Abstract
Despite the increasing popularity of total lumbar disc replacement (TDR) in predominantly young and active patients, no previous study has addressed possibilities, limitations and potential risks regarding athletic performance following TDR. Mechanical concerns remain and the implant’s resilience as regards its load-bearing capacity during sporting activities is unknown. Thirty-nine athletic patients fulfilled the inclusion criteria for this study. These patients participated in a large variety of different types of sport. Significant and lasting pain-relief was attained following TDR with a mean follow-up of 26.3 months (range 9–50.7 months; FU rate 97.4%). Sporting activity was resumed within the first 3 months (38.5%) to 6 months (30.7%) with peak performance being reached after 5.2 months. Thirty-seven patients (94.9%) achieved resumption of sporting activity. Athletic performance improved significantly in 33 patients (84.6%). Minor subsidence was observed in 13 patients (30%) within the first 3 months with no further implant migration thereafter in 12 patients. Participation in all types of sport recorded in this study was accessible for a high rate of patients up to the level of professional athletes as well as those participating in extreme sports. Preoperative participation in sport proved to be a strong positive predictor for highly satisfactory postoperative outcome following TDR. In a selected group of patients, however, preoperative inability to participate in sporting activities did not impair postoperative physical activity. Due to the young age of the patients and significant load increase exerted during athletic activities, persisting concerns regarding the future behaviour of the implant remain and will require longer follow-up, modified investigation techniques and larger patient cohorts.
Keywords: Total disc replacement, Spine arthroplasty, Sports, Clinical results, Prospective studies
Introduction
Over the last two decades, efforts have been made to preserve segmental mobility instead of fusing spinal motion segments for the treatment of lumbar degenerative disc disease (DDD). The first series of prospective, randomized FDA-controlled IDE-studies have shown comparable results between disc-arthroplasty and fusion procedures in a highly selected patient population [6, 16, 43, 58]. Following FDA-approval of the SB Charité III prosthesis (Waldemar-Link GmbH, Germany), a dramatic increase in the number of disc replacement procedures is expected over the next decades.
Due to increasing prevalence of previously defined contraindications to TDR with age [29], total lumbar disc replacement (TDR) is predominantly performed in younger patients engaged in varying physical and sporting activities and with high subjective expectations regarding their postoperative performance. Nevertheless, literature has not previously addressed possibilities, limitations or risks associated with postoperative physical activity or sporting performance following TDR. Presently, no consensus has been reached regarding the time frame in which patients can resume their sporting activities and no study has focussed on the ability of patients to participate in different sports.
Previous studies of in vivo intradiscal measurements reported significant shear and compressive forces exerted upon lumbar discs during activities of daily living [35, 57]. A rapid increase of forces is to be expected during sporting activities (Table 1). However, the subsequent impact arising from sporting activities such as repetitive axial/rotational stress or sudden exposure to high external forces to the implants has not been addressed at this stage. Mechanical concerns remain and the implant’s resilience as regards its load-bearing capacity during sporting activities is unknown.
Table 1.
Reported forces on lumbar discs for different sporting activities
| Activity | Force | Reporting authors |
|---|---|---|
| Walking speed (slow, preferred, fast speed) | 2.28×, 2.53×, 2.95× body-weight compressive force L5/S1 | Cheng [12] |
| Golf swing | 6,100–7,500 N compressive force L3/4 (amateur and professional players) | Hosea et al. [27] |
| Rowing | 6,100 N lumbar compressive forces | Hosea [26] |
| Rowing | 4.6× body weight compressive force L4/5 (shear force L4/5: 660 ± 117 N) | Morris [46] |
| Half-squat exercises with weights 0.8–1.6× body weight | 10× body weight compressive loads across L3/4 segment (70 kg person approx. 7,000 N) | Cappozzo [9] |
| Football linemen, blocking manoeuvres | >8,600 N peak compressive force 3,300 N average peak sagittal shear force L4/5 motion segment | Gatt [20] |
| Competitive weight-lifters | >17,000 N average compressive loads L4/5 motion segment | Cholewicki [13] |
In this study, we describe our experiences with TDR (ProDisc II; Synthes, Paoli, USA) in a selected group of athletic patients with varying sporting exposure and different biomechanical demands from the level of hobby athletes up to the level of professional athletes and patients practising extreme sports.
Materials and methods
Study protocol and patient selection
Patients included in this series are part of an ongoing prospective, non-randomized study, of which preliminary results as well as 3-year results for different indications have been published previously [42, 51]. Between July 2000 and November 2005 lumbar disc replacement with ProDisc II was performed in 215 patients. The majority of these patients participated in some kind of physical activity preoperatively such as swimming, cycling, walking or weight exercises as part of their conservative preoperative workout program. However, only 39 patients participating significantly in sporting activities fulfilled inclusion criteria for this study as outlined in Table 2.
Table 2.
Inclusion criteria: patients had to meet more than one of the following requirements
| Frequency of athletic performance ≥2/week pre- or postoperatively |
| Participation in contact sports (e.g. soccer) |
| Exposure to high impact external forces/extreme sports (e.g. parachute jumping, wild water rafting, etc.) |
| Athletic performance required for professional life (e.g. sports teacher, skiing instructor) |
| Professional athlete |
The minimum frequency for participation in athletic activity required was 2×/week either pre- or postoperatively. Changes in activity frequency and performance level were recorded and evaluated. Furthermore, patients who participated in a variety of extreme sports (e.g. marathon running, parachute jumping, wild-water rafting, etc.) with exposure to either repetitive forces or high-impact external loads as well as professional athletes and patients that relied on sporting activity for their income were included in this study.
All patient data was collected and patients were examined preoperatively as well as 3, 6 and 12 months postoperatively. Then followed routine annual examinations by an independent observer not involved in preoperative decision-making (C.J.S.). Standardized study documentation included visual analogue scale (VAS), the Oswestry-Disability-Low-Back-Pain-Questionnaire (ODI) [18] as well as other clinical and radiological parameters. All patients received a separate questionnaire which focussed on numerous sport-related issues regarding subjective evaluation of pre- versus postoperative sporting activity, athletic status, level of competition, personal limitations, etc. Patients subjectively evaluated the success of the disc replacement operation. The subjective outcome evaluation was divided into three categories, namely ‘completely satisfied’, ‘satisfied’ or ‘not satisfied’.
Preoperative diagnosis was based on lumbar X-rays and MRI-images. All patients underwent fluoroscopically guided diagnostic injections to exclude facet joint and/or sacroiliac joint pain preoperatively. Patients with significantly positive pain relief of >50% following these injections were excluded from this study and were not considered candidates for TDR.
