Abstract
Recurrent disc herniation is frequently observed due to leakage of nucleus pulposus through injured anulus fibrosus. There is no effective treatment to prevent recurrent disc herniation yet. In this study, we proposed to implant non-cell-based materials into the porcine disc to stimulate the growth of fibrous tissue and thereby increase the disc functional integrity. The disc herniation was simulated by anular punctures using the spinal needles. Four clinically used implantation materials, i.e., gelfoam, platinum coil, bone cement and tissue glue, were delivered into the discs via percutaneous spinal needles. Two months after the surgery, the swine were killed. The degree of disc integrity of intact, naturally healed and implanted discs, was examined by quantitative discomanometry apparatus. We found the disc injury could not recover after 2 months of healing, and the disc implantation affected the degree of disc integrity. The disc integrity of gelfoam-implanted discs was better than that of coil-, bone cement-, and glue-implanted discs. The implantation of non-cell-based material was proved to be a potentially clinically applicable method to recover the integrity of injured discs and to prevent recurrent disc herniation.
Keywords: Anular puncture, Nucleus implantation, Disc integrity, Anulus sealing
Introduction
The herniated intervertebral disc (HIVD) syndrome is a neurological symptom where the protrusion of nucleus pulposus (NP) compresses the nerve root in the spinal canal or the intervertebral foramen [10]. Conventional treatments include medication, steroid injection, physical therapy and surgery. When non-operative treatment fails, surgical intervention may be required. The surgical treatments of HIVD, for example, nucleotomy [26] or microdiscectomy [28], sometimes result in a recurrent herniation of disc material through the incompetent disc anulus fibrosus (AF). The revision rate of HIVD is 4.9% at a 5.25-year follow-up and 7.9% at a 10-year follow-up [6]. Recurrent disc herniation is a significant clinical problem. Methods for improving disc integrity after surgery can be of significant benefit.
Tissue engineering technology might be a prospective method of restoring intervertebral discs. The regeneration of disc tissue is in the beginning stages of development. Biological materials such as the growth factor, cells which carry growth factor [21] and tissue-engineered discs [2] have been used to stimulate the tissue growth or replace the disc. However, the use of non-cell-based materials to stimulate the growth of fibrous tissue has been less considered. Examination of outcome of disc implantation included the gross morphology, histological appearance, biochemical composition, and biomechanical properties. Biomechanical properties examination of disc implantation included the compressive stiffness [13, 14, 23] and height [1, 3, 31] of disc, the hydraulic permeability of AF [23], and the flexibility of motion segment [11, 18]. The effect of disc implantation on the disc integrity, i.e., the capability of maintaining the intradiscal pressure (IDP), however, has been less examined.
In this study, we proposed to implant non-cell-based materials into the disc, to stimulate the growth of fibrous tissue, in order to seal the injured disc. The result of disc implantation was evaluated by the degree of disc integrity using the quantitative discomanometry (QD) apparatus. Two hypotheses were raised in this study. First, the disc injury due to percutaneous stab injury cannot recover with the help of natural healing process. Second, the disc implantation will affect the degree of disc integrity. The goal of this study was to prove that the implantation of non-cell-based materials would increase the functional integrity of an injured disc, thus preventing the recurrent disc herniation.
Materials and methods
Animal species
Four Spotted Lanyu Miniature Swine [39] were used. These swine were 3-months old during surgery and 5-months old when killed. The average weight of the swine was 9 kg at 3-months and 15 kg at 5-months. The average size of the L2/L3 disc of the 3-month-old miniature swine was 17 mm wide, 12 mm deep and 2 mm thick. Twelve discs (from T8/T9 to L5/S1) were used for the experiments in each swine. Among these 12 discs, 2 discs remained intact, 2 discs served as control and the remaining 8 discs were implanted with 4 different materials (2 discs per material).
Anesthesia
Atropine sulfate (0.03 mg/kg body weight) was intramuscularly (IM) injected before anesthesia to prevent salivary secretion. Zoletil® (0.55–0.8 mg/kg body weight IM) was used for pre-anesthesia and then Citosol® (Thiamylal) (1.11–1.66 mg/kg body weight intravenous, IV) was slowly used for deep anesthesia. Buprenorphine (0.005–0.1 mg/kg IM) or Flunixin (1.0–2.2 mg/kg IM) was used for analgesics. Antibiotic Ceftiofur (3 mg/kg IM) was used to prevent bacterial infection.
