Abstract
The proinflammatory mediator (PIM) levels were assessed in surgically removed samples of herniated cervical intervertebral discs. The objective of this study was to investigate if there is a correlation between the levels of PIMs in disc material and myelopathy associated with cervical intervertebral disc herniation and spondylosis. The role of proinflammatory mediators in the degeneration of intervertebral disc and the inflammatory effects of disc herniations on radicular pain has been previously published. However, the possible relationship between PIMs and myelopathy related to cervical disc herniation and spondylosis has not been investigated before. Thirty-two patients undergoing surgery for cervical disc herniation and spondylosis were investigated. Surgically obtained disc materials, stored at 70°C, were classified into two groups: cervical disc herniation alone or with myelopathy. Biochemical preparation and solid phase enzyme amplified sensitivity immunoassay (ELISIA) analysis of the samples were performed to assess the concentration of mediators in the samples. Very similar values of interleukin-6 were found in both groups whereas the concentrations of mediators were significantly higher in myelopathy group. This study has demonstrated that PIMs are involved in cervical intervertebral disc degeneration with higher concentrations in the samples associated with myelopathy.
Keywords: Proinflammatory mediators, Degenerated cervical intervertebral disc herniation, Myelopathy, Intensity changes on MRI
Introduction
Possible biochemical mechanisms of lumbar intervertebral disc degeneration and its chemical effects on neural structures have been studied for many years [7, 9, 10, 13, 14, 17, 18]. However, it is not certain whether the same mechanism can be adjusted to cervical intervertebral discs. In literature, there are few studies, which analyzed the biochemistry of cervical disc herniation [1, 5, 9].
In this study, the authors aimed to investigate if there is a correlation between high PIM levels in degenerated cervical disc materials and myelopathy associated with cervical disc herniation and spondylosis. The results may provide a further insight to find new therapeutic targets to prevent their chemical effects on neural tissue.
Materials and methods
The study was carried out during the period from 2000 to 2003 on non-randomizedly selected 32 patients who had operations on cervical disc herniation and spondylosis with or without myelopathy. The patients include 12 women and 20 men ranging in age from 42 to 63 years. Twelve of the patients had clinical myelopathy findings, such as hyper-reflexia and spasticity in lower extremities (7 patients). Varying degrees of spondylotic changes in cervical spine were found in all patients besides cervical disc herniations. Spondylotic changes were confirmed with computerized tomography scans and plain roentgenograms. In addition to these imaging techniques, all patients were evaluated for associated myelopathy by using axial and sagittal T1 and T2 weighted 1,5 T MR scanner. The patients were divided into two groups: the ones without myelopathy (20 patients) (Group 1) and the ones with myelopathy in the adjacent spinal cord segments on MR images (12 patients) (Group 2) (Fig. 1). Patients with a history of previous cervical disc surgery, systemic inflammatory disease, neoplasm and trauma were not included.
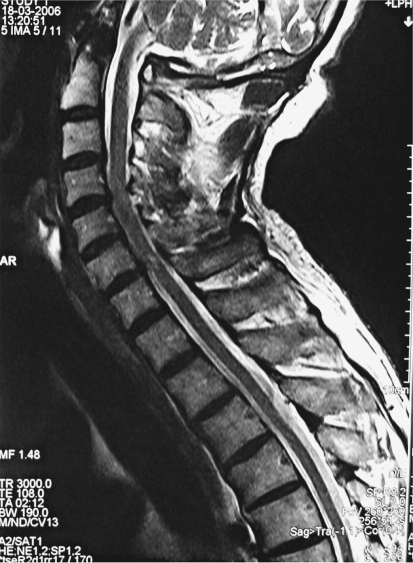
Fig. 1.
Cervical MR image of a 52-year-old woman presenting with clinical symptoms of myelopathy. Preoperative sagittal T2-weighted image shows intramedullary high signal intensity
The patients were operated via standard anterior approach. The appropriate level was confirmed with intraoperative fluoroscopy before disc removal. Meticulous blood control was achieved in the field before incising the anterior longitudinal ligament to prevent contamination of the disc material with blood and a sample was taken in the center of nucleus pulposus with a disc forceps. All biopsy samples were stored at −70°C.