The role of discography in identifying discogenic pain remains debated. Previous studies showed a high rate of false positive and negative results equally, [10] failure of the patient to distinguish between concordant and non-concordant pain, [11] 100% ‘memory pain’ in patients with abnormal psychometric testing [11] as well as 0.5% infection rate [5, 11]. Moreover, since there is no evidence-based data available regarding the potential predictive value of discography for clinical outcome, a discogram was not employed as a diagnostic tool in the present study.
At each follow-up (FU), radiographs of the lumbar spine were taken in ap- and lateral view as well as dynamic flexion/extension images. Images at last FU were digitalized and assessed for proper implant positioning, minor subsidence or dislocations. Two independent observers (C.J.S., M.F.K.) analysed the radiographic images using a custom-made software which enabled the measuring of angles and distances (Medimage, VEPRO AG, Pfungstadt, Germany). ‘Dislocation’ was defined as migration of the implant ≥6 mm with secondary functional impairment whilst the term ‘subsidence’ described minor migration (≤5 mm) and intact function of the implant on functional X-ray images.
Functional flexion/extension images were analysed for segmental range of motion (ROM) at the index level preoperatively and at the last FU examination. Standard Cobb measurements were used to determine the preoperative ROM at the instrumented levels [25]. Conversely, the spikes of the upper and lower prosthesis-keels were used as radiological landmarks to determine the postoperative segmental ROM. This method has been described and recommended in previous studies due to improved precision and inter/intra-observer reliability [8, 38, 39].
Low-back pain (LBP) was the leading complaint in all patients where extensive conservative therapy for a minimum of 6 months had proved unsuccessful. In cases of accompanying sciatica, the amount of LBP exceeded 80% of overall complaints. Contraindications for lumbar disc replacement are listed in Table 3.
Table 3.
Exclusion criteria/contraindications for total lumbar disc replacement
| Central or lateral spinal stenosis |
| Facet joint arthrosis/symptomatic facet joint problems |
| Spondylolysis/spondylolisthesis |
| Spinal instability (iatrogenic/altered posterior elements, e.g. following laminectomy) |
| Major deformity/curvature deviations (e.g. scoliosis) |
| Metabolic bone disease (e.g. manifest osteoporosis/osteomalacia) |
| Previous operation with severe scarring and radiculopathy |
| Compromised vertebral body (irregular endplate shape) |
| Previous/latent infection |
| Metal allergy |
| Spinal tumor |
| Post-traumatic segments |
The disc spaces were approached through a mini-open laparotomy as described previously. [41, 42] Implantation of the ProDisc II implant was performed according to the manufacturers guidelines [3].
Statistical analysis
All data was recorded using Microsoft Excel 2002 (Microsoft Inc., Redmond, WA) and transferred to SAS V9.1 (SAS Institute Inc., Cary, NC, USA) for statistical analysis. For group sample analysis, paired Student’s t test was performed. For comparison of different groups, two-tailed Mann–Whitney U–Wilcoxon rank sum test was performed. Statistical significance was defined and accepted as P < 0.05.
Results
Patient distribution
A total of 39 patients (21 male, 53.8%; 18 female, 46.2%) fulfilled inclusion criteria for this study as listed in Table 2. Preoperative diagnosis included degenerative disc disease with (n = 14) and without (n = 13) Modic changes, DDD with accompanying soft disc herniation (n = 2), and nine patients following previous discectomies. In one patient, lumbar disc replacement was performed at L5/S1 due to adjacent level degeneration at the lumbosacral junction 4.5 years after a previous fusion procedure at the L4/5 level.
The average age of the patients was 39.8 years (range 26.2–58 years). Forty-two disc replacements were performed in 39 patients. The operations were performed mono-segmentally (n = 36; 92.3%) and bisegmentally (n = 3; 7.7%). Monosegmental disc replacements were performed at the lumbosacral junction (L5/S1; n = 26; 66.7%) as well as at the level above the lumbosacral junction (L4/5 and L5/6; n = 10; 25.6 %). Bisegmental disc replacements were performed at the levels L4/5 and L5/S1.
Thirty-eight out of 39 patients were available for follow-up (FU rate 97.4%) with a mean follow-up of 26.3 months (range 9–50.7 months). One patient lost to follow-up was last seen 3 months postoperatively after she had sustained a traumatic L1-fracture following a fall during horse-riding. However, the patient was able to resume sporting activity after conservative treatment following the trauma and completed a telephone interview 52 months postoperatively.
Intraoperative/perioperative data
The overall operating time averaged 106 min for monosegmental operations (range 58–180 min) and 156 min for bisegmental procedures (range 103–185 min). The recorded volume of blood loss averaged 134 ml for both mono- and bisegmental TDR groups (range 10–600 ml, SD 125 ml). Access to the disc space was achieved through an anterior retroperitoneal access in 32 patients (82.1%), a transperitoneal approach was used in 7 patients (17.9%). Patients were mobilized from the first postoperative day on without additional support and a postoperative rehabilitation program was encouraged in all cases.
Peri/postoperative complications are outlined in Table 4 and included a total of 5 complications (12.8% overall complication rate). Two patients required revision surgery (5.1%). One revision was due to a haematoma of the abdominal wall, the other was due to an adjacent segment disc herniation. Postoperative participation in athletic activity was only temporarily negatively influenced by the occurrence of the listed complications.
Table 4.
Peri/postoperative complications following total lumbar disc replacement (TDR) in n = 39 patients
| n | % | Comment | |
|---|---|---|---|
| Intraoperative complications | |||
| Access related | |||
| Lesion of superior hypogastric plexus | 1 | 2.6 | Persisting sexual dysfunction with retrograde ejaculation |
| Postoperative complications | |||
| Surgery related | |||
| L5-radiculopathy due to extraforaminal disc protrusion following TDR | 1 | 2.6 | Spontaneous improvement in both patients upon conservative therapy including nerve root infiltrations |
| L5-radiculopathy of unknown reason | 1 | 2.6 | |
| Intra/postoperative complications (total) | 3 | 7.7 | |
| Reoperations | |||
| Haematoma of the abdominal wall | 1 | 2.6 | Revision surgery |
| Reoperations (non-index level) | |||
| Adjacent segment disc herniation | 1 | 2.6 | Microsurgical discectomy |
| Total revision surgery | 2 | 5.1 | |
| Overall complication rate | 5 | 12.8 | |
Clinical outcome
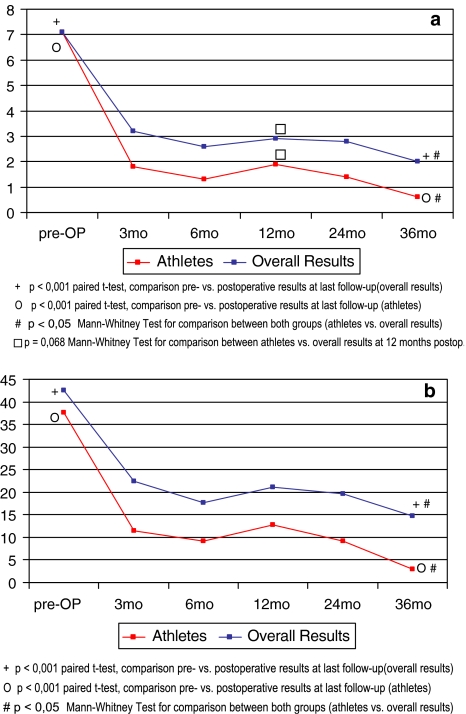
Average reduction for VAS from preoperative levels (VASpreop = 7.1) was 5.7 (range 0.8–9.1) and preoperative ODI of 37.7% was reduced on average by an absolute value of 30.0% (range 8–60%) at the last FU examination which represents a 79.6% relative improvement (Fig. 1a, b).
