Abstract
Corrosion affects spinal instrumentations and may cause local and systemic complications. Diagnosis of corrosion is difficult, and nowadays it is performed almost exclusively by the examination of retrieved instrumentations. We conducted this study to determine whether it is possible to detect corrosion by measuring metal levels on patients with posterior instrumented spinal fusion. Eleven asymptomatic patients, with radiological signs of corrosion of their stainless steel spinal instrumentations, were studied by performing determinations of nickel and chromium in serum and urine. Those levels were compared with the levels of 22 patients with the same kind of instrumentation but without evidence of corrosion and to a control group of 22 volunteers without any metallic implants. Statistical analysis of our results revealed that the patients with spinal implants without radiological signs of corrosion have increased levels of chromium in serum and urine (P < 0.001) compared to volunteers without implants. Corrosion significantly raised metal levels, including nickel and chromium in serum and urine when compared to patients with no radiological signs of corrosion and to volunteers without metallic implants (P < 0.001). Metal levels measured in serum have high sensibility and specificity (area under the ROC curve of 0.981). By combining the levels of nickel and chromium in serum we were able to identify all the cases of corrosion in our series of patients. The results of our study confirm that metal levels in serum and urine are useful in the diagnosis of corrosion of spinal implants and may be helpful in defining the role of corrosion in recently described clinical entities such as late operative site pain or late infection of spinal implants.
Keywords: Corrosion, Spinal implants, Metal levels
Introduction
Since the description of Harrington’s implant in 1962 [14], instrumented spine arthrodesis is the treatment of choice for severe spinal deformities and instability. There are multiple kinds of implants but most of them have one characteristic in common: they are made of stainless steel. Although theoretically these instrumentations are necessary only until arthrodesis is achieved, most of them are not removed. In fact, survivorship analyses reveal that approximately 80% of these remain in situ 10 years after the initial surgery [3].
Gradual degradation of metallic implants affects all kinds of instrumentations and alloys. Corrosion, a generally slow but progressive phenomenon, is undesirable for two reasons: it can lead to mechanical failure, and the release and dissemination of corrosion particles can produce adverse biological reactions in the host [15]. Also, specific localization of the corrosion site may determine other complications. In spinal implants, Tezer et al. [26] and Takahashi et al. [25] have reported delayed neurological symptoms caused by intraspinal metallosis, including radiculopathy and paraparesis.
Corrosion is not a recently described problem. In fact, Aulisa et al. [2] published, in 1982, a case of corrosion in Harrington instrumentation. Modern spinal instrumentations also suffer corrosion. Akazawa et al. [1] report macroscopic evidence of corrosion in 66.2% of rod junctions after long-term implantation. Vieweg et al. [27] found corrosion on pedicle screws and telescopic rods after a mean length of implantation of 10 months.
Kim et al. [19] studied serum levels of nickel and chromium after posterior spinal arthrodesis with implants made of stainless steel, without any evidence of corrosion. They observed that these levels rose after surgery and remained above normal levels 4 years after the surgical procedure.
Metal levels have been widely studied in relation to metal-on-metal hip arthroplasties. Some authors have observed that these levels increase after the implantation of this kind of bearing surface (as occurs with stainless steel spinal implants) [22]. Higher levels are observed on patients with loosened implants. This observation made Jacobs et al. [17] propose the idea that metal levels may be useful to monitor metal-on-metal hip arthroplasties. What has been observed in relation to this kind of hip arthroplasties may also occur in other types of implants like spinal instrumentations.
We observed radiological signs of corrosion in some asymptomatic patients with a posterior spinal instrumentation made of stainless steel. Our main objective, in this study, was to measure and compare metal levels, in serum and urine, on patients with radiological images of corrosion, patients with the same instrumentation without any evidence of corrosion and a control group of volunteers without implants.
