Abstract
The objective of this study was to assess the correlation between neurogenic intermittent claudication (NIC) in LSS and different positions as well as loading status, using the treadmill device. The study was a prospective clinical trial on lumbar spinal stenosis (LSS) using a treadmill equipment. The study population comprised of 80 LSS patients with a mean age of 61. The equipment included a treadmill, unloading station and loading vests. The patients were instructed to walk in five different positions. The initiation time of symptoms and total walking time were recorded. The examination was stopped after 20 min or at the onset of severe symptoms. In order to obtain pretest demographic data on subjects, visual analog scale, Roland–Morris questionnaire, pain disability index, and Beck depression index were used. The initiation time of symptoms (ITS) and total walking time (TWT) were measured during the test. Unloading provided a longer and loading a shorter ITS and TWT. Decline or incline positions did not affect ITS or TWT. The changes in posture had no correlation with the appearance of symptoms in LSS patients with NIC on a treadmill in this study, rather ITS and TWT were determined by axial loading and unloading.
Keywords: Lumbar spinal stenosis, Posture, Unloading, Treadmill
Introduction
Lumbar spinal stenosis (LSS) is a common condition in the elderly. Patients with LSS are most often at least 50 years of age with prolonged histories of low back pain and recent onset of unilateral or bilateral lower extremity pain [1, 17, 22]. The symptoms, which are posture-dependent, are worsened with extension of the lumbar spine or with weight bearing and decreased with flexion or nonweight bearing [5, 19, 21]. Neurogenic intermittent claudication (NIC) defined as pain, paresthesia, and cramping of the lower extremities, brought on by walking and relieved by sitting, frequently accompanies LSS [1, 22].
LSS has been attributed to structural narrowing of the spinal canal by facet joint arthrosis and hypertrophy, thickening and bulging of the ligamentum flavum, outward bulging of the intervertebral disk, and anterior displacement of the superior articulating process of the vertebral body due to lumbar spinal instability [8, 9, 18, 20]. Symptomatic LSS cannot be attributed solely to structural narrowing of the canal dimensions, however, as evidenced by the high prevalence of narrowing seen on magnetic resonance imaging (MRI) in individuals who have no symptoms, and by the poor correlation found between the severity of findings from imaging studies and the symptoms of patients with symptomatic LSS [1, 15].
There is a need to objectively define an LSS patient’s status both before and after treatment; however, traditional measures including clinical examination, radiographic findings, and questionnaires can neither accurately fulfill nor correlate with the symptoms of the patient [1, 11]. In this regard, treadmill testing was used and found to hold promise to produce symptoms, quantify them, detect responses to varying positions, and determine changes with treatment [2, 5]. However, these studies focused on position and the response to loading has never been studied using the treadmill device. With these characteristics in mind we undertook a study to assess the correlation of NIC in LSS with different positions and loading status by a practical method using the treadmill device.
Materials and methods
The study population comprised of 80 patients (17 men and 63 women) with a mean age of 61.55 ± 6.34 years. All had the clinical diagnosis of degenerative LSS confirmed by MRI, with intractable NIC defined as leg pain or paresthesias precipitated by walking and relieved by sitting or lying down. Each subject underwent a standardized examination including an assessment of subjective complaints and relevant medical history, lower-extremity flexibility, neurological status, gait abnormalities, and condition of soft tissues.
Patients were excluded if they presented with LSS secondary to neoplastic or metabolic causes, had lumbar spine or lower extremity surgery within last year, or were candidates for urgent low back or lower extremity surgery. Patients with peripheral vascular disease were excluded by means of clinical examination of peripheral pulses and noninvasive vascular studies, as indicated. No patient had a cardiopulmonary condition that would limit exercise capacity during the treadmill examination. The arthritis of the hip and knee limiting walking and the presence of diabetes mellitus were also among the exclusion criteria.