Fig. 1.
Comparison pre/postoperative results for a Visual Analogue Scale (VAS) and b Oswestry Disability Index (ODI) for athlete patients from this study in comparison to our overall results
Results from patients in this study were compared to those of patients from an ongoing prospective study [51] for which pre- or postoperative athletic activity was not a study inclusion criteria. Preoperative levels of VAS or ODI did not reveal any significant differences between the two cohorts. In both groups we were able to detect significant and lasting improvement throughout the entire postoperative course (Fig. 1a, b). However, greater improvement for both VAS and ODI was seen in patients from this cohort with athletic activity. Differences between both groups were significant for both VAS and ODI (P < 0.05) in favour of results from athletic patients at all postoperative stages with the exception of VAS at 12 months. This nevertheless revealed a tendency towards statistical significance (P = 0.068).
Subjective outcome evaluation
Patient satisfaction rates in this cohort were significantly superior compared to our previously reported overall results [42, 51]. Asked for their subjective evaluation of total disc replacement, 33 (84.6%) of the patients were ‘completely satisfied’ at the time of their last follow-up and recorded their result as ‘excellent’, 4 patients (10.3%) were satisfied and marked ‘good’ results, whilst 2 (5.1%) of the patients were not satisfied with their personal outcome. Thus, 94.9% of the patients were satisfied or highly satisfied overall.
High subjective satisfaction rates were reflected in the overall rate of patients being back in a working environment of 81.1% as compared to 68.1% from our overall results [51].
Back to work
Whilst 23 patients (59%) were actively professionally engaged on a full-time (n = 20; 51.3%) or part-time basis (n = 3; 7.7%) preoperatively, this number increased considerably to 79.5% (n = 31) postoperatively following total lumbar disc replacement. At last FU, 26 patients (66.7%) were employed on a full-time basis, 5 patients (12.8%) on a part-time basis, respectively. Four patients (10.3%) reorganized their professional life after surgery and found themselves in a new working environment.
Only one patient (2.6%) received worker’s compensation preoperatively in this highly selected patient cohort. However, the patient was not able to benefit from the disc replacement procedure with regards to the working status with ongoing worker’s compensation claims at last FU. Similarly, two patients that were already unemployed preoperatively did not benefit with regards their postoperative working status.
Resumption of sporting activity
Fourteen patients (35.9%) were disabled to an extent that did not allow any athletic activity preoperatively, whilst 25 patients (64.1%) participated in sport but at a reduced level up until the time of surgery. On average, preoperative duration of absence from sport due to LBP was 2.5 years (range 0–8 years).
The majority of patients (69.2%) resumed physical activity within the first 3 months (n = 15; 38.5%) and 3–6 months (n = 12, 30.7%) following TDR, respectively. According to the patient’s subjective evaluation, full recovery and peak fitness was achieved after 5.2 months (range 1.5–24 months) postoperatively.
Two patients (5.1%), both with disc replacements performed at the lumbosacral junction, were not able to participate in physical/athletic activity due to unsatisfactory results with persisting low-back pain, leaving an overall return to sport rate of 94.9%. Out of these, reduced athletic activity was recorded in four patients, in three patients due to reasons unrelated to surgery. Reduced athletic activity due to persisting LBP following TDR was therefore observed in three patients overall (7.7%).
Excluding one of the above-mentioned unsatisfactory results, the remaining 24 patients from the patient cohort that was still actively engaged in sports before surgery (n = 25) were able to resume athletic activity postoperatively (96% return to sport rate in this cohort).
Overall, participation frequency in sport increased 194% from 1.7×/week preoperatively to 3.3×/week postoperatively.
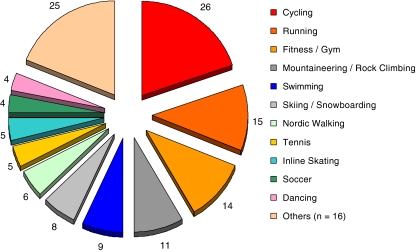
At last FU, the majority of patients participated in a variety of different athletic activities. On average, each patient was engaged in 3.3 different types of sport. Athletic activities and different sports were subdivided according to the patients’ preferences into ‘primary’ and ‘secondary’ sports, summarized and outlined in Table 5 and Fig. 2. Most popular athletic activities included cycling, running, fitness (gym), swimming as well as outdoor sports (mountaineering, rock climbing, skiing, snowboarding).
Table 5.
Participation and distribution in varying sports/athletic activities following total lumbar disc replacement
| Athletic activity/sport | Primary sport | Secondary sport | Total | |||
|---|---|---|---|---|---|---|
| n | % of patients | n | % of patients | n | % of all patients | |
| Cycling | 13 | 33.3 | 13 | 33.3 | 26 | 66.7 |
| Running | 7 | 17.9 | 8 | 20.5 | 15 | 38.5 |
| Fitness/gym | 9 | 23.1 | 5 | 12.8 | 14 | 35.9 |
| Mountaineering/rock climbing | 3 | 7.7 | 8 | 20.5 | 11 | 28.2 |
| Swimming | 2 | 5.1 | 7 | 17.9 | 9 | 23.1 |
| Skiing/snowboarding | 4 | 10.3 | 4 | 10.3 | 8 | 20.5 |
| Nordic walking | 1 | 2.6 | 5 | 12.8 | 6 | 15.4 |
| Tennis | 2 | 5.1 | 3 | 7.7 | 5 | 12.8 |
| Inline skating | 5 | 12.8 | 5 | 12.8 | ||
| Soccer | 3 | 7.7 | 1 | 2.6 | 4 | 10.3 |
| Dancing (Ballet, belly dancing) | 2 | 5.1 | 2 | 5.1 | 4 | 10.3 |
| Cross-country skiing | 3 | 7.7 | 3 | 7.7 | ||
| Horse riding | 3 | 7.7 | 3 | 7.7 | ||
| Golf | 2 | 5.1 | 1 | 2.6 | 3 | 7.7 |
| Badminton | 1 | 2.6 | 1 | 2.6 | 2 | 5.1 |
| Scuba diving | 1 | 2.6 | 1 | 2.6 | 2 | 5.1 |
| Table Tennis | 2 | 5.1 | 2 | 5.1 | ||
| Aerobics | 1 | 2.6 | 1 | 2.6 | ||
| Archery | 1 | 2.6 | 1 | 2.6 | ||
| Wild-water rafting/sea kajaking | 1 | 2.6 | 1 | 2.6 | ||
| Cross-country motor cycling (Enduro) | 1 | 2.6 | 1 | 2.6 | ||
| Parachute Jumping | 1 | 2.6 | 1 | 2.6 | ||
| Volleyball | 1 | 2.6 | 1 | 2.6 | ||
| Squash | 1 | 2.6 | 1 | 2.6 | ||
| Trampoline jumping | 1 | 2.6 | 1 | 2.6 | ||
| Diving | 1 | 2.6 | 1 | 2.6 | ||
| Archery | 1 | 2.6 | 1 | 2.6 | ||
| Total | 55 | 75 | 130 | |||
Fig. 2.
