Abstract
To better understand the role of facet joint degeneration in chronic neck and back pain epidemiological and morphological data are needed. For the cervical spine, however, such data are rare. Therefore, the aim of this study was to determine the degree of cartilage degeneration of cervical facet joints with respect to spinal level and age, to investigate whether any region of the joint surface is more often affected by degeneration and to determine the localisation of osteophytes. A total of 128 left-sided facet surfaces from 15 fresh frozen cervical spine specimens (59–92 years) including in maximum C2–C7 were inspected in a way to ensure a direct comparability to data reported for the lumbar spine. First, the macroscopic degree of cartilage degeneration was determined and correlated to spinal level and age. Then, each facet surface was divided into five regions (anterior, posterior, lateral, medial and central) to check whether cartilage degeneration occurs more often in any of these regions. Finally, the localisation of osteophytes was determined. The results showed that the mean degree of cartilage degeneration was 2.8 (±0.6) on a scale from Grade 1 (no degeneration) to 4 (severe degeneration). None of all 128 facet surfaces was classified as Grade 1. All spinal levels had about the same degree of degeneration (in mean 2.5–3.0). The youngest age group (<70 years) had a somewhat lower degree of degeneration (2.6) than the oldest (≥90 years) (3.1). Cartilage defects were found all over the joint surfaces, none of the five regions was more often affected than the others. Least osteophytes were found on the medial border of the facet joints. In conclusion, the prevalence of cervical facet joint degeneration is probably very high in individuals aged 50 years and more, with a tendency to increase in severity with age. All levels of the middle and lower cervical spine were affected to almost the same degree, whereas in the lumbar spine an increase in degeneration towards the lower levels was reported. Also, in the cervical spine in most cases the cartilage was evenly degenerated all over the joint surface while in the lumbar spine certain regions were reported to be affected predominantly.
Keywords: Cervical spine, Facet joint, Degeneration, Morphology
Introduction
There is still a controversial discussion on the role of facet joint degeneration in chronic neck and back pain. Yet, recently, implants have been developed to replace the posterior elements [17]. As a rationale for the treatment with such implants, and to better understand the role of facet joint degeneration in chronic neck and back pain epidemiological and morphological data are needed. In the past, the morphology of lumbar facet joint degeneration has been described by means of macroscopic inspection [11, 12, 18], histology [4] and clinical imaging techniques such as conventional tomography, computed tomography, magnetic resonance imaging or plain radiography [1, 7, 9, 15]. In these studies, the pathological changes attributed to facet joint degeneration are articular cartilage thinning, sclerosis of the subchondral bone, osteophyte formation or hypertrophy. Most recently, for the lumbar spine it was shown that the mean degree of facet joint degeneration in the population aged 50 years and more lies between 2.8 in the upper and 3.7 in the lower lumbar spine on a scale from 1 (no degeneration) to 4 (severe degeneration) [13]. From these results it was concluded that the prevalence of lumbar facet joint degeneration in the population aged 50 years and more is relatively high. In contrast, for the cervical spine only few morphological and epidemiological studies exist [6, 7, 10]. Furthermore, how the degenerative changes described in these studies correlate with age or spinal level and how these data are compared with the lumbar spine is sill unknown.
The aim of this study therefore was to determine the degree of cartilage degeneration of cervical facet joints with respect to spinal level and age, to investigate whether any region of the joint surface is more often degenerated than the others and to determine the localisation of osteophyte formation. Finally, the results will be compared with the data reported for the lumbar spine.
Materials and methods
A total of 128 left- and 127 right-sided facet surfaces from 15 fresh frozen cervical spine specimens (59–92 years) including in maximum C2–C7 were inspected (Table 1). None of these specimens showed radiographic signs of injuries or spinal diseases other than degeneration. The segments C0–1 and C1–2 were excluded from this study since their anatomical characteristics strongly differ from those of the middle and lower cervical segments.
Table 1.
