Abstract
Adenoviral evolution has generated mechanisms to resist host cell defense systems, but the biochemical basis for evasion of multiple antiviral pathways in the airway by adenoviruses is incompletely understood. We hypothesized that adenoviruses modulate airway epithelial responses to type I interferons by altering the levels and activation of specific Janus family kinase-signal transducer and activator of transcription (JAK-STAT) signaling components. In this study, specific effects of adenovirus type 5 (AdV) on selected JAK-STAT signal transduction pathways were identified in human tracheobronchial epithelial cells, with focus on type I interferon–dependent signaling and gene expression. We found that wild-type AdV infection inhibited IFN-α–induced expression of antiviral proteins in epithelial cells by blocking phosphorylation of the Stat1 and Stat2 transcription factors that are required for activation of type I interferon–dependent genes. These effects correlated with AdV-induced down-regulation of expression of the receptor-associated tyrosine kinase Jak1 through a decrease in Jak1 mRNA levels. Phosphorylation of Stat3 in response to IL-6 and oncostatin M was also lost in AdV-infected cells, indicating loss of epithelial cell responses to other cytokines that depend on Jak1. In contrast, IL-4– and IL-13–dependent phosphorylation of Stat6 was not affected during AdV infection, indicating that the virus modulates specific signaling pathways, as these Stat6-activating pathways can function independent of Jak1. Taken together, the results indicate that AdV down-regulates host epithelial cell Jak1 to assure inhibition of the antiviral effects of multiple mediators to subvert airway defense responses and establish a productive infection.
Keywords: JAK-STAT signaling, interferon, interleukin
CLINICAL RELEVANCE
This article reveals how adenovirus inhibits airway epithelial cell responses to multiple cytokines. These findings allow understanding of adenoviral subversion of host defense responses in the human airway.
Adenoviruses are nonenveloped, icosahedral, DNA viruses that cause a broad spectrum of infections (1). Several of the 51 recognized serotypes cause epidemic acute respiratory infections in humans that include pharyngitis, croup, bronchitis, pneumonia, and acute respiratory distress syndrome (2). Epithelial cells are the primary site of adenoviral replication in the airway, and the prototypic adenovirus type 5 (AdV) infects airway epithelia most efficiently from the basolateral cell surface by binding the coxsackie B and adenovirus type 2 and 5 receptor (CAR) (3). Adenoviral gene expression can be conceptually divided into two major overlapping phases based on the major functions of viral genes that are expressed in an ordered, temporal pattern during the replication cycle of the virus (4). During the early phase (the first 8–18 h of infection ending with viral DNA synthesis), specific adenoviral early genes (E1–4) sequester host cellular synthetic machinery for virus production while counteracting host cell defenses. During the late phase (12–36 h after the initiation of infection), adenoviral late genes (L1–5) direct the assembly of new virus and shut down nonessential host cell macromolecular synthesis. While adenoviral gene expression and replication have been relatively well studied, the mechanisms by which adenoviruses evade the multiple and complex antiviral defense systems in airway epithelia are incompletely understood.
A central feature of the host response to viral infection in the airway is activation of cellular genes that are important in innate and adaptive immunity by a potent group of mediators termed interferons. Type I interferons are produced by most nucleated cells primarily through multiple IFN-α and one IFN-β genes, and mediate host cell effects by binding to a specific receptor complex linked to a Janus family kinase-signal transducer and activator of transcription (JAK-STAT) signaling cascade (5, 6). Activation of the type I interferon–driven pathway is triggered by engagement and multimerization of the IFN-α receptor (IFNAR) by IFN-α or IFN-β, phosphorylation of IFNAR2-associated Jak1 and IFNAR1-associated Tyk2 tyrosine kinases, and then phosphorylation of IFNAR1 and IFNAR2 (7). Phosphorylation of the IFNAR1 chain of the IFN-α receptor results in recruitment, phosphorylation, and subsequent release of Stat2 and Stat1 from the receptor (8, 9). Activated Stat2 and Stat1 associate with interferon regulatory factor-9 to form the transcriptional activator complex IFN-stimulated gene factor 3, which translocates to the nucleus, binds specific DNA recognition sequences, and activates transcription of type I interferon–inducible genes (10). These genes include MxA, 2′,5′-oligoadenylate synthase, and protein kinase R (PKR), all of which have antiviral properties that are important for establishment of a host cell antiviral state (6).
The success of adenoviruses in establishing productive infections in human airway epithelia depends on the expression of viral gene products that mediate evasion of innate and acquired immune responses (11). Many of these products must be generated during active infection, after viral internalization, when adenoviral DNA is imported into the nucleus of host cells, thereby allowing viral gene expression to begin (12). In this report, we demonstrate that adenoviral evolution has generated a mechanism that efficiently blocks multiple antiviral systems, including type I interferon–dependent gene expression in human airway epithelial cells. We show that AdV infection results in a specific decrease in epithelial Jak1 expression with consequent blockade of type I interferon–dependent downstream signaling and gene expression. Furthermore, loss of Jak1 function in AdV-infected cells result in loss of epithelial responses to cytokines with JAK-STAT signal transduction pathways that require Jak1. Our results support the concept that inhibition of Jak1-dependent signaling results in modulation of immune response genes by adenoviruses, which may allow for evasion of the airway defense response and establishment of a productive infection.
MATERIALS AND METHODS
Airway Epithelial Cell Isolation, Culture, and Treatments
Human trachea and bronchial samples from multiple individuals without lung disease were obtained under a protocol approved by the University of Iowa Institutional Review Board. Airways were dissected from lung tissue, and primary human tracheobronchial epithelial (hTBE) cells from the surface of airway mucosa were isolated by enzymatic dissociation. Cells were cultured in Laboratory of Human Carcinogenesis (LHC)-8e medium on plates coated with collagen/albumin as described previously (13–15). Cells were treated with the following mediators: 1,000 U/ml recombinant human IFN-α2a (PBL Biomedical Laboratories, Piscataway, NJ) for 15 to 30 minutes to induce type I interferon JAK-STAT pathway activation and for 12 hours to induce antiviral gene mRNA and protein expression, 10 ng/ml of recombinant human interleukin(IL)-6 or oncostatin M (OSM; R&D Systems, Minneapolis, MN) for 30 minutes to induce Stat3 phosphorylation, or 10 ng/ml of recombinant human IL-4 or IL-13 (R&D Systems) for 30 minutes to induce Stat6 phosphorylation. In some experiments, cells were treated with control DMSO or the cell-permeable nonspecific proteasome inhibitor carbobenzoxy-L-leucyl-L-leucyl-L-leucinal (MG-132; Calbiochem, San Diego, CA). To assure reproducible and generalizable results, experiments were replicated two to five times using cells from different individuals, with key experiments performed on cells from at least three different individuals. Accordingly, hTBE cells obtained from 10 different individuals were used for this study.
