Abstract
Epidemiologic studies have associated exposure to airborne particulate matter (PM) with exacerbations of asthma. It is unknown how different sources of PM affect innate immunity. We sought to determine how car- and diesel exhaust–derived PM affects dendritic cell (DC) activation. DC development was modeled using CD34+ hematopoietic progenitors. Airborne PM was collected from exhaust plenums of Fort McHenry Tunnel providing car-enriched particles (CEP) and diesel-enriched particles (DEP). DC were stimulated for 48 hours with CEP, DEP, CD40-ligand, or lipopolysaccharide. DC activation was assessed by flow cytometry, enzyme-linked immunosorbent assay, and standard culture techniques. DEP increased uptake of fluorescein isothiocyanate–dextran (a model antigen) by DC. Diesel particles enhanced cell-surface expression of co-stimulatory molecules (e.g., CD40 [P < 0.01] and MHC class II [P < 0.01]). By contrast, CEP poorly affected antigen uptake and expression of cell surface molecules, and did not greatly affect cytokine secretion by DC. However, DEP increased production of TNF, IL-6, and IFN-γ (P < 0.01), IL-12 (P < 0.05), and vascular endothelial growth factor (P < 0.001). In co-stimulation assays of PM-exposed DC and alloreactive CD4+ T cells, both CEP and DEP directed a Th2-like pattern of cytokine production (e.g., enhanced IL-13 and IL-18 and suppressed IFN-γ production). CD4+ T cells were not functionally activated on exposure to either DEP or CEP. Car- and diesel-enriched particles exert a differential effect on DC activation. Our data support the hypothesis that DEP (and to a lesser extent CEP) regulate important functional aspects of human DC, supporting an adjuvant role for this material.
Keywords: asthma, allergy, innate immunity, Toll-like receptors, pollution
CLINICAL RELEVANCE
This study describes the immunological effects of diesel and car-enriched particulates on dendritic cell activation and their promotion of a Th2-like cytokine response by CD4+ T cells. This is a novel observation relevant to our understanding of the immune adjuvant effects of environmental pollutants and pathogenesis of allergic pulmonary diseases.
Exposure to airborne particulate matter (PM) is firmly linked with the morbidity and mortality from respiratory diseases (1, 2). Ambient airborne particles as well as road traffic pollution are associated with exacerbations of asthma, chronic obstructive pulmonary disease, and allergic rhinitis (1–4). It is hypothesized that airborne PM may contribute to the increased incidence, morbidity, or mortality from asthma in adults and children in urban areas (2). Increases in air pollution levels exacerbate existing airway diseases and are associated with the promotion of allergic airway diseases (3–7). Diesel exhaust particulates may direct an adjuvant effect by exerting pro-allergic sensitization to commonly found environmental allergens (8). Also, exposing mice to PM induces asthma-like conditions, airway responsiveness, and inflammation (4, 9). The mechanisms by which PM adversely affects human health are incompletely understood. Current thinking is that inhaled PM induces lung inflammation, possibly in an oxidative stress–dependent manner, by acting on respiratory epithelial or other cells (4).
Dendritic cells (DC) are the sentinel cells of the innate immune system, and evolved to rapidly translate diverse environmental cues into signals that activate T lymphocytes. Although they are poised to sense and respond to inhaled pollutants, surprisingly little is known about whether and how PM affects the function of DC. Immature DC respond efficiently to danger signals (e.g., endotoxin) such that a primary immune response is appropriately induced (10). Danger signals are transduced by pattern recognition receptors that include the Toll-like receptor (TLR) family (11). Occupation of the TLR by ligand (e.g., lipopolysaccharide [LPS] binding to TLR4) instructs DC to mature (12). DC can also be activated by T cell–dependent signals such as the ligand for the co-stimulatory receptor CD40 (CD40 ligand, or CD40L). DC maturation is defined by enhanced cell surface expression of co-stimulatory molecules, reduced endocytosis, enhanced antigen presentation, stimulation of T cell proliferation, and increased cytokine production (13–15).
Immature DC are specialized for taking up exogenous antigen by mechanisms that include endocytosis (14), yet they exhibit a poor ability to stimulate T cells unless activated (16). Lung DC are continuously repopulated from circulating progenitors such as monocytes and lineage-committed stem cells, and this can be augmented in response to environmental stimuli (17). Interestingly, PM itself may promote DC recruitment to the lung by inducing secretion of the chemokine MIP-3α (CCL20) from airway epithelial cells (18). Immature DC capture antigen and migrate to the draining lymph nodes. It is thought that maturation takes place during their migration, where DC are now defined as potent stimulators of T cell proliferation with a suppressed ability to take up exogenous antigen.
Other studies provide compelling evidence that allergic diseases are immune-mediated and directed by T cells against allergens or microbial byproducts (19). CD4+ type 2 T-helper (Th2) cells, which secrete IL-4 and IL-13, induce many of the pathologic features of allergic inflammation including IgE synthesis, suppressed IFN-γ production, eosinophilia, and tissue remodeling (19). It is unknown how DC translate allergen exposure into signals that initiate Th2 differentiation. In asthma, activated airway DC may induce Th2 polarization of allergen-specific T cells (20). In the current study, we speculated that DC may provide one link between exposure to particulate matter and a Th2-driven allergic immune response. This is consistent with the notion that DC are key sentinel cells that evolved to respond to diverse environmental stimuli (16). Here we provide a comprehensive analysis of DC functional activation following their interaction with either diesel or car exhaust particles, and we define a unique pattern of DC functional maturation as a consequence of these interactions.
MATERIALS AND METHODS
Reagents
Recombinant human cytokines used for DC culture were: stem cell factor (SCF), IL-4, Flt-3 Ligand (Flt-3L), and TNF-α, all from Peprotech Inc. (Rocky Hill, NJ). Granulocyte-macrophage colony-stimulating factor (GM-CSF) was a gift of Immunex (Seattle, WA). Lipopolysaccharide (LPS, Escherichia coli serotype 0.55:B5) and fluorescein isothiocyanate (FITC)-conjugated dextran (MW of 40,000), were obtained from Sigma-Aldrich (St. Louis, MO). Recombinant human trimeric CD40-Ligand (CD40L trimer) was a kind gift from Dr. John McDyer, Johns Hopkins University.
The following monoclonal antibodies were used for flow cytometry on activation of DC: FITC-conjugated antibodies were IgG1-κ (isotypic control, clone MOPC-21), HLA-DR (IgG2a-κ, clone G46–6), and CD206 (IgG1, clone 19.2); phycoerythrin (PE)-conjugated antibodies were IgG1-κ (isotypic control, clone MOPC-21), IgG2a-κ (isotypic control, clone G155–178), CD4 (IgG1-κ, clone RPA-T4), CD80 (IgG1, clone L307.4), CD83 (IgG1, clone HB15e), CD86 (IgG1, clone FUN-1), and CD14 (IgG2a, clone M5E2), all from PharMingen (San Diego, CA). The following PE-conjugated antibodies were obtained from Santa Cruz Inc. (Santa Cruz, CA); TLR-2 (IgG2a, clone TL2.3) and TLR4 (IgG2a, clone HTA125). FITC-conjugated CD40 (IgG1, clone HB14) was from Caltag (Burlingame, CA).
The following monoclonal antibodies were used for flow cytometry on activation of CD4+ T cells: FITC-conjugated antibodies were IgG2a-κ (isotypic control, clone G155–178) and HLA-DR (IgG2a-κ, clone G46–6, L243); PE-conjugated fluorochromes were IgG1-κ (isotypic control, clone MOPC-21), IgG2a-κ (isotypic control, clone G155–178), CD4 (IgG1-κ, clone RPA-T4), CD11b (IgG2a-κ, clone D12), CD31 (anti-PECAM1; IgG1-κ, clone L133.1), CCR7 (Rat IgG2a-κ, clone 3D12), and CD62L (L-selectin or LAM1, IgG1-κ, clone Dreg 56).
Preparation and Enrichment of Highly Pure CD4+ Allogeneic T Cells
The procedures used to enrich for these cells has been described (14). In assays of allogeneic T cell responses, CD4+ T cells were enriched from whole blood using the Rosettesep negative selection system according to the manufacturer's instructions (Stem Cell Technologies, Ltd, Vancouver, BC, Canada). This system depletes the contaminating cells by immunologically cross-linking those cells in whole blood to multiple red blood cells (immunorosettes) using an antibody cocktail. In this procedure, the density of the rosetted cells increases and they can be pelleted with free red blood cells by centrifugation over low endotoxin (< 0.12 EU/ml, where 1 EU = 100 pg/ml LPS) cell separation medium (Ficoll-Paque Plus, d = 1.077 g/cm3; GE Healthcare Biosciences AB, Uppsala, Sweden). Briefly, after rosetting cells for 20 minutes at room temperature, the blood sample was diluted with an equal volume of divalent cation-free PBS supplemented with 2% vol/vol FBS, mixed gently, layered over Ficoll-Paque and centrifuged at 800 × g for 30 minutes at room temperature. Enriched cells were harvested from the separation medium/upper plasma interface then washed twice in PBS/2% FBS buffer. By this procedure, the cells were 95% ± 4% viable by trypan blue dye exclusion assay and cell counting and 92% ± 4% enriched CD4+ T cells by staining with FITC-conjugated anti-human CD4 (clone RPA-T4, IgG1) and flow cytometry (data not shown). This method of CD4 T cell purification was chosen because of its high reproducibility of enrichments between donors.