Specimens inspected in this study sorted by age for females and males
| No. of specimens | Sex | Age (years) | n left side | n right side |
|---|---|---|---|---|
| 1 | f | 72 | 6 | 6 |
| 2 | f | 75 | 7 | 8 |
| 3 | f | 79 | 10 | 9 |
| 4 | f | 81 | 8 | 8 |
| 5 | f | 86 | 10 | 10 |
| 6 | f | 87 | 7 | 7 |
| 7 | f | 87 | 8 | 8 |
| 8 | f | 87 | 10 | 9 |
| 9 | f | 91 | 10 | 10 |
| 10 | f | 92 | 8 | 8 |
| 11 | m | 59 | 10 | 10 |
| 12 | m | 67 | 8 | 8 |
| 13 | m | 72 | 10 | 10 |
| 14 | m | 74 | 8 | 8 |
| 15 | m | 92 | 8 | 8 |
| 10 f:5 m | Mean = 80; SD = 10; median = 81; min = 59; max = 92 | Sum = 128 | Sum = 127 |
n number of left- and right-sided facet joint surfaces per specimen. The left-sided surfaces were finally evaluated. Their number varied from specimen to specimen since the length of the specimens differed and single facet surfaces were damaged during harvesting and could therefore not be included into the evaluation. SD standard deviation
After thawing the specimens at room temperature, the facet joints were exarticulated. Digital photographs of the joint surfaces were taken and evaluated on the computer monitor. The whole evaluation process was adapted to the study by Tischer et al. [13] on lumbar facet joint degeneration in order to allow a direct comparison with the lumbar spine. Also, similar to Tischer et al., two observers were asked to evaluate the joints surfaces together and to find a consensus in each case. The evaluation consisted of three parts.
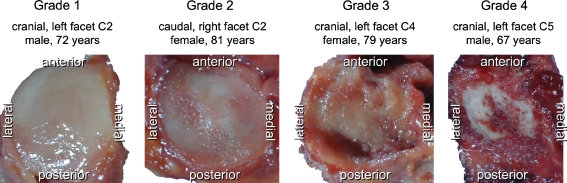
First, the degree of cartilage degeneration of each single joint surface was determined using the classification system proposed by Wang et al. [14]. This system is based on a macroscopic inspection of the cartilaginous surface of the facet joints and assigns one of the Grades 1–4 to each surface (Fig. 1).
1: Uniform thickness of cartilage with a smooth surface covering the articular surface completely.
2: Cartilage covers the entire articular surface but contains erosions or regions of irregularity.
3: Cartilage incompletely covers the articular surface, with the underlying bone exposed to the joint space and the cartilage present often irregular and discoloured.
4: Either complete absence of cartilage or only traces of cartilage evident on the articular surfaces.
Second, each facet surface was divided into five regions (antero-superior called anterior, posterio-inferior called posterior, lateral, medial, central) to check whether or not cartilage degeneration is distributed evenly all over the facet surface. This evaluation was adapted to Tischer et al. [13] and did not take into account the degree but only the presence or absence of cartilage degeneration.
Fig. 1.
Representative cervical facet joint surfaces with increasing cartilage degeneration in Grade 1 (no degeneration) to 4 (severe degeneration) according to Wang et al. Note that the example for Grade 1 is a superior facet of C2 which was not included in this study
Third, each joint was screened for osteophytes. Similar to Tischer et al. [13], their localisation was determined (medial, lateral, anterior, posterior) while the length of the osteophytes was not considered. Osteophytes were defined as local (but not generalised) bony apposition. Thus, facet hypertrophy was not included in this evaluation.
The Wilcoxon signed rank test was used to check whether the degree of degeneration of the left-sided facet joints significantly differed from those on the right side. If not, one of the two sides would be excluded from evaluation. In order to statistically compare the overall degrees of degeneration of the different age ranges with each other the Kruskal–Wallis test was used followed by the Wilcoxon rank sum test for pair-wise comparisons if necessary. These statistical tests were explorative and were not meant to prove a certain hypothesis. Therefore, the P values were not corrected for multiple comparisons.
Results
None of all the 128 left- and 127 right-sided facet joint surfaces (caudal facets of C2 to cranial facets of C7) had a degeneration Grade 1 according to Wang et al. [14], i.e. all inspected surfaces had at least some cartilage erosions or surface irregularities.
The degree of cartilage degeneration of all left-sided facet joints only slightly differed from those on the right side (P > 0.05). The difference between left and right side was in mean 0.0 (±0.7 standard deviation). Therefore, only one of the two sides—the left side—was included into the following evaluations.
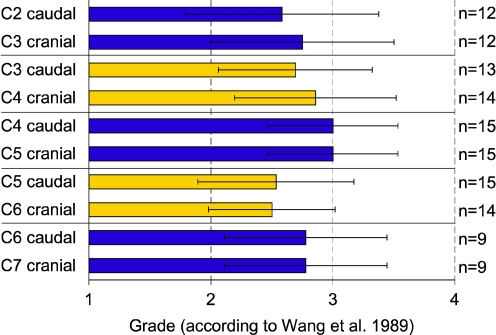
The degree of degeneration at different spinal levels from the caudal facets of C2 to the cranial facets of C7 was very similar (Fig. 2). The mean values varied between 2.5 and 3.0 on Wang’s scale from 1 (no degeneration) to 4 (severe degeneration). Due to these similarities statistical tests were not carried out. Instead, one single mean degree of degeneration was calculated for all 128 surfaces. This value was 2.8 (±0.6).