Viral Preparation and Infection
Wild-type adenovirus type 5 (AdV) and replication-deficient AdV-d312 with deletion in early region 1A were gifts from S. Brody (Washington University, St. Louis, MO). Adenoviruses were propagated and titered as described previously (13, 14) using human 293 cells (CRL-1573; American Type Culture Collection, Manassas, VA). Viruses were incubated with hTBE cells for 4 to 24 hours in LHC-8e media at 37°C in 5% CO2 at a multiplicity of infection (MOI) of 0 to 30 plaque-forming units/cell. Previous studies demonstrated that a MOI of 10 results in infection of greater than or equal to 98% of epithelial cells without significant cytotoxicity (13, 14).
Primary Antibodies
Chicken polyclonal IgY against human MxA was from BacLab (Muttenz, Switzerland); rabbit polyclonal IgG 9172 against total human Stat1, rabbit polyclonal IgG 3072 against human PKR, rabbit polyclonal IgG 9171 against phosphorylated human Stat1, rabbit polyclonal IgG 9321 against phosphorylated human Tyk2, rabbit polyclonal IgG 3331 against phosphorylated human Jak1, rabbit IgG mAb clone 58E12 against phosphorylated human Stat3, rabbit polyclonal IgG 9132 against total human Stat3, rabbit polyclonal IgG 9361 against phosphorylated human Stat6, and rabbit polyclonal IgG 9362 against total human Stat6 were from Cell Signaling Technology (Beverly, MA); mouse IgG2a mAb clone AC-74 against human β-actin was from Sigma-Aldrich (St. Louis, MO); mouse IgG1 mAb clone 1/Stat1 against human Stat1 and mouse IgG2b mAb clone 73 against human total Jak1 were from BD Biosciences (San Jose, CA); rabbit polyclonal IgG 07–224 against phosphorylated human Stat2, rabbit polyclonal IgG 06–502 against total human Stat2, and rabbit polyclonal IgG 06–638 against total human Tyk2 were from Upstate Biotechnology (Lake Placid, NY); mouse IgG2A mAb clone M73 against adenoviral E1A was from Oncogene Science (Cambridge, MA); mouse IgG1 mAb clone H-11 against human IFNAR1, and rabbit polyclonal IgG sc-704 against human IFNAR2 were from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoblot Analysis
Epithelial expression levels of specific proteins were assessed by immunoblot analysis using specific antibodies as described previously (14, 16, 17). Whole cell protein extracts were prepared by lysis of cell monolayers in 50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, a protease inhibitor cocktail (Roche Bioscience, Palo Alto, CA), and a phosphatase inhibitor panel (Calbiochem, San Diego, CA). Protein concentrations were determined using a commercial kit with the Folin phenol reagent (18) (Bio-Rad Laboratories, Hercules, CA) and equal amounts of cell protein were subjected to SDS-PAGE in 5 to 7.5% polyacrylamide. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Hybond; Amersham Biosciences, Piscataway, NJ), exposed to 5% nonfat milk or 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween-20 to block nonspecific antigens, and then incubated with antibodies against a specific cellular protein. Primary antibody binding was detected using rabbit antichicken IgY (Sigma-Aldrich), or goat antirabbit or antimouse IgG (Santa Cruz) conjugated to horseradish peroxidase and an enhanced chemiluminescence detection system (Amersham Biosciences). Reprobing of membranes with a different primary antibody was done after washing in Restore buffer (Pierce, Rockford, IL) for 15 minutes at 37°C.
Real-Time Reverse Transcription PCR mRNA Analysis
Total RNA was isolated using a commercial spin column isolation kit (Stratagene, La Jolla, CA), and 0.5 μg was reverse transcribed using a commercial kit (Ambion, Austin, TX). Equal aliquots of cDNA were subjected to PCR using an iCycler iQ Fluorescence Thermocycler (Bio-Rad) with SYBR Green I DNA dye (Molecular Probes, Eugene, OR) and iTaq DNA Polymerase (Bio-Rad). PCR conditions included denaturation at 95°C for 3 minutes, and then 45 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, followed by melting curve analysis. The following primers were used. (1) MxA forward 5′-GAAGCCCTGCAGAGAGAGAA-3′, and reverse 5′-AACTCGTGTCGGAGTCTGGT-3′; (2) Stat1 forward 5′-TGGTGAAATTGCAAGAGCTG-3′, and reverse 5′-AGAGGTCGTCTCGAGGTCAA-3′; (3) PKR forward 5′-TGCCTAATTCAGGACCTCCA-3′, and reverse 5′-GCCAATTGTTTTGCTTCCTG-3′; (4) Jak1 forward 5′-AGTGCCCTGAGCTACTTGGA-3′, and reverse 5′-AGGTCAGCCAGCTCCTTACA-3′; (5) Tyk2 forward 5′-CTGCAGCTGGTCATGGAGTA-3′, and reverse 5′-CAGCAGCTCATACAGGGTCA-3′; and (6) human hypoxanthine phosphoribosyltransferase (HPRT) forward 5′-TTGGAAAGGGTGTTTATTCTTC-3′, and reverse 5′-TCCCCTGTTGACTGGTCATT-3′. Fluorescence data was captured during annealing reactions, and specificity of amplification was confirmed using melting curve analysis. Data were collected and recorded by iCycler iQ software (Bio-Rad) and initially determined as a function of threshold cycle (Ct), the cycle at which the fluorescence intensity in a given reaction tube rises above background (calculated as 10 times the mean standard deviation of fluorescence in all wells over the baseline cycles). Levels of mRNA are expressed relative to control HPRT levels, calculated as 2ΔCt.
Immunofluorescence Microscopy
Cellular localization of Stat1 was detected in hTBE cells grown on a chamber slide system (Lab-Tek; Nalge Nunc International, Napersville, IL), similar to methodology described previously (13, 15, 16). Epithelial cells were fixed and permeabilized in 100% methanol, exposed to 2% fishgel (Sigma-Aldrich) to block nonspecific antibody binding, and incubated with rabbit polyclonal IgG against phosphorylated human Stat1. Antibody binding was detected by exposure to goat anti-rabbit IgG conjugated to Alexa Fluor 568 (Molecular Probes). Slides were mounted using Gel/Mount (Biomeda Corporation, Foster City, CA), and images were acquired using a fluorescence microscope linked to a digital camera system and interfaced with Leica Application Suite software version 2.4.0 (Leica Microsystems, Cambridge, UK).
Enzyme-Linked Immunoassay
IFN-β concentrations in cell culture media were determined using a commercial sandwich enzyme-linked immunosorbent assay kit (PBL Biomedical laboratories, Piscataway, NJ). This kit is highly selective for IFN-β and has a sensitivity of more than 250 pg/ml according to the manufacturer.
Flow Cytometry
Type I interferon receptor levels on the surface of hTBE cells were determined by fluorescence-activated flow cytometry as described previously (14, 19), with minor modifications. Cell monolayers were harvested in PBS with 1 mM EDTA, and cells were suspended in PBS containing 10% heat-inactivated FBS and 20 μg/ml of mouse mAb against human IFNAR1, rabbit polyclonal IgG against human IFNAR2, or matched control antibody. Primary antibody binding was detected using goat antimouse or antirabbit IgG conjugated to Alexa Fluor 488 (Molecular Probes), followed by analysis on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with a 15-mW argon-ion laser. Data were acquired using CELLQuest version 3.3 software and analyzed using CELLQuest version 4.0 software (Becton Dickinson) using 256-channel resolution over a 4-decade log range setting.
