Abstract
We conducted a cross-sectional study of 40 radiculopathy patients in comparison with norm data from healthy subjects using a new electrophysiological method. Early manifestations of dorsal root impairment escape objective diagnosis by conventional somatosensory-evoked potentials due to the overlapping innervation of the affected dermatome by thickly myelinated mechanoreceptive afferents projecting to adjacent intact roots. Evidence suggested less intersegmental overlap for thermonociceptive afferents rendering laser-evoked potentials (LEP) sensitive to monosegmental dorsal root damage. Therefore we used this new method to study acute manifestations of monosegmental dorsal root pathology. Dorsal root function was tested in 12 healthy subjects and 40 sciatica patients by intraindividual interside comparison. Mechanosensibility and thermosensibility were clinically investigated. LEP were induced by moderately painful laser stimuli. The LEP were evaluated by amplitude and latency of the averaged electroencephalogram. Normal interside differences of LEP for amplitude were ±22% (lower limb) and ±35% (upper limb) and ±15 to ±16% for latency. Twenty-six patients (65%) showed significant LEP changes, mainly amplitude decreases. Six of these patients exhibited latency prolongations. Clinical testing yielded more frequent pathological results for pain compared to mechanosensibility. The study confirmed our preliminary evidence of LEP sensitivity to objectively document dorsal root impairment in patients suffering from acute monosegmental radiculopathy. This result opens the perspective of electrophysiologically differentiating the presence or absence of dorsal root pathology in patients with similar clinical symptoms but possibly different prognoses, which require different therapies.
Keywords: Laser-evoked potential, Sciatica, Dorsal root impairment, Dermatomal-evoked potentials, Radiculopathy
Introduction
The radicular pain syndrome is a frequent disease arising from the dorsal root that is characterized by segmental pain or dysesthesia and paresis. Despite exhibiting a rather homogenous clinical symptomatology radiculopathies underly diverse pathophysiological mechanisms [11, 12, 25–27, 35, 48, 49]. Such diversity could have impact on the optimal time point and the kind of therapeutical interventions relevant for long-term prognosis. Up to 20–30% of sciatica patients have persisting symptoms longer than 3 months after its onset [5]. The current pathophysiological concepts of dorsal root damage differentiate between biochemical and mechanical processes that both interact with electrophysiological dorsal root properties significantly [11, 12, 26, 27, 42]. However, no electrophysiological or other objective methods are currently available to document and quantify degrees of dorsal root impairment and discriminate pathophysiological mechanisms [15, 16, 38, 47].
Past electrophysiological studies attempted to objectively document and grade dorsal root impairment by stimulation of the nerve stems or peripheral sensory endings supplying a dermatome of a given dorsal root and record the elicited somatosensory-evoked potentials (SEP) [1, 2, 14, 28]. However, results of these studies indicated limitations of these techniques in detecting monosegmental dorsal root impairment since neither the large nerve stems nor the cutaneous branches of the myelinated Aβ-fiber spectra represent only one single dorsal root, such that the afferent neuronal activation reaches the brain after entering the spinal cord along several adjacent dorsal roots, thereby by-passing the lesion [13, 17, 18, 20, 30, 39, 40, 43].
The neurosurgeon Foerster already pointed to the superior diagnostic value of the pain and temperature system over that of the mechanoreceptive system in differentiating a single dorsal root damage [17, 18], when he first described the herringbone pattern of the dermatomes at the beginning of the last century on the basis of surgical dissections of dorsal roots in patients with severe spastic torticollis. His findings led Lorenz et al. [31] to examine dorsal root function in a patient with a cervical monosegmental C7 radiculopathy by a systematic comparison of evoked potentials elicited by either electrical, mechanical or laser radiant heat stimuli, the latter giving rise to laser-evoked potentials (LEP) by stimulating selectively thermonociceptive afferents. Their results confirmed Foerster’s clinical descriptions for the first time by neurophysiological data in that only the LEP, as correlate of pain and temperature function, but not electrical and mechanical SEP, as correlates of mechanoreceptive function, indicated the affected dorsal root by a total loss of LEP. Obviously, and in accordance with Foerster’s suggestions, enter Aδ-fibers, activated by the laser, the spinal cord without the herringbone pattern of intersegmental overlap that is typical for Aβ-fibers. The latter contribute to SEP generation projecting spinally and supraspinally upon respective dorsal column and medial lemniscal tract fibers. In contrast nociceptive Aδ- and C-fibers contribute to LEP generation and synapse with crossing fibers of the spinothalamic tract that convey information to the thalamus and the primary and secondary somatosensory cortex as well as limbic structures such as the anterior cingulate cortex [9].
We recorded LEP in 40 patients with monosegmental painful dorsal root impairment and compared results with those obtained from the non-affected control side as well as with norm data from 12 healthy subjects tested in our laboratory. The potential significance of results with respect to a differentiation of pathophysiological processes at the dorsal root and their prognostic value for long-term outcome will be discussed, particularly with respect to early therapeutical interventions.
Methods
Healthy control subjects
Twelve male subjects (mean age 25.9 ± 3.1 years, mean height 183.2 ± 6.2 cm) were recruited for the study. No subjects had any history of back or leg pain, neurological, psychiatric or other diseases, as confirmed by anamnesis and clinical examination. The data of one subject was discarded due to excessive movement artifacts in the electroencephalogram (EEG).
