Abstract
A Neurometer device is an electrical nerve stimulator used to determine the current perception threshold (CPT) evoked by stimulating A-beta fibers at 2,000 Hz, A-delta fibers at 250 Hz and C fibers at 5 Hz. CPT evaluation is used for analyzing peripheral nerve dysfunction. In this study, the sensory disturbance of the lower-extremity was quantitatively analyzed using CPT testing before and after lumbar discectomy. In 33 patients (L4/5: 16 and L5/S: 17), as subjective evaluations, tactile sensation and leg pain were assessed before and 2 weeks after surgery. In the subjectively improved group (n = 22), significant decreases in CPT at 2,000 and 250 Hz were noted postoperatively, whereas in the unchanged group (n = 11), no significant changes in CPT at any frequencies was noted. The leg pain improved in all patients. Likewise, CPT at 5 Hz, which stimulated C fiber, decreased significantly for both improved and unchanged groups. CPT measured by a Neurometer is very useful in assessing lower-extremity sensory functions before and after surgery for lumbar disc herniation.
Keywords: Current perception threshold, Radiculopathy, Lumbar discectomy
Introduction
In routine clinical settings, lower-extremity sensory disturbances associated with lumbar spinal disease are typically assessed by testing tactile sensation with a brush or with a pinprick. However, these tests are subjective, and as tactile responses from test subjects can be ambiguous, quantifying and objectively evaluating the severity of sensory disturbance is difficult. A Neurometer allows selective stimulation of nerve fibers with different thick nesses, based on the concept that nerve fibers with different thick nesses have different depolarization times, and that depolarization depends on the frequency of sine waves due to electrical stimulation [5]. By stimulating A-beta fibers at 2,000 Hz, A-delta fibers at 250 Hz and C fibers at 5 Hz, current perception threshold (CPT) is quantified at each frequency. A-beta fibers are large myelinated fibers responsible for vibration and pressure. A-delta fibers are small myelinated fibers responsible for tactile sensation and sharp pain. C fibers are unmyelinated fibers responsible for sensing temperature and dull pain [8, 9]. CPT testing is convenient, rapid and noninvasive, and allows objective assessment of sensory disturbance.
We previously measured CPT values in 48 patients with lumbar disc herniation accompanied by unilateral lower-extremity lumbar radiculopathy, and reported correlations between sensory disturbance and leg pain [13]. The present study aimed to ascertain chronological changes in CPT values and lower-extremity sensory disturbance before and after lumbar discectomy.
Patients and methods
Subjects comprised 33 patients (19 men and 14 women) with single-level lumbar disc herniation and unilateral localized lower-extremity symptoms, who underwent initial surgery between 2002 and 2003. Mean age was 45.8 years (range 20–65 years. Patients with recurrent herniation, extraforaminal herniation, cauda equina syndrome, spondylolysis, spondylolisthesis or trauma were excluded from the present study.). According to the level and localization of disc herniation assessed by T1- and T2-weighted MRI, herniation was at the L4/5 (n = 16) or L5/S1 (n = 17) level. All patients had radicular pain in only the L5 or S1 sensory area to the corresponded herniated level. Microdiscectomy was performed under surgical microscopy. The same surgeon, who is very experienced in microdiscectomy, performed all surgeries. Nerve damage, dura mater damage, infection and any other severe complications were absent.
Lower-extremity sensory functions were assessed before and 2 weeks after surgery. As subjective evaluations, the sensory disturbance was assessed by evaluating tactile sensation with a brush, and the leg pain was assessed using the visual analogue scale (VAS; 10-point scale). Depending on the degree of change in subjective evaluations, patients were grouped as follows: improved; sensory disturbance clearly improved postoperatively; unchanged; sensory disturbance not improved postoperatively or no obvious sensory disturbance seen before or after surgery; and exacerbated; sensory disturbance exacerbated postoperatively.