Immunoprecipitation
Tyrosine phosphorylation of Jak1 was determined by immunoprecipitation of the protein from 1 mg of hTBE cell extracts that were prepared and concentrations determined as for immunoblot analysis. Cell protein extracts were first exposed to Protein G–conjugated to agarose beads (Sigma-Aldrich) to preclear nonspecific interacting proteins. Jak1 was precipitated from extracts using specific primary mouse mAb and Protein G–conjugated agarose beads, and detected by immunoblot analysis using Ab against phosphorylated or total Jak1 as described previously (14).
Statistical Analysis
Real-time reverse transcription PCR mRNA analysis was analyzed for statistical significance using a one-way ANOVA for a factorial experimental design. The multicomparison significance level for the ANOVA was 0.05. If significance was achieved by one-way analysis, post-ANOVA comparison of means was performed using Bonferroni's multiple comparison test (20).
RESULTS
AdV Infection Inhibits IFN-α–Dependent Gene Expression
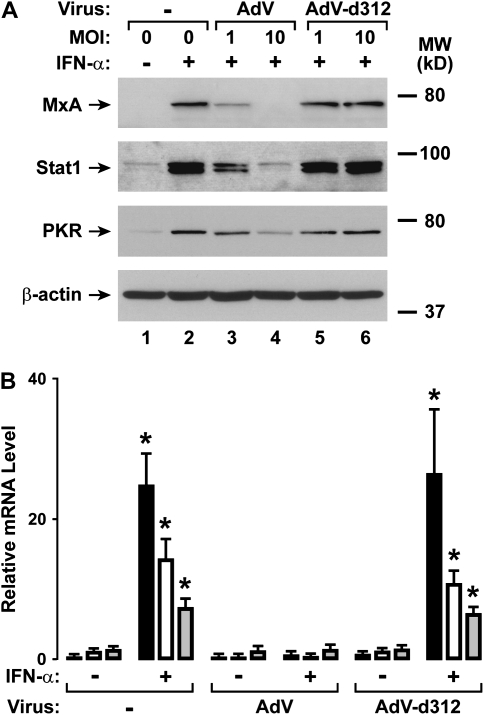
Human airway epithelial cells under uninfected conditions respond to type I interferons by expression of antiviral genes (16). To evaluate AdV effects on airway epithelial responses, we initially examined the expression of three well-characterized antiviral genes that are induced by type I interferons. MxA is a dynamin superfamily transport GTPase that exhibits antiviral activity against several RNA viruses (21). Stat1 is activated by both the type I (IFN-α and -β) and II (IFN-γ) interferon signaling cascades, but the overall expression levels of this transcription factor in the unactivated state may modify cellular responses to interferons (22, 23). PKR is a double-stranded RNA-activated serine-threonine kinase that inhibits protein synthesis and mediates other cellular responses to viral infection or inflammatory stimuli (21). We found that initial AdV infection inhibited subsequent IFN-α–induced MxA, Stat1, and PKR protein expression in hTBE cells, while infection by replication-deficient AdV-d312 did not have this capacity (Figure 1A). Similarly, AdV infection inhibited IFN-α–induced MxA, Stat1, and PKR mRNA expression in hTBE cells, while AdV-d312 had minimal effect (Figure 1B). AdV did not affect basal levels of expression of these antiviral genes. We have demonstrated previously that other signaling pathways remained functional during AdV infection, supporting our related finding that AdV did not cause significant epithelial cell cytotoxicity under these conditions (14). Taken together, the results were consistent with the hypothesis that AdV inhibits antiviral gene expression through effects on type I interferon signal transduction in airway epithelial cells. Therefore, subsequent experiments were directed at examining viral mechanisms for specifically modifying the type I interferon JAK-STAT pathway for antiviral gene expression.
Figure 1.
Adenovirus type V (AdV) infection inhibits IFN-α–dependent gene expression. (A) MxA, total Stat1, and protein kinase R (PKR) protein levels were assessed using immunoblot analysis of extracts from primary human tracheobronchial epithelial (hTBE) cell monolayers that were left uninfected or were infected with wild-type AdV or replication-deficient AdV-d312 at the indicated multiplicity of infection (MOI) for 6 hours, followed by incubation without or with IFN-α for 12 hours. The positions of MxA, PKR, total Stat1, and β-actin (to verify equivalency of protein isolation and loading) are indicated by arrows. (B) MxA (solid bars), total Stat1 (open bars), and PKR (shaded bars) mRNA levels were assessed using real-time RT-PCR analysis of total RNA from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for 6 hours, followed by incubation without or with IFN-α for 12 hours. Values are expressed as mean relative mRNA level compared with control hypoxanthine phosphoribosyltransferase mRNA ± SD (n = 3), and a significant increase from levels in cells not treated with IFN-α for each viral infection condition is indicated by an asterisk. Results in A and B are representative of three experiments.
