Abstract
EcoHIV/NL4-3 is a chimeric human immunodeficiency virus type 1 (HIV-1) that can productively infect mice. This study tests the utility of EcoHIV/NL4-3 infection to reveal protective immune responses to an HIV-1 vaccine. Immunocompetent mice were first immunized with VRC 4306 which encodes subtype B consensus sequences of gag, pol, and nef and then were infected by EcoHIV/NL4-3. Anti-Gag antibodies were sampled during immunization and infection. The extent of EcoHIV/NL4-3 infection in spleen cells and peritoneal macrophages was determined by quantitative real-time PCR (QPCR). Although antibody titres were not significantly different in control and vaccinated groups, VRC 4306 immunization induced protective responses that significantly reduced virus burden in both lymphocyte and macrophage compartments. These results indicate that EcoHIV/NL4-3 infection can be controlled by HIV-1 vaccine induced responses, introducing a small animal model to test vaccine efficacy against HIV-1 infection.
Keywords: HIV-1, mouse model, DNA vaccine
1. Introduction
As HIV-1 infection continues to spread worldwide the need for a safe and effective vaccine remains unmet. Extraordinary scientific efforts have been expended to identify the means to elicit protective immunity against HIV-1 [1,2]. This endeavor is impeded by the absence of immunocompetent experimental animals susceptible to HIV-1. Consequently, vaccine efficacy is tested for the ability to reduce the efficiency of HIV-1 transmission and the frequency of new infections only in human beings at risk.
To permit the use of conventional mice for investigation of HIV-1 replication and control, we switched the tropism of HIV-1 from human to rodent [3]. The regions encoding gp120 of subtype B NL4-3 [4] and subtype D NDK [5] were replaced with that of the gp80, the envelope of ecotropic murine leukemia virus (MLV). The resulting infectious chimeric viruses, EcoHIV/NL4-3 and EcoHIV/NDK, encode all HIV-1 proteins except gp120 and can infect conventional mice systemically, replicating in lymphocytes and macrophages, and inducing immune responses. Antiretroviral drugs in clinical use inhibited EcoHIV/NDK infection in mice, demonstrating that HIV-1 enzymes retain their native functions in mice [6]. Here we report proof of principle that EcoHIV infection of mice can be used to evaluate protective efficacy of potential vaccines, in this case VRC 4306, a DNA vaccine encoding subtype B consensus sequences of gag, pol, and nef [7].
2. Materials and methods
VRC 4306 was kindly provided by Dr. G. Nabel, NIAID-NIH. Adult female C57BL6/J mice were immunized by four intramuscular injections of 100 μg VRC 4306 or control plasmid pUC19 at two week intervals with five mice per group. Seven weeks and ten weeks after the first immunization, mice were infected with 5 × 106 pg p24 EcoHIV/NL4-3 by intraperitoneal injection of cell-free virus, prepared as described [3]. One week after the challenge EcoHIV/NL4-3 infection, mice were euthanized by carbon dioxide asphyxiation and blood, spleen, and peritoneal macrophages were collected. DNA was isolated from spleen and macrophages on the day of euthanasia using DNAzol. QPCR was conducted as described [6] using primers that amplify sequences with the MLV envelope: forward primer (5′-GGCCAAACCCCGTTCTG-3′), reverse primer (5′-ACTTAACAGGTTTGGGCTTGGA-3′) and probe (5′-CAGACCAACAGCCACT-3′). Custom primers and probe were obtained from ABI. Data are reported as number of viral DNA copies per 106 cells, cell numbers were obtained by amplification of glyceraldehyde phosphate dehydrogenase by QPCR as described [6]. Elisa was conducted to detect anti-Gag antibodies in mouse sera. Wells were coated with 50 ng HIV-1/LAV Gag p55, the recombinant protein was provided by the NIH AIDS Research and Reference Reagent Repository. Preimmune sera and sera obtained eight weeks and eleven weeks after immunization were tested at 50-fold dilutions. Bound antibody was detected using horseradish peroxidase conjugated anti-mouse IgG and the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine, wells were read at OD450.