Fig. 2.
Grade of facet joint degeneration according to Wang et al. with respect to spinal level. Mean values with standard deviation. Left-sided facet joints only. n number of joint surfaces per spinal level
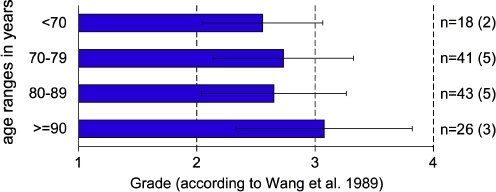
With increasing age the degree of cartilage degeneration tended to increase (Fig. 3). Yet, already for the youngest age-group <70 years the mean degree of degeneration was 2.6. For the oldest age group ≥90 years this value was 3.1. The Kruskal–Wallis test indicated some differences between the four age groups. Therefore, pair-wise comparisons were carried out, which revealed the lowest P values for the oldest compared to each of the three younger age groups (P below or only little above 0.05).
Fig. 3.
Grade of facet joint degeneration according to Wang et al. with respect to age. Mean values with standard deviation. All spinal levels of the left-sided facets were put together. n number of facet joint surfaces (number of donors in parentheses) per age range
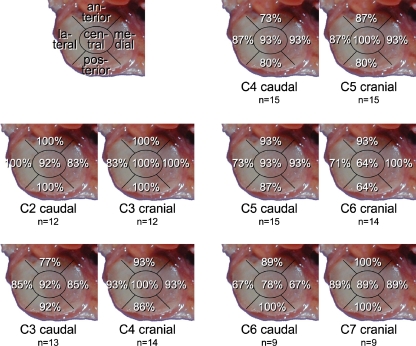
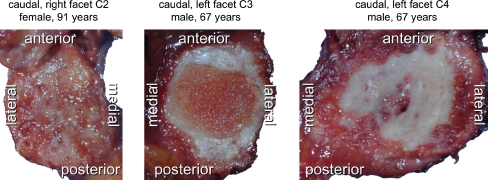
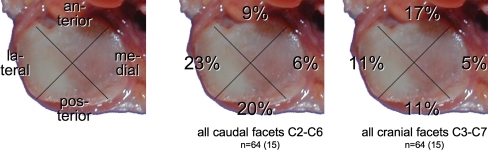
Cartilage defects were found all over the joint surfaces. None of the five regions was more often affected than the others at any spinal level (Fig. 4). However, while most facets had defects all over their surface, few had larger defects centrally and few had larger defects in the periphery (Fig. 5).
Fig. 4.
Percentage of all facet joint surfaces with cartilage defects in the respective region: anterior, posterior, lateral, medial and central. Left-sided facets only. n number of joint surfaces
Fig. 5.
Representative cervical facet joint surfaces with cartilage defects all over the joint surface (left, most cases), with defects, which are stronger centrally (middle, only few cases) and with defects, which are stronger in the periphery (right, only few cases)
Least osteophytes were found on the medial border of the facet joints (Fig. 6).
Fig. 6.
Percentage of all left-sided facet joint surfaces with osteophytes at the respective localisation: anterior, posterior, lateral and medial. n number of joint surfaces (number of donors in parentheses)
Discussion
In this study, the morphology of cervical facet joint degeneration was investigated and correlated to spinal level and age in a way that allowed direct comparison to data reported for the lumbar spine.
The prevalence of cervical facet joint degeneration in the sample inspected in this study was 100%. All 128 left- and 127 right-sided facet surfaces had at least degeneration Grade 2 (mild degeneration) according to Wang et al. but none a Grade 1 (no degeneration). The description of Fletcher et al. [2] of how the facet joint surfaces look like in specimens under 20 years of age matches well with Grade 1. Thus, Grade 1 can probably only be seen in younger individuals. Even though, in the present study, the number of specimens was only 15, this finding indicates that the prevalence of cervical facet joint degeneration in individuals older than 57 years is probably high. A similar trend was also reported for the lumbar spine [13]. In view of this probably high prevalence of cartilage thinning and erosions in the elderly population it could be suspected that these changes should not be considered pathologic but rather reflect the normal ageing process.