Materials and methods
Study design
After a case of a patient with corrosion of a stainless steel implant that presented paraparesis 14 years after the initial surgery [6], we started to re-evaluate patients that had undergone posterior instrumented spinal arthrodesis at our hospital between 1986 and 2004. Standing posterior–anterior and lateral radiographs were taken. Radiological signs of corrosion, similar to the ones observed in the initial case report, were detected in 11 asymptomatic patients (Group 2). Corrosion was observed as a progressive decrease in metal density of the rods (Fig. 1). Twenty-two patients with the same kind of instrumentation but no signs of corrosion were also analyzed. These patients were randomly selected from those that came for follow-up after fusion surgery (Group 3). None of these patients had any clinical or radiographic evidence of infection or pseudoarthrosis. The control group (Group 1) consisted of 22 volunteers with no implants. An informed consent was obtained from each patient. In Group 2, we explained the patients the relation between the observed decreased density of the rod and corrosion, and the potential complications of this phenomenon. Implant removal was recommended to all of them. Patients or volunteers with metallic implants in another location or alterations in renal function were excluded from the study.
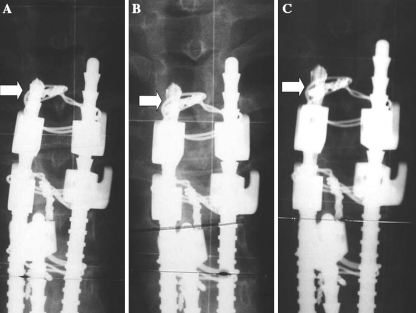
Fig. 1.
Postoperative anteroposterior view of the proximal end of a stainless steel spinal instrumentation (a). Corrosion is observed as a progressive decrease in metal density of the rod 1-year (b) and 7 years after surgery (c) (arrows)
In Group 1, 10 patients were men and 12 were women; in Group 2, 2 patients were men and 9 were women and in Group 3, 7 patients were men and 15 were women.
Idiopathic scoliosis was the cause of surgery in the majority of the patients (22 of 33). Other causes for posterior instrumentation were scoliosis in cerebral palsy, congenital scoliosis, post-tumoral resection and Scheuermann kyphosis.
Mean age at metal level determination was 27.2 years (range: 13–42) in Group 1, 31.8 years (range: 25–51) in Group 2 and 24.5 years (range: 14–79) in Group 3. Time from surgery to metal level determination was 170 months (range: 159–180) in Group 2 and 72 months (range: 33–129) in Group 3. Individual characteristics of patients in Group 2 are shown in Table 1.
Table 1.
Individual characteristics of patients with radiological signs of corrosion of their implants
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | F | F | F | F | F | F | M | F |
| Diagnosis | IS | SK | IS | AS | SK | IS | IS | IS | IS | CP | CS |
| Age at surgery (years) | 12 | 15 | 13 | 37 | 34 | 13 | 14 | 12 | 14 | 16 | 14 |
| Age at metal level determination (years) | 25 | 28 | 26 | 51 | 49 | 26 | 29 | 27 | 29 | 31 | 29 |
| Year of surgery | 1992 | 1992 | 1992 | 1991 | 1990 | 1992 | 1990 | 1991 | 1990 | 1991 | 1991 |
| Fusion level | T4L3 | T1L2 | T3L2 | T2L3 | T2L1 | T2L2 | T3L4 | T2L2 | T3L3 | T2L3 | T7L3 |
| Months from surgery to metal levels determination | 159 | 162 | 159 | 167 | 180 | 161 | 180 | 176 | 179 | 172 | 175 |
| Serum Ni (μg/l) | 3.8 | 1.5 | 3 | 5.6 | 2.8 | 3.6 | 5.3 | 3.5 | 3.9 | 9 | 5.2 |
| Urine Ni (μg/l) | 1.7 | 16 | 129 | 19 | 10 | 14.5 | 96.5 | 26.9 | 20.4 | 300 | 74.9 |
| Serum Cr (μg/l) | 4.8 | 33 | 26.4 | 11.1 | 22 | 10.5 | 23 | 9 | 2.2 | 1.6 | 7.2 |
| Urine Cr (μg/g) creatinine | 15.7 | 73.2 | 90.9 | 12 | 47.2 | 27.9 | 96.5 | 22.5 | 6 | 10 | 37.5 |
M Male; F female; IS idiopathic scoliosis; SK Sheuermann kyphosis; AS adult scoliosis; CP scoliosis in cerebral palsy; CS congenital scoliosis
Serum and urine levels of nickel and chromium were measured in all the patients. Spot urine and blood samples were collected in plastic tubes. These samples were sent to a blinded laboratory for metal level determination. Levels were measured by atomic absorption spectrophotometry.