Generation, Expansion, and Stimulation of CD34+ Peripheral Blood Progenitor Cell–Derived DC
DC were generated from CD34+ enriched peripheral blood progenitor cells (PBPC) using protocols that use cytokine-driven expansion and propagation of DC precursors (21). PBPC were obtained from an NIH core facility at the University of Washington (Seattle, WA) and were used as the starting material for the generation of myeloid DC. Enriched CD34+ PBPC were seeded into 6-well culture dishes at 2.5 × 105 cells/ml in a total volume of 4 ml DC culture medium in a 5% CO2/95% air fully humidified atmosphere. DC were cultured continuously for 14 days in DC culture medium that had been previously optimized in pilot experiments. For the culture of PBPC-derived DC, RPMI 1640 base culture medium (GIBCO/BRL, Invitrogen, Carlsbad, CA) was used and supplemented with 8% vol/vol FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 1% vol/vol nonessential amino acids (GIBCO-BRL), 1 μg/mL gentamicin sulfate, 100 IU/ml penicillin-G, 100 μg/ml streptomycin sulfate, 20 mM HEPES buffer, and 5 × 10−4 M 2-mercaptoethanol. All cell cultures were conducted at 37°C in a humidified atmosphere of 5% CO2 in air. Cultures of PBPC were pulsed with the following human cytokines on Days 0, 2, 5, 8, and 11: GM-CSF 25 ng/ml, SCF 25 ng/ml, Flt-3L 10 ng/ml, TPO 10 ng/ml, and TNF-α 2.5ng/ml. The addition of IL-4 at 20 ng/ml was delayed until Day 5 of culture to permit cell expansion and DC development. The additions of SCF, thrombopoietin (TPO), and Flt-3L were omitted from Day 8 onwards. DC were harvested at Days 14 to 16, washed twice in complete culture medium, and then seeded into 6-well dishes at 2.5 × 106 cells/well in a total volume of 2.5 ml. We would typically obtain a 50- to over 100-fold expansion of PBPC over a 14- to 16-day culture. Thus, when using a single vial of 5 × 106 PBPC hematopoietic progenitor cells at Day 0 of culture, we would obtain 2.5 × 108 to ∼ 5.5 × 108 immature DC at Day 16 of culture.
Car- and Diesel Exhaust–Derived Particulate Matter
Car exhaust– and diesel exhaust–derived particulate matter (PM) sampling stations were set up in the exhaust plenums of two bores of the Fort McHenry Tunnel, East Baltimore, MD. Airborne particulate matter was collected on Teflon filters (37 mm Teflo, with a pore size of 2 μm; Pallflex Pall Corporation, Ann Arbor, MI) using SKC Personal Environmental Monitors (PEM; SKC Inc., Eight Four, PA) collecting PM of 10 and 2.5 microns in aerodynamic diameter. Pre- and post-sampling masses were determined using a microbalance. One bore was subject to primarily passenger car particulate emissions (referred to as car PM tunnel) and the other bore was subject to both car and diesel truck traffic (referred to as the diesel PM tunnel). Hereafter these two sources of PM will be referred to as car exhaust PM (or CEP) and diesel-enriched PM (or DEP), respectively. Six DEP and CEP PM10 and PM2.5 filters were collected from each tunnel tube. Pre- and post-sampling masses were determined using a microbalance. Video recordings were reviewed and cars and large trucks were differentially counted. Results of traffic counts indicated that less than 2% of the vehicles in the car PM tunnel were trucks. In contrast, 22% of the vehicles in the diesel PM tunnel were trucks. The mean airborne PM2.5 concentration in the diesel PM tunnel was 30% greater than in the car PM tunnel. This increase in the airborne PM2.5 concentration was due to the enrichment from diesel exhaust emissions. CEP and DEP samples were paired by sampling date and time in six independent experiments, and were matched so that the mass of PM collected did not vary by more than 15% between each tunnel pair. Nonexposed blank filters were used to control for any effect of the Teflon filter alone. To exclude the possibility of endotoxin contamination of particulate matter embedded Teflon filters, replicate portions of the filters were assayed for contaminating endotoxin levels by the QCL-1000 Limulus amebocyte lysate assay (Cambrex Corporation, East Rutherford, NJ). Levels of endotoxin were detected but found to be less than 50 pg/ml, a concentration of endotoxin that was not found to activate human DC (data not shown).
Stimulation of DC Cultures with Filter-Immobilized Car or Diesel Particulate Matter
Before the addition of DC to the 12-well plates, CEP- or DEP-immobilized filters were placed in the 12-well plates. DC cultures were then carefully dispensed onto the surface of the filters using a sterile filter-barrier pipette tip to a final volume of 2.5 ml and 2.5 × 106 DC per well. Filter-immobilized particulate matter was used since this resulted in minimum manipulation of the test material and provided to the DC cultures the test material exactly as it was collected from the tunnels. In addition, immobilization of the particulate matter onto Teflon filters ensured that none of the particulate matter was carried over into subsequent experiments of DC and T cell co-cultures. By microscopic examination, we confirmed that particulate matter was not present in thoroughly washed cultures of CEP or DEP filter exposed DC that would subsequently be co-cultured with purified CD4+ T cells.
In control cultures, blank membranes that had not been exposed to particulate matter sampling were used as negative controls. These were used to test for the possibility of nonspecific interactions of the Teflon filter membrane and the cell surface of DC. Additional controls included LPS (a TLR4 agonist, serotype 055:B5, E. coli–derived endotoxin) used at a concentration of 100 ng/ml and CD40-ligand (CD40L, a non TLR agonist used at a concentration of 50 ng/ml). Cultures were incubated for 48 hours in a 5% CO2/95% air and fully humidified incubator. After stimulation, all cultures were washed three times in complete medium to prevent potential “carry-over” of the stimulus to co-culture experiments of stimulated DC and CD4+ T cells.
In addition, in dose-dependent studies of DC or CD4+ T cell exposure to DEP or CEP, nonimmobilized free particulate matter was collected from the same sampling stations that were set up in the exhaust plenums of two bores of the Fort McHenry Tunnel, East Baltimore, MD and used for such studies. In these investigations, DEP and CEP particulate matter was suspended in 10 mM HEPES-buffered divalent cation-free PBS pH 7.4 at 10 mg/ml and either used immediately or stored frozen at −86°C and used within 1 month of preparation. In a limited series of experiments, DC and CD4+ T cells were assayed (as described below) on activation by DEP or CEP over the concentration range of 100, 10, 1, and 0.1 μg/ml for a period of 48 hours. As described above, to exclude the possibility of endotoxin contamination of DEP or CEP particulate matter, the suspensions were assayed for contaminating endotoxin levels by the QCL-1000 Limulus amebocyte lysate assay (Cambrex Corporation). Levels of endotoxin were detected but found to be less than 33 pg/ml, a concentration of endotoxin that was not found to activate human DC (data not shown).
Phenotypic Analysis of Cell-Surface and Toll-like Receptor Expression by Flow Cytometry
DC were prepared for flow cytometric analyses as described previously (14). After washing DC cultures twice in fluorescence-activated cell sorter (FACS) buffer (divalent cation-free PBS, 20 mM HEPES, 2.5% vol/vol FBS, 0.02% wt/vol sodium azide), DC were pre-incubated with 5% vol/vol normal human AB serum to block nonspecific staining of test monoclonal antibodies against Fc-γ receptors, then washed twice in ice-cold FACS buffer. DC were subsequently stained with the appropriate antibodies as listed above. DC were aliquoted into labeled tubes at a density of 1 × 105 cells in a volume of 100 μl and incubated with the respective isotype-matched irrelevant control antibody or the specific test monoclonal antibody for 30 minutes, on ice, in the dark. Antibodies were titrated for optimal concentrations in pilot experiments. Also, the same lots of all monoclonal antibodies were used throughout the study. Samples were washed twice in FACS buffer by centrifugation at 400 × g for 6 minutes at 4°C and post-fixed in 2% vol/vol paraformaldehyde in FACS buffer before analysis. The samples were analyzed immediately on a FACScaliber flow cytometer using CellQuest 3.1 software (Becton Dickinson, San Jose, CA). The instrument had a standard optical filter configuration with band pass filters of 530/30-nm and 585/44-nm for FL1 (FITC-conjugated antibodies) and FL2 (PE-conjugated antibodies) data acquisition, respectively. For the analysis of forward-angle light scatter, side-angle light scatter, and cell surface receptor expression, data were acquired in real time. Data were recorded as geometric mean fluorescence intensity (MFI) and percent fluorescent-positive cells. The instrument was standardized before phenotypic analysis with Calibration beads (FluoroSpheres 6-Peak; Dako Cytomation, Carpinteria, CA) and cleaned with sequential washes of distilled water, 10% vol/vol hypochlorite and distilled water before data acquisition.