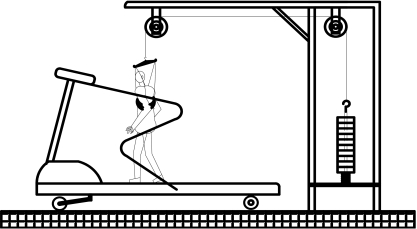
The equipment included a treadmill, unloading station, and loading vests (Fig. 1). The subjects performed a 5–10 min trial session of ambulation on the treadmill using various inclination, loads, and unloading to optimize the limits. The vest had anterior and posterior pouches to accept up to 10 kg of weight distributed equally. The unloading station involved the use of a traction harness and the application of vertical traction intended to reduce the gravitational force on the spine while the patient ambulated on a treadmill, which was adjusted to unload 1/5 of patient’s weight. The walking speed was fixed at 1.2 km/h. The patients were not allowed to hold on to the handrail during examination. The examination was stopped after 20 min or at the onset of severe symptoms. Severe symptoms were defined as the level of discomfort that would make the patient stop walking in usual life situations. No one was encouraged or prompted to continue walking beyond this point. A long rest (2 h) was permitted between trials to allow symptoms to return to baseline levels. The initiation time of symptoms (ITS) and total walking time (TWT) were recorded. Treadmill protocol was as follows:
Walking at 0° ramp incline
Walking at +10° ramp incline
Walking at −10° ramp incline
Walking at 0° ramp incline with 1/5 of body weight unloaded
Walking at 0° ramp incline loaded with 10 kg
The tools used to obtain basic data to reflect the pretrial condition of the patients were the visual analog scale (VAS) to assess level of pain (0 = no pain, 100 = worst pain imaginable), Roland–Morris questionnaire (RMQ) [16] to assess patient’s functional status, pain disability index (PDI) [7] to determine disability of the patient, and Beck depression index (BDI) [23] to assess depressive symptoms.
Fig. 1.
Line drawing of treadmill device and its equipments
Statistical analysis
Statistical evaluations were performed with the software program SPSS 11.0. The data were expressed as a mean ± standard error of the mean. The data were compared by using a repeated-measure analysis of variance and paired t test as appropriate. Statistical significance was set at 0.005 by using the Bonferroni correction.
Results
The treadmill examination was well tolerated, and there were no complications or patient complaints.
The data on patient demograhics and study findings are summarized in Tables 1 and 2, in Figs. 2 and 3.
Table 1.
Data on patients
| Age (year) | 61.55 ± 6.34 |
| Weight (kg) | 79.4 ± 11.90 |
| Body mass index (kg/m2) | 32.11 ± 9.89 |
| VAS (mm) | 70.29 ± 11.67 |
| Oswestry low back pain disability questionnaire (OPQ) | 27.39 ± 7.68 |
| Pain disability index | 36.84 ± 11.56 |
| Roland Morris functional capacity index | 16.83 ± 4.12 |
| Beck depression index | 13.85 ± 7.16 |
Table 2.
Data on walking time at different posture and loading conditions
| 0° incline | +10° incline | −10° incline | 1/5 unloading at 0° incline | 10 kg loading at 0° incline | |
|---|---|---|---|---|---|
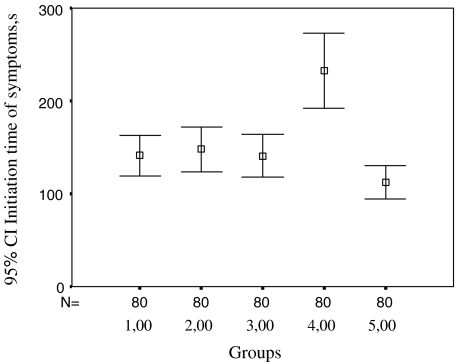
| ITS (s) | 141.06 ± 98.71 | 147.78 ± 106.27 | 141.00 ± 103.39 | 232.45 ± 182.13 | 112.40 ± 82.68 |
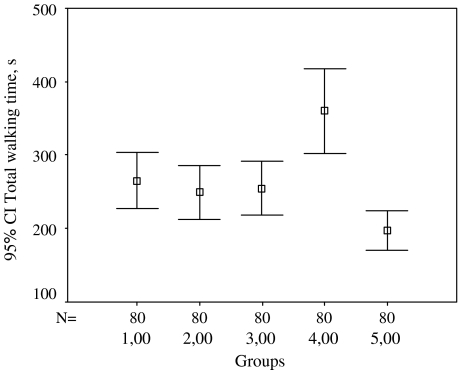
| TWT (s) | 265.51 ± 171.35 | 249.22 ± 167.44 | 255.54 ± 162.72 | 359.95 ± 258.88 | 197.27 ± 120.27 |
ITS Initiation time of first symptoms; TWT total walking time; s second
Fig. 2.
Time for initiation of symptoms according to different walking types, n = 80. 1 Walking at 0° ramp incline, 2 walking at +10° ramp incline, 3 walking at −10° ramp incline, 4 walking with 1/5 of body weight unloading, 5 walking loaded with 10 kg. s Second
Fig. 3.