Patients
Forty patients were included in the study (mean age 35.1 ± 6.8 years, mean height 179.5 ± 11.3 cm) who suffered from sciatica due to a lesion of one nerve root (2 × L4-syndrome, 14 × L5-syndrome, 24 × S1-syndrome). The following clinical inclusion criteria were defined: monodermatomal radicular pain down to the distal third of the lower leg and pain intensity of 3–8 on the numerical rank scale (NRS from 0 = no pain to 10 = highest imaginable pain) and radiculopathy for the first time. In addition, one of the following symptoms must have been present: positive sign of Lasègue at least at a flexion below 50°, monosegmental paresis (grade III or less) of the affected myotome, monodermatomal dysesthesia or paresthesia. Morphological correlates of dorsal root impairment were proven by magnetic resonance imaging in all patients. The following exclusion criteria were defined for healthy subjects and patients: Any previous spine surgery or surgery of the central nervous system (CNS); infectious, inflammatory or neoplastic disease; epidural injections within 1 week before the LEP-application, bilateral symptoms, any metabolic disease affecting central or peripheral conduction velocity, disease of the CNS or the peripheral nervous system.
Eligible patients were recruited consecutively into the study after providing written informed consent. Participation in the study was voluntary and patients were assured in writing that refusal to participate would not affect their care in any way. The results of the LEP did not influence the clinical workflow or any therapeutical decisions in the patients. Ethical approval of the protocol was obtained by the ethics committee of Hamburg. The study was conducted in accordance with the Declaration of Helsinki and under International Conference on Harmonisation, Good Clinical Practice and Good Epidemiological Practice guidelines.
Clinical neurological test procedure
Healthy subjects and patients were examined in a sound shielded air-conditioned room (22–24°C) of constant humidity. Mechanosensibility was tested using a tuning folk (128 Hz) for vibration threshold and calibrated Semmes Weinstein nylon filaments for pressure sensibility. Thermosensibility was tested by short (0.5 s) and long (3 s) contacts with cold (22°C) and warm (43°C) test tubes. Each test modality was repeated ten times. Wrong results were counted. Results were documented as follows: 9–10 correctly detected trials = normal, 7–8 correctly detected trials = low degree reduction, 5–6 correctly detected trials = middle degree reduction, less than 5 correctly detected trials = high degree reduction. Testing down the order of filaments the thinnest Semmes Weinstein filament detected correctly was rated. Semmes Weinstein filaments are graduated as follows: ≤2.83N = normal; 3.22N–3.61N = low degree reduction; 3.84N–4.08N = middle degree reduction, 4.17N–4.31N = high degree reduction, ≥4.56N = next to loss of pressure sensibility.
Laser method
A commercial thulium YAG laser stimulator was used (Neurolaser, wavelength 1,960 nm, pulse duration 1 ms, 5 mm diameter; Starmedtec GmbH, Starnberg, Germany). This infrared wavelength is fully absorbed in superficial skin layers without influence of skin pigmentation [9]. Stimuli were given in blocks of 40 (10 min). Stimulus intensity was adjusted to the individual pain threshold (ip) and set to 1.5× and 2× ip. The interstimulus intervals were randomized from 10 to 20 s (mean 15 s). Three seconds (s) after each stimulus a weak tone prompted the subjects to rate his/her sensations on a NRS ranging from 0 (no sensation) to 10 (maximum pain) with 4 denoting pain threshold. Pain threshold intensity (PT) in the healthy dermatome was determined by three series of stimuli ascending in steps of 30 mJ from below sensation threshold to 90 mJ above pain threshold and back again to below sensation threshold. Thus, PT was the average of the six values at which the laser pulse was either first noted as a pinprick-like pain sensation (NRS 4) during ascending series or as no longer painful (NRS < 4) during descending series. We also used the criterion of two standard deviation above the normal mean as cut off criterion for abnormal pain threshold.
In healthy subjects stimuli were applied to identical dermatomes of both legs and both distal arms. For each experiment one of the segments L4 to S1 and C5 to C8 were selected in a randomized order. In patients stimuli were applied to the affected and to the corresponding contralateral dermatome. Each test side was examined twice. The sequence of blocks was balanced over both dermatomes such that the area tested first was repeated last (healthy: side 1–side 2–side 2–side 1, patients: affected–control–control–affected). This procedure minimizes a decrement of vigilance over the long lasting session (habituation; for details see [9, 41]). The EEG was recorded using a Nicolet SM 2000 amplifier with Ag/AgCl electrodes over Fz, Cz, Pz, T3 and T4 according to the 10-20 electrode positioning system and referenced against linked ear lobes. We present only the data from the vertex position (Cz), where pain-related LEP are known to be maximal [9]. The interelectrode impedance was kept below 5 kΩ. The band pass was 0.016–70 Hz (−12 dB/octave cut off). Peristimulus EEG segments of 2.6 s (100 ms before and 2,500 ms after stimulus onset) were controlled for ocular artifacts by visual inspection of a vertical electrooculogram recorded in parallel with electrodes placed above and below the left eye. Data was digitized (200 Hz) and averaged over the stimulus blocks according to stimulus intensity and stimulus side. The resulting LEP were evaluated for amplitude differences between the large vertex negativity (N2) and the following positivity (P2) [44].