Functional Assay of DC Endocytosis of Low-Molecular-Weight FITC-Conjugated Dextran
This assay was conducted as described (14). Samples that had been exposed to CEP, DEP, LPS, or blank membranes (as described above) were washed in warm complete culture medium and 100-μl aliquots were distributed into a series of tubes to assay for the time-dependent uptake of FITC-labeled dextran (MW, 40,000) at a final concentration of 0.5 mg/ml (14) as detailed in Results. Cells were also incubated with FITC-dextran for the final 30 minutes of this assay at 4°C to assess background surface binding (without internalization). Test cells were incubated at 37°C to assess the kinetics of endocytosis at defined time-points as indicated in the text. At the conclusion of the assay, samples were washed with ice-cold FACS buffer three times and resuspended in 250 μl of FACS buffer before analysis on a FACSCaliber flow cytometer equipped with Cellquest software. Data were recorded as geometric MFI and the instrument was set up and calibrated as detailed above. The test time–dependent MFI data represents the mean geometric MFI with the background surface binding mean geometric MFI subtracted from those values.
Assay of Cytokine Secretion by Cultures of Resting and Stimulated DC
Cultures of DC were stimulated with CEP, DEP, LPS (100 ng/ml), CD40L (50 ng/ml), or culture medium alone (resting DC) for a period of 48 hours at 37°C in a fully humidified cell culture incubator. Cell-free culture supernatants were prepared by centrifugation at 600 × g for 10 minutes at 4°C and stored at −86°C until assayed for IL-12p40, TNF-α, IFN-γ, IL-6, IL-18, and VEGF. Since this assay system used a pure population of DC, data were recorded as picograms of secreted product per 106 total DC.
Assay of Cytokine Secretion by Co-Cultures of Allogeneic T Cells and DC
To determine the allogeneic stimulatory function of DC, mixed leukocyte assays were designed and performed in 24-well tissue culture plates. Cultures of immature (nonstimulated) DC, stimulated DC, and purified CD4+ T cells were cultured alone at 5 × 104 cells/well/ml in duplicate (in the case of dendritic cells) and 2.5 × 105 cells/well/ml in duplicate (in the case of T cells) to assay for spontaneous production of cytokines. In addition, in co-culture experiments, CD4+ T cells (the responders) were seeded at a density of 2.5 × 105 cells/well/ml of culture medium and co-cultured with DC (the stimulators) at a stimulator to responder ratio of 1:5 such that 5 × 104 DC/well/ml were co-cultured with the T cells.
This ratio of DC to T cells was found to give maximal stimulation of CD4+ T cells in preliminary experiments. The total volume of this co-culture was 2 ml per well. Before co-culture, DC were first treated with 25 μg/ml mitomycin C for 30 minutes at 37°C, then washed four times in complete culture medium to block their potential nonspecific proliferation and interference in the assay. However, we have never found this to be the case, since after 12 to 14 days of propagation CD34+ progenitor cell–derived DC do not proliferate and are instead primed for functional activation and differentiation (14). The co-cultures were incubated for 48 hours at 37°C in a humidified atmosphere of 5% CO2 in air. Cell-free supernatants from the co-cultures and DC or T cells cultured alone were pooled and assayed for IFN-γ, IL-13, IL-12p70, IL-6, and IL-4. Since these assays were of a mixed cell culture, data were recorded as picograms of cytokine per milliliter of the co-culture system. However, in pilot experiments of CD34+ progenitor cell–derived DC cultures, we have not found levels of IL-13 or IL-4 secreted by DC above assay sensitivity of the enzyme-linked immunosorbent assay (ELISA) platforms used. By contrast, IFN-γ can be secreted by activated DC as well as appropriately stimulated CD4+ T cells. However, we did not observe production of IFN-γ by DC under any of the stimulatory conditions reported in this manuscript.
Assays to Determine T Cell Activation by DEP or CEP
It is possible that after the exposure of DC to CEP or DEP, any particulate matter taken up by DC can be carried over to the CD4+ T cell co-culture assay either as particulates attached to the cell or as endocytosed/phagocytosed material that could be subsequently released. Although this indirect effect of DC-transferred particulate matter seems unlikely, we have nonetheless conducted studies to test this concern. Therefore, we assessed modulation of the cell surface expression of T cell–associated activation markers including CD4, CD11b, CD31, CD62L, CD71, CCR7, and MHC class II (HLA-DR) by a flow cytometric approach. This was done after we had exposed CD4+ T cells to a separate series of Teflon membrane–immobilized DEP or CEP in identically designed experiments that we had conducted with myeloid DC as described above. For flow cytometric assays only, we compared responses of CEP- or DEP-exposed CD4+ T cells to the stimulatory effects of ambient particulate matter (PM10) collected from Baltimore City as we had previously described (22).
We measured cytokine secretion by CEP or DEP exposed CD4+ T cells using commercially available ELISA kits to measure TNF-α and IFN-γ. CD4+ T cells were exposed to Teflon membrane–immobilized DEP or CEP for 48 hours, and cell-free culture supernatants were harvested and stored at −86°C until assayed. As positive control, we stimulated CD4+ T cells with rHuIL-2 (50 IU/ml) for 48 hours followed by phorbol 12-myristate, 13-acetate (PMA) for the final 4 hours of culture at 5ng/ml to nonspecifically promote cytokine-producing responses by the enriched CD4+ T cells.
Since exposure of T cells to particulate matter may affect their viability, we measured uptake of 0.2% wt/vol trypan blue, a vital stain taken up by nonviable cells that can be distinguished from viable cells (exlusion of trypan blue) by conventional light microscopy. In this assay, T cells were stimulated with DEP or CEP for 18 hours, washed twice in PBS supplemented with 10 mM HEPES, pH 7.4, and incubated with 0.2% trypan blue for 5 minutes. CD4+ T cells were assessed for % cell death using brightfield light microscopy and manual counting to distinguish viable from nonviable cells.
Biometry and Statistical Measurements
Data are expressed as mean ± 1 SD or 1 SEM as described. A minimum of n = 6 independent experiments were completed as described in the text. Comparisons between data were tested for significance using Student's t test and post hoc correction according to the Bonferroni method. Statistical significance was set an α value of at least P < 0.05 and as indicated in the text.
RESULTS
Modulation of Macrophage-Mannose Receptor CD206 and CD83 Expression by DC
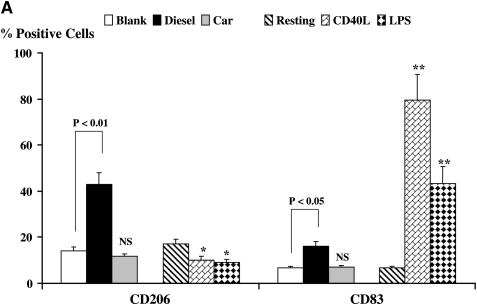
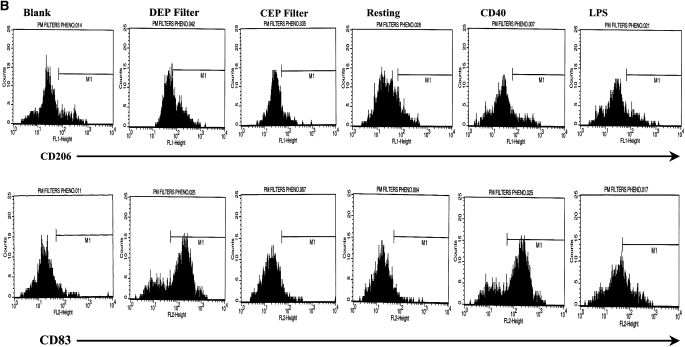
We compared the effects of DC exposure to Teflon filter–immobilized CEP and DEP with two known DC activators: CD40L and LPS, representing T cell– and TLR-dependent pathways of DC activation, respectively (Figures 1A and 1B). We used flow cytometry to determine the cell surface expression of functionally important molecules on resting and activated DC. The expression of CD206, a mannose receptor that plays an important role in endocytosis of extracellular antigens by DC, was down-regulated when DC were stimulated with either CD40L or LPS (Figure 1A, P < 0.05), consistent with a normal pattern of DC maturation. However, an unexpected observation was the marked augmentation in CD206 expression when DC were exposed to DEP (Figures 1A and 1B, P < 0.01). This is inconsistent with a conventional pattern of DC activation and was not observed when DC were exposed to either CEP or blank filters. We also measured the level of expression of the maturation-associated marker CD83 (Figures 1A and 1B). As expected, CD83 expression was enhanced when DC were stimulated with either CD40L or LPS (P < 0.01), an observation consistent with conventional maturation. However, while neither blank filters nor CEP affected the expression of CD83, DEP induced a smaller but significant enhancement of CD83 expression (P < 0.05), a feature of maturing and activated DC.