The biochemist (NG) performing the laboratory studies was blinded about the patients’ clinical and radiological findings. Biochemical preparation and analysis of the samples were performed as described by Nygaard et al. [13]. To determine the concentrations of PIMs in the samples, the assay systems based on solid-phase enzyme amplified sensitivity immunassay (EASIA) (for interleukins-1β, −6, −8, interferon-gamma and tumor necrosis factor-alpha) and competitive enzyme immunoassay (EIA) (for thromboxane-B2 and leukotriene-B4) were used. The EASIA (Biosource Europe S.A., Nivelles, Belgium, with the catalogue numbers of KAC 1211, 1261, 1301, 1231 and 1751, respectively) and EIA kits (Cayman Chemical Company, MI 48108, USA) are commercially available.
Statistical analysis
The collected data were encoded into SPSS (Statistical Package for Social Sciences)/PC version 10.0 and analyzed. The association between the levels of PIMs and cervical disc herniation with or without myelopathy was assessed by means of Mann–Whitney U test. The results were calculated in 95% confidence interval and the level of significance was set at P < 0.05, two tailed.
Results
The values of PIMs in samples are shown in Table 1. The following results were obtained from the distribution of values.
Very similar values of interleukin−6 were found in both groups.
Higher values of mediators (IL-1β, IL-8, IFN-γ, TNF-α, TX-B2 and LT-B4) were found in Group 2.
Table 1.
The mean values of PIMs in cervical intervertebral disc samples
| Group 1 | Group 2 | P | |
|---|---|---|---|
| IL-1 β | 449.45 ± 211.99 | 882.25 ± 262.97 | 0.0001 |
| IL-6 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.6872* |
| IL-8 | 10.7 ± 5.44 | 32.08 ± 1.98 | 0.00001 |
| IFN-GAMA | 3.12 ± 2.83 | 20.83 ± 5.92 | 0.00001 |
| TX-B2 | 94.57 ± 72.9 | 1,008.08 ± 341.08 | 0.00001 |
| LT-B4 | 16.74 ± 8.9 | 104.17 ± 110.51 | 0.00001 |
| TNF-ALFA | 117.2 ± 18.83 | 134.88 ± 27.88 | 0.0042 |
* Statistically not significant (P > 0.05)
Discussion
The inflammatory aspect of intervertebral disc degeneration and radiculopathy has been investigated for many years. Although their roles in the pathogenesis of such conditions were supported by many studies, it could not have been totally proven because of some conflicting results. Most of the studies have been performed in lumbar region, but a few of them pertain to cervical intervertebral discs [2, 6, 9, 13, 14]. To our knowledge, there is no study addressing the role of inflammatory mediators in myelopathy associated with cervical spondylosis.
Inflammatory process can be triggered by a biomechanical trauma or even by environmental factors such as stress, body weight, and smoking. They exert their degenerative effects on intervertebral discs through matrix metalloproteinases, which have been implicated in the breakdown of extracellular matrix components during disc degeneration [3, 4, 11, 12, 16, 19, 20].
It is also suggested that PIMs, such as cytokines and chemokines, are responsible for the chemical pathomechanism of nerve root injury [2, 8–10, 13, 14, 17, 18].
The exact pathomechanism by which an intervertebral disc herniation and spondylosis lead to neural tissue damage within the spinal cord has not been determined. But, it is obvious that there must be more than a mechanical mechanism. It was experimentally demonstrated that autologous nucleous pulposus could cause histologic and functional changes in spinal nerve roots when applied epidurally [2]. Kawakami et al. [10] also demonstrated experimentally that the application of autologous nucleous pulposus to the nerve root produces transient mechanical hyperalgesia, which is completely eliminated by epidurally injected phospholipase A2 inhibitor and therefore postulated the possible relation between the mechanical hyperalgesia and the production of arachidonic acid and its metabolites.
Patients with non-contained intervertebral disc herniation have more severe clinical appearance than that of patients with contained herniation. Nygaard et al. [13] reported that they observed higher levels of LT-B4 and TX-B2 in non-contained disc herniations when compared with the levels in contained disc herniations. Therefore, they suggest the possible effects of these mediators on nerve root. Even a small amount of inflammatory mediators are capable of further inflammatory response with the recruitment of cytokine-producing cells and upregulation of genes for PIMs [10, 14, 15, 18].