In the youngest age range investigated in this study (<70 years), the mean degree of degeneration was already 2.6. This value slightly increased towards the oldest age range (≥90 years), which indicates that facet joint degeneration tends to increase in severity. This finding is supported by Fletcher et al. [2] who studied the cervical facet joints of 20 cadavers with magnetic resonance imaging, computed tomography, cryomicrotomy and histologic sections to determine the anatomic changes that occur with age. Their results showed that the articular processes of individuals under 20 years of age were covered by a layer of glistening homogeneous ivory-white cartilage while individuals older than 37 had less articular cartilage. In contrast, for the lumbar spine, Tischer et al. [13] could not detect any age-dependent increase of the severity of degeneration even though a very similar age range (Tischer et al.: 52–97 years; present study: 59–92 years) was investigated. This could be explained by the higher overall degree of degeneration reported for the lumbar compared to the cervical spine. Already in the youngest age range Tischer et al. reported in mean Grade 3.1 for males and 2.8 for females, while in the present study this value was 2.6 for males and females together. Whether the facet joints of the lumbar spine really tend to be more severely degenerated than those of the cervical spine at one and the same age is difficult to answer since lumbar and cervical spine were rated by different observers. Unfortunately, the interobserver reliability of the system of Wang et al. [14] is unknown but at least part of the difference between lumbar and cervical spine are probably attributable to this source of error. Also, the specimens used by Tischer et al. were fixed with formalin whereas the specimens inspected in the present study were fresh frozen. Formalin fixation is known to change the colours of the tissue and therefore could have influenced the grading.
There was almost no difference in the mean degree of degeneration between the middle and lower cervical spine while for the lumbar spine an increase in degeneration towards the lower lumbar levels was described [13]. Thus, even though intervertebral disc degeneration is known to affect the segments of the lower cervical spine more often than those of the middle cervical spine [3], this is not the case for the facet joints. This observation again raises the question whether disc degeneration is really one of the main causes of facet joint degeneration or whether facet joint degeneration may also occur independently from disc degeneration as suspected in literature [4, 11].
Another difference between the lumbar and cervical spine is the type of distribution of the cartilage defects on the joints surface. In the cervical spine the facet joints tend to degenerate evenly all over their surface while in the lumbar spine the superior region of the cranial facets and the inferior region of the caudal facets are reported to be more often affected [13]. Other authors also showed that the cartilage of the lumbar facet joints tends to degenerate locally rather than evenly [11, 12]. This difference between the lumbar and cervical spine could be explained by differences in facet orientation and shape. While in the lumbar spine the facets are oriented almost in cranio-caudal direction and are convex (caudal facets) respectively concave (cranial facets) in shape, the facets of the cervical spine are oriented obliquely from antero-superior to postero-inferior and are almost plane [8]. These morphological characteristics of the cervical facets together with the larger range of motion compared to the lumbar spine [16] could be responsible for an almost even stress distribution all over the facet surface. However, as soon as the facets are not completely plane, but somewhat convex or concave, the stresses might mainly act in the middle or in the periphery of the surfaces as seen in few of the facets inspected in this study (Fig. 5). In contrast, for the lumbar spine, Tischer et al. [13] hypothesised that during flexion/extension movements only certain regions of the surfaces have to bear maximum load since the caudal facets slide upwards and tilt anteriorly relative to the cranial facets. These are all assumptions, but they could be correct since an influence of facet joint angle on facet joint degeneration was already shown [5].
Osteophytes were found all around the cervical facet joints, however, least were detected medially. This was reported to be the same in the lumbar spine [13]. It is difficult to answer why this is the case, yet as an advantage the neuroforamen become less narrowed. In this study the assessment of osteophytes proved to be difficult since the contour of the facet joints can physiologically be irregular. An irregular contour can therefore not generally be considered as a sign of osteophyte formation. Also, it has to be considered that hypertrophy was not included into this evaluation. Thus, the percentages given in Fig. 6 just show where definite, local osteophytes were found. In cases where no osteophytes were found there could still be a hypertrophy. However, hypertrophy as well is difficult to quantify since the border between normal and hypertrophic is not always clear especially in cases where a comparison with a “healthy” facet is not possible.
Conclusions
In conclusion, the prevalence of cervical facet joint degeneration in individuals aged 50 years and more is probably high, with a tendency to increase in severity with age. Compared to the lumbar spine similarities and differences were found. For example, in both the cervical and lumbar spine, least osteophytes were found on the medial rim of the joint surfaces. Furthermore, the overall degree of degeneration was only little higher in the lumbar (2.8–3.7 for the different levels) than in the cervical spine (2.5–3.0), a difference, which should not be overestimated as discussed above. On the other hand, in the cervical spine all levels of the middle and lower cervical spine were affected to almost the same degree whereas in the lumbar spine an increase in degeneration towards the lower segments was reported. Also, in the cervical spine in most cases the cartilage was evenly degenerated all over the joint surface while in the lumbar spine most defects of the cranial facets were reported to be present in the superior region and most defects of the caudal facets in the inferior region.