Statistical analysis
We analyzed the norm data of LEP latencies and amplitudes and the pain ratings from the 11 healthy subjects by repeated-measures analyses of variance (2 × 2 × 2 × 2 factorial ANOVA) entering ‘limb’ (hand vs. foot), ‘side’ (left vs. right), ‘intensity’ (low vs. high) and ‘block’ (first vs. second) as within-subjects effects. Sources of significant main or interaction effects were explored by paired t-tests. The percentage side-to-side differences were calculated for the LEP N2–P2 peak-to-peak amplitude and the latencies of N2 and P2 (according to the formula higher value − lower value/higher value × 100). Due to the fact that absolute values, particularly amplitudes, yield large interindividual variability we used the percentage mean side-to-side differences of healthy subjects ±2 times standard deviation to discriminate pathological changes in the individual patient data. This definition of abnormal values was carried out in comparable SEP studies hence allowing a comparison to these results.
Results
Healthy subjects
Clinical neurological test procedure
The clinical test results for mechano- and thermosensibility as well as nociception yielded normal findings and no obvious side differences in all healthy subjects.
Laser method
The average normal pain threshold (±1 standard deviation) following laser stimuli was 360 ± 32.86 and 355 ± 39.87 mJ at respective right and left hands and 445 ± 97.52 and 405 ± 49.30 mJ at respective right and left feet.
Pain ratings
Repeated-measures ANOVA of the pain ratings obtained after each laser pulse during the EEG blocks yielded significant main effects of intensity (F1,10 = 86.7; P < 0.001), site (F1,10 = 12.7; P < 0.01), body side (F1,10 = 5.2; P < 0.05) and block (F1,10 = 16.2; P < 0.01). These results were due to higher pain ratings for high compared to low laser intensity (t = 9.3; P < 0.001), for hand compared to foot (t = 3.6; P < 0.01), for left compared to right body side (t = 2.3; P < 0.05) and for first compared to second block (t = 4.0; P < 0.01) stimulations, respectively. There were no significant interactions between these factors.
Latency
As expected, the mean peak latency of the N2-component of the LEP, averaged over both intensities, body sides and blocks, appeared significantly earlier (t = 4.2; P < 0.01) following hand (230 ms; 1 SD = 28.8 ms) compared to foot stimulation (276 ms; 1 SD = 45.9 ms). Furthermore, intensity exhibited a significant effect upon N2 latency (F = 12.0; P < 0.01) due to shorter latency following high compared to low intensity (t = 3.5; P < 0.01). Similarly, site influenced the P2-latency (F = 9.4; P < 0.05) due to longer latency following foot (435 ms; 1 SD = 62.1 ms) than hand stimulation (388 ms; 1 SD = 46.8 ms; t = 3.1; P < 0.05). Intensity had no effect on P2-latency and there were no effects of body side or block on either N2- or P2-latency. The mean intraindividual side-to-side difference for latency of the N2- and P2-component of LEP, calculated for the high intensity stimuli, was 5.0 ± 4.7 and 6.0 ± 4.7% yielding an upper limit of normal interside differences of N2 and P2 latencies at 15 or 16%, respectively.
Amplitude
Repeated-measures ANOVA of the N2–P2 peak-to-peak data yielded significant main effects of intensity (F1,10 = 19.67; P = 0.001), site (F1,10 = 12.01; P < 0.01) and block (F1,10 = 22.87; P = 0.001). These results were due to higher N2–P2 amplitudes for high compared to low laser intensity (t = 4.4; P < 0.001), for hand compared to foot (t = 3.5; P < 0.01) and for first compared to second block (t = 4.8; P < 0.001) stimulations, respectively. There were no significant interactions between these factors (for mean values see Table 1). An illustration of typical individual data is shown in Fig. 1.
Table 1.
Summary of LEP results in healthy subjects
| Hand | Foot | |||
|---|---|---|---|---|
| Right | Left | Right | Left | |
| Mean + SD | Mean + SD | Mean + SD | Mean + SD | |
| N2 latency | 226.4 ± 32.0 | 214.1 ± 18.6 | 267.2 ± 50.6 | 258.2 ± 47.6 |
| P2 latency | 397.7 ± 47.8 | 395.5 ± 57.1 | 430.9 ± 62.8 | 435.9 ± 66.3 |
| N2P2 amplitude low stimulation | 24.4 ± 8.45 | 28.68 ± 14.27 | 19.3 ± 6.6 | 20.0 ± 6.62 |
| N2P2 amplitude high stimulation | 37.9 ± 14.1 | 43.0 ± 15.2 | 28.6 ± 13.1 | 29.6 ± 10.8 |
| N2 amplitude low stimulation | −10.76 ± 4.45 | −11.66 ± 10.04 | −8.20 ± 8.87 | −6.12 ± 7.23 |
| N2 amplitude high stimulation | −15.64 ± 10.57 | −16.83 ± 10.64 | −14.65 ± 10.86 | −13.17 ± 11.33 |
| P2 amplitude low stimulation | 13.63 ± 6.11 | 17.02 ± 6.49 | 9.97 ± 6.32 | 11.92 ± 7.10 |
| P2 amplitude high stimulation | 22.30 ± 9.85 | 26.12 ± 9.53 | 13.94 ± 10.27 | 16.46 ± 9.53 |
| Skin temperature | 33.8 ± 0.9 | 33.5 ± 1.0 | 31.5 ± 1.37 | 31.3 ± 1.2 |
| Rating low stimulation | 3.89 ± 1.46 | 4.31 ± 1.19 | 3.16 ± 1.02 | 3.39 ± 1.08 |
| Rating high stimulation | 5.26 ± 1.49 | 5.41 ± 1.38 | 4.50 ± 0.96 | 4.85 ± 1.10 |
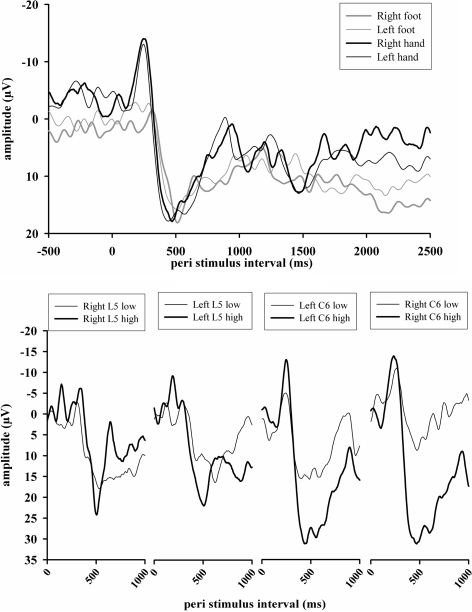
Fig. 1.