Figure 1.
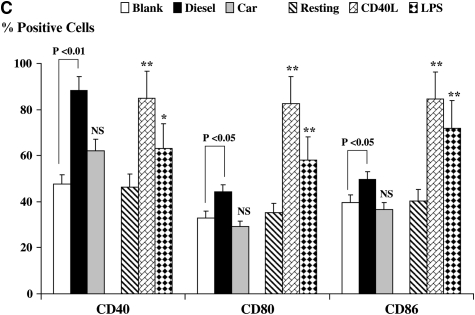
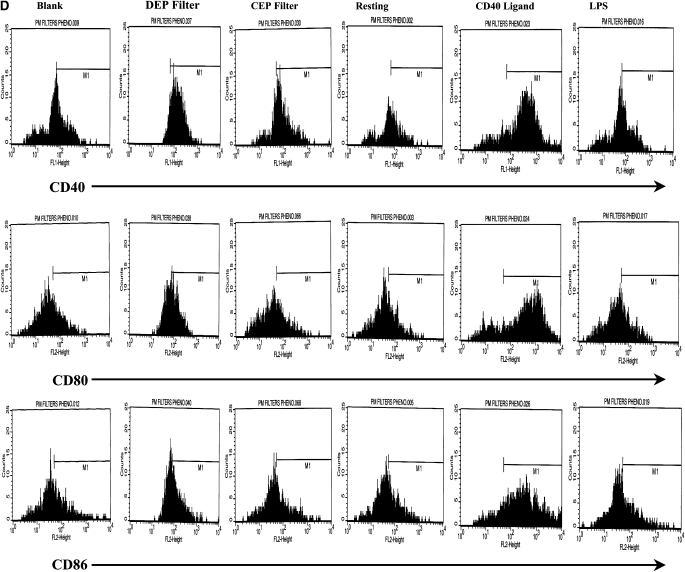
Flow cytometric quantitation of the macrophage-mannose (endocytosis) receptor CD206 and CD83 (A) expression and flow cytometric histograms of this data from a representative experiment (B) on the cell surface of DC. Also shown is the expression of the co-stimulatory molecules CD40, CD80, and CD86 (C) and flow cytometric histograms of this data from a representative experiment (D.) Data for these series of experiments is recorded as % positive cells ± SD. The levels of significance between car-enriched particulate matter (CEP)- and diesel-enriched particulate matter (DEP)-treated dendritic cells (DC) are shown in the figure. *P < 0.05 and **P < 0.01 indicates differences between CD40L- or lipopolysaccharide (LPS)-treated DC as compared with resting DC. NS, not statistically significant.
Modulation of Co-Stimulatory Molecule Expression by DC
The expression of CD40 by DC was enhanced after stimulation with CD40L (Figures 1C and 1D, P < 0.01) or LPS (P < 0.05), as compared with resting DC. This is consistent with normal maturation of DC. The expression of CD40 was also augmented by DEP as compared with blank filters (Figures 1C and 1D, P < 0.01), while CEP had no effect. The expression of CD80 and CD86 were similarly enhanced when DC were stimulated with CD40L or LPS as compared with resting DC (Figures 1C and 1D, P < 0.01). Moreover, DEP but not CEP augmented the expression of CD80 and CD86 (Figures 1C and 1D, P < 0.05). The enhanced expression of CD40, CD80, and CD86 by DEP-stimulated DC is consistent with conventional maturation of activated DC.
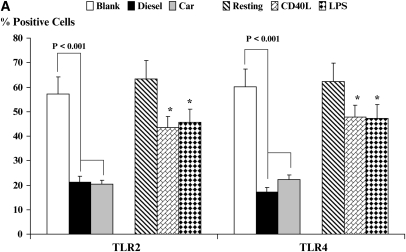
Toll-Like Receptors TLR2 and TLR4 Are Markedly Down-Regulated by PM-Stimulated DC
Toll-like receptors are important in directing the recognition of microbial signals by DC and initiating immune responses to danger signals such as infection. We next determined how the expression of these receptors could be modulated by DEP as compared with classical TLR signals such as LPS or activation of DC by CD40L (Figures 2A and 2B). The expression of TLR2 by DC was partially down-regulated after stimulation with CD40L or LPS (Figure 2, P < 0.05). In contrast, there was a dramatic diminution of TLR2 expression on activation of DC by CEP or by DEP (Figure 2, P < 0.001). The expression of TLR4 (Figure 2) followed the same pattern of response to CD40L and LPS as well as to CEP and DEP as was observed for TLR2. The importance of reduced TLR2 and TLR4 expression with regard to the known functions of DC in host immunity is considered further below (see Discussion).
Figure 2.
Flow cytometric quantitation of the cell-surface expression of the pattern-recognition receptors TLR2 and TLR4 for resting and stimulated DC (A). Data are recorded as % positive cells ± SD. Levels of significance between CEP- and DEP-treated DC are shown in the figure. *P < 0.05 indicates differences between CD40L- or LPS-treated DC compared with resting DC. A series of flow histograms from one experiment is shown (B). Data are recorded as % positive cells and geometric mean fluorescence intensity.
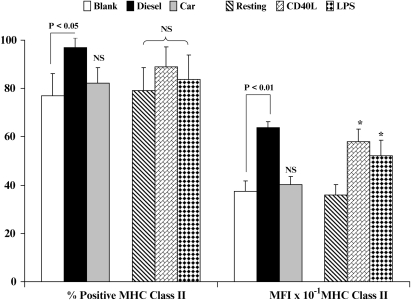
Modulation of MHC Class II (HLA-DR) Expression by DC
The expression of MHC Class II by DC was enhanced after stimulation with CD40L or LPS, another effect that is consistent with normal cellular maturation of DC as compared with resting DC (Figure 3, P < 0.05). While CEP did not alter the expression of MHC Class II, DEP directed a significant upregulation in the expression of this receptor (Figure 3, P < 0.01).
Figure 3.
Flow cytometric quantitation of MHC class II expression. Data on the left show % positive cells, data to the right show geometric mean fluorescence intensity (MFI) ± SD for resting and stimulated DC. Levels of significance between CEP- and DEP-treated DC are shown; *P < 0.05 indicates differences between resting and CD40L- or LPS-treated DC. NS, not statistically significant.
Taken together, these data indicate that CEP and DEP induce distinct patterns of DC activation. DEP exhibited qualitatively similar patterns of surface molecule expression as CD40L and LPS, with the exception of marked up-regulation of CD206. In contrast, the effects of CEP appeared to be restricted to TLR2 and TLR4 expression.
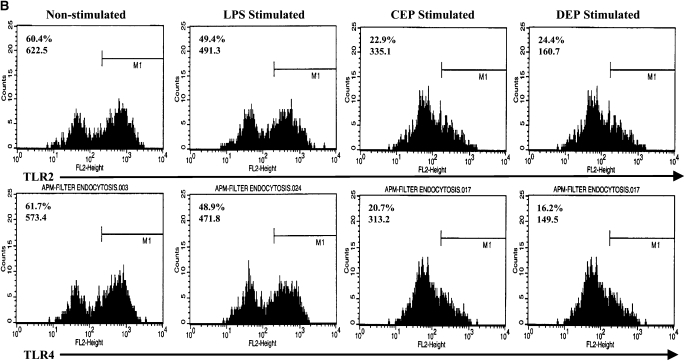
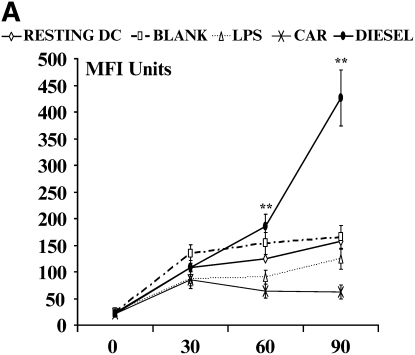
Alteration of the Endocytic Function of DC
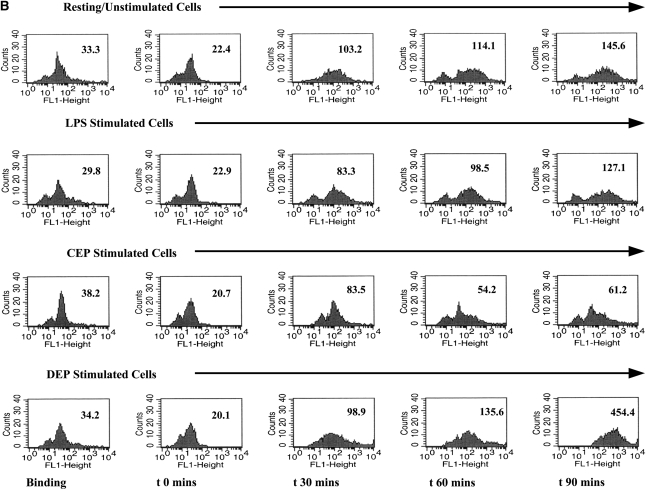
One of the most important functions of immature DC is their ability to take up extracellular antigens by endocytosis: this involves binding of antigens to surface receptors including the mannose receptor CD206. Endocytosis tends to be lost in favor of antigen presentation by DC undergoing conventional maturation. To determine the functional significance of the enhanced levels of CD206 seen on DC stimulated with DEP, we modeled the functional ability of DC to take up extracellular antigen using FITC-dextran (Figures 4A and 4B). Consistent with this phenomenon, we observed that immature DC exhibited time-dependent uptake of FITC-dextran (Figure 4). As expected, mature DC, for example after stimulation with CD40L, exhibited suppressed uptake of FITC-dextran (Figure 4). Consistent with enhanced cell surface expression of CD206, we observed that DEP-exposed DC exhibited a potent uptake of FITC-dextran (Figure 4). An important observation was that DEP-exposed DC exhibited a greater ability to take up FITC-dextran than resting DC (Figure 4, P < 0.01, ANOVA). Neither CEP nor blank filters altered the ability of DC to take up FITC-dextran.