Surgical procedure
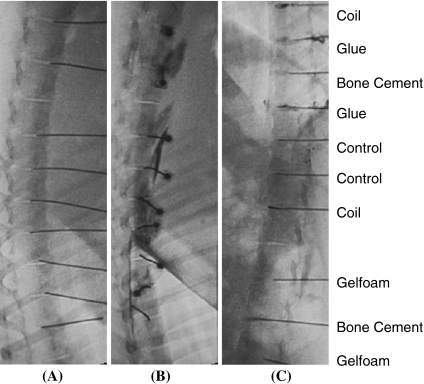
After the swine was anesthetized, a 22 G spinal needle was placed in the center of the disc through the left posterolateral site, under fluoroscopic guidance (LCX, GE Healthcare) (Fig. 1a). An 18 G spinal needle, with larger coaxial diameter, was used to replace the 22 G spinal needle to produce a larger injury on the AF. The discography of contrast medium leakage confirmed the AF injury (Fig. 1b). Four implantation materials were delivered into the center of the disc via the 18 G spinal needles. The four implantation materials were: gelfoam (0.5 ml, absorbable gelatin sponge, USP, Pharmacia & Upjohn Company, Kalamazoo, USA), platinum coil (2 mm × 4 mm, VORTX, Fibered Platinum Coil-18, Boston Scientific Cork Ltd, Cork, Ireland), bone cement (0.5 ml, CMW 1 Radiopaque, DePuy CMW, Johnson–Johnson company, Lancashire, England), and nonbiologic tissue glue (0.5 ml, NBCA Glue, Ingenor, Paris, France). Six groups of discs were randomly assigned in each swine. The six disc groups are: intact (no injury), controlled (injured disc, naturally-healed) and four implantation groups. The six disc groups are mentioned hereafter as: Intact, Control, Gelfoam, Coil, Bone Cement and Glue. The fluoroscopic images were examined to assure that the implantation materials were in the center of the discs (Fig. 1c).
Fig. 1.
Fluoroscopic images of a percutaneous anular punch, b the leakage of contrast medium indicating the anulus fibrosus injury, and c the implantation materials in the center of the disc. Gelfoam absorbable gelatin sponge, Coil platinum coil, Glue nonbiologic tissue glue, Control injured disc, no implantation
Quantitative discomanometry apparatus
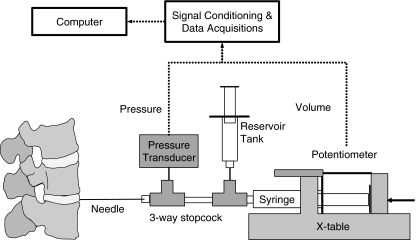
The disc integrity was evaluated by the QD apparatus. The QD was found to be a reproducible technique [12, 25] that measures both the volume injected and pressure developed within the disc, thus quantifying the degree of disc integrity. The pressure tolerance of QD apparatus for testing the dysfunction [37] or degenerated [5, 30] disc was only 0.6 MPa. Since the IDP of normal disc is often up to 2 MPa during daily activities [38], a high-pressured QD apparatus was designed to test the intact disc in comparison with injured and healed disc. The high-pressured QD apparatus was composed of a spinal needle (22 G, 90 mm, BD Yale Spinal, Madrid, Spain), a high-pressured syringe (1 ml, Madallion, Merit Medical Systems, Inc., UT, USA), a programmable X-table (Sintek Automation Co., Taiwan) that produced 110 N horizontal thrust, a pressure transducer (American Sensor Technologies, Inc., Landing, NJ, USA) for pressure measurement, a linear potentiometer (50 mm, Gefran Inc., Italy), for volume measurement, and a computer for data acquisition. High-pressure 3-way stopcocks (MX4331R, Medex, Dublin, OH, USA), were used for component connections. The system was calibrated by a precision digital pressure gage (Dwyer Instruments, Inc., Michigan City, IN, USA). The maximum pressure of the system was 5 MPa. The linear range of the system was 4.5 MPa and the error of linearity was under 0.5%. The pressure and volume resolution of the system was 0.01 MPa and 0.002 ml. In the pilot study, the fluid leaked when the injection volume was less than 0.1 ml, and pressure reached saturation when the injection volume was less than 0.5 ml, hence 0.5 ml was set as the injection volume. The injection rate was fixed at 0.5 ml/min (Fig. 2).