Representative results of LEP (above: Cz electrode, mean over high and low stimuli and both stimulus blocks; below: separately for high and low intensity stimulation) of a single healthy subject. The chart above summarizes the LEP (Cz amplitude) for each stimulus location, intensity and both stimulus blocks over all 80 stimuli. Obviously one can see the significant amplitude and latency differences between hand and foot (right foot—N = 305 ms/−1.8 μV, P = 520 ms/18.4 μV; left foot—N = 295 ms/−3.2 μV, P = 530 ms/16.0 μV; right hand—N = 255 ms/−12.5 μV, P = 475 ms/19.3 μV; left hand—N = 255 ms/−13.8 μV, P = 460 ms/17.9 μV). The chart below gives the results for the same subject, separately for high and low stimuli and all stimulus locations
Further analysis showed a higher intraindividual stability and reproducibility of LEP after the stimulation with the high intensities of approximately two times individual pain threshold (600 mJ) compared to the low intensity equivalent to approximately 1.5 times pain threshold (450 mJ). For this reason, the patients’ interside comparisons were performed by analysis of the LEP after high intensity stimulation.
The normal mean interside amplitude difference of LEP is 10.9 ± 5.5% stimulating the lower and 14.4 ± 10.3% stimulating the upper extremity. Considering an upper limit of normal interside difference at normal mean plus 2 SD, interside differences above 22 and 35% were regarded as cut-off criterion for abnormality in lower and upper limbs, respectively.
An overview of the psychophysical and evoked potential results in healthy subjects is presented in Table 1.
Patients
Clinical neurological test procedure
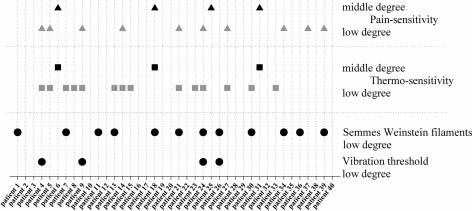
Figure 2 summarizes the test results of mechano- and thermosensitivity for the affected dermatome. For mechanosensitivity (Aβ-fiber function), only low degree reductions were seen at all. Twelve patients showed a slight decrease of sensitivity to pressure of Semmes Weinstein filaments and four patients had a slightly increased vibration threshold. Two of the patients (#24, #26) had impairments of both of these test modalities. In contrast, and as expected, higher incidences of impairment were seen for pain- and thermosensitivity (Aδ-fiber function). Fourteen patients had a mild reduction and another three patients a moderate reduction of sensitivity to thermal stimuli.
Fig. 2.
Results of the neurophysiological test procedure in patients. Pathological findings are indicated by symbols for each patient and each tested modality. Impairment of mechanosensibility was seen in 35% of the patients, at a low degree exclusively. Thermo- and pain sensitivity were affected in 53% of the patients, 20% of those with a middle degree
A mild reduction of pain sensitivity was found in ten patients and a moderate reduction in another four patients. Hence a total number of 21 patients showed clinically an impaired Aδ-fiber function. Fourteen of the 21 patients with impaired Aδ-fiber function showed significant LEP changes (67%) as well. All patients with moderate reductions of pain- or thermosensitivity had significant LEP changes.
Laser method
A normal laser pain threshold was documented in all patients for the unaffected dermatome. The subjective sensation after laser stimulation is expressed by the pain ratings. Pain ratings were analyzed for both stimulus sides and intensities. A significant difference between healthy and affected side was seen after high intensity stimulation in four patients. Three out of these four showed a significant (as defined above) LEP-amplitude reduction. In contrast, after low intensity stimulation a significant rating difference appeared more often (ten patients). Eight of these patients also had significant amplitude decreases (Table 2).
Table 2.
