100% and 200% increase in SP-A and -D, respectively. This finding is consistent with observations in humans with pulmonary alveolar proteinosis as well as other mouse models of defective surfactant clearance, where SP-D was found to be disproportionately increased compared with other surfactant proteins (39, 50). The reason for this is not known, but it has been speculated that an increase in SP-D may be a compensatory response to increased phospholipid levels (39). Mouse models have also demonstrated the important role of SP-D in maintaining surfactant homeostasis (24). Thus, SP-D may play an accessory role in regulating aspects of pulmonary homeostasis and in turn, its levels may be differentially regulated by many other local signals from resident lung cells.
In summary, our data indicate that the activation of latent TGF-β1 by the αvβ6 integrin is central to maintaining normal basal levels of phospholipids and collectins in the alveolar space. Based on the sum of alveolar epithelial cell and macrophage data from Itgb6−/− mice, we speculate that a depletion of active TGF-β leads to decreased catabolism of phospholipids and collectins in the lung. These in vivo findings in adult mice call attention to the importance of αvβ6 integrin and active TGF-β in maintaining normal levels of phospholipids and SP-A and -D, and identify new potential targets in diseases involving surfactant dysregulation. In addition, our findings have implications for treatments of fibrotic lung diseases, such as idiopathic pulmonary fibrosis, where therapeutics may target and inhibit TGF-β1 activity in the lung.
Supplementary Material
Acknowledgments
The authors thank Cindy Brown, Jess Edmonson, and Tom Shimotake for their expert assistance with surfactant analysis, and Jeffrey A. Whitsett, Bruce C. Trapnell, and Prescott Woodruff for critical review of the manuscript.
This work was supported by the National Institutes of Health HL04465 and HL072915 (L.L.K.), the American Thoracic Society, and the American Lung Association.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2006-0428OC on July 19, 2007
Conflict of Interest Statement: D.G.M. is now a paid employee of Roche Palo Alto, LLC. This work was performed before his taking that position. D.S. is a co-owner of a patent covering blockade of integrin αvβ6 for the treatment of pulmonary fibrosis and acute lung injury. He also has had a sponsored research agreement with BiogenIdec to cover work on anti-integrin antibodies in pulmonary fibrosis and acute lung injury for $150,000/year (total costs) since January 2002. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. FASEB J 1994;8:957–967. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol 2002;64:775–802. [DOI] [PubMed] [Google Scholar]
- 3.Rider ED, Ikegami M, Jobe AH. Localization of alveolar surfactant clearance in rabbit lung cells. Am J Physiol 1992;263:L201–L209. [DOI] [PubMed] [Google Scholar]
- 4.Wright JR. Clearance and recycling of pulmonary surfactant. Am J Physiol 1990;259:L1–L12. [DOI] [PubMed] [Google Scholar]
- 5.Nogee LM. Genetics of pediatric interstitial lung disease. Curr Opin Pediatr 2006;18:287–292. [DOI] [PubMed] [Google Scholar]
- 6.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–2539. [DOI] [PubMed] [Google Scholar]
- 7.Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 2003;31:20–27. [DOI] [PubMed] [Google Scholar]
- 8.Erpenbeck VJ, Schmidt R, Gunther A, Krug N, Hohlfeld JM. Surfactant protein levels in bronchoalveolar lavage after segmental allergen challenge in patients with asthma. Allergy 2006;61:598–604. [DOI] [PubMed] [Google Scholar]
- 9.Gunther A, Schmidt R, Nix F, Yabut-Perez M, Guth C, Rosseau S, Siebert C, Grimminger F, Morr H, Velcovsky HG, et al. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur Respir J 1999;14:565–573. [DOI] [PubMed] [Google Scholar]
- 10.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994;91:5592–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida M, Ikegami M, Reed JA, Chroneos ZC, Whitsett JA. GM-CSF regulates protein and lipid catabolism by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2001;280:L379–L386. [DOI] [PubMed] [Google Scholar]
- 12.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994;264:713–716. [DOI] [PubMed] [Google Scholar]
- 13.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, Thomassen MJ. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol 2003;285:L1132–L1136. [DOI] [PubMed] [Google Scholar]
- 14.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 1995;268:233–239. [DOI] [PubMed] [Google Scholar]
- 15.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992;69:11–25. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol 2000;19:203–209. [DOI] [PubMed] [Google Scholar]
- 17.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 18.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin avb6-mediated TGF-b activation causes MMP-12-dependent emphysema. Nature 2003;422:169–173. [DOI] [PubMed] [Google Scholar]