Fig. 2.
Apparatus of quantitative discomanometry (QD). An industrial X-table was used to drive the syringe at constant flow rate. The potentiometer was used to record the volume of saline injected into the disc. The pressure transducer was used to record the developed pressure. The syringes and 3-way stopcocks were able to hold the pressure up to 5 MPa
Protocol of QD test
Two months after the surgery, the swine were killed. The spinal columns were dissected preserving the osteoligamentous structure. All specimens were examined by X-ray, before the QD test. The 22 G spinal needle was carefully placed in the center of the disc using a lateral injection with the help of a plastic stopper/indicator around the needle. Each disc was tested five times. To assure the disc biomechanical function, the QD tests were finished within 10 h after killing. The implantation was delivered through posterolateral site and the QD test was conducted through lateral site after 2 months of healing. There was no potential interference between the implantation and the QD test.
Data analysis
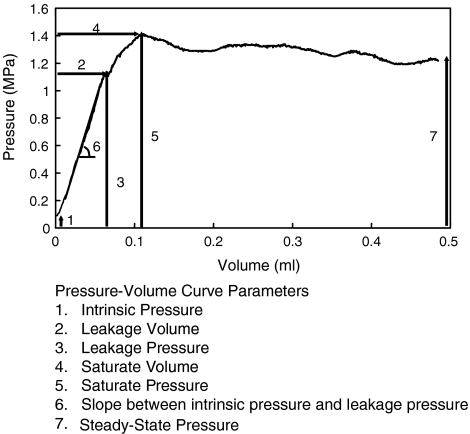
Seven parameters were obtained from each pressure–volume (PV) curve. The seven parameters were: the intrinsic pressure, leakage volume, leakage pressure, saturate volume, saturate pressure, slope between intrinsic and leakage pressure and steady-state pressure (Fig. 3) [5]. The intrinsic pressure was the disc pressure before injection. The leakage volume and pressure were the volume and pressure of the linear threshold of the PV curve. The saturate volume and pressure were the volume and pressure of the maximum pressure during the injection. The slope between intrinsic and leakage pressure corresponded to the elasticity of the AF ring. The steady-state pressure was defined as the pressure at 0.5 ml. The effect of testing sequence was examined. Only the first and second test of intrinsic pressure, leakage volume and slope were different. The rest of the tests were not affected by the testing sequences. Since the effect of testing sequence was minimal, all five QD tests of each disc were included for analysis.
Fig. 3.
Schematics of a pressure–volume curve obtained using QD apparatus and the seven QD parameters [37]
Statistical analysis
Forty-eight discs were tested by QD apparatus. One-way analysis of variance (ANOVA) was used to find the significant QD parameters affected by the disc treatments. Post hoc multiple pair-wise comparison tests (Fisher’s least significant difference) were performed to determine the level differences among intact, controlled and implanted discs at the significance level of α = 0.05.
Results
The QD parameters statistically significantly affected by the disc treatment included the leakage volume (P = 0.000), leakage pressure (P = 0.000), saturate pressure (P = 0.000), slope (P = 0.000) and steady-state pressure (P = 0.000). The intrinsic pressure (P = 0.069) showed a tendency to be affected by disc treatment, and the saturate volume (P = 0.191) was not affected by disc treatment.