Total walking time according to different walking types, n = 80. 1 Walking at 0° ramp incline, 2 walking at +10° ramp incline, 3 walking at −10° ramp incline, 4 walking with 1/5 of body weight unloading, 5 walking loaded with 10 kg. s Second
The data on ITS revealed the following:
There were no differences between walking at 0° ramp incline and walking at +10° ramp incline or walking at −10° ramp incline (P > 0.05 and P > 0.05).
There was no difference between walking at +10° ramp incline and walking at −10° ramp incline (P > 0.05).
Walking at 0° ramp incline with 1/5 of body weight unloaded provided a longer ITS compared to walking at 0° ramp incline (P < 0.001).
Walking at 0° ramp incline loaded with 10 kg shortened ITS compared to walking at 0° ramp incline (P < 0.001).
Walking at 0° ramp incline with 1/5 of body weight unloaded prolonged ITS compared to walking at +10° ramp incline (P < 0.001).
Walking at 0° ramp incline loaded with 10 kg shortened ITS compared to walking at +10° ramp incline (P < 0.001).
Walking at 0° ramp incline with 1/5 of body weight unloaded prolonged ITS compared to walking at −10° ramp incline (P < 0.001).
Walking at 0° ramp incline loaded with 10 kg shortened ITS compared to walking at −10° ramp incline (P < 0.005).
The data on total walking time revealed the following:
Walking at 0° ramp incline had no difference compared to walking at +10 and −10° ramp incline (P > 0.05).
TWT was shorter with walking at 0° ramp incline compared to walking at 0° ramp incline with 1/5 of body weight unloaded (P < 0.001).
TWT was longer with walking at 0° ramp incline compared to walking at 0° ramp incline loaded with 10 kg (P < 0.001).
Walking at +10° ramp incline decreased the TWT compared to walking at 0° ramp incline with 1/5 of body weight unloaded and increased TWT compared to walking at 0° ramp incline loaded with 10 kg (P < 0.001). Walking at +10° ramp incline had no difference from other walking types (P > 0.05).
Walking at −10° ramp incline decreased the TWT compared to walking at 0° ramp incline with 1/5 of body weight unloaded and increased the TWT compared to walking at 0° ramp incline loaded with 10 kg (P < 0.001).
As a summary, for both ITS and TWT variables, the results were similar. Both variables in the loaded condition were significantly shorter than they were in the other four conditions. In the unloaded condition they were significantly longer than the other four conditions. There were no significant differences between the 0, +10 and −10° incline conditions.
Discussion
In LSS patients with NIC, initiation time of first symptoms (ITS) and total walking time (TWT) were influenced more by the loading/unloading status more than the inclination in our study.
The pathophysiology of symptoms in LSS is poorly known, but it has become apparent that it is multifactorial. There are two major factors believed to act on the diameter/surface area of the vertebral canal in LSS. They are position and loading.
The patients with NIC frequently complain of posture-related pain, saying that they are more comfortable walking in a stooped-posture and that when they can no further continue walking they will bend forward, lean against a wall, or fasten a shoe-lace until the symptoms settle. Often, they are more comfortable walking uphill than downhill in lordosis [4]. In a normal spine, the cross-sectional area is reduced by 9% during extension, but the reduction increases to 67% with severe stenosis [18]. The changes with motion in the dimensions of stenotic subarachnoid space have been shown by dynamic computerized tomography–myelography and support the above mentioned findings [10, 19]. There are also studies with contrast findings like the Iversen’s [11], in which the most common activity associated with pain in the lower extremities was walking uphill. Although the posture assumed for this activity is generally one of flexion, this increase in patients with uphill walking may result from increased compressive forces on the spine [18, 20]. These controversies indicate the role of many dynamic factors in the pathogenesis of NIC.
In addition to lumbar extension, loading of the spine through the compressive force associated with a weight-bearing posture reduces the cross-sectional area of the spinal canal. Schonstrom et al. [20] found that compressive loading had a slightly greater effect on decreasing the dimensions of the canal than did lumbar extension. In another study, in 66 of the investigated 84 patients, there was a statistically significant reduction of the dural sac cross-sectional area in at least one site during axial compression in slight extension. In 11 patients investigated with MRI, there was narrowing of the lateral recess in 13 sites, during axial compression in slight extension [24].
The utility of exercise treadmill test in the diagnosis of LSS has also been described and found useful in the confirmation of NIC and walking capacity in LSS [3, 5, 12, 14]. Following the introduction of treadmill test into clinical practice, several issues have aroused: the speed and inclination of the device as well as its effect on lumbar lordosis and the variables to measure. Several protocols used various speeds and the conclusion was that NIC could better be demonstrated by ambulation at a slow and constant speed. Increases in speed emphasized cardiopulmonary function over neuromusculoskeletal performance and patients had to stop walking before they experience leg pain, rendering the test ineffective for LSS.