In the present study, PIM levels were detected by using solid phase enzyme amplified sensitivity immunoassay (ELISIA) analysis. We aimed to investigate the possible correlation between high PIM levels and myelopathy associated with cervical spondylosis. Since the myelopathy group was compared with non-myelopathy group, a control group was not designed.
Because there are a few studies on inflammatory mediators in cervical intervertebral discs, we needed to consider the results of lumbar studies, although cervical spine has different anatomical structure and biomechanical features. The results of the present study contrary to the studies, which failed to demonstrate inflammation in chronic disc herniation demonstrate higher values of PIMs in Group 2 and similar IL-6 values in both groups. Our results may indicate ongoing inflammatory process in cervical spondylosis [6]. Furthermore, these remarkable results may suggest biochemical inducement of myelopathy in intervertebral disc herniation and spondylosis.
Conclusion
The present study has demonstrated that PIMs are involved in cervical intervertebral disc degeneration with higher concentrations in the nucleus pulposus associated with myelopathy. However, there is need for further studies to prove their role in pathogenesis of myelopathy. In such condition, inhibition of some certain PIMs may become a therapeutic target for prevention of myelopathy resulting from cervical disc herniation and spondylosis.
References
- 1.Baba H, Maezawa Y, Furusawa N, Fukuda M, Uchida K, Kokubo Y, Imura S. Herniated cervical intervertebral discs: histological and immunohistochemical characteristics. Eur J Histochem. 1997;41(4):261–270. [PubMed] [Google Scholar]
- 2.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujita K, Nakagawa T, Hirabayashi K, Nagai Y. Neutral proteinases in human intervertebral disc. Spine. 1993;18:1766–1773. doi: 10.1097/00007632-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Furusawa N, Baba H, Miyoshi N, Maezawa Y, Uchida K, Kokubo Y, Fukuda M. Herniation of cervical intervertebral disc: immunohistochemical examination and measurement of nitric oxide production. Spine. 2001;26(10):1110–1116. doi: 10.1097/00007632-200105150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Goupille P, Jayson MIV, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum. 1998;28(1):60–71. doi: 10.1016/S0049-0172(98)80029-2. [DOI] [PubMed] [Google Scholar]
- 7.Gruber H, Hanley EN. Analysis of aging and degeneration of the human intervertebral disc: comparison of surgical specimens with normal controls. Spine. 1998;23(7):751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Jimbo K, Park JS, Yokosuka K, Sato K, Nagata K. Positive feedback loop of interleukine-1β upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2005;2:589–595. doi: 10.3171/spi.2005.2.5.0589. [DOI] [PubMed] [Google Scholar]
- 9.Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostoglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami M, Matsumoto T, Tamaki T. Roles of thromboxane A2 and leukotriene B4 in radicular pain induced by herniated nucleus pulposus. J Orthop Res. 2001;19:472–477. doi: 10.1016/S0736-0266(00)90032-9. [DOI] [PubMed] [Google Scholar]
- 11.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the disc pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier CA, Bobbioni E, Gabay C, et al. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87:1184–1188. doi: 10.1210/jc.87.3.1184. [DOI] [PubMed] [Google Scholar]
- 13.Nygaard ØP, Mellgren S, Østerud B. The inflammatory properties of contained and non-contained lumbar disc herniation. Spine. 1997;22(21):2484–2488. doi: 10.1097/00007632-199711010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Sang-Ho A, Yoon-Woo C, Myun-Whan A, Sung-Ho J, Yoon-Kyung S, Hee-Sun K. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27(9):911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Scuderi GJ, Brusovamik VG, Greg Anderson D, Dunham CJ, Vaccaro AR, Demeo RF, Hallab N. Cytokine assay of the epidural space lavage in patients with lumbar intervertebral disc herniation and radiculopathy. J Spinal Disord Tech. 2006;19(4):266–269. doi: 10.1097/01.bsd.0000204501.22343.99. [DOI] [PubMed] [Google Scholar]
- 16.Shen B, Melrose J, Ghosh P, Taylor TKF. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposes cells grown in three-dimensional agarose gel culture by interleukin-1β: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66–75. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- 17.Sobajima S, Shimer A, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine. 2004;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi H, Suguro T, Okajima Y, Motegi M, Okada Y, Kakjuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Tzusaki M, Guyton G, Garrett W, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 20.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. Expression of matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]