Frequency and distribution of participation in various athletic activities
A subjective evaluation of the patient’s individual postoperative athletic performance is outlined in Table 6. Overall, 17 patients (43.6%) reported improved physical performance with only minor complaints during athletic activities whilst another 16 patients (41.0%) felt completely unrestricted when participating in sport. At last FU the duration of athletic training averaged 6.1 h/week with 12 patients (30.1%) practising 5–10 h/week and 6 patients (15.4%) exceeding a duration of 10 h/week of athletic performance.
Table 6.
Subjective patient evaluation of postoperative athletic performance
| Athletic performance Compared to preoperatively, the patients’ subjective evaluation to participate in sporting activities was judged | |||||||
|---|---|---|---|---|---|---|---|
| Not possible | Worse | Unchanged | Better | Completely unrestricted | |||
| n = 2 (5.1%) | n = 1 (2.6%) | n = 3 (7.7%) | n = 17 (43.6%) | n = 16 (41.0%) | |||
| LBP during athletic performance Following surgery, patients reported low back pain during athletic activity | |||||||
|---|---|---|---|---|---|---|---|
| Sport not possible | Only with strong pain | With mild pain | Occassional/recurrent pain | Never, completely pain-free | |||
| n = 2 (5.1%) | n = 1 (2.6%) | n = 6 (15.4%) | n = 16 (41.0%) | n = 14 (35.9%) | |||
| Level of competition/athletic performance Patients were asked for change in level of competition compared with before surgery | |||||||
|---|---|---|---|---|---|---|---|
| Stopped—reasons unrelated to surgery/LBP | Stopped—moderate/significant problems | Lower—moderate/significant problems | Lower—none or insignificant problems/reasons unrelated to LBP | Not changed—moderate/significant problems | Not changed—none or insignificant problems | Higher—moderate/significant problems | Higher—none or insignificant problems |
| n = 0 (0%) | n = 2 (5.1%) | n = 1 (2.6%) | n = 3 (7.7%) | n = 1 (2.6%) | n = 6 (15.4%) | n = 5 (12.8%) | n = 21 (53.8%) |
Level of competition
Professionals
Five patients were either professional athletes or relied on full-time athletic activity for their income: one professional soccer player (>20 games/season), one professional dancer (ballett), one fitness-instructor (Gym), one instructional teacher for back exercises and one sports teacher (high school). Another patient was seasonally employed on a part-time basis as a skiing instructor. Overall, six patients (15.4%) relied on professional athletic activity for their income. With the exception of the high-school teacher who was already on sick leave 4 years prior to surgery and claiming workers compensation, all professional athletes were able to fully resume their athletic activity.
Semi-professionals/competitive athletes
A further six patients (15.4%) considered themselves as highly motivated and ambitious athletes: two marathon runners, one half-marathon runner, one patient involved in mountaineering/skiing (>10 h/week) and one tennis player. A former golf professional retired 5 years prior to surgery and did not return to his previous level of professional activity due to reasons unrelated to disc replacement surgery. However, the patient continued playing golf on a semi-professional competitive level.
One runner participated in a marathon 3 months postoperatively despite medical restrictions and reported five times participation in marathon competitions following TDR at 51 months FU. Furthermore, one patient practising archery and one practising cross-country motor biking (‘Enduro Racing’) similarly participated in athletic competitions.
Overall, nine patients practised sport at a competitive level with an average of 6.5 competitions/year.
Hobby athletes
The majority of patients in this study regarded themselves as hobby athletes (n = 27; 69.2%) and participated in a wide variety of different activities (Fig. 2, Table 5). Average participation in sporting activities in this group was 2.7×/week, the majority of patients (56.5%) practising ≤5 h/week.
Varying sporting activities
Contact sports
No specific type of sport was detected as unsuitable following TDR. However, two patients previously involved in contact sport at a high level (karate, wrestling) did not achieve their previous level of activity. Whilst the patient participating in wrestling reported reasons unrelated to surgery, a former black-belt karate-instructor complained of acute onset of LBP from sudden rotational high impact movements limiting his athletic performance which led to a modification of his athletic activities.
Parachute/trampoline jumping
One patient, participating in parachute jumping as a secondary sport, performed first free-fall jumps from >10,000 feet altitude at 3 months postoperatively without medical permission and described only minor temporary LBP. X-ray follow-up did not reveal any pathological findings and no influence on implant positioning.
Another patient resumed occasional diving/trampoline jumping as a secondary sport 6 months postoperatively. Follow-up in this patient to evaluate the impact of his secondary sport (9 months FU) was too short. However, at the time this article was written, the patient did not report any complaints.
High-impact sports
Other high impact sports included soccer, cross-country motor biking (‘Enduro Racing’), wild-water rafting and sea-kajaking and were performed with no or only minor restrictions up to a competitive level.
Analysis of different motion patterns
When asked which motions were most difficult to perform following disc replacement surgery the majority of patients marked jumping, remaining in fixed position (each n = 12; 30.8%) and rotational movements (n = 10; 25.6%) as most limiting (multiple answering allowed). Another six patients (15.4%) felt limitations during running whilst two patients (5.1%) marked heavy lifting as most limiting. Interestingly, only three patients (7.7%) marked limitations for flexion/extension movements.