AdV Infection Rapidly Inhibits IFN-α–Induced STAT Phosphorylation
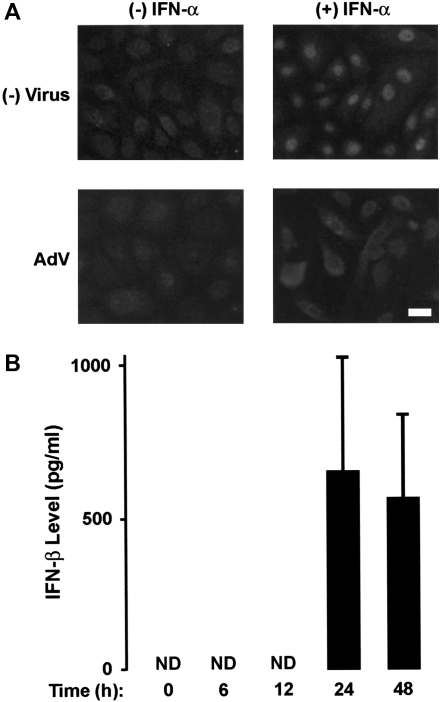
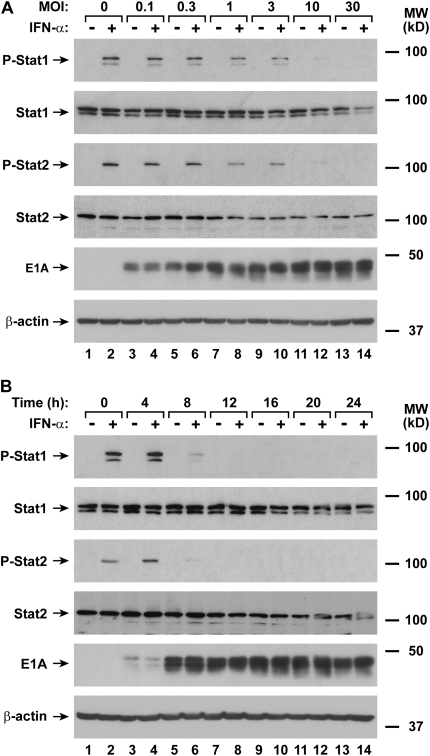
Type I interferon–induced transcription of antiviral genes in airway epithelial cells requires nuclear translocation of Stat1 driven by tyrosine phosphorylation of this transcription factor (10). AdV infection resulted in inhibition of IFN-α–dependent nuclear localization of phosphorylated Stat1 in hTBE cells, suggesting that viral infection caused a defect in Stat1 activation (Figure 2A). Epithelial cells also produce variable amounts of IFN-β in response to AdV infection, but detection of interferon release required greater than 12 hours of infection (Figure 2B). To confirm inhibition of Stat function by AdV and assess the timing of this effect, immunoblot analysis was used to detect type I interferon–activated STAT proteins in hTBE cells. AdV infection at an inoculum level that inhibited antiviral gene expression also inhibited IFN-α–induced phosphorylation of Stat1 and Stat2 (Figure 3A). At an MOI of 10, minimal Stat1 and Stat2 phosphorylation was detected, correlating with previous results indicating infection of greater than or equal to 98% of epithelial cells at this inoculum (13, 14). Using hTBE cells infected with this inoculum level for varying durations, we found that minimal IFN-α–induced Stat1 and Stat2 phosphorylation was detected after 8 to 12 hours of AdV infection, and this effect persisted for at least 24 hours (Figure 3B). Total Stat1 and Stat2 levels also decreased after AdV infection, but these effects were relatively small, inconsistent, and occurred both at higher AdV inoculum levels and later than AdV inhibition of STAT activation. The decline in detectable Stat1 and Stat2 activation correlated temporally with adenoviral E1A protein expression, and occurred before IFN-β release by the epithelial cells. Thus, it is likely that AdV inhibition of type I interferon–dependent activation of Stat1 and Stat2 allows viral subversion of antiviral defenses beginning in the early stages of viral infection and replication.
Figure 2.
AdV infection inhibits IFN-α–induced nuclear translocation of phosphorylated Stat1, but causes type I interferon release. (A) Phosphorylated Stat1 cell location was determined using immunofluorescence staining of hTBE cell monolayers that were left uninfected or were infected with AdV at MOI 10 for 18 hours, followed by incubation without or with IFN-α for 30 minutes. Scale bar, 20 μm. (B) Released IFN-β protein levels were determined using an enzyme-linked immunoassay with media from hTBE cell monolayers that were left uninfected or infected with AdV at MOI 10 for the indicated durations. Values are expressed as mean level ± SD (n = 3); ND, none detected. Results in A and B are representative of four to five experiments.
Figure 3.
AdV infection rapidly inhibits IFN-α–induced STAT phosphorylation. (A) Phosphorylated and total Stat1 and Stat2, adenoviral E1A, and β-actin protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were left uninfected or were infected with AdV at the indicated MOI for 20 hours, followed by incubation without or with IFN-α for 15 minutes. The positions of phosphorylated and total Stat1 and Stat2, E1A, and β-actin are indicated by arrows. (B) Phosphorylated and total Stat1 and Stat2, E1A, and β-actin protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were left uninfected or were infected with AdV at MOI 10 for the indicated duration, followed by incubation without or with IFN-α for 15 minutes. Results in A and B are representative of three to four experiments.
AdV Infection Inhibits IFN-α Signaling Inside Cells
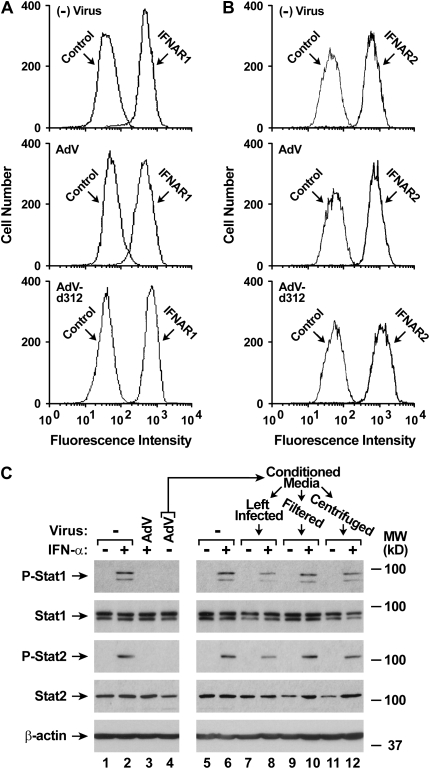
Because AdV infection appeared to inhibit the IFN-α signaling cascade at or before Stat1 phosphorylation, we examined the effects of AdV on the upstream type I interferon signal transduction complex. Using flow cytometry, no clear differences were observed in the expression of the two cell surface components of the type I interferon receptor, IFNAR1 (Figure 4A) and IFNAR2 (Figure 4B), after AdV infection. We also questioned whether cells infected with AdV might release a soluble extracellular factor that could inhibit type I interferon signaling. To assess this possibility, media from hTBE cells infected with AdV for 24 hours was transferred to uninfected epithelial cells, and IFN-α–dependent Stat1 and Stat2 activation in cells exposed to the transferred media was determined. In these experiments, no evidence of release of a soluble extracellular inhibitor of interferon signaling was found (Figure 4C). The results suggest that AdV affects IFN-α–dependent gene expression through modulation of the type I interferon JAK-STAT pathway inside infected epithelial cells.
Figure 4.
AdV infection inhibits IFN-α–induced signal transduction inside cells. (A) IFNAR1 surface protein levels were determined using flow cytometry with hTBE cell monolayers that were left uninfected or infected with AdV or AdV-d312 at MOI 10 for 20 hours. (B) IFNAR2 surface protein levels were determined using flow cytometry with hTBE cell monolayers that were left uninfected or infected with AdV or AdV-d312 at MOI 10 for 20 hours. (C) Potential release of an extracellular inhibitor of IFN-α–dependent Stat1 phosphorylation was assessed using immunoblot analysis with extracts from two sets of hTBE cell monolayers. In the first set, extracts were isolated from monolayers that were initially left uninfected or were infected with AdV at MOI 10 for 24 hours, followed by incubation without or with IFN-α for 15 minutes. Conditioned media from monolayers infected with AdV but not incubated with IFN-α (the monolayers used for lane 4) were harvested after completion of the incubation period and residual viral particles in aliquots were removed by filtration through a 100-kD pore size membrane (Filtered) or centrifugation at 175,000 × g for 18 hours (Centrifuged). Conditioned media aliquots were then placed on a second set of uninfected hTBE cell monolayers, which were incubated with IFN-α for 15 minutes, followed by isolation of cellular proteins for immunoblot analysis. Results in A–C are representative of two experiments.