Summary of patients with a significant difference of pain ratings at the affected dermatome
| Patient | Healthy side high | Sick side high | Healthy side low | Sick side low | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Patient 6* | 2.05 | 1.77 | 0.70 | 1.09 | 1.60 | 1.63 | 0.45 | 0.88 |
| Patient 11* | 3.95 | 1.45 | 3.45 | 0.96 | 4.28 | 0.60 | 2.87 | 1.54 |
| Patient 12 | 2.88 | 1.09 | 1.90 | 0.78 | 2.30 | 1.32 | 1.85 | 0.98 |
| Patient 13 | 3.85 | 1.17 | 3.48 | 1.24 | 3.18 | 1.63 | 1.58 | 1.96 |
| Patient 21* | 4.85 | 0.75 | 4.90 | 0.64 | 5.05 | 1.05 | 4.15 | 0.75 |
| Patient 22* | 3.00 | 1.28 | 2.43 | 1.39 | 2.88 | 1.11 | 1.75 | 1.35 |
| Patient 27* | 3.58 | 0.90 | 4.05 | 0.45 | 3.15 | 1.17 | 3.78 | 0.62 |
| Patient 31* | 3.68 | 1.16 | 3.40 | 1.65 | 3.45 | 1.11 | 2.35 | 1.56 |
| Patient 32* | 3.77 | 1.65 | 2.15 | 2.66 | 3.65 | 1.28 | 2.48 | 1.97 |
| Patient 33* | 3.86 | 1.16 | 2.86 | 1.15 | 2.95 | 1.08 | 1.95 | 0.96 |
| Patient 36* | 3.98 | 1.70 | 2.75 | 1.15 | 2.30 | 1.47 | 1.38 | 0.87 |
| Patient 39 | 4.98 | 1.27 | 0.55 | 0.75 | 3.48 | 1.06 | 0.10 | 0.30 |
Asterisk indicates patients with a significant amplitude decrease, bold type indicates the significant results, either after high or low stimulation or both
Figure 3 demonstrates typical plots of the evoked potential waveform at the vertex electrode (Cz).
Fig. 3.
Cz curve of patient #36 with a S1 radiculopathy (34% amplitude reduction and normal N2 and P2 latency). Note the good correspondence of curve morphology between healthy and sick body side
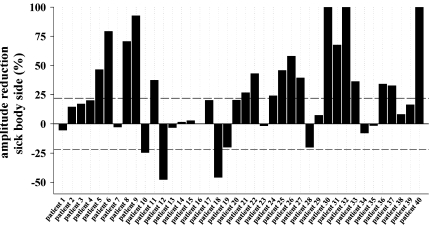
Significant LEP-amplitude changes of the affected side appeared in 21 of 40 patients (53%; Fig. 4).
Fig. 4.
Summary of amplitude differences for each patient (% difference sick vs. healthy side). The dashed lines mark the ±22% interval which is the normal interside difference for dermatomal LEP of the lower limbs. Twenty-one of 40 patients show relevant amplitude differences
Over all any significant LEP changes appeared in 26 out of 40 patients (65%). A pathological decrease of LEP amplitude at the affected side was found in 18 of the patients (45%). Eight patients (20%) showed an amplitude decrease of more than 50% and three patients showed a complete loss of LEP (7.5%). Comparatively, pathological changes of latency were less frequently noticed [eight patients (20%), Table 3]. More often the N2 latency (six patients) was altered compared to P2 latency (two patients). Of the six patients showing significant N2 latency changes two had a decrease and the remaining four increases. Three patients (patients #11, #25, #33) showed significant disturbances for both, LEP amplitude and latency. One (#11) out of the eight patients with significant latency changes also showed an impaired mechanosensitivity and five (patients #14, #15, #23, #25, #33) an impaired thermo- or pain sensitivity.
Table 3.
Summary of patients with a significant difference of latency, compared to the normal data in this study
| Patient | N2 latency healthy (ms) | N2 latency sick (ms) | P2 latency healthy (ms) | P2 latency sick (ms) |
|---|---|---|---|---|
| Patient 2 | 330.00 | 420.00 | 420.00 | 480.00 |
| Patient 11 | 195.00 | 210.00 | 305.00 | 385.00 |
| Patient 14 | 240.00 | 285.00 | 340.00 | 370.00 |
| Patient 15 | 185.00 | 140.00 | 415.00 | 395.00 |
| Patient 17 | 255.00 | 310.00 | 450.00 | 515.00 |
| Patient 23 | 295.00 | 285.00 | 360.00 | 420.00 |
| Patient 25 | 210.00 | 250.00 | 480.00 | 495.00 |
| Patient 33 | 220.00 | 185.00 | 365.00 | 370.00 |
Bold type indicates significant results, either for the N2 or the P2 component of the LEP
Discussion
This study compared dermatomal LEP in healthy subjects with those of patients suffering early stages of a lumbar radiculopathy, using an experimental setting as described in earlier studies [9, 31]. LEP norm data were defined intraindividually by the degree of side-to-side differences of amplitude and latency measures, which limits variability by interindividual effects, such as gender or age, which we were not able to address in this study due to the comparably small sample size of our control group. LEP amplitudes have been reported by other studies to significantly decrease with age, whereas latency and the interside difference of amplitude are not influenced by age. Similarly, gender was reported to have no effect on both latency and amplitude of LEP [45]. Our general finding of LEP amplitude increases according to stimulus intensity or decreases due to habituation are in good accordance with the results of other groups [29, 44]. Generally lower LEP amplitude following foot compared to hand stimulation in our study is also described by Spiegel et al. [41]. The healthy subject data allowed to define a tentative upper limit of normal interside difference for the LEP amplitude of 22% (lower limbs) or 35% (upper limbs) above the normal mean, by using a two standard deviation criterion. According to this criterion we identified 21 patients exceeding the amplitude criterion. Higher cut off criteria were reported by Beydoun et al. [7] (28%), Hansen and Treede [19] (35%) or by Truini et al. [45] (39%) using similar stimuli. Even if we apply the 40% criterion there were still 13 patients with pathological side-to-side amplitude differences.