In the patients with radiological signs of corrosion that removed their implants, samples for pathological and microbiological study were obtained during surgery. Routine blood analyses were performed including white blood cell count, erythrocyte sedimentation rate and C-reactive protein. The level of corrosion was determined. All the retrieved instrumentations were sent for alloy composition analysis by inductively coupled plasma emission spectrometry.
Statistical analysis
With the technique used, the lowest detection limit was 1 μg/l for serum nickel. Levels under this limit are informed as <1 μg/l and were considered as 0.99 μg/l for statistical analysis. The same values and units were used for urine nickel. In the case of serum chromium, the lowest detection limit was 0.2 μg/l. Levels under this limit are informed as <0.2 μg/l and were considered as 0.19 μg/l for statistical analysis. For urine chromium the lowest detection limit was 0.1 μg/g creatinine. Levels under this limit are informed as <0.1 μg/l and were considered as 0.09 μg/g creatinine for statistical analysis.
As some values were under the lowest limit of detection we did not use mean values and standard deviation. Values are expressed in median and interquartil range. We performed a non-parametric analysis using the Kruskal–Wallis test followed by the Dunn–Sidak correction method.
ROC curves were used to determine the optimal value for diagnosis of corrosion by combining the greatest sensibility and specificity for each metal level.
Results
In Group 1, of the 22 patients analyzed 17 were under the lowest limit of detection for serum nickel, 16 for urine nickel, 5 for serum chromium and 8 for urine chromium. In Group 3, 10 of the 22 patients analyzed were under the lowest limit of detection for serum nickel, 9 for urine nickel, 1 for serum chromium and no patient was under this limit for urine chromium. No patient with radiological evidence of implant corrosion (Group 2) was under the lowest limit of detection for any determination.
Metal levels in serum and urine are summarized in Table 2. We observed a statistically significant (P < 0.001) elevation of nickel and chromium in patients with corrosion of their spinal implants compared to patients with the same instrumentation but without radiological signs of corrosion (Group 3) and compared to volunteers without implants (Group 1).
Table 2.
Comparison of metal levels in serum and urine in the three groups analyzed
| Metal | Group | Median | Q1 | Q3 | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Serum nickel (μg/l) | Group 1 (n = 22) | 0.99 | 0.99 | 0.99 | 0.99 | 1.40 |
| Group 2 (n = 11)a | 3.80 | 3.00 | 5.30 | 1.50 | 9.00 | |
| Group 3 (n = 22) | 1.00 | 0.99 | 1.42 | 0.99 | 2.00 | |
| P | <0.001 | |||||
| Urine nickel (μg/l) | Group 1 (n = 22) | 0.99 | 0.99 | 1.55 | 0.99 | 3.20 |
| Group 2 (n = 11)a | 20.40 | 14.50 | 96.50 | 1.70 | 300.00 | |
| Group 3 (n = 22) | 1.30 | 0.99 | 2.45 | 0.99 | 6.80 | |
| P | <0.001 | |||||
| Serum chromium (μg/l) | Group 1 (n = 22) | 0.40 | 0.20 | 0.50 | 0.19 | 0.90 |
| Group 2 (n = 11)a | 10.50 | 4.80 | 23.00 | 1.60 | 33.00 | |
| Group 3 (n = 22)b | 1.00 | 0.48 | 1.33 | 0.19 | 2.70 | |
| P | <0.001 | |||||
| Urine chromium (μg/g creatinine) | Group 1 (n = 22) | 0.20 | 0.09 | 0.50 | 0.09 | 1.60 |
| Group 2 (n = 11)a | 27.90 | 12.00 | 73.20 | 6.00 | 96.50 | |
| Group 3 (n = 22)b | 2.70 | 1.70 | 5.10 | 0.50 | 12.70 | |
| P | <0.001 | |||||
Q Quartiles
aStatistically significant elevation compared to the other two groups (Dunn–Sidak test)
bStatistically significant elevation compared to the control group (Group 1) (Dunn–Sidak test)
We did not observe any increase in the levels of nickel in serum or urine when comparing Group 3 with Group 1, but there was a statistically significant increase (P < 0.001) in the level of chromium, in serum and urine, when these two groups were compared.