Accordingly, a walking speed of 1.2 km/h was adopted in most of the latest studies [2]. In a study, two-staged treadmill test was used and the patients walked in both extension (on level) and in flexion (15° inclined). The symptoms started earlier in extension and the walking distance was improved with flexion [5]. In Dong et al.’s study [4], subjects were conducted to walk in 30° flexion posture and 8 out of 19 patients had no improvement in walking tolerance. Only 15.8% of their patients with NIC had less symptoms walking uphill than downhill. So, the limits for the inclination that would help the symptoms to disappear are not clear. We utilized 10° of inclination, which was found to be more appropriate for our deconditioned LSS patients because of long-term physical inactivity. Most of the patients had to quit before the appearance of symptoms and the end of time limit in the trial session of ambulation at 15°. The query for the degree of inclination or the lumbar flexion that will improve symptoms is not very well clear and yet brings out another issue to be answered: Does the inclination of the treadmill really provide lumbar flexion? Fritz et al. [5] demonstrated that both stenotic and nonstenotic subjects walked in greater spinal flexion, by an average of 8°, when walking on an inclined treadmill at a 15° grade. In another study, walking on an inclined treadmill was shown to increase spinal flexion [3]. The relation between lumbar flexion in LSS patients and inclination of the treadmill can be dealt within two categories based on a number of different dynamic factors that are important in the pathogenesis of NIC. In some patients who have LSS and degenerative changes that has stabilized the spinal segments, bending forward will take place largely at the hips, and flexion of the lumbar spine will be more apparent than real. The posture then is not likely to affect the space within the vertebral canal, and stooping forward will not improve claudication distance. Other LSS patients, however, may have some segmental instability in the sagittal plane, when lumbar extension is likely to aggravate the stenosis. For these patients, posture will influence walking distance to a variable degree. As we are trying to establish a universal test applicable to all LSS patients we cannot propose pretest determination of lumbar flexibility and include only the patients with motion in lumbar region. Our study results showing the irrelevancy between NIC and inclination of the treadmill can be explained by the above-mentioned factors, relating the lack of lumbar flexibility and the reaction to inclined treadmill testing.
In our study, 20% reduction of body weight by way of traction provided a longer, whereas, addition of 10 kg resulted in a shorter walking time. Lumbar traction provides distraction of vertebral bodies, widening of intervertebral foramina, decreased venous congestion, and increased axoplasmic flow. Thus, theoretically we may expect a decrease in the symptoms of LSS patients if we could decrease the compressive forces on the lumbar spine and an increase in total walking distance. The harness-supported treadmill ambulation or unloading’s use in LSS rehabilitation has only been reported in a case report [6]. The current study is the first to incorporate unloading as well as loading to treadmill testing. Fritz et al. [6] proved this hypothesis by demonstrating improvement in the functional status of two patients with LSS after a course of treadmill exercise using unloading. For subjects with increased pain symptoms with walking and relief of symptoms with sitting the recommended amount of unloading is 13–25% of body weight [13]. So, an average value of 20% unloading was used in our study. On the other hand, we did not have previous test values for loading in LSS, in the literature. That is why we used 10 kg of loading in this study, as it was the average maximum tolerated weight by the subjects in the trial session of ambulation on the treadmill [13].
In LSS patients with NIC axial-loading decreases while unloading increases the walking time. On the other hand, postural changes in small degrees do not affect the walking time. The impact of axial loading is more profound than changes in posture on NIC.