The statistical significance of differences in virus burden or anti-Gag titres between groups was determined by t Test, the significance of the difference in frequency of anti-Gag positive mice between different groups was also determined by Chi-square Test.
3. Results
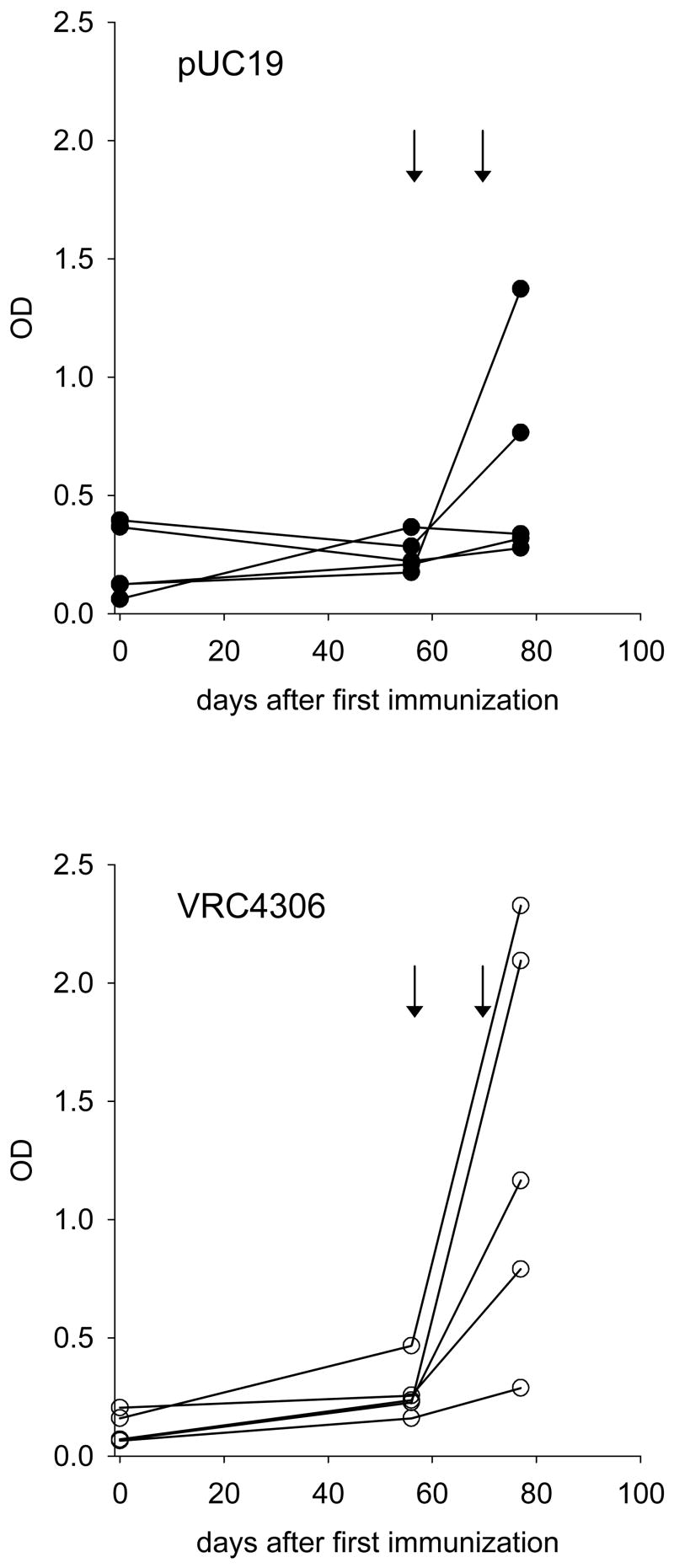
This study was conducted to determine whether EcoHIV/NL4-3 infection of mice can be reduced by immune responses induced by a known immunogenic HIV-1 vaccine, VRC 4306 [7]. Mice were immunized either with a control plasmid, pUC19, or VRC 4306. Immunization using HIV-1 DNA vaccines is generally most efficient when it is followed by boosting responses through infection with a viral vector of the antigens [8]. In this case, the viral antigenic boost was provided by an initial infection by EcoHIV/NL4-3 itself. Then efficacy of responses was tested in a subsequent challenge infection. Serological responses to HIV-1 Gag were measured as a simple readout for immunization. Most infected mice mounted immune responses to HIV-1 Gag one week after the challenge infection; two of five control mice mounted responses, indicating that EcoHIV/NL4-3 infection itself induces humoral responses, as previously observed [3] (Figure 1). VRC 4306 immunized mice had higher titres than the control group and four out of five mounted responses but the differences did not reach statistical significance (by t Test p=0.07 or by Chi-square test, p=0.197). These results suggest that this DNA vaccine immunization did not induce extensive humoral responses to Gag.