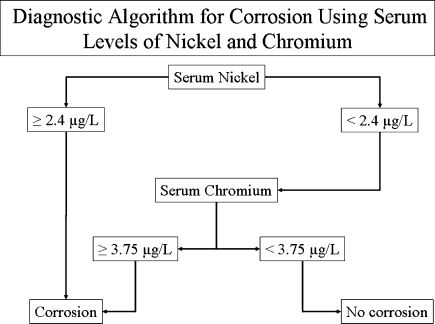
According to ROC curves’ results, the optimal values considered as the lowest limit for detection of corrosion are 2.4 μg/l for serum nickel [ABC (ROC) = 0.981 (IC95%: 0.941; 1.000)], 8.4 μg/l for urine nickel [ABC (ROC) = 0.959 (IC95%: 0.877; 1.000)], 3.75 μg/l for serum chromium [ABC (ROC) = 0.981 (IC95%: 0.946; 1.000)] and 8.65 μg/g creatinine for urine chromium [ABC (ROC) = 0.983 (IC95%: 0.950; 1.000)]. Values over these limits are highly suggestive of corrosion. For our patients the diagnosis of corrosion by metal level analysis proved possible, with no false positives or negatives, by combining the determination of nickel and chromium serum levels (Fig. 2).
Fig. 2.
This diagnostic algorithm using serum levels of nickel and chromium detects corrosion in all our patients without any false negatives or positives
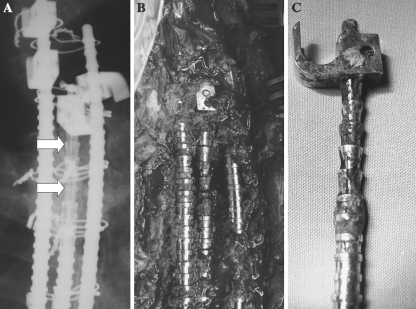
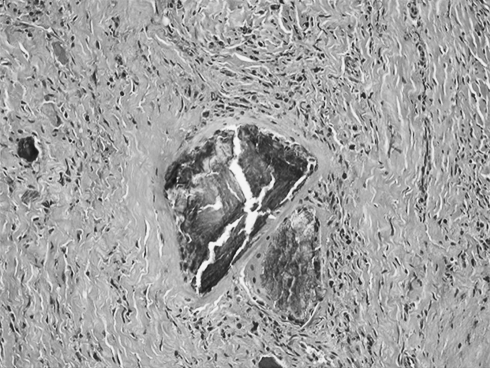
Eight of the 11 asymptomatic patients with radiological evidence of corrosion had their implants removed, 2 were lost to follow-up and 1 is still pending. During surgery, macroscopic corrosion, with partial destruction of the rod, was observed in all these patients (Fig. 3). In these eight cases histological analysis of the samples obtained during surgery confirmed metallosis (Fig. 4). One patient had a positive intraoperative culture for Propionibacterium acnes. The 11 patients of Group 2 had routine blood analyses, including white blood cell count, erythrocyte sedimentation rate and C-reactive protein within the normal values. Ten of the 11 patients presented corrosion at the ends of the rods, in the rod-hook junction area. Alloy composition analysis of the retrieved instrumentations is shown in Table 3. All implants were made of austenitic stainless steel.
Fig. 3.
Radiological signs of corrosion in the proximal end of the rod (a) (arrows). Macroscopic corrosion of the implant was evident during surgery (b) and in the examination of the retrieved instrumentation (c)
Fig. 4.
Microscopic photography (H&E stain, ×100) that shows a multinucleated giant cell with foreign bodies (metallic debris)
Table 3.