References
- 1.Amundsen T, Weber H, Lilleas F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis: clinical and radiologic features. Spine. 1995;20(10):1178–1186. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Deen HG, Jr, Zimmerman RS, Lyons MK, McPhee MC, Verheijde JL, Lemens SM. Test–retest reproducibility of the exercise treadmill examination in lumbar spinal stenosis. Mayo Clin Proc. 2000;75:1002–1007. doi: 10.4065/75.10.1002. [DOI] [PubMed] [Google Scholar]
- 3.Deen HG, Zimmerman RS, Lyons MK, McPhee MC, Verheijde JL, Lemens SM. Use of the exercise treadmill to measure baseline functional status and surgical outcome in patients with severe lumbar spinal stenosis. Spine. 1998;23:244–248. doi: 10.1097/00007632-199801150-00019. [DOI] [PubMed] [Google Scholar]
- 4.Dong GX, Porter RW. Walking and cycling tests in neurogenic and intermittent claudication. Spine. 1989;14(9):965–969. doi: 10.1097/00007632-198909000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Fritz JM, Erhard RE, Delitto A, Welch WC, Nowakowski PE, Nowakowski PE. Preliminary results of the use of a two-stage treadmill test as a clinical diagnostic tool in the differential diagnosis of lumbar spinal stenosis. J Spinal Disord. 1997;10(5):410–416. doi: 10.1097/00002517-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Fritz JM, Erhard RE, Vignovic M. A nonsurgical treatment approach for patients with lumbar spinal stenosis. Phys Ther. 1997;77(9):962–973. doi: 10.1093/ptj/77.9.962. [DOI] [PubMed] [Google Scholar]
- 7.Gronblad M, Hupli M, Wennerstrand P, Jarvinen E, Lukinmaa A, Kouri JP, Karaharju EO. Intercorelation and test–retest reability of the pain disability index (PDI) and the Oswestry disability questionnaire (ODQ) and their correlation with pain intensity in low back pain patients. Clin J Pain. 1993;9:189–195. doi: 10.1097/00002508-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa T, An HS, Haughton VM, Nowicki BH. Lumbar foraminal stenosis, critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am. 1995;77-A:32–38. [PubMed] [Google Scholar]
- 9.Herzog RJ, Kaiser JA, Saal JA, Saal JS. The importance of posterior epidural fat pad in lumbar central canal stenosis. Spine. 1991;16(Suppl):227S–233S. doi: 10.1097/00007632-199106001-00010. [DOI] [PubMed] [Google Scholar]
- 10.Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion–extension movement. Spine. 1996;21:2412–2420. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Iversen MD, Katz JN. Examination findings and self-reported walking capacity in patients with lumbar spinal stenosis. Phys Ther. 2001;81:1296–1306. [PubMed] [Google Scholar]
- 12.Jensen OH, Schmidt-Olsen S. a new functional test in the diagnostic evaluation of neurogenic intermittent claudication. Clin Rheumatol. 1989;8:363–367. doi: 10.1007/BF02030349. [DOI] [PubMed] [Google Scholar]
- 13.Joffe D, Watkins M, Steiner L, Pfeifer BA. Treadmill ambulation with partial body weight support for the treatment of low back and leg pain. J Orthop Sports Phys Ther. 2002;32(5):202–215. doi: 10.2519/jospt.2002.32.5.202. [DOI] [PubMed] [Google Scholar]
- 14.Johnsson KE, Willner S, Pettersson H. Analysis of operated cases with lumbar spinal stenosis. Acta Orthop Scand. 1981;52:427–433. doi: 10.3109/17453678109050123. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson B, Annertz M, Sjoberg C, Stromqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part 1: clinical features related to radiographic findings. Spine. 1997;22:2932–2937. doi: 10.1097/00007632-199712150-00016. [DOI] [PubMed] [Google Scholar]
- 16.Kucukdeveci AA, Tennant A, Elhan AH, Niyazoglu H. Validation of the Turkish version of the Roland–Morris disability questionnaire for use in low back pain. Spine. 2001;26(24):2738–2743. doi: 10.1097/00007632-200112150-00024. [DOI] [PubMed] [Google Scholar]
- 17.Onel D, Sari H, Dönmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Spine. 1993;18:291–298. doi: 10.1097/00007632-199302000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Penning L. Functional pathology of lumbar spinal stenosis. Clin Biomech. 1992;7:3–17. doi: 10.1016/0268-0033(92)90002-L. [DOI] [PubMed] [Google Scholar]
- 19.Penning L, Wilmink JT. Posture-dependent bilateral compression of L4–L5 nerve roots in facet hypertrophy. Spine. 1987;2(5):491. doi: 10.1097/00007632-198706000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Schonstrom N, Lindahl S, Willen J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989;7:115–121. doi: 10.1002/jor.1100070116. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Miyazaki T, Takino T, Matsui T, Tomita K. Epidural pressure measurements: relationship between epidural pressure and posture in patients with lumbar spinal stenosis. Spine. 1995;20:650–653. doi: 10.1097/00007632-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis: an attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Wade DT (1990) Measures of emotion and social interaction. In: Measurement neurological rehabilitation. Oxford University Press, Oxford, pp 259–284
- 24.Willen J, Danielson B, Gaulitz A, Niklason T, Schonstrom N, Hansson T. Dynamic effects on the lumbar spinal canal. Spine. 1997;24:2968–2976. doi: 10.1097/00007632-199712150-00021. [DOI] [PubMed] [Google Scholar]