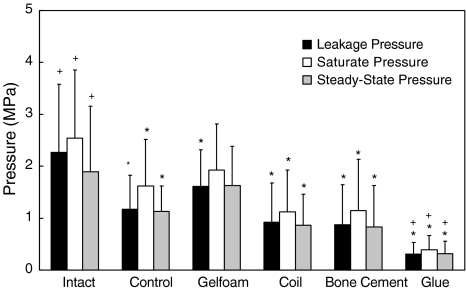
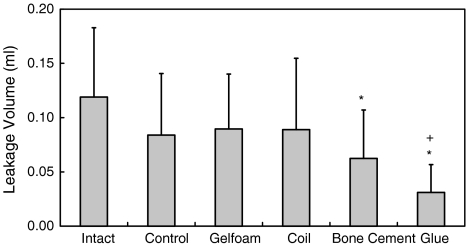
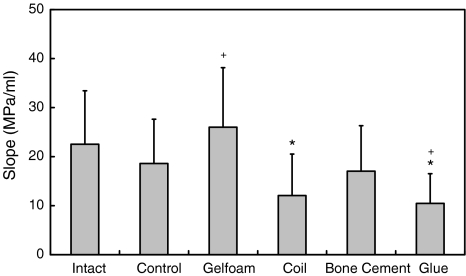
Higher QD pressure means better disc integrity. The QD pressures of Intact discs were higher than the ones of Control, Coil, Bone cement and Glue (all P < 0.05). The saturate pressure (P = 0.07) and steady-state pressure (P = 0.739) of Intact were not statistically significantly different from those of Gelfoam. The QD pressures of Control were lower than those of Intact (all P < 0.05), but higher than of Glue (all P < 0.05) (Fig. 4). Larger leakage volume means either better compliance of AF or larger space of NP. The leakage volume of Intact was larger than of Bone Cement (P < 0.001) and Glue (P < 0.001). The leakage volume of Control was larger than of Glue (P = 0.003) (Fig. 5). A disc with a higher PV slope can be interpreted as a disc with a stiffer AF ring. The slope of Intact was higher than of Coil (P < 0.001) and Glue (P < 0.001). The slope of Control was lower than of Gelfoam (P = 0.048), but higher than of Glue (P = 0.023) (Fig. 6).
Fig. 4.
Values of the leakage, saturate and steady-state pressures of intact, controlled and implanted discs after 2 months of healing. The “Intact” is the disc without injury, while the “Control” is the injured disc without implantation, i.e., naturally healed. * Significantly different from Intact, P < 0.05. + Significantly different from Control, P < 0.05. Error bars represent ±standard deviation (SD)
Fig. 5.
Values of the leakage volume of intact, controlled, and implanted discs after 2 months of healing. * Significantly different from Intact, P < 0.05. + Significantly different from Control, P < 0.05. Error bars represent ±SD
Fig. 6.
Values of the slope of intact, controlled, and implanted discs after 2 months of healing. * Significantly different from Intact, P < 0.05. + Significantly different from Control, P < 0.05. Error bars represent ±SD
Among the four implantation groups, the Gelfoam was the best one in maintaining the disc integrity. All the QD pressures of Gelfoam were higher than of Coil, Bone Cement, and Glue (all P < 0.05). The Gelfoam was also the only group in which, saturate and steady-state pressures were not statistically significantly different from the ones of the intact disc group (Fig. 4).
Discussion
We proved our hypotheses that the integrity of an injured disc cannot naturally recover within 2 months of healing and that disc integrity can be altered by disc implantation. With the proof of the two hypotheses, we may eventually find materials that seal the disc perfectly. The current study only tested four materials and found that the outcome of gelfoam-implanted disc was better than the naturally healed disc. Many more non-cell-based and fiber-inducing materials, for example: the copolymer-platinum coil, absorbable copolymer, biologic fibrin-based tissue glue, silk, etc., can be tried to prevent the disc leakage in future studies.
The implantation materials in this study were limited to ones that can be delivered by spinal needle. This gives the potential of treating disc injury/degeneration using percutaneous techniques but not invasive open surgery. The selected non-cell-based materials are widely and safely used in medical applications. For example, gelfoam and tissue glue are used for hemostasis, platinum coil is used for embolization, and bone cement is used for porous bone enhancement. Consequently, there is no worry of unexpected neoplasm using these materials.
A significant injury of the porcine disc was created. The average disc height of tested specimen was 2 mm. The outside diameter of 18 G spinal needle used to create the disc injury was 1.26 mm. The results showed that disc injury of this size could not recover, even after 2 months of healing. The age of sexual maturity of tested mini swine was 134 (SD 22) days [39]. It is expected that injuries to adolescent swine (3–5 months old) should recover faster than the injuries to fully matured or aged swine. The 2 months’ healing period of adolescent swine should be long enough to represent the chronic stage of a healing process. In addition, 2 months was also the time used for the subjective and objective measurement of disability after decompression surgery of HIVD [20].