Level of activity
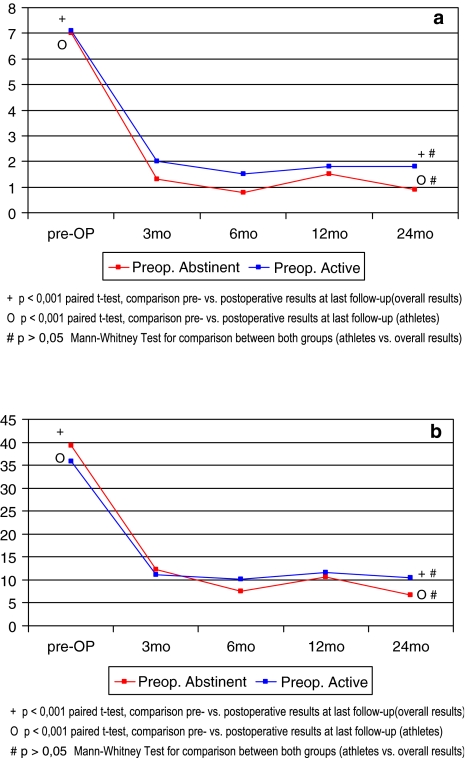
Whilst 14 patients had to abstain from athletic performance in the months prior to surgery due to intractable LBP, the remaining 25 patients were still actively involved in sport to a varying extent up until the time of surgery. Interestingly, postoperative outcome was not necessarily negatively influenced by preoperative abstinence from sport in this preselected group of patients (Fig. 3a, b). Both groups showed significant and maintained improvement throughout the entire FU period (P < 0.001) and no significant difference for functional outcome parameters VAS and ODI was detected between them (P > 0.05).
Fig. 3.
Comparison pre/postoperative results for a VAS and b ODI between patients with preoperative abstinence from sport due to low-back pain and patients still participating in athletic activity until lumbar disc replacement surgery
Radiological evaluation
Implant migration
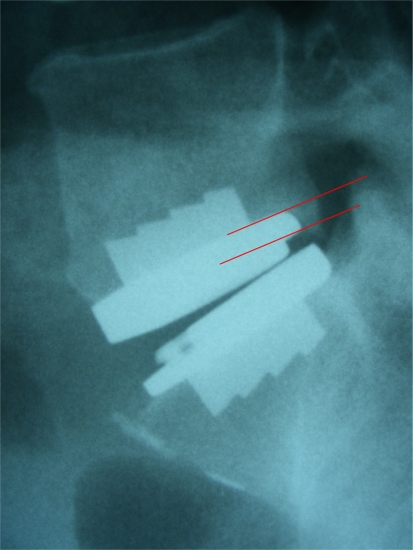
According to our previously described definition of the terms ‘disclocation’ and ‘subsidence’, we did not observe any implant dislocations. Subsidence was noted in n = 13 patients (30%) overall. In 11 patients, minor subsidence of 2–3 mm was recorded, whilst subsidence ≥4 mm was observed in 2 patients (Fig. 4). Subsidence occurred within the first 3 months following mobilization of the patients. No further implant migration was noted thereafter. However, migration of the implant slowly continued in one patient with recorded subsidence of 4 mm at 44.6 months follow-up.
Fig. 4.
Implant subsidence of 4 mm was observed within the first three postoperative months following total lumbar disc replacement at L5/S1. Despite continued physical activity (cycling, mountaineering) no further migration of the implant was noted thereafter
Range of motion
Preoperatively, ROM averaged 5.9° (range 0°–19.3°) at the index level. Following TDR, average postoperative flexion/extension ROM was maintained but remained virtually unchanged with 6.5° ROM at the instrumented segment (range 0°–14.5°).
ROM was 5.9° (range 0°–14.5°) for disc replacements performed at the lumbosacral junction (L5/S1, n = 26) and 7.2° (range 0°–13.2°) for L4/5 TDR (n = 7), respectively. Out of three bisegmental disc replacement procedures (L4/5/S1), two patients showed satisfactory range of motion (mean 13.4° at L4/5 and 9.9° at L5/S1), whilst one patient showed virtually no motion (< 1°) at both operated segments.
Overall, a ROM of ≤5° was observed in n = 15 out of 39 patients (38.5%). Eleven (28.2%) were observed following lumbosacral disc replacement procedures, which represents a total of 42.3% of all TDRs performed at L5/S1 (n = 26). Two TDRs performed at L4/5 similarly showed a ROM of ≤5°, representing 28.6° (n = 7) of all L4/5 disc replacement procedures, respectively.
Discussion
Published rates of low back pain (LBP) in athletes have been reported to range from 1 to 30% depending on gender, type, frequency, intensity of athletic activity and technique during athletic performance [2, 21, 23, 50, 55].
According to a literature review by Bono [7] participation in sporting activities seems to be a risk factor for the development of disc degeneration with conservative treatment representing the mainstay of treatment of discogenic LBP in the athlete.
Over the past two decades, disc arthroplasty has become increasingly popular for the treatment of lumbar degenerative disc disease without true deformity or instability. FDA-controlled investigational device exemption (IDE) studies have shown comparable results between disc replacement with Charité III and lumbar fusion procedures performed with BAK cages at short- to mid-term follow-up [6, 16, 43, 58]. The authors reported a faster postoperative mobilization and shorter recovery time for patients with artificial disc replacements.
Due to an increasing prevalence of commonly accepted contraindications with age [29], TDR is predominantly performed in young and active patients. Despite increasing popularity of disc arthroplasty, current literature has not addressed postoperative physical activity or sporting performance following TDR.
We have observed good and excellent results in 94.9% of a selected group of athletic patients following TDR. However, in a young population with high subjective expectations as presented here, definition of the term ‘successful result’ must take into consideration whether patients will be able to resume sporting activities on a satisfactory level. We could show that 84.6% of the patients reported improved (43.6%) or unlimited (41.0%) athletic activity up to the level of professional sports and extreme sports. Patients in this study participated in a variety of different activities, each with unique physical requirements and distinct patterns of movements and forces acting on the lumbar spine and the implant. We did not find a specific sport that was less tolerated by the patients. However, high contact sports in particular such as karate or wrestling require further evaluation with longer follow-up and larger patient numbers.
The ability to perform and participate in physical activity up until the time of surgical intervention proved to be a highly positive predictor for excellent postoperative results which is also reflected in significantly superior results from patients in this cohort compared to our previously published overall results for patients in which athletic activity was not an inclusion criteria [42, 51]. Similar to our findings, Wang [56] also reported favourable results for a cohort of college athletes following discectomy in comparison to results from the general population. Interestingly, in a recent study, Le Huec [31] correlated postoperative outcome with the degree of fatty posterior muscle degeneration and found better outcome for patients with muscle degeneration grades 1 and 2 compared to grades 3 and 4 according to the Goutallier classification system.
As was further shown in this study, however, preoperative absence from athletic activity due to LBP did not necessarily impair resumption of sporting activity. Excellent postoperative results were similarly achieved in patients that were preoperatively physically active and patients that were not able to participate in sport before surgery in a preselected group of patients (Fig. 3a, b). This emphasizes the role of postoperative rehabilitation and mobilization as a key factor for clinical success as has been mentioned previously [15, 22]. In a radiographic analysis Cinotti [15] reported greater mobility at the operated levels in patients who began to exercise 1 week after surgery than those who wore a corset for 3 months. Similarly, other authors adhere to the philosophy of an early and active rehabilitation following TDR [22].