Figure 1.
HIV-1 specific IgG. The upper panel shows serum anti-HIV-1 Gag levels over time in mice immunized with the control plasmid pUC19, the lower panel shows the responses of mice immunized with VRC 4306, each symbol represents the average titre of an individual mouse. The arrows indicate the times of infection by EcoHIV/NL4-3.
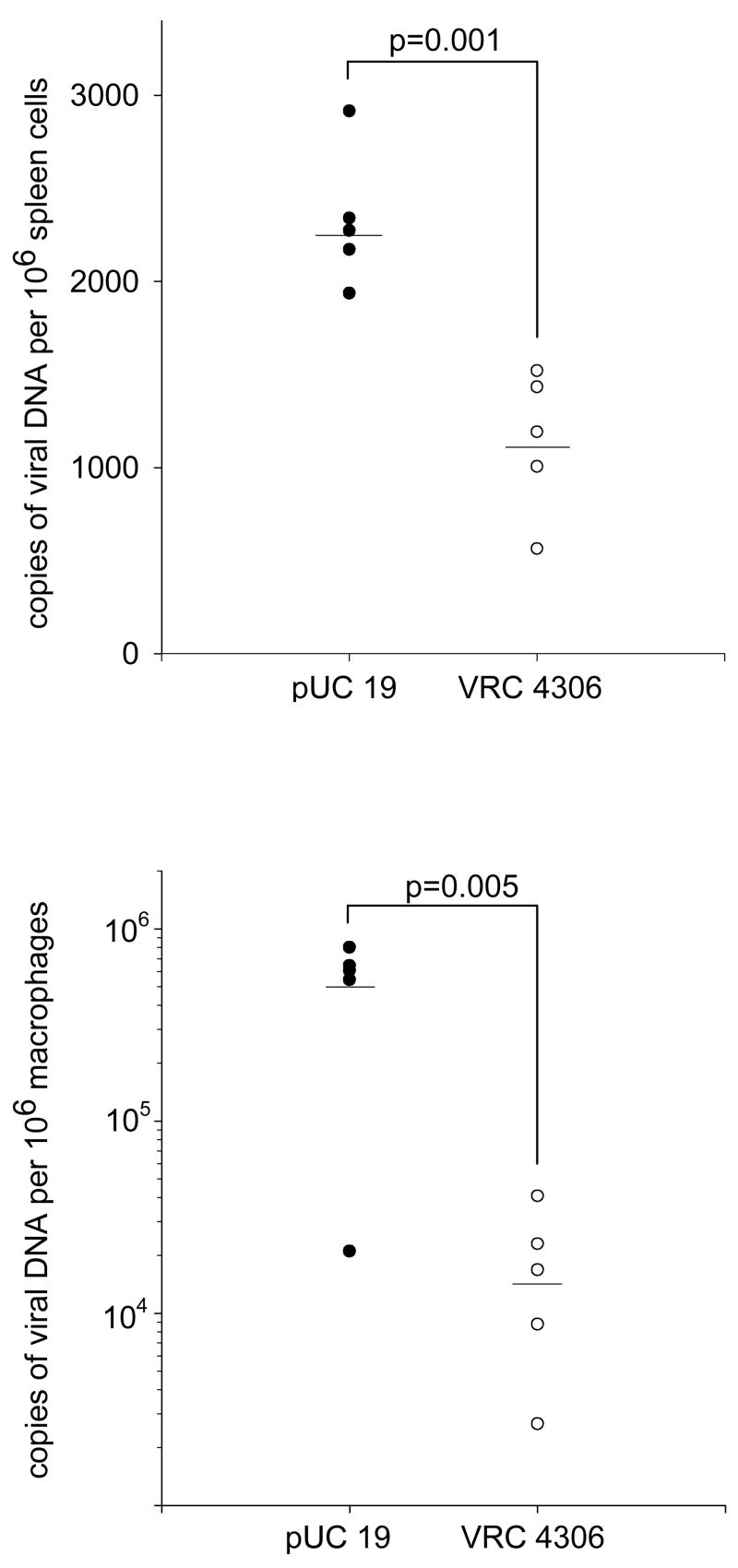
Immunization with VRC 4306 has been shown to induce CD4 and CD8 T lymphocyte responses to HIV-1 Gag [7], suggesting that both T helper cells and cytotoxic effector cells were primed. To investigate whether these potential protective immune responses could control EcoHIV/NL4-3 infection, we measured the viral burdens in VRC 4306 and control immunized mice after the challenge virus infection. To distinguish newly synthesized EcoHIV/NL4-3 DNA from the gag, pol, and nef DNA vaccine, we amplified a region in the chimeric envelope consisting of MLV sequences that is absent from VRC 4306. Viral burdens in splenic lymphocytes and peritoneal macrophages were determined by QPCR amplification, normalized by amplification of a cellular gene (Figure 2). The EcoHIV/NL4-3 burden was significantly reduced in VRC 4306 immunized compared to control mice; virus detected in both the lymphoid and macrophage compartments was sensitive to the immune responses induced by vaccination. These findings provide clear evidence that the extent of EcoHIV/NL4-3 infection in mice is a legitimate measure for the generation of protective immunity by an HIV-1 vaccine.
Figure 2.