Gelfoam, together with fat grafts and sodium hyaluronate, were examined to find their ability to inhibit peridural fibrosis in dogs [29]. The gelfoam was found to increase rather than to inhibit fibrous tissue. The consequence of this observation is consistent with our assumption. Gelfoam was used as a matrix for disc cell [8] and tumor cell [34] implantation as well as a drug delivery system [24]. It is safe to use gelfoam of different compositions, sizes, and geometries for future studies.
The biological function of the platinum coil is to produce a thrombus and later a fibrosis to fill the cavity of the aneurysm. The length of time from thrombus to fibrosis is controversial. It was reported that the fibroblast was observed in the aneurysm 4 days after coil placement in a canine model [32]. However, in a rabbit model, the fibrous cell infiltration was not observed even 12 weeks after coil placement [15]. The mechanical strength of thrombus is 0.5 MPa [36], which is less than the strength needed to hold the IDP during daily activities (up to 2 MPa) [38]. This study reveals that the integrity of the coil-implanted disc was less than an intact disc.
The bone cement (polymethylmethacrylate, PMMA), often used to secure a metal prosthesis to living bone or to augment the strength of osteoporotic bone, merely elicits a fibrous reaction [9]. This is consistent with our observation that the bone cement cannot induce the fibrous tissue in the AF to maintain the disc integrity. There is no operation that purposely implants bone cement into the disc yet. However, the bone cement sometimes accidentally leaks into the disc during vertebroplasty. If this happens, the risk of adjacent vertebral fracture (AVF) increases [19]. This phenomenon may be explained by our observation that the low disc integrity induced by the bone cement leakage would result in an unstable spinal column, thereby increasing the risk of AVF [16].
The nonbiologic tissue glue (Histoacryl®, a cyanoacrylate adhesive) polymerizes water substances and creates a bonding on the contact surface. It is widely used for skin closure and vessel embolism. The implantation of nonbiologic glue will result in inflammation [7] and foreign body giant cell reaction [4]. Some researchers have tried to apply the glue in tendon repair [33], bone adhesion [22] and blepharoplasty [35], but without much success. Our results also showed that the nonbiologic tissue glue could not seal the AF. The biologic fibrin-based tissue glue, which consists of naturally occurring molecules and proteins [7], may be the candidate to induce the fibrous tissue in the AF.
The implantation of an artificial nucleus has recently become clinically practical [17]. The purpose of implanting an artificial nucleus is to replace the nucleus to withstand the external loading. One of the failure mechanisms of an artificial nucleus was migration of implantation. The purpose of disc implantation in this study was not to replace the NP, but to induce fiber to recover the biomechanical function of AF. Although this study did not find the migration of implantation materials after 2 months of healing, there is potential that the implantation materials may protrude out of the disc. The effect and outcome of implantation migration needs further investigation.
The QD method was used to test disc function instead of traditional spine testing methods; for example, compressive test of disc and the flexibility test of motion segment. The biomechanical properties of disc integrity, disc compressive strength and spine flexibility are connected with each other. The AF holds the IDP, therefore the disc can sustain the loading and the motion segment can provide the stability. A disc of poor integrity would lead to a weak disc, hence an unstable motion segment. The advantage of using the proposed QD method is that the QD method directly tests the anatomic biomechanical function of AF, i.e. the capability of holding IDP. The QD test can be approached through either the endplate [12, 27] or the disc [5, 30]. The endplate approach produced no injury to the disc, but it also needs one complete motion segment for the testing of one disc. The current QD test used the disc approach method. Our internal study showed that the use of 22 G spinal needle would not produce a significant injury that changed the disc functional integrity. Furthermore, the disc-approached QD test is easier and potentially possible for the in vivo test.
In this study, we tested the hypotheses of implanting non-cell-based materials to recover the disc integrity. The results showed that disc injuries cannot be recovered within a 2-month healing period and the disc implantation did affect the disc integrity. This result indicated that the implantation of non-cell-based materials might be a clinically useful procedure. The best material to prevent the recurrent disc herniation needs further investigation.
Acknowledgment
This study was supported by NTU Hospital, NTU Animal Hospital, the National Science Council, Taiwan (NSC 92-2320-B-002-091, NSC 94-2320-B-002-035), and National Health Research Institute, Taiwan (NHRI-EX94-9425EI). The use of animals was approved by the Animal Care and Use Committee, National Taiwan University.