The collective evidence from previous studies in patients with different peripheral or central lesions suggests a superior use of LEP over the standard SEP technique whenever there is a dissociated loss of thermonociceptive sensitivity [9]. A different degree of overlapping entry along adjacent dorsal roots into the spinal cord renders the LEP method also more sensitive than the SEP method in the documentation of sensory deficits in monosegmental dorsal root impairment [31]. We confirmed these suggestions based, for the first time, on a larger sample of patients with monosegmental radiculopathies. Eighteen of 40 patients had LEP-amplitude reductions, 9 of them reductions in laser pain rating and 12 out of those showed a decrease in pain- and thermosensitivity as tested with clinical methods.
Another finding was a prolongation of latency observed in six patients, two of them combined with amplitude reduction and none of them with a rating reduction. Thus, 26 out of 40 patients (65%) exhibited either pathological amplitude or latency changes or both. Standard electrical SEP elicited by mixed nerve stem stimuli typically fail to indicate a monosegmental dorsal root lesion [16], whereas some degree of higher diagnostic sensitivity can be achieved using segmental and dermatomal SEP [2, 15, 39, 43]. All SEP techniques yield much lower sensitivity compared to LEP.
Although a distinction between amplitude and latency alterations of evoked potentials is generally considered to indicate differential damage of the axon or the myelin sheath, respectively, it remains unclear whether these measures obtained by the LEP method also indicate different pathological processes of the dorsal root.
Mechanical compression can lead to fibrosis and total functional loss of the affected nerve fibers. This causes a reduced number of fibers in animals [22, 23]. It is therefore likely reducing neuronal impulse synchronization or inducing a complete conduction block. Such condition would lead to reduction or loss of amplitude of postsynaptic fields causing the evoked potentials over stimulus repetition in relay stations downstream the lesion, rendering decreased LEP amplitudes most likely a correlate of mechanical dorsal root damage.
An alternative relevant factor of radiculopathy is a local metabolic effect on nerve roots or root sleeves by substances leaking from the degenerated intervertebral discs [10]. Of particular importance is that nucleus pulposus material possess inflammatory properties as indicated by leukotaxis and increase of vascular permeability [4, 8, 21, 32, 36]. One major experimental electrophysiological finding of studies dealing with these inflammatory changes was a decrease in conduction velocity of nerve roots which was antagonized by anti-inflammatory substances [33, 34, 37]. It is therefore possible that patients with a predominant biochemical lesion yield prolonged LEP latencies, consistent with a predominant decrease in nerve conduction velocity described in animal experiments under these conditions [4, 12].
Finally, there is also a possible role of neuropathic hyperexcitability that increases the spontaneous neuronal discharge rates of the affected dorsal root fibers [3, 24, 46]. Current concepts of chronic pain consider neuroplastic changes of dorsal horn neurones associated with continuous discharges of afferent fibers and resulting into a prolonged state of hypersensitivity [6, 50]. Evoked potentials resulting from transient and synchronous discharge generation will decrease if interfered by concurrent ongoing and asynchronous activity. Such phenomena can well contribute to LEP-amplitude decrements. It would therefore be highly interesting whether different types and grades of LEP changes are associated with different risks for the development of chronic pain syndromes on the basis of radiculopathies which would allow earlier and differentiated therapeutical interventions.
In conclusion, LEP represent a reliable and sensitive method to objectively document pathological dorsal root function in early monosegmental radiculopathies. Therefore, LEP are superior to other EP. Based on pathophysiological concepts we think that the impairment of dorsal root function correlates with the degree of dorsal root damage and might have prognostical relevance. Future studies may, therefore, combine the LEP method with electrophysiological techniques to test the ventral root, such as electromyography and motor-evoked potentials to gain information about both pathways. Further prospective studies may address the important question whether LEP abnormalities in radiculopathies can serve as predictors for a long-term clinical outcome, in particular the incidence of a chronic disabling pain syndrome, which is more likely the consequence of dorsal rather than ventral root pathology. In addition there is a potential value of LEP to allot different therapies due to the interaction between different pathophysiological processes of dorsal root impairment and LEP results.
Acknowledgments
We thank Dr G. Weidemann from Starmedtec GmbH, Starnberg, Germany, for technical support during the study. The study is supported by the Deutsche Forschungsgemeinschaft (Qu 156/1-1).