Protection against EcoHIV/NL4-3 infection in VRC 4306 immunized mice. The upper panel shows the virus burden obtained by real-time PCR in spleen cells from mice immunized with VRC 4306 and pUC19 and infected by EcoHIV/NL4-3, the lower panel shows the virus burden in peritoneal macrophages from the same mice. Each symbol represents the average number of viral DNA copies, normalized by amplification of a cellular gene in tissue of an individual mouse. The horizontal line indicates the mean virus burden in each group
4. Discussion
The results from this small study indicate that EcoHIV/NL4-3 infection of conventional mice can be controlled by immune responses to an HIV-1 vaccine, forming a suitable system to test vaccine efficacy. In this model, HIV-1 proteins expressed by infected lymphocytes and macrophages assemble to form infectious HIV-1 [3], a system closely analogous to the infected human host. Like infected humans, infected mice mount serological responses to HIV-1 proteins. This observation indicates that HIV-1 proteins are immunogenic to mice in their native form in infected cells or virions. The DNA vaccine, VRC 4306, has several features that may contribute to induction of protective immune responses that can be recalled by live virus infection [7]. It is a codon optimized vector encoding consensus subtype B Gag-Pol-Nef, thus it carries sequences closely related to EcoHIV/NL4-3 that are optimized for expression. In addition, point mutations were introduced into protease, reverse transcriptase, and integrase to inactivate enzyme function and minimize cytotoxicity, permitting efficient expression of antigens by transduced cells in the mouse.