References
- 1.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K (2005) Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 30:25–31; discussion 31–32 [DOI] [PubMed]
- 2.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86–S92. doi: 10.1097/00007632-200308011-00015. [DOI] [PubMed] [Google Scholar]
- 3.Aota Y, An HS, Homandberg G, Thonar EJ, Andersson GB, Pichika R, Masuda K. Differential effects of fibronectin fragment on proteoglycan metabolism by intervertebral disc cells: a comparison with articular chondrocytes. Spine. 2005;30:722–728. doi: 10.1097/01.brs.0000157417.59933.db. [DOI] [PubMed] [Google Scholar]
- 4.Forseth M, O’Grady K, Toriumi DM. The current status of cyanoacrylate and fibrin tissue adhesives. J Long Term Eff Med Implants. 1992;2:221–233. [PubMed] [Google Scholar]
- 5.Fye MA, Southern EP, Panjabi MM, Cholewicki J. Quantitative discomanometry: technique and reproducibility in vitro. J Spinal Disord. 1998;11:335–340. doi: 10.1097/00002517-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Gaston P, Marshall RW. Survival analysis is a better estimate of recurrent disc herniation. J Bone Joint Surg Br. 2003;85:535–537. doi: 10.1302/0301-620X.85B4.13813. [DOI] [PubMed] [Google Scholar]
- 7.Gosain AK, Lyon VB. The current status of tissue glues: part II. For adhesion of soft tissues. Plast Reconstr Surg. 2002;110:1581–1584. doi: 10.1097/00006534-200211000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hamadouche M, Sedel L. Ceramics in orthopaedics. J Bone Joint Surg Br. 2000;82:1095–1099. doi: 10.1302/0301-620X.82B8.11744. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama J, Yamagata M, Ogata S, Shimizu K, Ikeda Y, Takahashi K. Relationship between low-back pain, muscle spasm and pressure pain thresholds in patients with lumbar disc herniation. Eur Spine J. 2006;15:41–47. doi: 10.1007/s00586-004-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitchon PW, Eichholz K, Barry C, Rubenbauer P, Ingalhalikar A, Nakamura S, Follett K, Lim TH, Torner J. Biomechanical studies of an artificial disc implant in the human cadaveric spine. J Neurosurg Spine. 2005;2:339–343. doi: 10.3171/spi.2005.2.3.0339. [DOI] [PubMed] [Google Scholar]
- 12.Iencean SM. Lumbar intervertebral disc herniation following experimental intradiscal pressure increase. Acta Neurochir (Wien) 2000;142:669–676. doi: 10.1007/s007010070111. [DOI] [PubMed] [Google Scholar]
- 13.Joshi A, Fussell G, Thomas J, Hsuan A, Lowman A, Karduna A, Vresilovic E, Marcolongo M. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials. 2006;27:176–184. doi: 10.1016/j.biomaterials.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Joshi A, Mehta S, Vresilovic E, Karduna A, Marcolongo M. Nucleus implant parameters significantly change the compressive stiffness of the human lumbar intervertebral disc. J Biomech Eng. 2005;127:536–540. doi: 10.1115/1.1894369. [DOI] [PubMed] [Google Scholar]
- 15.Kallmes DF, Helm GA, Hudson SB, Altes TA, Do HM, Mandell JW, Cloft HJ. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology. 1999;213:217–222. doi: 10.1148/radiology.213.1.r99oc16217. [DOI] [PubMed] [Google Scholar]
- 16.Kayanja MM, Ferrara LA, Lieberman IH. Distribution of anterior cortical shear strain after a thoracic wedge compression fracture. Spine J. 2004;4:76–87. doi: 10.1016/j.spinee.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Klara PM, Ray CD. Artificial nucleus replacement: clinical experience. Spine. 2002;27:1374–1377. doi: 10.1097/00007632-200206150-00022. [DOI] [PubMed] [Google Scholar]
- 18.Kotani Y, Cunningham BW, Abumi K, Dmitriev AE, Ito M, Hu N, Shikinami Y, McAfee PC, Minami A. Multidirectional flexibility analysis of cervical artificial disc reconstruction: in vitro human cadaveric spine model. J Neurosurg Spine. 2005;2:188–194. doi: 10.3171/spi.2005.2.2.0188. [DOI] [PubMed] [Google Scholar]
- 19.Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol. 2004;25:175–180. [PMC free article] [PubMed] [Google Scholar]