- 19.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996;133:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 1995;121:1845–1854. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 1999;18:1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 1959;234:466–468. [PubMed] [Google Scholar]
- 23.Hawgood S, Akiyama J, Brown C, Allen L, Li G, Poulain FR. GM-CSF mediates alveolar macrophage proliferation and type II cell hypertrophy in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 2001;280:L1148–L1156. [DOI] [PubMed] [Google Scholar]
- 24.Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA 1998;95:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodruff PG, Koth LL, Yang YH, Rodriguez MW, Favoreto S, Dolganov GM, Paquet AC, Erle DJ. A distinctive alveolar macrophage activation state induced by cigarette smoking. Am J Respir Crit Care Med 2005;172:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moloney WC, McPherson K, Fliegelman L. Esterase activity in leukocytes demonstrated by the use of naphthol AS-D chloroacetate substrate. J Histochem Cytochem 1960;8:200–207. [DOI] [PubMed] [Google Scholar]
- 27.Reed JA, Ikegami M, Cianciolo ER, Lu W, Cho PS, Hull W, Jobe AH, Whitsett JA. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am J Physiol 1999;276:L556–L563. [DOI] [PubMed] [Google Scholar]
- 28.Yam LT, Li CY, Crosby WH. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol 1971;55:283–290. [DOI] [PubMed] [Google Scholar]
- 29.Ding A, Nathan CF, Graycar J, Derynck R, Stuehr DJ, Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol 1990;145:940–944. [PubMed] [Google Scholar]
- 30.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature 1988;334:260–262. [DOI] [PubMed] [Google Scholar]
- 31.Kumar AS, Gonzales LW, Ballard PL. Transforming growth factor-beta(1) regulation of surfactant protein B gene expression is mediated by protein kinase-dependent intracellular translocation of thyroid transcription factor-1 and hepatocyte nuclear factor 3. Biochim Biophys Acta 2000;1492:45–55. [DOI] [PubMed] [Google Scholar]
- 32.Beers MF, Solarin KO, Guttentag SH, Rosenbloom J, Kormilli A, Gonzales LW, Ballard PL. TGF-beta1 inhibits surfactant component expression and epithelial cell maturation in cultured human fetal lung. Am J Physiol 1998;275:L950–L960. [DOI] [PubMed] [Google Scholar]
- 33.Maniscalco WM, Sinkin RA, Watkins RH, Campbell MH. Transforming growth factor-beta 1 modulates type II cell fibronectin and surfactant protein C expression. Am J Physiol 1994;267:L569–L577. [DOI] [PubMed] [Google Scholar]
- 34.Whitsett JA, Budden A, Hull WM, Clark JC, O'Reilly MA. Transforming growth factor-beta inhibits surfactant protein A expression in vitro. Biochim Biophys Acta 1992;1123:257–262. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Zhu NL, Tan RC, Ballard PL, Derynck R, Minoo P. Transforming growth factor-beta inhibits pulmonary surfactant protein B gene transcription through SMAD3 interactions with NKX2.1 and HNF-3 transcription factors. J Biol Chem 2002;277:38399–38408. [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993;90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992;359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest 1996;97:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed JA, Ikegami M, Robb L, Begley CG, Ross G, Whitsett JA. Distinct changes in pulmonary surfactant homeostasis in common beta-chain- and GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L1164–L1171. [DOI] [PubMed] [Google Scholar]
- 40.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA III, Hudson LD, Maunder RJ, Crim C, Hyers TM. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 1991;88:1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005;5:58–68. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt R, Meier U, Markart P, Grimminger F, Velcovsky HG, Morr H, Seeger W, Gunther A. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 2002;283:L1079–L1085. [DOI] [PubMed] [Google Scholar]
- 43.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 2004;125:754–765. [DOI] [PubMed] [Google Scholar]
- 44.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol 2004;172:4275–4284. [DOI] [PubMed] [Google Scholar]
- 45.Ohnishi H, Miyata T, Yasuda H, Satoh Y, Hanatsuka K, Kita H, Ohashi A, Tamada K, Makita N, Iiri T, et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem 2004;279:8873–8878. [DOI] [PubMed] [Google Scholar]
- 46.Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J 2006;393:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol 1996;270:L650–L658. [DOI] [PubMed] [Google Scholar]
- 48.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001;15:557–567. [DOI] [PubMed] [Google Scholar]
- 49.Ikegami M, Jobe AH, Huffman Reed JA, Whitsett JA. Surfactant metabolic consequences of overexpression of GM-CSF in the epithelium of GM-CSF-deficient mice. Am J Physiol 1997;273:L709–L714. [DOI] [PubMed] [Google Scholar]
- 50.Crouch E, Persson A, Chang D. Accumulation of surfactant protein D in human pulmonary alveolar proteinosis. Am J Pathol 1993;142:241–248. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.