Valuable information about possible postoperative load increase was gained from patients that did not comply with medical restrictions and continued athletic activity from an early postoperative phase. Based on these results and the findings of this current study, the postoperative rehabilitation program was therefore modified over time. Following an uneventful disc replacement procedure, patients in our institution are mobilized from the first postoperative day with physiotherapeutic assistance and without additional external support. Early resumption of physical activity is encouraged on a moderate level in non-contact sports (e.g. swimming, cycling) within the first 3 months following a short rehabilitation period. Solid osteointegration of the implants allows for further load increase and participation in preoperative sporting activities from 3 to 6 months postoperatively. In an uneventful postoperative course, participation even in highly demanding physical contact sports/extreme sports (e.g. marathon running, parachute jumping, soccer) has been shown to be accessible and may be resumed from 4 to 6 months postoperatively.
One possible explanation for excellent postoperative results in this study might have been the high rate of monosegmental disc replacement procedures (92.3%). While other authors described equivalent results for monosegmental and multiple level disc replacement procedures [53], our own results could not confirm such findings [51, 52]. Wang [56] described 90% return to sport rate in elite college athletes following monosegmental discectomies, whilst the authors stated two-level disease might be associated with a less favourable outcome. Unfortunately, the limited number of patients with bisegmental TDR in this study did not allow for statistical comparison between the two groups.
Furthermore, factors such as age, concomitant litigation or workers compensation, previously associated with negative influence on postoperative outcome [32, 40] were significantly less frequent in this athletic patient cohort as compared to our overall results.
The exact number of ‘relevant’ spine cycles experienced by a healthy adult remains a matter of speculation. Previous authors have published a wide range of an estimated 100,000–10,000,000 spine cycles per year [24, 34, 49]. Due to the young age of the patients and a considerable number of spine cycles over a lifetime period, additional wear rates from severe stress in competitive athletics must be addressed carefully. Forces on lumbar discs during activities of daily living from direct in vivo measurements have been described previously [35, 57], and multiple times increase of forces with presently unknown influence on the UHMWE-inlay and implants are to be expected during athletic activities (Table 1). Our radiological evaluation did not reveal any implant dislocations as a consequence of excessive external forces and we were not able to note any radiological macroscopic signs of UHMWPE-wear on conventional X-ray images. However, well-established methods for measurement of polythylene wear after total hip replacement such as computer assisted edge-detection techniques or even radiostereometric analysis are still lacking for lumbar disc replacements and were thus not available in this study [44]. Persisting concerns regarding the future of the implant therefore remain a matter of debate.
To date, there is no clear definition of the terms implant-‘subsidence’ and ‘dislocation’. No radiological investigations which would allow a more detailed classification of different stages of implant migration are available.
According to our own definition, a ‘dislocation’ was referred to as a migration of the implant ≥6 mm with secondary functional impairment whilst the term ‘subsidence’ described minor migration (≤5 mm) and intact function of the implant. No migration beyond 6 mm was observed in this study, whilst subsidence was noted in 30% of the patients. It has been reported previously that once subsidence occurs it will probably progress [33]. Various authors have reported on their concerns towards subsidence of implants [1, 48], previously attributed to implant size and large difference in material properties such as elasticity-modulus and stiffness at the implant–endplate interface under axial compression [47, 49]. In the present study 12 out of 13 cases of subsidence occurred during the first 3 months following TDR and mobilization of the patients with no further implant migration thereafter. Similarly, Tropiano [54] reported a 31% rate of minor subsidence (<2 mm) in a cohort of non-athletes with no negative influence on postoperative results. Bertagnoli [4] described one incidence of minor subsidence 3 days following TDR at the index-level, with no increase thereafter and no negative clinical impact. We therefore believe that subsidence in this study was observed due to reasons unrelated to physical activity. However, longer follow-up evaluations will be required to further assess the possibility of implant migration due to excessive loads associated with athletic activities and decreasing bone mineral density in the aging patient.
Published reports show that the results of the new procedure are inconsistent with respect to restoration of segmental motion. A wide range of data has been reported with regards to flexion/extension ROM following TDR at the index and adjacent level [14, 16, 28, 36, 37].
Results of this study show that segmental motion was maintained following lumbar disc replacement procedure in comparison to preoperative levels. Overall, the average ROM remained virtually unchanged with 5.9° (range 0°–19.3°) preoperatively and 6.5° ROM (range 0°–14.5°) postoperatively at the instrumented segment. However, previous results from other study groups indicated significantly higher ROM values for healthy adult volunteers [17, 19].
Recently, various authors have reported low ROM-values for sagittal flexion/extension movements following TDR procedures [28, 36]. It has been mentioned previously that a low ROM may be attributed to unsatisfactory postoperative outcome [30]. However, in this particular preselected cohort of active patients we observed a high subjective patient satisfaction rate. Similarly, Leivseth [36] reported that low ROM-measurements following TDR may only partly be attributed to unsatisfactory postoperative outcome and persisting symptoms.
According to the FDA IDE study protocol, a ROM of less than 5° has been defined as a criterion for solid fusion. In a recent study, Lim [39] reported that due to measurement error, a ROM of at least 4.6° must be observed in order to be certain that an implanted artificial disc has any sagittal motion. Huang [28, 30] previously reported that 5° of motion may represent a protective threshold against adjacent level degeneration.
Subsequently, we conducted further investigations and supplemented results obtained from all patients with flexion- extension ROM of ≤5°. Overall, 38.5% (n = 15/39) of all patients showed a segmental ROM of ≤5°. This was seen in 28.6% (n = 2) of all TDRs performed at L4/5 and in 42.3% (n = 11) of all TDRs performed at L5/S1, respectively. Similarly, a ROM of ≤5° was observed in 39.3% of all patients following TDR with Charité III in the US FDA IDE study [45].
However, the ideal range and quality of motion of a lumbar disc prosthesis remains yet unestablished. The question as to whether adjacent segment degeneration can be avoided following TDR by maintaining adequate and sufficient motion will therefore require longer FU and further investigation.
Conclusion
Athletic patients treated with total lumbar disc replacement for lumbar degenerative disc disease showed highly satisfactory results. Patients were able to perform a variety of different sporting activities up to the level of competitive sports, extreme sports and professional athletics. Preoperative ability to participate in sporting activity proved to be a strong positive predictor for satisfactory postoperative results. However, preoperative absence from sporting activities due to LBP did not imply inability to resume sport on a satisfactory level following disc replacement procedure in a preselected group of patients.
Minor implant subsidence was observed in 30% of patients during the first 3 months with no further implant migration thereafter and was therefore not attributed to sporting activity. No evidence of implant wear was seen in radiological follow-up evaluations. However, due to the young age of the patients and significant load increase during athletic activities, concerns about the future of the implant remain a matter of debate that will require larger patient cohorts, longer follow-up evaluations and modified examination techniques.