AdV Infection Decreased Jak1 Expression
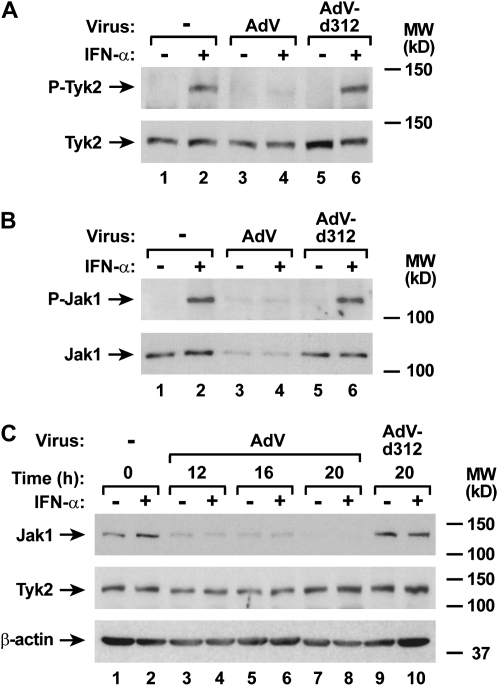
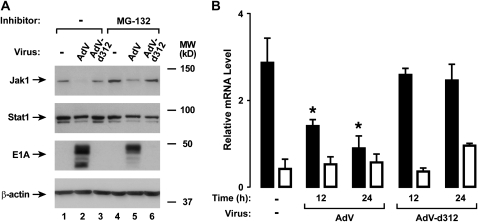
To complete evaluation of potential adenoviral effects on components of the type I interferon receptor complex, we examined expression and activation of the JAK kinases that link receptor activation to STAT protein phosphorylation. AdV infection did not significantly affect epithelial cell Tyk2 expression, but did inhibit subsequent IFN-α–dependent phosphorylation of Tyk2 (Figure 5A). In contrast, experiments in which Jak1 was isolated from airway epithelial cell lysates using immunoprecipitation suggested there was a marked decrease in Jak1 levels in AdV-infected cells, which correlated with loss of detectable phosphorylated Jak1 in the same samples (Figure 5B). Decreased Jak1 expression in AdV-infected cells was confirmed using immunoblot analysis with hTBE cells infected for 12 hours, and this effect persisted through 20 hours of infection (Figure 5C). Although proteasome-dependent degradation of signaling proteins is a common mechanism for viral modulation of signaling proteins, pretreatment of hTBE cells with MG-132 under conditions previously shown to inhibit epithelial cell proteasome function did not clearly alter AdV effects on Jak1 levels (Figure 6A) (16, 17, 24). However, decreased Jak1 mRNA was detected in epithelial cells infected with AdV, indicating that AdV modulated Jak1 gene transcription or mRNA stability to regulate its level (Figure 6B). In comparison, AdV infection had smaller and inconsistent effects on Stat1 and Stat2 mRNA levels (unpublished observation) and no clear effect on Tyk2 mRNA level.
Figure 5.
AdV infection decreases Jak1 protein expression. (A) Phosphorylated and total Tyk2 protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for 20 hours, followed by incubation without or with IFN-α for 15 minutes. The positions of phosphorylated and total Tyk2 are indicated by arrows. (B) Phosphorylated and total Jak1 protein levels were assessed by immunoprecipitation of Jak1 in extracts from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for 20 hours, followed by incubation without or with IFN-α for 15 minutes. For each condition, anti-Jak1 antibody and protein G–agarose were mixed with cell extract to generate an immune complex that was then subjected to immunoblot analysis using Ab against phosphorylated or total Jak1. The positions of phosphorylated and total Jak1 are indicated by arrows. (C) Total Jak1, Tyk2, and β-actin protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for the indicated duration, followed by incubation without or with IFN-α for 15 minutes. Results in A–C are representative of three to four experiments.
Figure 6.
AdV infection down-regulates Jak1 mRNA expression. (A) Total Jak1 and Stat1, E1A, and β-actin protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were pretreated with control DMSO or 2.1 μM MG-132. Cells were then left uninfected or were infected with AdV or AdV-d312 at MOI 10 for 20 hours. (B) Jak1 (solid bars) and Tyk2 (open bars) mRNA levels were assessed using real-time RT-PCR analysis of total RNA from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for the indicated duration. Values are expressed as mean relative mRNA level compared with control hypoxanthine phosphoribosyltransferase mRNA ± SD (n = 3), and a significant decrease from levels in uninfected cells is indicated by an asterisk. Results in A and B are representative of two experiments.
AdV Infection Specifically Inhibits Jak1-Dependent Signaling Pathways
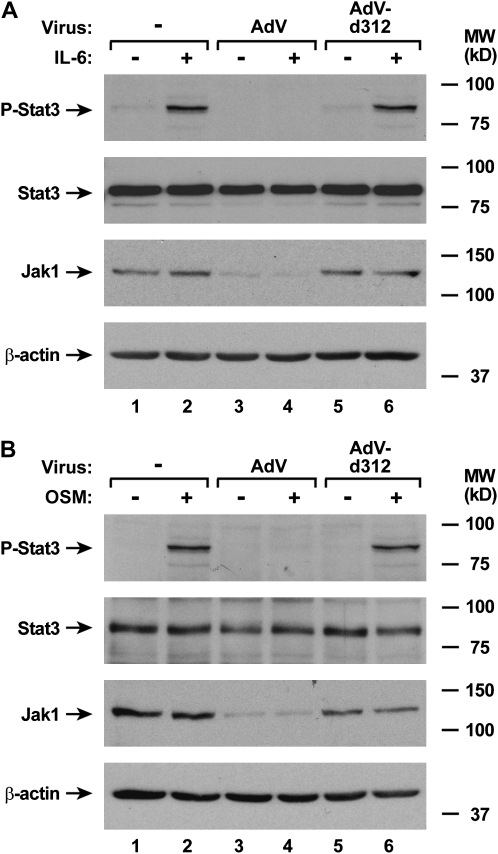
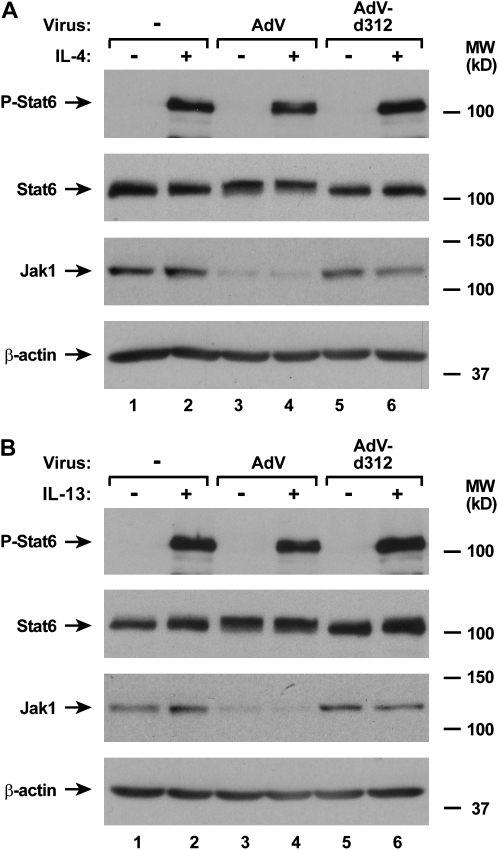
Due to the well-established requirement for Jak1 in signal transduction pathways for multiple cytokines, combined with our observation of adenoviral down-regulation of Jak1 expression, we reasoned that other Jak1-dependent signaling pathways would be affected by AdV infection (25, 26). To test this hypothesis, we examined AdV effects on activation of STAT protein in response to specific cytokines. We found that AdV had the capacity to block IL-6– and OSM-dependent phosphorylation of Stat3 in airway epithelial cells (Figure 7A and 7B). This correlates with the requirement for Jak1 for signal transduction in response to these cytokines (27). Although Jak1 has been reported to participate in IL-4– and IL-13–dependent signal transduction, it appears that some cells respond to these cytokines without Jak1 function (28–30). Consistent with these findings, we confirmed the previous report that AdV-infected epithelial cells maintain the capacity for IL-4–dependent activation of Stat6 (Figure 8A) (14). In addition, IL-13–induced phosphorylation of Stat6 was unchanged by AdV infection (Figure 8B). Taken together, it appears that multiple, but not all, JAK-STAT signaling pathways are inhibited in airway epithelial cells by adenoviral infection.