As objective evaluations, CPT values were measured using a Neurometer at room temperature (22°C) by the automated forced double-blind method as follows: first of all, electrodes for CPT detection were applied to the skin above the first and fifth metatarsals, representing the proper dermatomes for L5 and S1. The stimulation output was increased from 0 mA to a maximum of 9.99 mA until the test subject (patient) perceived the stimulus at the point where the electrode was placed. When the test subject reported a prickly electric stimulus on the skin, the stimulation was stopped, and then the stimulation output was decreased in 0.10 mA increments. The level of output where the test subject could no longer feel the stimulus was set as the stimulation output at the start of CPT testing. In each CPT testing session, the Neurometer automatically adjusted output levels and randomized the order of stimuli, and once sufficient responses were obtained, the final CPT value was determined by averaging the minimal stimulation intensities. Consistency and accuracy of CPT values were maintained by the randomness of the double-blind test. In other words, neither the subject nor the researcher could discern the type of output from the machine, so no room was available for subjective judgment in the test results, thus allowing objective and quantitative assessment of sensory functions [3]. This series of procedures was performed at three frequencies: 2,000, 250 and 5 Hz.
The following were investigated: (1) CPT values at bilateral legs before and after surgery in all patients; (2) correlation between changes in CPT values and subjective sensory evaluations before and after surgery and (3) correlation between changes in CPT values and VAS scores before and after surgery. A t test was used for statistical analyses, and P values < 0.05 was considered significant.
Results
Leg pain improved in all patients, with the mean VAS score improving from 8.3 points preoperatively to 1.9 points postoperatively. Postoperatively, subjective sensory disturbance improved in 22 patients (improved group) and remained unchanged in 11 patients (unchanged group), but was not exacerbated in any of the patients. Of the 11 unchanged patients, sensory disturbance did not improve in 8 patients, and sensory disturbance was not seen before or after surgery in 3 patients.
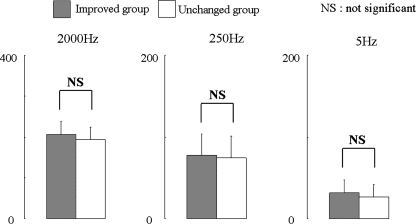
There was no significant change in CPT of noninvolved segment of the other leg as control at each frequency between improved and unchanged groups preoperatively (Fig. 1).
Fig. 1.
Current perception threshold (CPT) values in unaffected legs (n = 11). CPT values of the noninvolved segment of the other legs as control at each frequency between the improved and unchanged groups preoperatively
CPT values in all patients
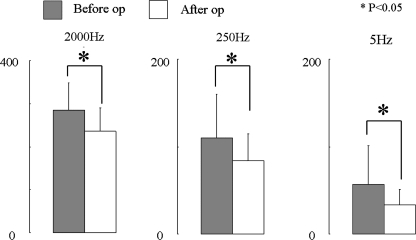
Current perception threshold offers an objective indicator for sensory disturbance, and all patients displayed significant postoperative decreases at 2,000, 250 and 5 Hz (Fig. 2).
Fig. 2.
Current perception threshold values in all cases (n = 33). CPT values in all cases at each frequency before and after surgery
Relationships between hypesthesia and CPT before and after surgery
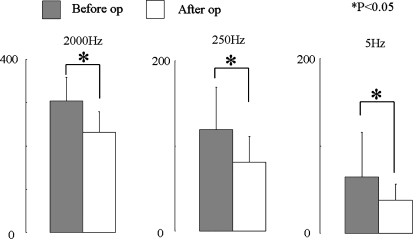
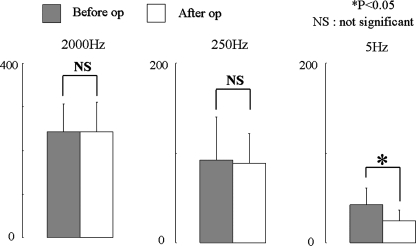
Among patients with improved sensory disturbance, significant decreases in CPT at 2,000 and 250 Hz were noted postoperatively (Fig. 3). Among unchanged patients, no significant changes in CPT were seen at 2,000 and 250 Hz frequencies (Fig. 4). On the other hand, CPT at 5 Hz significantly decreased both in the improved and unchanged groups (Figs. 3, 4).