References
- 1.Anderson PA, Rouleau JP. Intervertebral disc arthroplasty. Spine. 2004;29:2779–2786. doi: 10.1097/01.brs.0000146460.11591.8a. [DOI] [PubMed] [Google Scholar]
- 2.Bahr R, Andersen SO, Loken S, Fossan B, Hansen T, Holme I. Low back pain among endurance athletes with and without specific back loading—a cross-sectional survey of cross-country skiers, rowers, orienteerers, and nonathletic controls. Spine. 2004;29:449–454. doi: 10.1097/01.BRS.0000096176.92881.37. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnoli R, Marnay T, Mayer HM (eds) (2003) Spine Solutions GmbH. The ProDisc Book, Tuttlingen
- 4.Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, Kershaw T, Husted DS. The treatment of disabling multilevel lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2192–2199. doi: 10.1097/01.brs.0000181061.43194.18. [DOI] [PubMed] [Google Scholar]
- 5.Block AR, Vanharanta H, Ohnmeiss DD, Guyer RD. Discographic pain report. Influence of psychological factors. Spine. 1996;21:334–338. doi: 10.1097/00007632-199602010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R Jr, Regan JJ, Ohnmeiss DD (2005) A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine 30:1565–1575; discussion E1387–E1591 [DOI] [PubMed]
- 7.Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A:382–396. doi: 10.2106/00004623-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Cakir B, Richter M, Puhl W, Schmidt R. Reliability of motion measurements after total disc replacement: the spike and the fin method. Eur Spine J. 2006;15:165–173. doi: 10.1007/s00586-005-0942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappozzo A, Felici F, Figura F, Gazzani F. Lumbar spine loading during half-squat exercises. Med Sci Sports Exerc. 1985;17:613–620. [PubMed] [Google Scholar]
- 10.Carragee EJ, Tanner CM, Yang B, Brito JL, Truong T. False-positive findings on lumbar discography. Reliability of subjective concordance assessment during provocative disc injection. Spine. 1999;24:2542–2547. doi: 10.1097/00007632-199912010-00017. [DOI] [PubMed] [Google Scholar]
- 11.Carragee EJ, Chen Y, Tanner CM, Hayward C, Rossi M, Hagle C. Can discography cause long-term back symptoms in previously asymptomatic subjects? Spine. 2000;25:1803–1808. doi: 10.1097/00007632-200007150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Cheng CK, Chen HH, Chen CS, Lee SJ. Influences of walking speed change on the lumbosacral joint force distribution. Biomed Mater Eng. 1998;8:155–165. [PubMed] [Google Scholar]
- 13.Cholewicki J, McGill SM, Norman RW. Lumbar spine loads during the lifting of extremely heavy weights. Med Sci Sports Exerc. 1991;23:1179–1186. [PubMed] [Google Scholar]
- 14.Chung SS, Lee CS, Kang CS, Kim SH. The effect of lumbar total disc replacement on the spinopelvic alignment and range of motion of the lumbar spine. J Spinal Disord Tech. 2006;19:307–311. doi: 10.1097/01.bsd.0000208255.14329.1e. [DOI] [PubMed] [Google Scholar]
- 15.Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine. 1996;21:995–1000. doi: 10.1097/00007632-199604150-00015. [DOI] [PubMed] [Google Scholar]
- 16.Delamarter RB, Fribourg DM, Kanim LE, Bae H. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine. 2003;28:S167–S175. doi: 10.1097/01.BRS.0000092220.66650.2B. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak J, Panjabi MM, Chang DG, Theiler R, Grob D. Functional radiographic diagnosis of the lumbar spine. Flexion-extension and lateral bending. Spine. 1991;16:562–571. doi: 10.1097/00007632-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 19.Frobin W, Brinckmann P, Leivseth G, Biggemann M, Reikeras O. Precision measurement of segmental motion from flexion-extension radiographs of the lumbar spine. Clin Biomech (Bristol, Avon) 1996;11:457–465. doi: 10.1016/S0268-0033(96)00039-3. [DOI] [PubMed] [Google Scholar]
- 20.Gatt CJ, Jr, Hosea TM, Palumbo RC, Zawadsky JP. Impact loading of the lumbar spine during football blocking. Am J Sports Med. 1997;25:317–321. doi: 10.1177/036354659702500308. [DOI] [PubMed] [Google Scholar]
- 21.Granhed H, Morelli B. Low back pain among retired wrestlers and heavyweight lifters. Am J Sports Med. 1988;16:530–533. doi: 10.1177/036354658801600517. [DOI] [PubMed] [Google Scholar]
- 22.Guyer RD, Ohnmeiss DD. Intervertebral disc prostheses. Spine. 2003;28:S15–S23. doi: 10.1097/00007632-200308011-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hainline B. Low back injury. Clin Sports Med. 1995;14:241–265. [PubMed] [Google Scholar]
- 24.Hallab N, Link HD, McAfee PC. Biomaterial optimization in total disc arthroplasty. Spine. 2003;28:S139–S152. doi: 10.1097/01.BRS.0000092214.87225.80. [DOI] [PubMed] [Google Scholar]
- 25.Harrison DE, Harrison DD, Cailliet R, Janik TJ, Holland B. Radiographic analysis of lumbar lordosis: centroid, Cobb, TRALL, and Harrison posterior tangent methods. Spine. 2001;26:E235–E242. doi: 10.1097/00007632-200106010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Hosea TM, Boland AL. Rowing Injuries. Postgrad Adv Sports Med. 1989;III:1–17. [Google Scholar]
- 27.Hosea TM, Gatt CJ, Jr, McCarthy KE, Langrana NA, Zawadsky JP. Analytical computation of rapid dynamic loading of the lumbar spine. Trans Orthop Res Soc. 1989;14:358. [Google Scholar]
- 28.Huang RC, Girardi FP, Cammisa FP, Jr, Tropiano P, Marnay T. Long-term flexion-extension range of motion of the prodisc total disc replacement. J Spinal Disord Tech. 2003;16:435–440. doi: 10.1097/00024720-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Huang RC, Lim MR, Girardi FP, Cammisa FP., Jr The prevalence of contraindications to total disc replacement in a cohort of lumbar surgical patients. Spine. 2004;29:2538–2541. doi: 10.1097/01.brs.0000144829.57885.20. [DOI] [PubMed] [Google Scholar]
- 30.Huang RC, Girardi FP, Cammisa FP, Jr, Lim MR, Tropiano P, Marnay T. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine. 2005;30:1407–1411. doi: 10.1097/01.brs.0000166528.67425.0e. [DOI] [PubMed] [Google Scholar]