Figure 7.
AdV infection specifically inhibits Jak1-dependent signaling pathways. Phosphorylated and total Stat3, total Jak1, and β-actin protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for 20 hours, followed by incubation without or with IL-6 (A) or OSM (B) for 15 minutes. The positions of phosphorylated and total Stat3, total Jak1, and β-actin are indicated by arrows. Results in A and B are representative of two to four experiments.
Figure 8.
AdV infection does not inhibit all Jak-STAT signaling pathways. Phosphorylated and total Stat6, total Jak1, and β-actin protein levels were assessed using immunoblot analysis of extracts from hTBE cell monolayers that were left uninfected or were infected with AdV or AdV-d312 at MOI 10 for 20 hours, followed by incubation without or with IL-4 (A) or IL-13 (B) for 15 minutes. The positions of phosphorylated and total Stat6, total Jak1, and β-actin are indicated by arrows. Results in A and B are representative of three experiments.
DISCUSSION
While epithelial cells are often targeted for respiratory virus infection in the airway, these cells also actively participate in the antiviral response by responding to interferons and other mediators through induction of a host cell antiviral state, participation in immune cell recognition of viruses, recruitment and activation of leukocytes, and other effects that are required for effective antiviral defense. Because interferon-dependent immunity is critical for limiting and clearing viral infections, it has been proposed that a prerequisite for successful viral invasion and replication in host cells is a mechanism for avoiding the effects of interferons (21, 31). Our results indicate that AdV inhibits the type I interferon JAK-STAT signaling pathway in human airway epithelial cells, resulting in reduced antiviral gene expression. AdV effects on type I interferon signaling appear to be mediated through down-regulation of Jak1 mRNA and protein expression. Precedent for this viral mechanism has been established previously, as human cytomegalovirus also appears to decrease Jak1 levels in human endothelial cells and fibroblasts, although this appears to occur through virus-induced Jak1 protein degradation (32). AdV effects on Jak1 and type I interferon signaling occur within the first 12 hours of infection, and before epithelial cell IFN-β release. Although other cells in the airway respond to AdV by type I interferon release, it is likely that initial release is initiated by epithelial cells infected by the virus (33, 34). Interestingly, since Jak1 is required for several signaling pathways, AdV has the capacity through this mechanism to modulate the epithelial response to other soluble mediators in the airway. Therefore, AdV effects on these signaling pathways appear during initial airway infection, likely being established before type I interferon and other Jak1-dependent antiviral systems have been activated by virus-induced mediator release.
The capacity to inhibit interferon effects in host cells has coevolved in several pathogens, reflecting the importance of interferons for antiviral defense, and this remains an area of intense research (6). Viruses use several targeted strategies to modify antiviral effects of interferons. These strategies can involve suppression of interferon production, release of soluble inhibitory factors, blockade of expression of function of interferon signaling cascade components, or direct modulation of specific interferon-inducible antiviral proteins (6, 21). For adenoviruses, elevated interferon levels may be encountered in the airway during infection, and thus adenoviral evolution has resulted in multiple potent strategies to inhibit interferon-dependent signal transduction (34–36). AdV expresses mechanisms to inhibit type II interferon signaling that include: (1) inhibition of type II interferon receptor complex activation through decrease in the level of the IFN-γ receptor 2 component of the IFN-γ receptor; (2) direct interaction between adenoviral E1A oncoprotein and Stat1; and (3) direct interaction between E1A and the Stat1 coactivator proteins CBP and p300 (11, 13, 14). For type I interferons, AdV also uses multiple mechanisms to inhibit the type I interferon–dependent JAK-STAT pathway, which include decreasing Jak1 expression (as described in this article), as well as direct E1A effects on Stat1, CBP, and p300. Taken together, this information suggests that direct E1A effects on transcription factors are insufficient, and additional mechanisms that block JAK-STAT signaling are required for subversion of these antiviral systems. Effects on interferon-activated JAK-STAT pathways may be needed later in the viral replication cycle to assist in diversion of host cellular energy toward production of viral progeny or may be required when adenoviral early gene (i.e., E1A) expression and its direct immune inhibitory effects are decreasing (13, 14). The ability of adenoviruses to inhibit interferon-inducible gene expression and function by multiple mechanisms with a distinct temporal relationship is presumably designed to assure a dampened antiviral response during all phases of the viral replication cycle.
Like most large mammalian DNA viruses, adenoviruses express several gene products that modulate host antiviral responses independent of effects on interferon signal transduction. These gene products include a 19-kD glycoprotein encoded in the adenoviral E3 region (E3-19K) that inhibits constitutive and IFN-γ–inducible major histocompatibility complex (MHC) class I molecule transport to the cell surface for immune cell recognition (13, 37, 38). In addition, a virally encoded 159 nucleotide RNA called VA-RNAI binds to constitutive and IFN-α/β–inducible PKR and prevents its shutdown of protein synthesis that occurs by phosphorylation of eukaryotic initiation factor 2 (39). Multiple proteins encoded in adenoviral early region genes inhibit apoptosis in infected cells through effects that include acting as a Bcl-2 functional homolog (E1B-19K), inactivating p53 (E1B-55K, E4-orf6), preventing TNFR1 internalization (E3-14.7K), interacting with caspase-8, FIP-3, and possibly other proapoptosis proteins (E3-14.7K), or decreasing cell surface EGF receptor, Fas (CD95), and other TNF receptor family molecules (E3-10.4K, E3-14.5K) (40–48). Furthermore, the E3-10.4K and E3-14.5K complex inhibits TNF-induced translocation of cytosolic phospholipase A2, decreasing inflammatory eicosanoid synthesis and TNF-induced infected cell death (49). Our report indicates that AdV also inhibits infected cell responses to IL-6 and OSM, and likely other Jak1-dependent epithelial cell responses. Thus, a significant portion of the AdV genome is devoted to expression of factors that evade host defenses.