Alloy analysis of the eight retrieved instrumentations with radiological signs of corrosion
| Patients | C (%) | Si (%) | Mn (%) | S (%) | P (%) | Cr (%) | Ni (%) | Mo (%) | N (%) | Definition |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 0.093 | 0.40 | 1.23 | 0.005 | 0.019 | 16.9 | 7.63 | 0.12 | 0.051 | AISI 301 |
| Patient 2 | 0.10 | 0.63 | 1.92 | 0.36 | 0.030 | 17.4 | 7.95 | 0.35 | 0.071 | AISI 303 |
| Patient 3 | 0.019 | 0.55 | 1.76 | <0.005 | 0.018 | 17.4 | 13.4 | 2.77 | 0.080 | AISI 316L |
| Patient 4 | 0.02 | 0.56 | 1.76 | <0.005 | 0.019 | 17.6 | 13.3 | 2.75 | 0.080 | AISI 316L |
| Patient 5 | 0.085 | 0.63 | 1.99 | 0.37 | 0.025 | 17.6 | 7.92 | 0.34 | 0.071 | AISI 303 |
| Patient 6 | 0.056 | 0.52 | 1.97 | 0.37 | 0.030 | 17.6 | 8.05 | 0.36 | 0.077 | AISI 303 |
| Patient 7 | 0.064 | 0.62 | 2.07 | 0.38 | 0.025 | 17.4 | 8.09 | 0.35 | 0.075 | AISI 303 |
| Patient 8 | 0.067 | 0.41 | 1.05 | <0.005 | 0.029 | 18.2 | 8.13 | 0.18 | 0.057 | AISI 304 |
AISI American Iron and Steel Institute; C carbon; Si silicon; Mn manganese; S sulfur; P phosphorus; Cr chromium; Ni nickel; Mo molybdenum; N nitrogen
Discussion
Corrosion has been observed in most types of spinal instrumentations used in the last 40 years, including Harrington, Isola, TSRH, CD and others [1, 2, 8, 25, 27, 29]. In general, production of particulate debris has been associated with modularity of implants [18]. Modern spinal instrumentations can be considered “the most modular” implants used in orthopedic surgery. Every junction is a potential site of fretting or crevice corrosion. Probably, as more years pass since we began using modern instrumentations, more cases of corrosion will be observed.
With the spinal instrumentation we use, radiographic signs of corrosion are very easy to diagnose if specifically looked for, as we did for this study. This is clearly seen in Figs. 1 and 3a. Localized decreased density of the rod when compared to adjacent rods is evident. In fact, corrosion was confirmed during surgery in the eight cases that underwent implant removal. But with other types of implants this is not observed. Looking at the cases reported by Tezer [26] and Takahashi [25], with neurological symptoms in relation to corrosion, no radiological signs of corrosion are seen in plain radiographies. It is possible that for other kinds of instrumentations, like CD or TSRH, in which corrosion has been reported in the literature, metal levels can be more useful than looking for radiographic signs of corrosion.
The local effects of metal particles produced by corrosion include neurological symptoms [25, 26], osteolysis [16], cellular toxicity [11] and alterations in muscle microcirculation [20]. There is concern about the systemic effects of these particles, mainly of the teratogenic and carcinogenic potential of metal ions [9], but no direct association has ever been proven. Also, Hallab et al. [13] have observed an increase in metal hypersensitivity in patients with poorly functioning implants.
Even though metal levels in serum and urine in patients with corrosion are far from toxic levels [4, 5], it has to be taken into account that most of the patients with modern spinal instrumentations are fertile young women, who underwent fusion for idiopathic scoliosis and most of them will bear their implants for long periods of time, if not for life. Corrosion, especially in these patients, must be diagnosed as early as possible and solved by implant removal.