Fig. 3.
Current perception threshold values in the improved group (n = 22). CPT values in the improved group at each frequency before and after surgery
Fig. 4.
Current perception threshold values in the unchanged groups (n = 11). CPT values in the unchanged group at each frequency before and after surgery
Discussion
Leg pain, sensory disturbance and muscle weakness represent the main symptoms of lumbar disc herniation. However, accurately ascertaining these conditions is difficult, and quantifying the severity of leg pain and sensory disturbance has generally been considered impossible. While pain has been quantitatively assessed using the VAS, likely sensory disturbance was evaluated using electrophysiological tests by measuring latency. These techniques, however, are insufficient to objectively evaluate those symptoms. In CPT testing using a Neurometer, nerve functions are quantified based on sensory threshold values. Moreover, this represents a revolutionary test method that can test pathways between the central nervous system and electrode [3].
In the past, the Neurometer has been used to assess the sensory function of patients with diabetic peripheral nerve disorders [1, 8, 9, 11, 12], carpal tunnel syndrome [6, 7], hand-arm vibration syndrome [10] or toe-to-digit transplantation [2]. At present, the Neurometer has been used in pain clinics for diagnosis, prognostication and course evaluation. Because quantitative electrophysiological diagnosis offers a means to evaluate the function of selective nerve fibers, a Neurometer has been used as an auxiliary diagnostic technique for allodynia, dysesthesia and malingering [4]. Selective evaluation of A-delta and C fiber functions has been difficult, but is possible with a Neurometer. Assessment of the functions of these nerve fibers involved in acute and chronic pain allows accurate evaluation of pain [1], which is clinically most problematic for patients, thus contributing greatly to improved diagnosis and treatment.
Previously, we objectively evaluated sensory function in 48 patients with lumbar disc herniation by measuring CPT values. The results showed that CPT values at 2,000 and 250 Hz were significantly increased in patients with sensory disturbance and that tactile sensation findings using a brush reflected functions of A-beta and A-delta fibers [13]. In the present study, in patients for whom tactile sensation clearly improved postoperatively, CPT values at 2,000 and 250 Hz significantly decreased. Conversely, in the unchanged group, no significant changes in CPT values were noted at 2,000 or 50 Hz. Preoperatively, there was no significant change in CPT values of the noninvolved segment of the other leg as control at each frequency between improved and unchanged groups, although it is a fact that CPT values vary individually. Thus, those findings suggest that CPT values at 2,000 and 250 Hz are useful as relative indicators for postoperative improvement in sensory functions. Meanwhile, preoperative CPT values at 2,000 and 250 Hz in the improved group were significantly higher than those in the unchanged group. Consequently, the cases with more severe magnitudes of sensory disturbance, that is to say, higher CPT values, effectively improved postoperatively. One possible explanation for this is that sensory disturbance is caused by not only mechanical compression of the spinal nerve roots, but also inflammation observed frequently with relatively acute lumbar disc herniation.
On the other hand, in both improved and unchanged groups, CPT value at 5 Hz decreased significantly. It is possible that CPT values at 5 Hz are not related to sensory disturbance in the involved lower leg. The previous study reported that CPT at 5 Hz for patients with severe leg pain was significantly increased compared to the contralateral [13]. In the present study, all patients who had severe leg pain with preoperative VAS scores ≥7 points showed pain relief postoperatively. Therefore, these findings suggest that C fibers activated with 5 Hz stimulation (CPT value) may somehow be involved in the radiculopthy onset of lower-extremity sciatica in lumbar disc herniation.