Jak1 is a member of the Janus family of protein tyrosine kinases that are characterized by the presence of a second phosphotransferase-related or pseudokinase domain near the functional kinase domain (25). It is a relatively large, receptor-associated, ubiquitously expressed, phosphoprotein involved in the signal transduction of type I and type II interferons, IL-10, and granulocyte colony-stimulating factor, as well as cytokines whose receptors share the γc (including IL-2, IL-4, IL-7, IL-9, IL-15, IL-21) or gp130 (including IL-6, IL-11, OSM, ciliary neurotrophic factor, leukemia inhibitory factor) subunits (26, 50). Jak1 is obligatory for phosphorylation and activation of Tyk2 in the type I interferon receptor complex, explaining the loss of Tyk2 phosphorylation (and other downstream activation events) observed after AdV infection (51). Previous studies using primary hTBE cells isolated from a small number of individuals had shown that AdV infection led to small decreases in Jak1 levels, leading us to conclude that AdV did not substantially affect Jak1 expression in these cells (14). In subsequent experiments using additional primary hTBE cells isolates, it became evident that primary airway epithelial cells from the majority of individuals demonstrate a large decrease in Jak1 expression after AdV infection, and this decrease leads to a loss in the function of multiple Jak1-dependent pathways. This is a specific effect of adenoviral infection in airway epithelial cells, as infection of airway epithelial cells at the inoculum and for durations used in these studies do not result in detectable cytotoxicity (14). In the present study, we also examined several Jak1-independent JAK-STAT pathways, and found that some (e.g., IL-12) do not function in airway epithelial cells (unpublished observation). However, Stat6 activation after IL-4 and IL-13 treatment of airway epithelial cells was maintained after AdV infection. This fits with the observation that many cells respond to IL-4 and IL-13 independent of Jak1, and illustrates that not all JAK-STAT pathways in airway epithelial cells are affected by AdV infection (28–30).
Components of the pulmonary response to adenovirus include: (1) an antigen-nonspecific, cytokine-dependent response resulting in acute inflammation, which may have a neurogenic component and cause systemic toxicity; (2) an MHC class I-restricted, cytotoxic (CD8+) T lymphocyte-dependent response directed at cells expressing viral antigens, resulting in chronic inflammation and infected cell clearance; and (3) a helper (CD4+) T lymphocyte-dependent response directed at adenoviral capsid proteins, resulting in the production of neutralizing antibodies (11). It is not surprising that the host response to adenoviruses occurs through multiple, interrelated mechanisms because a multicomponent system can adapt for host defense against a variety of pathogens. However, by directly reducing Jak1 levels, AdV effectively blocks multiple components of this airway antiviral response. This includes responses to cytokines that participate in the acute response to infection such as IL-6, as well as pathways that activate immune recognition and cellular antiviral responses such as interferons (1).
Our results indicating that AdV infection leads to a decrease in Jak1 mRNA expression in human airway epithelial cells suggest that the virus modulates Jak1 levels by effects on Jak1 gene transcription or mRNA stability. Similar mechanistic possibilities can be proposed for AdV effect on the IFN-γ receptor 2 component of the IFN-γ receptor (14), and we speculate that AdV likely affects the transcription rates of these genes. Precedent for this mechanism may be found in adenoviral E1A effects on the function of several transcription factors (13). Regulation of the human Jak1 gene has not been well characterized, but the 5′-flanking region of the Jak1 gene contains putative binding sites for transcription factors that could be modulated by E1A (unpublished observation). Identification of the mechanisms through which AdV decreases Jak1 expression will require additional study.
We speculate that AdV evolution was driven to generate a mechanism to decrease Jak1 levels and thereby inhibit interferon signaling to counter the higher airway levels of type I interferons and possibly other cytokines that are commonly induced by the virus and that could inhibit viral replication. Although it is efficient to inhibit multiple antiviral pathways though modulation of a single protein inside infected cells, the evolutionary mechanisms and advantages of down-regulation of Jak1 versus other JAK-STAT pathway components are unclear. A better understanding of viral mechanisms for altering interferon-dependent immune responses may allow for the development of therapeutic strategies that block this viral capacity during infection of airway epithelium.
Acknowledgments
The authors gratefully acknowledge the Cell Culture and Tissue Core Facility of the University of Iowa Center for Gene Therapy for procurement of airway epithelial cells, S. Brody for generous gifts of adenoviruses, and M. McCormick and J. Zabner for helpful discussion.
This research was supported by grants from the National Heart, Lung, and Blood Institute (grant number R01HL075559 to D.C.L.), the Cystic Fibrosis Foundation, and the American Lung Association. The University of Iowa Center for Gene Therapy is supported by grants from the National Institutes of Health and the Cystic Fibrosis Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0158OC on July 19, 2007
Conflict of Interest Statement: DL received $5,000 in 2006 from Altana Pharma as a research grant for participating in a multicenter clinical trial. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Nazir SA, Metcalf JP. Innate immune response to adenovirus. J Investig Med 2005;53:292–304. [DOI] [PubMed] [Google Scholar]
- 2.Klinger JR, Sanchez MP, Curtin LA, Durkin M, Matyas B. Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med 1998;157:645–649. [DOI] [PubMed] [Google Scholar]
- 3.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 1999;274:10219–10226. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg HS, Prince GA. The molecular basis of adenovirus pathogenesis. Infect Agents Dis 1994;3:1–8. [PubMed] [Google Scholar]
- 5.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-α/β revisited. Nat Rev 2001;2:378–386. [DOI] [PubMed] [Google Scholar]
- 6.Katze MG, He Y, Gale MJ. Viruses and interferon: a fight for supremacy. Nat Rev Immunol 2002;2:675–687. [DOI] [PubMed] [Google Scholar]
- 7.Platanias L, Uddin S, Colamonici OR. Tyrosine phosphorylation of the α and β subunits of the type I interferon receptor. J Biol Chem 1994;269:17761–17764. [PubMed] [Google Scholar]
- 8.Leung S, Qureshi SA, Kerr IM, Darnell JE Jr, Stark GR. Role of Stat2 in the alpha interferon signaling pathway. Mol Cell Biol 1995;15:1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi SA, Leung S, Kerr IM, Stark GR, Darnell JE Jr. Function of Stat2 protein in transcriptional activation by alpha interferon. Mol Cell Biol 1996;16:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath CM, Stark GR, Kerr IM, Darnell JE Jr. Interactions between STAT and non-STAT proteins in the interferon-stimulated gene factor 3 transcription complex. Mol Cell Biol 1996;16:6957–6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Look DC, Brody SL. Engineering viral vectors to subvert the airway defense response. Am J Respir Cell Mol Biol 1999;20:1103–1106. [DOI] [PubMed] [Google Scholar]
- 12.Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol 1999;144:657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look DC, Roswit WT, Frick AG, Gris-Alevy Y, Dickhaus DM, Walter MJ, Holtzman MJ. Direct suppression of Stat1 function during adenoviral infection. Immunity 1998;9:871–880. [DOI] [PubMed] [Google Scholar]
- 14.Joseph TD, Look DC. Specific inhibition of interferon signal transduction pathways by adenoviral infection. J Biol Chem 2001;276:47136–47142. [DOI] [PubMed] [Google Scholar]
- 15.Aldallal N, McNaughton EE, Manzel LJ, Richards AM, Zabner J, Ferkol TW, Look DC. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am J Respir Crit Care Med 2002;166:1248–1256. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy M, Shi L, Monick MM, Hunninghake GW, Look DC. Specific inhibition of type I interferon signal transduction by respiratory syncytial virus. Am J Respir Cell Mol Biol 2004;30:893–900. [DOI] [PubMed] [Google Scholar]
- 17.Ramaswamy M, Shi L, Varga SM, Barik S, Behlke MA, Look DC. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology 2006;344:328–339. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Look DC, Keller BT, Rapp SR, Holtzman MJ. Selective induction of intercellular adhesion molecule-1 by interferon-γ in human airway epithelial cells. Am J Physiol 1992;263:L79–L87. [DOI] [PubMed] [Google Scholar]
- 20.Hassard TH. Understanding biostatistics. St. Louis: Mosby-Year Book; 1991.