Late drainage after instrumented arthrodesis affects up to 4.3% of the patients with stainless steel implants [24]. Its primary etiology is not completely clear. While some authors believe it may be caused by infection [7], others believe it is due to fretting corrosion and the associated granulomatous reaction [10]. Late operative site pain (LOSP) is an important cause of implant removal that remains not fully understood. It was the cause for reoperation in 14 of 182 patients (8%) in the study of Cook et al. [8]. In nine of these patients macroscopic evidence of corrosion was observed during surgery. As in the case of late drainage, there is controversy whether these symptoms are caused by soft tissue reaction to corrosion or by bacterial infection. Although corrosion could be related to these two clinical entities, there has not been, until now, a simple way to determine the prevalence of corrosion in patients with or without any symptoms.
Some authors recommend implant removal to all patients once fusion is achieved [1, 2], but this surgical procedure has some potential complications like progressive loss of correction [23] or vertebral body compression fractures [28]. The economic cost of removing implants to all patients with posterior spinal instrumentations is also quite considerable. Perhaps determination of metal levels will prove to be useful in determining more precisely which patients require implant removal.
Only one of our patients had a positive culture for Propionibacterium acnes. There are reports in the literature about late implant infection by low-virulent skin flora [6, 12]. Our patient was completely asymptomatic. White blood cell count was 6.9 × 109/l, the erythrocyte sedimentation rate (ESR) was 3 mm in the first hour and C-reactive protein was 2 mg/l, all within the normal range. We consider this patient to be colonized by the bacteria, rather than infected. In this case hematogenous seeding is the most probable way for colonization. Another possible cause for this finding is contamination during implant removal.
The causes of corrosion in our implants are difficult to determine. Corrosion was almost always observed at the ends of the rods (10 of 11 cases), in relation to the areas of rod-hook junctions. The analysis of retrieved instrumentations revealed that not all of them were made of 316L stainless steel, but at least two patients with this kind of alloy presented corrosion. Corrosion appears to be a multifactorial phenomenon related to fretting, implant alloy and individual host characteristics.
Although 24-h urine analysis is recommended [21], it is much more difficult to obtain than spot urine samples. This is probably less precise, but collection is simpler. Statistical analysis of our results suggests that serum levels are enough to perform the diagnosis of corrosion, although this has to be validated on other series of patients. An important number of our patients were under the limit of detection, mainly in Groups 1 and 3. This may be a problem for statistical analysis, but real values would have probably made the differences observed in metal levels more significant, considering that no patient in Group 2 was under the lowest limit of detection.
Conclusions
As demonstrated by Kim et al. [19], metal levels in serum increase regularly after instrumented spinal arthrodesis. It may be recommendable to establish basal metal levels in patients with specific kinds of stainless steel instrumentations and to perform metal concentration analysis as a part of the periodical clinical evaluation. This may allow an early diagnosis of corrosion, preventing local and systemic complications. Also, metal levels may be useful in defining the role of corrosion in late operative site pain and late infection of spinal implants.
Our results offer a simple way to diagnose corrosion, the clinical relevance of these findings and the final recommendations of how to follow up and treat these patients have to be studied further.
References
- 1.Akazawa T, Minami S, Takahashi K, Kotani T, Hanawa T, Moriya H. Corrosion of spinal implants retrieved from patients with scoliosis. J Orthop Sci. 2005;10:200–205. doi: 10.1007/s00776-004-0867-3. [DOI] [PubMed] [Google Scholar]
- 2.Aulisa L, Benedetto A, Vinciguerra A, Lorini G, Tranquilli-Leali P. Corrosion of the Harrington’s instrumentation and biological behaviour of the rod-human spine system. Biomaterials. 1982;3:246–248. doi: 10.1016/0142-9612(82)90028-X. [DOI] [PubMed] [Google Scholar]