Acknowledgment
The authors gratefully acknowledge the Deutsche Arthrose-Hilfe e.V. for financial support.
References
- 1.Demaerel P, Wilms G, Goffin J, Baert AL. Osteoarthritis of the facet joints and its role in low-back pain: evaluation with conventional tomography. J Belge Radiol. 1992;75(2):81–86. [PubMed] [Google Scholar]
- 2.Fletcher G, Haughton VM, Ho KC, Yu SW. Age-related changes in the cervical facet joints: studies with cryomicrotomy, MR, and CT. AJR Am J Roentgenol. 1990;154(4):817–820. doi: 10.2214/ajr.154.4.2107682. [DOI] [PubMed] [Google Scholar]
- 3.Friedenberg ZB, Miller WT. Degenerative disc disease of the cervical spine. J Bone Joint Surg Am. 1963;45:1171–1178. [PubMed] [Google Scholar]
- 4.Gries NC, Berlemann U, Moore RJ, Vernon-Roberts B. Early histologic changes in lower lumbar discs and facet joints and their correlation. Eur Spine J. 2000;9(1):23–29. doi: 10.1007/s005860050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grogan J, Nowicki BH, Schmidt TA, Haughton VM. Lumbar facet joint tropism does not accelerate degeneration of the facet joints. AJNR Am J Neuroradiol. 1997;18(7):1325–1329. [PMC free article] [PubMed] [Google Scholar]
- 6.Kellgren JH, Jeffrey MR, Ball J (1963) The epidemiology of chronic rheumatism. Atlas of standard radiographs of arthritis, vol II. Blackwell Scientific Publications, Oxford, pp 14–19
- 7.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panjabi MM, Oxland T, Takata K, Goel V, Duranceau J, Krag M. Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine. 1993;18(10):1298–310. doi: 10.1097/00007632-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164(1):227–30. doi: 10.1148/radiology.164.1.3588910. [DOI] [PubMed] [Google Scholar]
- 10.Silberstein CE. The evolution of degenerative changes in the cervical spine and an investigation into the “joints of Luschka”. Clin Orthop. 1965;40:184–204. [PubMed] [Google Scholar]
- 11.Swanepoel MW, Adams LM, Smeathers JE. Human lumbar apophyseal joint damage and intervertebral disc degeneration. Ann Rheum Dis. 1995;54(3):182–188. doi: 10.1136/ard.54.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanno I, Oguma H, Murakami G, Sato S, Yamashita T. Which portion in a facet is specifically affected by articular cartilage degeneration with aging in the human lumbar zygapophysial joint? Okajimas Folia Anat Jpn. 2003;80(1):29–34. doi: 10.2535/ofaj.80.29. [DOI] [PubMed] [Google Scholar]
- 13.Tischer T, Aktas T, Milz S, Putz RV. Detailed pathological changes of human lumbar facet joints L1–L5 in elderly individuals. Eur Spine J. 2006;15(3):308–315. doi: 10.1007/s00586-005-0958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Yu S, Haughton VM. Age-related changes in the lumbar facet joints. Clin Anat. 1989;2:55–62. doi: 10.1002/ca.980020202. [DOI] [Google Scholar]
- 15.Weishaupt D, Zanetti M, Boos N, Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28(4):215–219. doi: 10.1007/s002560050503. [DOI] [PubMed] [Google Scholar]
- 16.White AA, Panjabi MM (1990) Clinical biomechanics of the spine, 2nd edn. J.B. Lippincott Company
- 17.Wilke HJ, Schmidt H, Werner K, Drumm J, Claes L. Biomechanical evaluation of a new total posterior-element replacement system. Spine. 2006;31(24):2790–2796. doi: 10.1097/01.brs.0000245872.45554.c0. [DOI] [PubMed] [Google Scholar]
- 18.Ziv I, Maroudas C, Robin G, Maroudas A. Human facet cartilage: swelling and some physicochemical characteristics as a function of age. Part 2: age changes in some biophysical parameters of human facet joint cartilage. Spine. 1993;18(1):136–146. doi: 10.1097/00007632-199301000-00020. [DOI] [PubMed] [Google Scholar]