References
- 1.American Academy of Neurology’s Therapeutics and Technology Assessments Subcommittee (1997) Assessment: dermatomal somatosensory evoked potentials. Report of the American Academy of Neurology’s Therapeutics and Technology Assessments Subcommittee. Neurology 49:1127–1130 [PubMed]
- 2.Aminoff MJ, Goodin DS. Dermatomal somatosensory evoked potentials in lumbosacral root compression. J Neurol Neurosurg Psychiatry. 1988;51:740–742. doi: 10.1136/jnnp.51.5.740-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anzai H, Hamba M, Onda A, Konno S, Kikuchi S. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine. 2002;27:E50–E55. doi: 10.1097/00007632-200202010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Aoki Y, Rydevik B, Kikuchi S, Olmarker K. Local application of disc-related cytokines on spinal nerve roots. Spine. 2002;27:1614–1617. doi: 10.1097/00007632-200208010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Balague F, Nordin M, Sheikhzadeh A, Echegoyen AC, Brisby H, Hoogewoud HM, Fredman P, Skovron ML. Recovery of severe sciatica. Spine. 1999;24:2516–2524. doi: 10.1097/00007632-199912010-00014. [DOI] [PubMed] [Google Scholar]
- 6.Berthele A, Schadrack J, Castro-Lopes JM, Conrad B, Zieglgansberger W, Tolle TR. Neuroplasticity in the spinal cord of monoarthritic rats: from metabolic changes to the detection of interleukin-6 using mRNA differential display. Prog Brain Res. 2000;129:191–203. doi: 10.1016/S0079-6123(00)29014-6. [DOI] [PubMed] [Google Scholar]
- 7.Beydoun A, Morrow TJ, Shen JF, Casey KL. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr Clin Neurophysiol. 1993;88:173–181. doi: 10.1016/0168-5597(93)90002-7. [DOI] [PubMed] [Google Scholar]
- 8.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol. 1998;107:227–253. doi: 10.1016/S0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 10.Byrod G, Rydevik B, Nordborg C, Olmarker K. Early effects of nucleus pulposus application on spinal nerve root morphology and function. Eur Spine J. 1998;7:445–449. doi: 10.1007/s005860050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornefjord M, Olmarker K, Farley DB, Weinstein JN, Rydevik B. Neuropeptide changes in compressed spinal nerve roots. Spine. 1995;20:670–673. doi: 10.1097/00007632-199503150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Cornefjord M, Olmarker K, Rydevik R, Nordborg C. Mechanical and biochemical injury of spinal nerve roots: a morphological and neurophysiological study. Eur Spine J. 1996;5:187–192. doi: 10.1007/BF00395512. [DOI] [PubMed] [Google Scholar]
- 13.Dvonch V, Scarff T, Bunch WH, Smith D, Boscardin J, Lebarge H, Ibrahim K. Dermatomal somatosensory evoked potentials: their use in lumbar radiculopathy. Spine. 1984;9:291–293. doi: 10.1097/00007632-198404000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak J. Neurophysiologic tests in diagnosis of nerve root compression caused by disc herniation. Spine. 1996;21:39S–44S. doi: 10.1097/00007632-199601010-00009. [DOI] [PubMed] [Google Scholar]
- 15.Eisen A, Hoirch M. The electrodiagnostic evaluation of spinal root lesions. Spine. 1983;8:98–106. doi: 10.1097/00007632-198301000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Fisher MA. Electrophysiology of radiculopathies. Clin Neurophysiol. 2002;113:317–335. doi: 10.1016/S1388-2457(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 17.Foerster O. The dermatomes in man. Brain. 1933;56:139–152. doi: 10.1093/brain/56.1.1. [DOI] [Google Scholar]
- 18.Foerster O (1938) Symptomatologie der Erkrankungen des Rückenmarks und seiner Wurzeln. In: Bumke O, Foerster O, Handbuch der Neurologie, Bd.V. Springer, Berlin Heidelberg New York, pp 1–104
- 19.Hansen C, Treede R. Laser-evozierte Potentiale: eine neue klinisch neurophysiologische Untersuchungsmethode für die Schmerzbahnen. EEG Labor. 1995;17:76–85. [Google Scholar]
- 20.Inouye Y, Buchtal F. Segmental sensory innervation determined by potentials recorded from cervical spinal nerves. Brain. 2003;100:731–748. doi: 10.1093/brain/100.4.731. [DOI] [PubMed] [Google Scholar]
- 21.Iwabuchi M, Rydevik B, Kikuchi S, Olmarker K. Effects of anulus fibrosus and experimentally degenerated nucleus pulposus on nerve root conduction velocity: relevance of previous experimental investigations using normal nucleus pulposus. Spine. 2001;26:1651–1655. doi: 10.1097/00007632-200108010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Jou IM, Lai KA. Neuromonitoring of an experimental model of clip compression on the spinal nerve root to characterize acute nerve root injury. Spine. 1998;23:932–939. doi: 10.1097/00007632-199804150-00017. [DOI] [PubMed] [Google Scholar]