Figure 4.
Flow cytometric quantitation of the time-dependent functional uptake of FITC-dextran by DC. Data are recorded as geometric mean fluorescence intensity units from n = 6 separate experiments. (A) This assay measures total shift (increases) in fluorescence intensity and not the percent of positive cells. Thus, with enhanced uptake of FITC-dextran there is an associated increase in geometric MFI. A series of flow histograms demonstrating the alterations in fluorescence intensity from one experiment is shown (B). Data described in the individual flow histograms are geometric MFI.
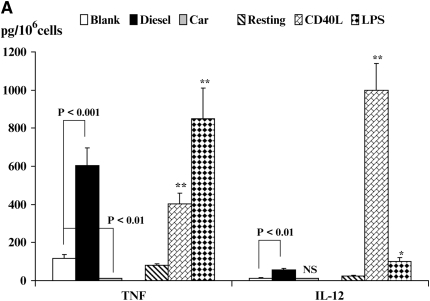
Inflammatory Cytokine Production by DC
Cytokine secretion by activated DC contributes to inflammation and influences Th cell polarization. Therefore, we next investigated cytokine production after stimulation of DC by CD40L, LPS, DEP, or CEP (Figures 5A–5C). We noted minimal spontaneous secretion of TNF by resting DC (Figure 5A). However, activation of DC by CD40L or LPS enhanced production of TNF (Figure 5A, P < 0.01). Similarly, exposure of DC to DEP, but not to CEP, increased production of TNF at levels that were similar to those for CD40L- and LPS-stimulated DC (Figure 5A, P < 0.01). Secretion of the Th1-promoting cytokine IL-12 by DC followed a similar pattern, albeit the magnitude of responses were different with maximal production observed in response to CD40L (Figure 5A, P < 0.01) and much smaller but statistically significant increases observed in response to LPS (P < 0.05) and DEP (Figure 5A, P < 0.01). CEP did not affect production of IL-12.
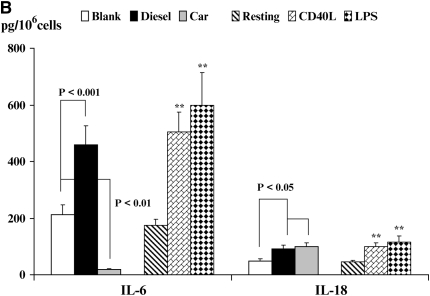
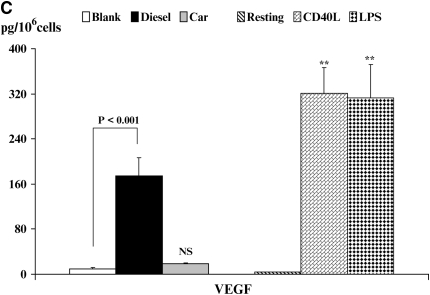
Figure 5.
Quantitation of cytokine secretion by DC. Production of TNF-α and IL-12p40 (A), IL-6 and IL-18 (B), or VEGF (C) is shown. Data are recorded as pg/million cells ± SEM. The levels of significance between CEP- and DEP-treated DC are shown. *P < 0.05 and **P < 0.01 indicates differences between CD40L- or LPS-treated DC compared with resting DC. NS, not statistically significant.
We also investigated the production of the immunoregulatory cytokines IL-6 and IL-18 by DC (Figure 5B). Resting DC secreted minimal levels of IL-6. However, DC that were stimulated with CD40L or LPS secreted enhanced levels of IL-6 (P < 0.01) as compared with resting DC. By contrast, while CEP actually suppressed production of IL-6 (P < 0.01), stimulation of DC with DEP significantly increased production of IL-6 (Figure 5B, P < 0.001), although the absolute levels were not as great as those found for CD40L- or LPS-stimulated DC. IL-18 plays an important role in T cell polarization. Although resting DC produced IL-18 constitutively, both CD40L and LPS augmented secretion of this cytokine from DC (Figure 5C, P < 0.01). In addition, DC that were stimulated with either CEP or DEP displayed a modest increase in IL-18 secretion when compared with blank membranes (P < 0.05).
Finally, we measured the production of VEGF by DC (Figure 5C), a cytokine that exhibits immune-modulating functions. Resting DC secreted minimal levels of VEGF that could be enhanced on activation of DC with either CD40L or LPS (Figure 5C, P < 0.01). Of interest, we found that while CEP did not alter production of VEGF by DC, stimulation of DC with DEP induced marked increases in VEGF production (P < 0.001) as compared with blank membranes. The role that secretion of VEGF plays in the context of inflammatory cytokine production by DC warrants additional investigation.
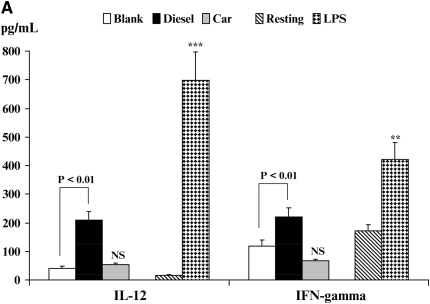
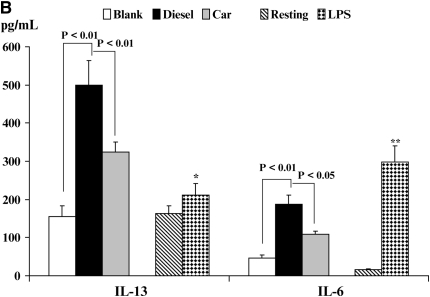
Inflammatory Cytokine Production in an Allogeneic DC/CD4+T Cell Co-Stimulation Assays
The hallmark of an activated DC is its ability to stimulate alloreactive T cells. Therefore, we finally studied how exposure to either CEP or DEP altered the ability of DC to induce T cell cytokine production using DC:T cell co-cultures (Figures 6A–6C; see Materials and Methods). The production of Th1-like cytokines (IL-12p70 and IFN-γ), the Th2-like cytokine IL-13, and the immunoregulatory cytokine IL-6 (Figures 6A and 6B) were studied. It should be noted that DC (as well as monocytes and neutrophils) exclusively produce the inflammatory cytokine IL-12p70, whereas T cells do not (23). Because we did not detect the secretion of IL-13 or marked alterations in IFN-γ production in experiments using isolated DC stimulated under different conditions (data not shown), we reasoned that secreted IFN-γ or IL-13 would be derived from T cells in these assays.
Figure 6.
Quantitation of immunoreactive cytokine secretion from DC/CD4+ allogeneic T cell co-cultures by enzyme-linked immunosorbent assay. The secretion of IL-12p70 and IFN-γ (A) and IL-13 and IL-6 (B) is shown. Data are recorded as pg/ml ± SEM. Levels of significance are *P < 0.05, **P < 0.01, and ***P < 0.001 as compared with resting DC. NS, not statistically significant.