- 3.Bago J, Ramirez M, Pellise F, Villanueva C. Survivorship analysis of Cotrel–Dubousset instrumentation in idiopathic scoliosis. Eur Spine J. 2003;12:435–439. doi: 10.1007/s00586-001-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barceloux DG. Chromium. J Toxicol Clin Toxicol. 1999;37:173–194. doi: 10.1081/CLT-100102418. [DOI] [PubMed] [Google Scholar]
- 5.Barceloux DG. Nickel. J Toxicol Clin Toxicol. 1999;37:239–258. doi: 10.1081/CLT-100102423. [DOI] [PubMed] [Google Scholar]
- 6.Beguiristain J, Rio J, Duart J, Barroso J, Silva A, Villas C. Corrosion and late infection causing delayed paraparesis after spinal instrumentation. J Pediatr Orthop B. 2006;15:320–323. doi: 10.1097/01202412-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine. 1999;24:1909–1912. doi: 10.1097/00007632-199909150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cook S, Asher M, Lai SM, Shobe J. Reoperation after primary posterior instrumentation and fusion for idiopathic scoliosis. Toward defining late operative site pain of unknown cause. Spine. 2000;25:463–468. doi: 10.1097/00007632-200002150-00012. [DOI] [PubMed] [Google Scholar]
- 9.Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996;329:S187–S205. doi: 10.1097/00003086-199608001-00017. [DOI] [PubMed] [Google Scholar]
- 10.Dubousset J, Shufflebarger H, Wenger D. Late “infection” with C-D instrumentation. Orthop Trans. 1994;18:121. [Google Scholar]
- 11.Granchi D, Cenni E, Ciapetti G, Savarino L, Stea S, Gamberini S, Gori A, Pizzoferrato A. Cell death induced by metal ions: necrosis or apoptosis? J Mater Sci Mater Med. 1998;9:31–37. doi: 10.1023/A:1008878527233. [DOI] [PubMed] [Google Scholar]
- 12.Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J. 2005;14:783–788. doi: 10.1007/s00586-004-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallab N, Merrit K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A:428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Harrington PR. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am. 1962;44:591–610. [PubMed] [Google Scholar]
- 15.Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80:268–282. doi: 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19:S59–S65. doi: 10.1016/j.arth.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JJ, Urban RM, Gilbert JL, Skipor AK, Black J, Jasty M, Galante JO. Local and distant products from modularity. Clin Orthop Relat Res. 1995;319:94–105. [PubMed] [Google Scholar]
- 19.Kim YJ, Kassab F, Berven SH, Zurakowski D, Hresko MT, Emans JB, Kasser JR. Serum levels of nickel and chromium after instrumented posterior spinal arthrodesis. Spine. 2005;30:923–926. doi: 10.1097/01.brs.0000158872.42802.be. [DOI] [PubMed] [Google Scholar]
- 20.Kraft CN, Burian B, Diedrich O, Wimmer MA. Implications of orthopedic fretting corrosion particles on skeletal muscle microcirculation. J Mater Sci Mater Med. 2001;12:1057–1062. doi: 10.1023/A:1012854325474. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004;19:S12–S16. doi: 10.1016/j.arth.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;406:282–296. doi: 10.1097/00003086-200301000-00039. [DOI] [PubMed] [Google Scholar]
- 23.Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J. 2004;13:645–651. doi: 10.1007/s00586-004-0694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine. 2001;26:1990–1996. doi: 10.1097/00007632-200109150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi S, Delecrin J, Passuti N. Intraspinal metallosis causing delayed neurologic symptoms after spinal instrumentation surgery. Spine. 2001;26:1495–1498. doi: 10.1097/00007632-200107010-00024. [DOI] [PubMed] [Google Scholar]
- 26.Tezer M, Kuzgun U, Hamzaoglu A, Ozturk C, Kabukcuoglu F, Sirvanci M. Intraspinal metalloma resulting in late paraparesis. Arch Orthop Trauma Surg. 2005;125:417–421. doi: 10.1007/s00402-005-0802-x. [DOI] [PubMed] [Google Scholar]
- 27.Vieweg U, Roost D, Wolf HK, Schyma CA, Schramm J. Corrosion on an internal spinal fixator system. Spine. 1999;24:946–951. doi: 10.1097/00007632-199905150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Waelchli B, Min K, Cathrein P, Boos N. Vertebral body compression fracture after removal of pedicle screws: a report of two cases. Eur Spine J. 2002;11:504–506. doi: 10.1007/s00586-002-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wimmer C, Gluch H. Aseptic loosening after CD instrumentation in the treatment of scoliosis: a report about eight cases. J Spinal Disord. 1998;11:440–443. [PubMed] [Google Scholar]