- 23.Jou IM, Hsu CC, Chern TC, Chen WY, Dau YC. Spinal somatosensory evoked potential evaluation of acute nerve-root injury associated with pedicle-screw placement procedures: an experimental study. J Orthop Res. 2003;21:365–372. doi: 10.1016/S0736-0266(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 24.Kasai M, Mizumura K. Effects of PGE(2) on neurons from rat dorsal root ganglia in intact and adjuvant-inflamed rats: role of NGF on PGE(2)-induced depolarization. Neurosci Res. 2001;41:345–353. doi: 10.1016/S0168-0102(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Yoshizawa H. Effect of mechanical compression on the vascular permeability of the dorsal root ganglion. J Orthop Res. 2002;20:730–739. doi: 10.1016/S0736-0266(01)00170-X. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res. 2004;22:170–179. doi: 10.1016/S0736-0266(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 2: morphological and immunohistochemical changes of dorsal root ganglion. J Orthop Res. 2004;22:180–188. doi: 10.1016/S0736-0266(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 28.Kraft GH, Aminoff MJ, Baran EM, Litchy WJ, Stolov WC. Somatosensory evoked potentials: clinical uses. AAEM Somatosensory Evoked Potentials Subcommittee. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1998;21:252–258. doi: 10.1002/(SICI)1097-4598(199802)21:2<252::AID-MUS17>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Lefaucheur JP, Brusa A, Creange A, Drouot X, Jarry G. Clinical application of laser evoked potentials using the Nd:YAG laser. Neurophysiol Clin. 2002;32:91–98. doi: 10.1016/S0987-7053(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 30.Liguori R, Krarup C, Trojaborg W. Determination of the segmental sensory and motor innervation of the lumbosacral spinal nerves. An electrophysiological study. Brain. 1992;115:915–934. doi: 10.1093/brain/115.3.915. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz J, Hansen HC, Kunze K, Bromm B. Sensory deficits of a nerve root lesion can be objectively documented by somatosensory evoked potentials elicited by painful infrared laser stimulations: a case study. J Neurol Neurosurg Psychiatry. 1996;61:107–110. doi: 10.1136/jnnp.61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Olmarker K, Rydevik B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine. 2001;26:863–869. doi: 10.1097/00007632-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 34.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. doi: 10.1097/00007632-199318110-00005. [DOI] [PubMed] [Google Scholar]
- 35.Olmarker K, Byrod G, Cornefjord M, Nordborg C, Rydevik B. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine. 1994;19:1803–1808. doi: 10.1097/00007632-199408150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Olmarker K, Blomquist J, Stromberg J, Nannmark U, Thomsen P, Rydevik B. Inflammatogenic properties of nucleus pulposus. Spine. 1995;20:665–669. doi: 10.1097/00007632-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 37.Otani K, Arai I, Mao GP, Konno S, Olmarker K, Kikuchi S. Nucleus pulposus-induced nerve root injury: relationship between blood flow and motor nerve conduction velocity. Neurosurgery. 1999;45:614–619. doi: 10.1097/00006123-199909000-00034. [DOI] [PubMed] [Google Scholar]
- 38.Pape E, Eldevik P, Vandvik B. Diagnostic validity of somatosensory evoked potentials in subgroups of patients with sciatica. Eur Spine J. 2002;11:38–46. doi: 10.1007/s005860100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seyal M, Sandhu LS, Mack YP. Spinal segmental somatosensory evoked potentials in lumbosacral radiculopathies. Neurology. 1989;39:801–805. doi: 10.1212/wnl.39.6.801. [DOI] [PubMed] [Google Scholar]
- 40.Slimp JC, Rubner DE, Snowden ML, Stolov WC. Dermatomal somatosensory evoked potentials: cervical, thoracic, and lumbosacral levels. Electroencephalogr Clin Neurophysiol. 1992;84:55–70. doi: 10.1016/0168-5597(92)90068-M. [DOI] [PubMed] [Google Scholar]
- 41.Spiegel J, Hansen C, Treede RD. Clinical evaluation criteria for the assessment of impaired pain sensitivity by thulium-laser evoked potentials. Clin Neurophysiol. 2000;111:725–735. doi: 10.1016/S1388-2457(99)00297-7. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi N, Yabuki S, Aoki Y, Kikuchi S. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine. 2003;28:435–441. doi: 10.1097/00007632-200303010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Tokuhashi Y, Satoh K, Funami S. A quantitative evaluation of sensory dysfunction in lumbosacral radiculopathy. Spine. 1991;16:1321–1328. doi: 10.1097/00007632-199111000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Treede RD, Lorenz J, Baumgartner U. Clinical usefulness of laser-evoked potentials. Neurophysiol Clin. 2003;33:303–314. doi: 10.1016/j.neucli.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Truini A, Galeotti F, Romaniello A, Virtuoso M, Iannetti GD, Cruccu G. Laser-evoked potentials: normative values. Clin Neurophysiol. 2005;116:821–826. doi: 10.1016/j.clinph.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 47.Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1998;21:1612–1631. doi: 10.1002/(SICI)1097-4598(199812)21:12<1612::AID-MUS2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Yoshizawa H, Kobayashi S, Kubota K. Effects of compression on intraradicular blood flow in dogs. Spine. 1989;14:1220–1225. doi: 10.1097/00007632-198911000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Yoshizawa H, Kobayashi S, Morita T. Chronic nerve root compression. Pathophysiologic mechanism of nerve root dysfunction. Spine. 1995;20:397–407. doi: 10.1097/00007632-199502001-00001. [DOI] [PubMed] [Google Scholar]
- 50.Zieglgansberger W, Berthele A, Tolle TR. Understanding neuropathic pain. CNS Spectr. 2005;10:298–308. doi: 10.1017/s1092852900022628. [DOI] [PubMed] [Google Scholar]