The effect of DC exposure to CEP, DEP, and LPS on the secretion of both IL-12 and IFN-γ in DC/CD4+ T cell co-cultures is shown in Figure 6A. In co-cultures of resting DC with T cells, we saw only minimal production of IL-12. However, IL-12 production was increased when LPS-stimulated DC were co-cultured with T cells (P < 0.001). By contrast, DEP-stimulated DC only modestly elevated IL-12 secretion in the co-culture at levels that were at least 3-fold less than was seen for LPS-stimulated DC (Figure 6A). CEP exposure did not affect IL-12 production. In these experiments, we also observed minimal spontaneous production of IFN-γ (Figure 6A) that was elevated when CD4+ T cells were co-cultured with LPS-activated DC (P < 0.01). We also saw enhanced production of IFN-γ when CD4+ T cells were co-cultured with DEP activated DC (Figure 6A, P < 0.01), while CEP exhibited no effect when compared with blank membranes. However, the levels of IFN-γ detected in the LPS-stimulated DC co-cultures far exceeded those observed in DEP-stimulated DC co-cultures.
By contrast, IL-13 secretion exhibited an inverse effect when compared with observations made for the secretion of IFN-γ (Figure 6B). While production of IL-13 was augmented in co-culture with LPS-stimulated DC (P < 0.05), the production of IL-13 from both DEP (P < 0.01)- and CEP (P < 0.05)-stimulated DC co-cultures was substantially greater as compared with co-cultures of blank membrane–exposed DC (Figure 6B). Secretion of IL-4 in these experiments was variable between donors and much lower than IL-13, a generally consistent finding using allogeneic DC/T cell co-cultures (data not shown). The secretion of IL-6 followed a pattern similar to that of IL-12 production where we saw minimal levels of IL-6 spontaneously secreted in the resting DC/T cell co-culture (Figure 6B). However, IL-6 production was enhanced when LPS-stimulated DC were co-cultured with T cells (P < 0.01). Both DEP- and CEP-stimulated DC elevated IL-6 production in the co-culture (Figure 6B), although the levels seen were somewhat lower than the levels secreted in LPS-stimulated DC/CD4+ T cell co-culture (Figure 6B).
Direct Stimulation of CD4+ T Cells by DEP or CEP
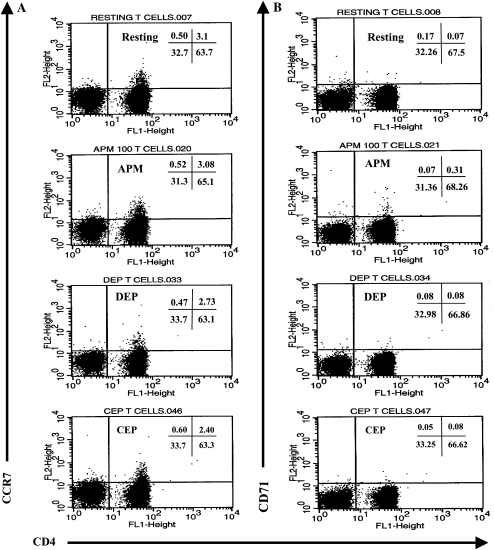
We were interested in excluding the possibility that DC could be responsible for indirectly delivering (carry-over) particulate matter to the CD4+ T cell/DC co-culture. Therefore, in a separate series of experiments, we directly stimulated CD4+ T cells with Teflon membrane–immobilized CEP or DEP for 48 h at 37oC in a fully humidified incubator providing an atmosphere of 5% CO2 in air. We then immediately quantitated alterations in CD4+ T cell surface phenotype, inflammatory cytokine production, and the sensitivity of CD4+ T cells to any toxic effect that was mediated by exposing T cells to Teflon membrane–immobilized DEP or CEP by trypan blue exclusion assay.
Exposing T cells to either CEP or DEP did not significantly alter expression of the activation markers CCR7 or CD71 on CD4+ T cells (Figures 7A and 7B, respectively). The expression of CD11b or MHC class II also remained unaltered (Figures 7C and 7D, respectively). These are two markers on CD4+ T cells that one would expect to be up-regulated on activated human T cells. In addition, CD4 expression remained consistent between treatments (Figures 7A and 7B). We also did not observe any alteration in the cell surface expression of either CD31 or CD62L (data not shown). Thus, by flow cytometric quantitation of membrane-expressed activation markers, neither CEP- nor DEP-activated CD4+ T cells.
Figure 7.
Flow cytometric determination of cell-surface expression of the chemokine receptor and activation marker CCR7 (A), the transferring receptor CD71 (B), the β2-integrin CD11b (C), and MHC class II/HLA-DR (D) on activation of CD4+ T cells by CEP or DEP. Neither CEP nor DEP significantly altered the cell surface expression of any of these markers by normal human CD4+ T cells. Data described in the flow cytometric dot plots and histograms are defined as % positive cells and/or geometric MFI as shown.
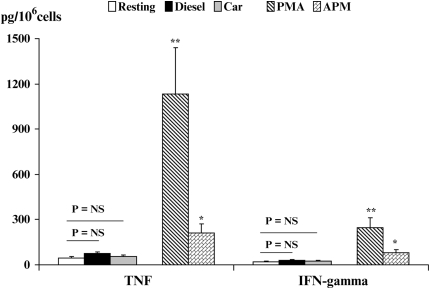
CD4+ T cell culture supernatants were assayed for the release of pro-inflammatory cytokines by commercially available ELISA kits after stimulation with either DEP or CEP for 48 hours at 37°C. The secretion of TNF and IFN-γ was not altered at all by exposing CD4+ T cells to particulate matter (Figure 8). By contrast, both TNF and IFN-γ were markedly enhanced on activation by PMA (Figure 8). This informed us that the lack of responsiveness of CD4+ T cells on stimulation with either CEP or DEP was not because they were refractory to stimulation, since they responded well to PMA. Although used as an independent control in out studies, we observed that ambient particulate matter (APM) also enhanced production of both TNF and IFN-γ (P < 0.05) as compared with resting CD4+ T cells, but at levels that were much lower than those seen with PMA.
Figure 8.
Determination of the effects of CEP or DEP in promoting either TNF (A) or IFN-γ (B) secretion by CD4+ T cells as compared with IL-2 and PMA co-stimulated CD4+ T cells. Data are described as pg of cytokine produced per million CD4+ T cells. CEP and DEP were without effect and did not promote any cytokine production by normal human CD4+ T cells.
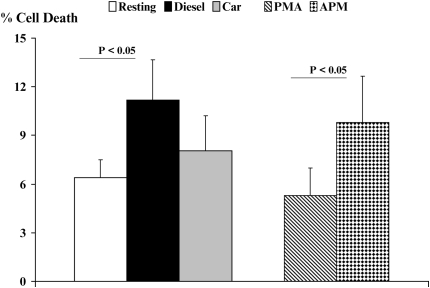
Finally, we assessed the viability of CD4+ T cells by trypan blue exclusion after the activation of T cells with PMA as compared with exposure to CEP or DEP (Figure 9). Although we observed no cell death on activation by PMA, we did observe an 8% decrease in cell viability on exposure of CD4+ T cells to CEP and an 11% decrease in viability of CD4+ T cells in response to DEP exposure. Since our CD4+ T cell cultures were exposed directly to particulate matter–impacted Teflon filters, we do not believe that DC are likely to promote this level of cell death by carry-over into the DC/CD4+ T cell co-cultures after the DC had been exposed to either CEP or DEP.
Figure 9.
Quantitation of percent cell viability by exclusion of the vital dye trypan blue by viable CD4+ T cells by brightfield light microscopy. Human CD4+ T cells were exposed to CEP or DEP for 18 hours and the percentage of cells that were nonviable was determined.
DISCUSSION
The numerous mechanisms that innate immunity employs to respond to inhaled environmental pollutants are poorly understood. A major concern in environmental public health is the global increase in roadside traffic pollution, but the potential links between exposure to the particulates contained in car and diesel exhaust emissions and immune dysregulation require further study. There is growing evidence that inhalable particulate matter derived from diesel and automotive exhaust particles in cities and surrounding urban areas contribute to the reported increases in the incidence of asthma, allergic conditions, respiratory infections, pulmonary infections, and mortality in the infant and adult populations (1–7). Furthermore, it is increasingly apparent that different types of air pollution can have qualitatively different effects on human health (3).
Dendritic cells are the key sentinels of the innate immune system that evolved to rapidly translate diverse environmental cues into signals that activate adaptive immunity. Moreover, the identification and characterization of different human DC subpopulations in the lung indicates to us their importance as key antigen-presenting cells in an appropriate anatomical setting to respond to inhaled particulate matter (24). In this study we assessed the effects of motor vehicle particulate emissions on DC functional activation using a comprehensive approach to compare and contrast the effects of two distinct sources of PM. We show that both CEP and especially DEP activate DC, but do so in a manner distinct from the classical DC activator LPS. Furthermore, CEP and DEP also had unique effects on the phenotype and function of DC. In the current study, we used well-established protocols that are thought to yield immature DC representative of those at mucosal sites in vivo (10, 13, 20). Since respiratory tract DC are rapidly derived from circulating precursors (16, 20), we believe our experiments provide a reasonable approximation of how DC and car or diesel exhaust PM interact in vivo. DC are richly interdigitated throughout the respiratory epithelium, and may even directly sample the bronchial lumen (20). Furthermore, PM-exposed epithelial cells secrete the DC chemoattractant MIP-3α (18). Therefore, one might expect that DC would be among the first cells to interact with and respond to inhaled PM.