In the present study, immunization with VRC 4306 DNA alone was sufficient to prime responses to virus infection. EcoHIV/NL4-3 burden was reduced in both spleen cells and macrophages in VRC 4306 immunized mice compared to controls. Using magnetic bead purification of CD4 bearing T lymphocytes from spleens of infected mice, we have previously shown that lymphocytes account for the majority of Eco/NL4-3 infected cells in the spleen [3], however we cannot rule out the possibility that some of the infection observed in unfractionated spleen arises from macrophages. The magnitude of the reduction of virus burden with immunization shown here was greater in macrophages than in spleen cells. We have previously shown that macrophages from infected mice express more viral RNA than do lymphocytes [6], suggesting that more viral antigen may be present in macrophages for targeting by immune effectors.
In this initial study we focused upon providing proof of principle that vaccine induced immune responses control EcoHIV/NL4-3 challenge infection and did not investigate the mechanism of protection. It is likely that cytotoxic T lymphocyte responses directed against the immunogens, Gag, Pol, and/or Nef mediated the reduction in virus burden. This view is based on the observation that mice immunized with VRC 4306 mount both CD4 and CD8 T lymphocyte responses to Gag [7]. In addition, antiviral CD8 responses are believed to be responsible for successful vaccination in the SIV macaque system [9] and for protection in HIV-1 exposed uninfected sex workers [10]. Although responses to the MLV envelope may be induced by EcoHIV/NL4-3 infection, they are unlikely to be responsible for the protection observed. Both VRC 4306 and pUC19 immunized mice were only exposed to the MLV envelope by EcoHIV/NL4-3 infection, so responses to this antigen cannot account for the differences in virus burden between groups.
A recent study by Rollman et al. evaluated vaccine efficacy in HLA-transgenic mice testing several immunization protocols for their ability to induce specific immune responses [11]. In that study, vaccinated mice reduced virus burden in mouse lymphocytes that had been infected by pseudotyped HIV-1 in culture and implanted into the peritoneal cavity. It is notable that the best protection against virus challenge in that model was achieved through immunization with DNA alone, the method employed here. Taken together, Rollman et al.’s investigation of immunization protocols and HLA-restricted responses and our study of the efficacy of vaccination to reduce systemic HIV-1 infection hold promise for the utility of evaluation of future HIV-1 vaccines for protective efficacy in immunocompetent mice.
Acknowledgments
We wish to thank Dr. G. Nabel for kindly supplying VRC 4306; DAIDS, NIAID/NIH for supplying HIV-1 p55 through the AIDS Research Reagent Repository, Wei Chao for advice in QPCR, and all the members of the Molecular Virology Division for discussion. This work was sponsored by PHS grants to MJP and DJV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMichael A. HIV Vaccines. Ann Rev Immunol. 2006;24:227–255. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 2.Letvin N. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6:930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 3.Potash MJ, Chao W, Bentsman G, et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci USA. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi A, Gendelman HE, Koenig S, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellrodt A, Barré-Sinoussi F, Le Bras P, et al. Isolation of human T-lymphotropic retrovirus (LAV) from Zairian married couple, one with AIDS, one with prodromes. Lancet. 1984;1(8391):1383–1385. doi: 10.1016/s0140-6736(84)91877-4. [DOI] [PubMed] [Google Scholar]
- 6.Hadas E, Borjabad A, Chao W, et al. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS. 2007;21:905–909. doi: 10.1097/QAD.0b013e3281574549. [DOI] [PubMed] [Google Scholar]
- 7.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77:12764–12772. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24(19):4062–4081. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Barouch D, Santra S, Schmitz J, et al. Control of Viremia and Prevention of Clinical AIDS in Rhesus Monkeys by Cytokine-Augmented DNA Vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 10.Rowland-Jones S, Dong T, Fowke KR, et al. Cytotoxic T cell responses to multiple conserved epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rollman E, Mathy N, Bråve A, et al. Evaluation of immunogenicity and efficacy of combined DNA and adjuvanted protein vaccination in a human immunodeficiency virus type 1/murine leukemia virus pseudotype challenge model. Vaccine. 2007;25:2145–2154. doi: 10.1016/j.vaccine.2006.10.057. [DOI] [PubMed] [Google Scholar]