- 21.Sen GC. Viruses and interferons. Annu Rev Microbiol 2001;55:255–281. [DOI] [PubMed] [Google Scholar]
- 22.Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 2006;107:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam NW, Ishii T, Li S, Wong AH, Cuddihy AR, Koromilas AE. Upregulation of STAT1 protein in cells lacking or expressing mutants of the double-stranded RNA-dependent protein kinase PKR. Eur J Biochem 1999;262:149–154. [DOI] [PubMed] [Google Scholar]
- 24.Young DF, Didcock L, Goodbourn S, Randall RE. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology 2000;269:383–390. [DOI] [PubMed] [Google Scholar]
- 25.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene 2000;19:5662–5679. [DOI] [PubMed] [Google Scholar]
- 26.Yamaoka K, Saharinen P, Pesu M, Holt VET, Silvennoinen O, O'Shea JJ. The Janus kinases (Jaks). Genome Biol 2004;5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark GR, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J 1995;14:1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata T, Obiri NI, Puri RK. Human ovarian carcinoma cell lines express IL-4 and IL-13 receptors: comparison between IL-4- and IL-13-induced signal transduction. Int J Cancer 1997;70:230–240. [DOI] [PubMed] [Google Scholar]
- 29.Murata T, Husain SR, Mohri H, Puri RK. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol 1998;10:1103–1110. [DOI] [PubMed] [Google Scholar]
- 30.Roy B, Cathcart MK. Induction of 15-lipoxygenase expression by IL-13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J Biol Chem 1998;273:32023–32029. [DOI] [PubMed] [Google Scholar]
- 31.Barnard P, McMillan NAJ. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-α. Virology 1999;259:305–313. [DOI] [PubMed] [Google Scholar]
- 32.Miller DM, Rahill BM, Boss JM, Lairmore MD, Durbin JE, Waldman WJ, Sedmak DD. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med 1998;187:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitkaranta A, Hovi T. Induction of interferon in human leukocyte cultures by natural and pathogenic respiratory viruses. J Interferon Res 1993;13:423–426. [DOI] [PubMed] [Google Scholar]
- 34.Alsharifi M, Regner M, Blanden R, Lobigs M, Lee E, Koskinen A, Mullbacher A. Exhaustion of type I interferon response following an acute viral infection. J Immunol 2006;177:3235–3241. [DOI] [PubMed] [Google Scholar]
- 35.Xing Z, Zganiacz A, Wang J, Divangahi M, Nawaz F. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-gamma release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J Immunol 2000;164:2575–2584. [DOI] [PubMed] [Google Scholar]
- 36.Huarte E, Larrea E, Hernandez-Alcoceba R, Alfaro C, Murillo O, Arina A, Tirapu I, Azpilicueta A, Hervas-Stubbs S, Bortolanza S, et al. Recombinant adenoviral vectors turn on the type I interferon system without inhibition of transgene expression and viral replication. Mol Ther 2006;14:129–138. [DOI] [PubMed] [Google Scholar]
- 37.Burgert HG, Maryanski JL, Kvist S. “E3/19K” protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci USA 1987;84:1356–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox JH, Bennink JR, Yewdell JW. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its abilty to block antigen presentation. J Exp Med 1991;174:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews MB, Shenk T. Adenovirus virus-associated RNA and translation control. J Virol 1991;65:5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tollefson AE, Stewart AR, Yei SP, Saha SK, Wold WS. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus form a complex and function together to down-regulate the epidermal growth factor receptor. J Virol 1991;65:3095–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yew PR, Berk AJ. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature 1992;357:82–85. [DOI] [PubMed] [Google Scholar]
- 42.Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl Acad Sci USA 1996;93:11295–11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Branton PE, Yang E, Korsmeyer SJ, Shore GC. Adenovirus E1b 19-kDa death suppressor protein interacts with Bax but not with Bad. J Biol Chem 1996;271:24221–24225. [DOI] [PubMed] [Google Scholar]
- 44.Shisler J, Yang C, Walter B, Ware CF, Gooding LR. The adenovirus E3–10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol 1997;71:8299–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P, Tian J, Kovesdi I, Bruder JT. Interaction of the adenovirus 14.7 kDa protein with FLICE inhibits Fas ligand-induced apoptosis. J Biol Chem 1998;273:5815–5820. [DOI] [PubMed] [Google Scholar]
- 46.Wold WSM, Doronin K, Toth K, Kuppuswamy M, Lichtenstein DL, Tollefson AE. Immune respones to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr Opin Immunol 1999;11:380–386. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Kang J, Friedman J, Tarassishin L, Ye J, Kovalenko A, Wallach D, Horwitz MS. Identification of a cell protein (FIP-3) as a modulator of NF-κB activity and as a target of an adenovirus inhibitor of tumor necrosis factor α-induced apoptosis. Immunology 1999;96:1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider-Brachert W, Tchikov V, Merkel O, Jakob M, Hallas C, Kruse M, Groitl P, Lehn A, Hildt E, Held-Feindt J, et al. Inhibition of TNF receptor 1 internalization by adenovirus 14.7K as a novel immune escape mechanism. J Clin Invest 2006;116:2901–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimitrov T, Krajcsi P, Hermiston TW, Tollefson AE, Hannink M, Wold WS. Adenovirus E3–10.4K/14.5K protein complex inhibits tumor necrosis factor-induced translocation of cytosolic phospholipase A2 to membranes. J Virol 1997;71:2830–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol 2000;37:1–11. [DOI] [PubMed] [Google Scholar]
- 51.Gauzzi MC, Velazquez L, McKendry R, Mogensen KE, Fellous M, Pellegrini S. Interferon-alpha-dependent activation of Tyk2 requires phosphorylation of positive regulatory tyrosines by another kinase. J Biol Chem 1996;271:20494–20500. [DOI] [PubMed] [Google Scholar]