An interesting and unexpected observation of our studies was that activation of DC by DEP enhanced the expression of CD206, a receptor that plays an important role in antigen uptake (10, 14). We confirmed the functional significance of CD206 up-regulation by showing that FITC-dextran uptake, a marker of fluid-phase endocytosis, was also increased in DEP-exposed DC. This contrasted with conventional maturation of DC by LPS and CD40L, which, as expected, resulted in diminished antigen uptake and enhanced antigen presentation (10, 14). In addition, the DC maturation marker CD83 and the co-stimulatory molecules CD40, CD80, and CD86 were also up-regulated on DEP stimulated DC, consistent with a conventional maturation pathway. We are not aware of other DC-activating signals that result in persistent antigen uptake as well as up-regulated antigen presentation: this appears to be an unusual feature of DEP exposure. The functional consequences of this for DC biology are unknown.
Exposure of DC to DEP enhanced the expression of MHC class II molecules, concordant with conventional activation of DC by LPS or CD40L. By contrast, CEP did not significantly alter expression of CD206, CD83, the co-stimulatory molecules or MHC class II. However, CEP markedly down-regulated the ability of DC to take up exogenous FITC-dextran. This would be an expected outcome when DC are conventionally activated by agents that drive a normal pattern of maturation characterized by augmented expression of co-stimulatory molecules, MHC class II, CD83 and down-regulated uptake of antigen. Clearly, there are features of both CEP- and DEP-stimulated DC that does not fit the conventional pattern of DC functional activation. These are novel observations that hitherto, and to the best of our knowledge, have not been reported. However, one previous report has explored the cellular responses of T cells and monocyte-derived DC to stimulation by DEP (25). The particles were collected directly from a diesel-engine vehicle where the particulate matter had not been exposed to the ambient environment (25). This study found that DEP suppressed IFN-γ mRNA and protein expression but spared expression of IL-4 or IL-5 by T cells. By contrast, production of IL-12p70 and IL-12p40 by DC was suppressed indicating that DEP could promote a Th2-type response by both T cells and DC (25). Many of these observations were consistent with the data that we report herein. Moreover, our data suggest that stimulation of DC with either DEP or CEP is functionally distinct from that provided by LPS or CD40L, and that DEP and CEP do not represent classical “danger” signals.
We found that in the resting state, myeloid DC expressed moderate levels of both cell-surface TLR2 and TLR4. Our observations were concordant with the work of others who have shown that under resting or unstimulated conditions, human myeloid DC express mRNA transcripts for TLR2 and TLR4 (26). Of relevance to our study, they showed that by flow cytometric analyses, human myeloid DC expressed high levels of TLR2 and moderate levels of TLR4 (26).
In our current study, the diminution in expression of TLR2 and TLR4 were consistent and unexpected observations after exposure of DC to either CEP or DEP. It is well known that both TLR2 and TLR4 serve critical functions in linking innate and adaptive immunity and since they recognize microbial products they initiate immune responses to a broad array of microbial challenge (27–30). Thus, the lack of expression TLR2 and TLR4 on human monocyte–derived DC after stimulation with DEP or CEP has several potential consequences. For example, the notion that exposure to LPS early in life redirects the developing immune system away from a Th2-bias at birth is a key provision of the hygiene hypothesis (31). Moreover, it has been shown that TLR2 and TLR4 agonists suppress allergic responses (30). In addition, expression of both TLR2 and TLR4 may be necessary to mount appropriate responses to bacterial agonists under conditions that may protect against allergic diseases such as asthma (30). Consistent with these hypotheses, our data suggest that exposure to car or diesel PM during childhood may be harmful, since the protective effects of LPS (or other danger signals that operate through TLR2 or TLR4) would be attenuated. This revision of the hygiene hypothesis would need to be addressed in carefully controlled experiments.
It is also plausible that down-regulation of TLR expression could render DC hypo-responsive to subsequent exposure to infectious agents. Others have suggested that PM may be immunosuppressive from studies in animal models (32–34). In one such study, exposure of already infected mice to inhaled airborne environmental particulate matter (PM10) both worsened the local infection and altered local pulmonary and systemic immunity (34). Because exacerbations of asthma or chronic obstructive pulmonary disease are usually driven by airway infections, our data suggest that one link between PM exposure and exacerbations of these conditions could be due to a relative immune suppression and predisposition to infections, although this hypothesis awaits determination. Alternatively, the diminution in expression of TLRs in response to DEP and CEP could be an inherent mechanism to limit the scope of pro-inflammatory airway hyperactivity and subsequent cellular damage due to oxidative stress.
Also, in murine models of airway inflammation, it has been shown that TLR4 is required for airway hyperactivity that is seen after subchronic ozone inhalation (35). In addition, in controlled exposure experiments in human subjects, exposure to an ambient concentration of DEP caused airway inflammation that was characterized by activated neutrophil influx and enhanced exhalation of carbon monoxide, which implicated oxidative stress being involved in the observed airway inflammation (36). Although TLRs were not studied, the authors confirmed that diesel exhaust particles provoke an inflammatory response in normal human subjects (36).
Myeloid DC are not by default pro-Th1 or pro-Th2; rather it is the nature of the stimulus (the “danger signal”) received by the DC that defines their ability to alter T cell differentiation programs (34, 37). In general, DC that secrete high levels of IL-12 are considered pro-Th1, whereas DC may more likely promote a Th2 T cell differentiation program should they exhibit suppressed IL-12 production. The reasons for this are unclear, but are thought to involve poor signaling through TLRs (27, 28).
We found that stimulation of DC with DEP provoked a pattern of cytokine secretion that in some ways was similar to but in other ways distinct from that seen with either LPS or CD40L. For example, both LPS and DEP elicited similar levels of TNF-α secretion, while CEP actually suppressed TNF-α production relative to both blank filter–stimulated DC and resting DC. However, when considering the production of IL-12, DEP induced levels of this cytokine that were augmented as compared with resting or blank filter–exposed DC but somewhat lower than levels produced by LPS-stimulated DC and much lower than the levels of IL-12 seen after stimulation of DC with CD40L. CEP did not significantly alter the production of IL-12 by DC. However, in co-cultures of T cells and DEP-exposed DC, we saw decreased production of both IL-12 and IFN-γ relative to LPS-stimulated DC, but elevated secretion of IL-13, a key cytokine in asthma. Therefore, exposure of DC to DEP results in a mixed pattern of T cell activation with elevated production of both Th1 (IFN-γ and IL-12) and Th2 (IL-13) cytokines. Given the increasing evidence that asthma is a disease with mixed Th1 and Th2 features (38), our results suggest that inhaled PM can contribute to immune activation in asthma by activating DC.
DEP-stimulated DC also secreted IL-6 and IL-18, consistent with that found for either LPS- or CD40L-stimulated DC. CEP-stimulated DC gave a suppressed production of IL-6 but an enhanced secretion of IL-18. IL-18 is a unique cytokine that may promote either Th1 or Th2 immune responses. In association with IL-12, IL-18 promotes Th1-mediated immunity that is characterized by enhanced production of IFN-γ. However, IL-18 is also capable of assisting a Th2-type immune response as well as allergic inflammation, and enhanced production of IL-18 has also been reported in individuals with allergic disorders (39, 40). Thus, by enhancing DC-dependent production of IL-18, inhaled PM could also augment airway inflammation in asthma.
VEGF exhibits both immune-modulating functions (39) as well as having an important role in vascular and extravascular remodeling and lung obstruction (40, 41). We showed that DEP (but not CEP) enhanced VEGF production at levels that were approximately 50% lower than those levels seen after DC were stimulated with LPS or CD40L. The presence of this cytokine is capable of skewing immune responses to a Th2-type adaptive inflammation (40). Although more work is warranted to appreciate the contributions made by VEGF in the model system that we have reported herein, the fact that DEP induced its production from DC could be important to early events occurring during Th differentiation. The role that the immune-modulating cytokine VEGF may play in this type of immune response also warrants further investigation.
Finally, we also confirmed that neither CEP nor DEP affected the functional activation of CD4+ T cells. We looked at a comprehensive panel of cell surface–expressed activation markers by flow cytometry as well as the secretion of inflammatory cytokines and cellular viability as enumerated by trypan blue exclusion. We found that the only parameter to be somewhat affected by direct interaction with either CEP or DEP was the percent of cells that survived interaction with the particulate matter, and this parameter of cellular behavior was only marginally compromised (∼ 10% of cells did not survive their interaction with DEP). However, we would contest that it would be extremely unlikely that this level of cell death was experienced by the CD+ T cells in a DC co-culture system should DC be responsible for any carry-over affect of endogenously taken up and subsequently released particulate matter. Other have also found that normal human CD4+ T cells are unaffected by diesel particulate matter, yet by contrast, T cells from those subjects with allergies and asthma exhibit responsiveness to stimulation by DEP (42). Since our CD4+ T cells were enriched from donor subjects known not to be allergic or atopic, our findings are concordant with that study (42).