- 31.Le Huec JC, Basso Y, Aunoble S, Friesem T, Bruno MB. Influence of facet and posterior muscle degeneration on clinical results of lumbar total disc replacement: two-year follow-up. J Spinal Disord Tech. 2005;18:219–223. [PubMed] [Google Scholar]
- 32.Junge A, Frohlich M, Ahrens S, Hasenbring M, Sandler A, Grob D, Dvorak J (1996) Predictors of bad and good outcome of lumbar spine surgery. A prospective clinical study with 2 years’ follow up. Spine 21:1056–1064; discussion 1064–1055 [DOI] [PubMed]
- 33.Kleuver M, Oner FC, Jacobs WC. Total disc replacement for chronic low back pain: background and a systematic review of the literature. Eur Spine J. 2003;12:108–116. doi: 10.1007/s00586-002-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostuik JP (1997) Intervertebral disc replacement. Experimental study. Clin Orthop Relat Res (337):27–41 [DOI] [PubMed]
- 35.Ledet EH, Tymeson MP, DiRisio DJ, Cohen B, Uhl RL. Direct real-time measurement of in vivo forces in the lumbar spine. Spine J. 2005;5:85–94. doi: 10.1016/j.spinee.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Leivseth G, Braaten S, Frobin W, Brinckmann P. Mobility of lumbar segments instrumented with a ProDisc II prosthesis: a two-year follow-up study. Spine. 2006;31:1726–1733. doi: 10.1097/01.brs.0000224213.45330.68. [DOI] [PubMed] [Google Scholar]
- 37.Lemaire JP, Carrier H, Ali el HS, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353–359. doi: 10.1097/01.bsd.0000172361.07479.6b. [DOI] [PubMed] [Google Scholar]
- 38.Lim MR, Girardi FP, Zhang K, Huang RC, Peterson MG, Cammisa FP., Jr Measurement of total disc replacement radiographic range of motion: a comparison of two techniques. J Spinal Disord Tech. 2005;18:252–256. [PubMed] [Google Scholar]
- 39.Lim MR, Loder RT, Huang RC, Lyman S, Zhang K, Sama A, Papadopoulos EC, Warner K, Girardi FP, Cammisa FP., Jr Measurement Error of Lumbar Total Disc Replacement Range of Motion. Spine. 2006;31:E291–E297. doi: 10.1097/01.brs.0000216452.54421.ea. [DOI] [PubMed] [Google Scholar]
- 40.Lotters F, Hogg-Johnson S, Burdorf A. Health status, its perceptions, and effect on return to work and recurrent sick leave. Spine. 2005;30:1086–1092. doi: 10.1097/01.brs.0000161484.89398.48. [DOI] [PubMed] [Google Scholar]
- 41.Mayer HM, Wiechert K. Microsurgical anterior approaches to the lumbar spine for interbody fusion and total disc replacement. Neurosurgery. 2002;51:S159–165. doi: 10.1097/00006123-200211002-00022. [DOI] [PubMed] [Google Scholar]
- 42.Mayer HM, Wiechert K, Korge A, Qose I. Minimally invasive total disc replacement: surgical technique and preliminary clinical results. Eur Spine J. 2002;2(11Suppl):S124–S130. doi: 10.1007/s00586-002-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham BW. SB Charite disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech. 2003;16:424–433. doi: 10.1097/00024720-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 44.McCalden RW, Naudie DD, Yuan X, Bourne RB. Radiographic methods for the assessment of polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2323–2334. doi: 10.2106/JBJS.E.00223. [DOI] [PubMed] [Google Scholar]
- 45.Mirza SK. Point of view: commentary on the research reports that led to Food and Drug Administration approval of an artificial disc. Spine. 2005;30:1561–1564. doi: 10.1097/01.brs.0000171806.30401.40. [DOI] [PubMed] [Google Scholar]
- 46.Morris FL, Smith RM, Payne WR, Galloway MA, Wark JD. Compressive and shear force generated in the lumbar spine of female rowers. Int J Sports Med. 2000;21:518–523. doi: 10.1055/s-2000-7409. [DOI] [PubMed] [Google Scholar]
- 47.Noailly J, Lacroix D, Planell JA. Finite element study of a novel intervertebral disc substitute. Spine. 2005;30:2257–2264. doi: 10.1097/01.brs.0000182319.81795.72. [DOI] [PubMed] [Google Scholar]
- 48.Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. J Spinal Disord Tech. 2003;16:369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Polly DW., Jr Adapting innovative motion-preserving technology to spinal surgical practice: what should we expect to happen? Spine. 2003;28:S104–S109. doi: 10.1097/01.BRS.0000092208.09020.16. [DOI] [PubMed] [Google Scholar]
- 50.Ranson CA, Kerslake RW, Burnett AF, Batt ME, Abdi S. Magnetic resonance imaging of the lumbar spine in asymptomatic professional fast bowlers in cricket. J Bone Joint Surg Br. 2005;87:1111–1116. doi: 10.1302/0301-620X.87B8.16405. [DOI] [PubMed] [Google Scholar]
- 51.Siepe CJ, Mayer HM, Wiechert K, Korge A. Clinical results of total lumbar disc replacement with ProDisc II: three-year results for different indications. Spine. 2006;31:1923–1932. doi: 10.1097/01.brs.0000228780.06569.e8. [DOI] [PubMed] [Google Scholar]
- 52.Siepe CJ, Mayer HM, Heinz-Leisenheimer M, Korge A (2006) Total lumbar disc replacement: different results for different levels. Spine (accepted for publication) [DOI] [PubMed]
- 53.Tropiano P, Huang RC, Girardi FP, Marnay T. Lumbar disc replacement: preliminary results with ProDisc II after a minimum follow-up period of 1 year. J Spinal Disord Tech. 2003;16:362–368. doi: 10.1097/00024720-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Tropiano P, Huang RC, Girardi FP, Cammisa FP, Jr, Marnay T. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005;87-A:490–496. doi: 10.2106/JBJS.C.01345. [DOI] [PubMed] [Google Scholar]
- 55.Videman T, Sarna S, Battie MC, Koskinen S, Gill K, Paananen H, Gibbons L. The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine. 1995;20:699–709. doi: 10.1097/00007632-199503150-00011. [DOI] [PubMed] [Google Scholar]
- 56.Wang JC, Shapiro MS, Hatch JD, Knight J, Dorey FJ, Delamarter RB. The outcome of lumbar discectomy in elite athletes. Spine. 1999;24:570–573. doi: 10.1097/00007632-199903150-00014. [DOI] [PubMed] [Google Scholar]
- 57.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 58.Zigler JE. Clinical results with ProDisc: European experience and U.S. investigation device exemption study. Spine. 2003;28:S163–S166. doi: 10.1097/00007632-200310151-00009. [DOI] [PubMed] [Google Scholar]