- 20.Mannion AF, Dvorak J, Muntener M, Grob D. A prospective study of the interrelationship between subjective and objective measures of disability before and 2 months after lumbar decompression surgery for disc herniation. Eur Spine J. 2005;14:454–465. doi: 10.1007/s00586-004-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 22.Maurer P, Bekes K, Gernhardt CR, Schaller HG, Schubert J. Comparison of the bond strength of selected adhesive dental systems to cortical bone under in vitro conditions. Int J Oral Maxillofac Surg. 2004;33:377–381. doi: 10.1016/j.ijom.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno H, Roy AK, Zaporojan V, Vacanti CA, Ueda M, Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2005;27:362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Negvesky GJ, Butrus SI, Abifarah HA, Lee YC, Yalkowsky SH. Ocular gelfoam disc-applicator for pupillary dilation in humans. J Ocul Pharmacol Ther. 2000;16:311–315. doi: 10.1089/jop.2000.16.311. [DOI] [PubMed] [Google Scholar]
- 25.Panjabi M, Brown M, Lindahl S, Irstam L, Hermens M. Intrinsic disc pressure as a measure of integrity of the lumbar spine. Spine. 1988;13:913–917. doi: 10.1097/00007632-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Saruhashi Y, Mori K, Katsuura A, Takahashi S, Matsusue Y, Hukuda S. Evaluation of standard nucleotomy for lumbar disc herniation using the Love method: results of follow-up studies after more than 10 years. Eur Spine J. 2004;13:626–630. doi: 10.1007/s00586-004-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schechtman H, Robertson PA, Broom ND. Failure strength of the bovine caudal disc under internal hydrostatic pressure. J Biomech. 2006;39:1401–1409. doi: 10.1016/j.jbiomech.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Solberg TK, Nygaard OP, Sjaavik K, Hofoss D, Ingebrigtsen T. The risk of “getting worse” after lumbar microdiscectomy. Eur Spine J. 2005;14:49–54. doi: 10.1007/s00586-004-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Songer MN, Ghosh L, Spencer DL. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine. 1990;15:550–554. doi: 10.1097/00007632-199006000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Southern EP, Fye MA, Panjabi MM, Patel TC, Cholewicki J. Disc degeneration: a human cadaveric study correlating magnetic resonance imaging and quantitative discomanometry. Spine. 2000;25:2171–2175. doi: 10.1097/00007632-200009010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, Masuda K. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–238. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Tenjin H, Fushiki S, Nakahara Y, Masaki H, Matsuo T, Johnson CM, Ueda S. Effect of Guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke. 1995;26:2075–2080. doi: 10.1161/01.str.26.11.2075. [DOI] [PubMed] [Google Scholar]
- 33.Trail IA, Powell ES, Noble J, Crank S. The role of an adhesive (Histoacryl) in tendon repair. J Hand Surg [Br] 1992;17:544–549. doi: 10.1016/S0266-7681(05)80239-6. [DOI] [PubMed] [Google Scholar]
- 34.Uzvolgyi E, Katona A, Kertai P. Tumor cell implantation with the use of Gelaspon gelatin sponge disc. Cancer Lett. 1990;51:1–5. doi: 10.1016/0304-3835(90)90222-J. [DOI] [PubMed] [Google Scholar]
- 35.Veloudios A, Kratky V, Heathcote JG, Lee M, Hurwitz JJ, Kazdan MS. Cyanoacrylate tissue adhesive in blepharoplasty. Ophthal Plast Reconstr Surg. 1996;12:89–97. doi: 10.1097/00002341-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Wang DH, Makaroun M, Webster MW, Vorp DA. Mechanical properties and microstructure of intraluminal thrombus from abdominal aortic aneurysm. J Biomech Eng. 2001;123:536–539. doi: 10.1115/1.1411971. [DOI] [PubMed] [Google Scholar]
- 37.Wang JL, Panjabi MM, Kato Y, Nguyen C. Radiography cannot examine disc injuries secondary to burst fracture: quantitative discomanometry validation. Spine. 2002;27:235–240. doi: 10.1097/00007632-200202010-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 39.Wu MC, Hsiao CT (2003) Breeds of the laboratory swine in ISO certified swine farm and their characteristics. In: Chinese Society of Laboratory Animal Sciences Annual Meeting, Taipei