We conclude that DEP- and to a lesser degree CEP-exposed DC exhibit a nonclassical and differential mode of activation that is phenotypically and functionally distinct from DC activated by classical stimuli such as LPS or CD40L. Moreover, DC activated by DEP direct a complex pattern of cytokine responses that in some ways are similar to but in other ways distinct from LPS-stimulated DC in term of both DC themselves and the T cells with which they react. Our DC and T cell co-culture data suggests that PM-exposed DC induces a mixed pattern of cytokine production, with enhanced production of both IL-13 and IFN-γ. Future studies will focus on the molecular and cellular mechanisms of action of DEP and CEP at the level of DC activation. It is anticipated that such work will contribute to a greater understanding of the role of subverted DC function in the setting of allergic diseases, and may open the way for targeted pharmacologic therapy to interrupt the detrimental effects of particulate matter on normal host innate immunity.
Acknowledgments
The authors thank Dr Peyton Eggleston and his team at The Johns Hopkins NIEHS Center for Childhood Asthma in the Urban Environment for their enthusiastic support. Dr. John McDyer (Johns Hopkins University) provided trimeric CD40 ligand.
This research was supported in part by a New Investigator Project from the Center for Childhood Asthma in the Urban Environment (funded by the NIEHS and EPA, 2P01ES09606 to S.G. and M.A.W.), research grants from the NIH (R01 HL073952 and HL071933 to S.G.), and a pilot project grant from the NIH (NIEHS P30 ES03819 to M.A.W.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0199OC on July 13, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities Study. Am J Respir Crit Care Med 2006;173:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A 2005;68:1071–1092. [DOI] [PubMed] [Google Scholar]
- 3.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol 2005;116:279–284. [DOI] [PubMed] [Google Scholar]
- 4.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction of between diesel exhaust particles and the immune system. J Allergy Clin Immunol 1998;102:539–554. [DOI] [PubMed] [Google Scholar]
- 5.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol 2005;115:221–228. [DOI] [PubMed] [Google Scholar]
- 6.Saxon A, Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat Immunol 2005;6:223–226. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich J, Wichman HE. Traffic related pollutants in Europe and their effect on allergic disease. Curr Opin Allergy Clin Immunol 2004;4:341–348. [DOI] [PubMed] [Google Scholar]
- 8.Miyabara Y, Ichinose T, Takano H, Lim HB, Sagai M. Effects of diesel exhaust on allergic inflammation in mice. J Allergy Clin Immunol 1998;102:805–812. [DOI] [PubMed] [Google Scholar]
- 9.Walters D, Breysse P, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med 2001;164:1438–1443. [DOI] [PubMed] [Google Scholar]
- 10.Mellman I, Steinman RM. Dendritic cells:specialized and regulated antigen processing machines. Cell 2001;106:255–258. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol (Paris) 2003;21:335–376. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B. Inferences, questions and possibilities in Toll-like receptor signaling. Nature 2004;430:257–263. [DOI] [PubMed] [Google Scholar]
- 13.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol 2001;13:291–298. [DOI] [PubMed] [Google Scholar]
- 14.Williams MA, Trout S, Spector SA. HIV-1 gp120 modulates the immunological function and expression of accessory and co-stimulatory molecules of monocyte-derived dendritic cells. J Hematother Stem Cell Res 2002;11:829–847. [DOI] [PubMed] [Google Scholar]
- 15.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol 2000;1:311–316. [DOI] [PubMed] [Google Scholar]
- 16.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005;172:530–551. [DOI] [PubMed] [Google Scholar]
- 17.McWilliam AS, Nelson D, Thomas J, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med 1994;179:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003;28:648–654. [DOI] [PubMed] [Google Scholar]
- 19.Wills-Karp M. Immunological basis of antigen-induced airway hyperresponseiveness. Annu Rev Immunol 1999;17:255–281. [DOI] [PubMed] [Google Scholar]
- 20.Lambrecht BN, Hammad H. Taking out breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol 2003;3:994–1003. [DOI] [PubMed] [Google Scholar]
- 21.Taussig D, Williams MA, Wiggins C, Feakins RM, Newland AC, Kelsey SM. Clinical regression and remission of primary refractory lymphoma following tumour peptide antigen-charged dendritic cells after high-dose chemotherapy and autologous stem cell rescue. Hematology 1998;3:277–289. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Porter M, Guo J, Roman J, Williams D, Breysse P, Georas SN. Ambient particulate matter directs non-classical immunological activation of dendritic cells and promotes Th2-like polarization. J Allergy Clin Immunol 2007;119:488–497. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003;3:133–146. [DOI] [PubMed] [Google Scholar]
- 24.Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol 2005;32:177–184. [DOI] [PubMed] [Google Scholar]
- 25.Ohtani T, Nakagawa S, Kurosawa M, Mizuashi M, Ozawa M, Aiba S. Cellular basis of the role of diesel exhaust particles in inducing Th2-dominant response. J Immunol 2005;174:2412–2419. [DOI] [PubMed] [Google Scholar]
- 26.Demedts IK, Bracke KR, Maes T, Joos GF, Bruselle GG. Different roles for human lung dendritic cell subsets in pulmonary immune defense mechanisms. Am J Respir Cell Mol Biol 2006;35:387–393. [DOI] [PubMed] [Google Scholar]
- 27.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol 1997;15:297–322. [DOI] [PubMed] [Google Scholar]
- 28.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88. J Immunol 2004;172:4527–4534. [DOI] [PubMed] [Google Scholar]
- 30.Velasco G, Campo M, Manrique OJ, Bellou A, He H, Arestides RS, Schaub B, Perkins DL, Finn PW. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am J Respir Cell Mol Biol 2005;32:218–224. [DOI] [PubMed] [Google Scholar]
- 31.Sabroe I, Parker LC, Wilson AG, Whyte MKB, Dower SK. Toll-like receptors: their role in allergy and non-allergic inflammatory disease. Clin Exp Allergy 2002;32:984–989. [DOI] [PubMed] [Google Scholar]
- 32.Aranyi C, Graf JL, O'Shea WJ, Graham JA, Miller FJ. The effects of intratracheally administered coarse mode particles on respiratory tract infection in mice. Toxicol Lett 1983;19:63–72. [DOI] [PubMed] [Google Scholar]
- 33.Hatch GE, Slade R, Boykin E, Hu PC, Miller FJ, Gardner DE. Correlation of effects of inhaled versus intratracheally injected males on susceptibility to respiratory infection in mice. Am Rev Respir Dis 1981;124:167–173. [DOI] [PubMed] [Google Scholar]
- 34.Jakab GJ. The toxicologic interactions resulting from inhalation of carbon black and acrolein on pulmonary antibacterial and antiviral defenses. Toxicol Appl Pharmacol 1993;121:167–175. [DOI] [PubMed] [Google Scholar]
- 35.Hollingsworth JW II, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med 2004;170:126–132. [DOI] [PubMed] [Google Scholar]
- 36.Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, Chung KF, Barnes PJ, Ashmore M, Newman-Taylor A. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med 2000;162:161–166. [DOI] [PubMed] [Google Scholar]
- 37.Zelikoff JT, Chen LC, Cohen MD, Fang K, Gordon T, Li Y, Nadziejko C, Schlesinger RB. Effects of inhaled ambient particulate matter on pulmonary antimicrobial immune defense. Inhal Toxicol 2003;15:131–150. [DOI] [PubMed] [Google Scholar]
- 38.Williams MA, Georas SN. Gene expression patterns and susceptibility to allergic responses. Expert Review of Clinical Immunology 2006;2:59–73. [DOI] [PubMed] [Google Scholar]
- 39.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med 2003;197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Mezzein RE, Matsumoto T, Nomiyama H, Miike T. Increased secretion of IL-18 in vitro by peripheral blood mononuclear cells of patients with bronchial asthma and atopic dermatitis. Clin Exp Immunol 2001;126:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boissel N, Rousselot P, Raffoux E, Cayuela JM, Maarek O, Charron D, Degos L, Dombret H, Toubert A, Rea D. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia 2004;18:1656–1661. [DOI] [PubMed] [Google Scholar]
- 42.Mamessier E, Nieves A, Vervloet D, Magnan A. Diesel exhaust particles enhance T cell activation in severe asthmatics. Allergy 2006;61:581–588. [DOI] [PubMed] [Google Scholar]