The present study showed that CPT measured by a Neurometer is very useful in assessing lower-extremity sensory functions before and after surgery for lumbar disc herniation. The Neurometer is a less invasive test that can be performed anywhere on the body in a simple manner. The test can be performed without any limitations in location and so is useful for assessing sensory functions in various spinal diseases (cervical spondylotic myelopathy and lumbar spinal canal stenosis) accompanied by neuropathy.
Acknowledgment
This study was supported by the Yuasa Memorial Foundation (2003 Research Grant).
References
- 1.Chado HN. The current perception threshold evaluation of sensory nerve function in pain management. Pain Digest. 1995;5:127–134. [Google Scholar]
- 2.Chu N-S. Current perception threshold in toe-to-digit transplantation and digit-to-digit replantation. Muscle Nerve. 1996;19:183–186. doi: 10.1002/(SICI)1097-4598(199602)19:2<183::AID-MUS9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Katims JJ, Long DM, Ng LK. Transcutaneous nerve stimulation: frequency and waveform specificity in humans. Appl Neurophysiol. 1986;49:86–91. doi: 10.1159/000100133. [DOI] [PubMed] [Google Scholar]
- 4.Katims JJ, Mitsuhata H, Miyazaki T. Electrodiagnostic evaluation of sensory nerve function in the patient with pain: neuroselective current perception threshold (CPT) and pain tolerance threshold (PTT) Pain Clinic. 1998;19:535–549. [Google Scholar]
- 5.Katims JJ, Naviasky EH, Rendell MS, Ng LK, Bleecker ML. Constant current sine wave transcutaneous nerve stimulation for the evaluation of peripheral neuropathy. Arch Phys Med Rehabil. 1987;68:210–213. [PubMed] [Google Scholar]
- 6.Katims JJ, Rouvelas P, Sadler BT, Weseley SA. Reproducibility and comparison with nerve conduction in evaluation of carpal tunnel syndrome. Trans Am Soc Artif Intern Organs. 1989;35:280–283. [PubMed] [Google Scholar]
- 7.Katims JJ, Patil AS, Rendell MS, et al. Current perception threshold screening for carpal tunnel syndrome. Arch Environ Health. 1991;46:207–212. doi: 10.1080/00039896.1991.9937449. [DOI] [PubMed] [Google Scholar]
- 8.Masson EA, Boulton AJM. The Neurometer: validation and comparison with conventional tests for diabetic neuropathy. Diab Med. 1991;8:563–566. doi: 10.1111/j.1464-5491.1991.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 9.Masson EA, Veves A, Fernando D, Boulton AJM. Current perception threshold: A new, quick, and reproducible method for assessment of peripheral neuropathy in diabetes mellitus. Diabetology. 1989;32:724–728. doi: 10.1007/BF00274531. [DOI] [PubMed] [Google Scholar]
- 10.Pelmear PL, Kusiak R. Clinical assessment of hand-arm vibration syndrome. Nagoya J Med Sci. 1994;57(Suppl):27–41. [PubMed] [Google Scholar]
- 11.Pitei DL, Watkins PJ, Stevens MJ, Edmonds ME. The value of the Neurometer CPT in assessing diabetic neuropathy by measurement of the current perception threshold. Diab Med. 1994;11:872–876. doi: 10.1111/j.1464-5491.1994.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 12.Rendell MS, Dovgan DJ, Bergman TF, O’Donnel GP, Drobny EP, Katims JJ. Mapping diabetic sensory neuropathy by current perception threshold testing. Diab Care. 1989;12:636–640. doi: 10.2337/diacare.12.9.636. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita T, Kanaya K, Sekine M, Takebayashi T, Kawaguchi S, Katahira G. A quantitative analysis of sensory function in lumbar radiculopathy using current perception threshold testing. Spine. 2002;27:1567–1570. doi: 10.1097/00007632-200207150-00016. [DOI] [PubMed] [Google Scholar]