Abstract
Eating represents a choice among many alternative behaviors. The purpose of this review is to provide an overview of how food reinforcement and behavioral choice theory are related to eating and to show how this theoretical approach may help organize research on eating from molecular genetics through treatment and prevention of obesity. Special emphasis is placed on how food reinforcement and behavioral choice theory are relevant to understanding excess energy intake and obesity and how they provide a framework for examining factors that may influence eating and are outside of those that may regulate energy homeostasis. Methods to measure food reinforcement are reviewed, along with factors that influence the reinforcing value of eating. Contributions of neuroscience and genetics to the study of food reinforcement are illustrated by using the example of dopamine. Implications of food reinforcement for obesity and positive energy balance are explored, with suggestions for novel approaches to obesity treatment based on the synthesis of behavioral and pharmacological approaches to food reinforcement.
Keywords: food reinforcement, food intake, choice, dopamine, behavioral genetics
Choice is ubiquitous. There are many choices that people make that influence their eating and body weight, beginning with how early to get up, to exercise before breakfast or to sleep in, what to have for breakfast, and so on. These choices are made in the context of alternatives, many of which are pleasurable and exert considerable influence to do these behaviors rather than others. The morning is a good time for many people to choose to exercise, but a warm bed and a little extra sleep time represent a powerful alternative that even the most enthusiastic exerciser confronts and often succumbs to. Similarly, eating a good breakfast starts the day off right, but a pastry and coffee may be particularly inviting alternative foods that taste very good and may tempt many away from healthy cereal and fruit. This article provides a theoretical framework for understanding motivation to eat, how people make choices about eating versus engaging in other behaviors, and how these theoretical approaches can provide insights into obesity, mechanisms that regulate food choice, and implications of choice theory for interventions. One of the unique aspects of food reinforcement and behavioral theories of choice is the way in which they can be used to understand factors that influence eating at multiple levels.Table 1 provides examples of the multiple levels of analysis, using the framework of micro to environmental levels of analysis. This framework will be used to organize the literature that is reviewed.
Table 1.
Levels of Analysis of Behavioral Economics of Food Reinforcement
| Level of analysis | Research area |
|---|---|
| Genetic | |
| Molecular | Identify genes associated with excess energy intake |
| Behavioral | Use co-twin design to control for the influence of genetics and test the influence of the environment on food reinforcement |
| Physiological | |
| Neurobiological | Identify brain mechanisms associated with food as a reinforcer and substitution of nonfood for food reinforcers |
| Metabolic | Determine the role of insulin and glucose in food reinforcement |
| Behavioral | |
| Nutritional | Compare food reinforcement value for carbohydrates, fat, and protein |
| Moderators of food reinforcement | Identify role of deprivation and satiation on food reinforcement value |
| Clinical | |
| Pharmacological | Test agents to reduce reinforcing value of food |
| Behavioral | Identify alternatives to food as a reinforcer |
| Policy | |
| Schools | Increase variety for healthy foods and reduce variety for less healthy foods |
| Pricing | Increase prices (taxes) on high energy dense, low nutrient dense foods, and/or reduce prices (subsidies) on low energy dense, high nutrient dense foods |
The Reinforcing Value of Food
A reinforcer is a stimulus that increases the rate of a behavior that it follows. For example, when a dog learns a trick to get a dog treat, the treat is the reinforcer. The reinforcing value, or reinforcer efficacy (Bickel, Marsch, & Carroll, 2000), of a stimulus refers to how much behavior the stimulus will support, or the response-strengthening function of reinforcement. Strong reinforcers can motivate a lot of behavior, whereas weaker reinforcers do not support very much behavior. It is not surprising to consider that many drugs of abuse are strong reinforcers as drug addicts will engage in a substantial amount of behavior to gain access to these strong reinforcers. Food is a strong reinforcer and, in some contexts, may be a more powerful reinforcer than are drugs of abuse (Hursh & Bauman, 1987).
Consumption of some reinforcers may be regulated, as the patterns of responding adjust to match the consumption of a regulated reinforcer. For example, when the dose of alcohol is varied the number of alcohol self-administrations is inversely related to dose, so that alcohol consumption can be regulated (Karoly, Winger, Ikomi, & Woods, 1978). An analogy to food regulation might be that if subjects are working for a slice of pizza as the reinforcer and the portion size is reduced in half, then subjects would double the amount of work they are willing to do to maintain the same amount of pizza. On the other hand, responding may also be a function of the concentration of the substance. Rats will increase responding in a dose–response manner as the concentration of sucrose increases (Sclafani & Ackroff, 2003). If the animals were working to gain a regulated amount of sucrose, then they would respond less, rather than more, as the concentration increased.
The process of presenting a reinforcer contingent on behavior and observing increases in the rate of the behavior is called positive reinforcement. Positive reinforcement is one example of the law of effect, which is defined by the more general rule that the rate of instrumental behavior depends on its effect on the environment (Bouton, 2007). The process of a behavior producing a positive consequence is also called reward learning, and the positive events can also be called rewards. In many cases, reward and reinforcer have similar meanings, but we have tried to use the terms reinforcement or reward consistently with the author’s use of the terms. The behavior that is associated with presentation of the contingent event can be described as instrumental or operant behavior. In situations in which we refer to the differences in the amount of work that subjects engage in to get access to reinforcers, we refer to these behaviors as motivated behaviors.
Behavioral Theories of Choice: Relative Reinforcing Value and Choice Among Multiple Alternatives
Reinforcer efficacy, or reinforcer value, can be defined in terms of how many responses are made to obtain food (Epstein & Saelens, 2000). Schedules of reinforcement establish how much work is needed to gain access to the reinforcer. The most common method for evaluating the efficacy of a single reinforcer, which is referred to as absolute (rather than relative) reinforcing value, is to arrange for a subject to gain access to a reinforcer by responding on progressive ratio schedules of reinforcement. In progressive ratio schedules, the schedule requirements are progressively increased after a reinforcer is obtained. For example, a subject may have to make 10 responses to obtain the first reinforcer, 100 to get the second, 1,000 to get the third, and so on. Reinforcer efficacy is most commonly defined in terms of the breakpoint—the point at which subjects stop responding. A reinforcer that maintains a higher breakpoint is considered to be more reinforcing than is a commodity for which subjects terminate responding earlier (Richardson & Roberts, 1996). The amount of behavior that a specific reinforcer will support can be compared across substances just as the abuse liability of drugs can be compared in animals (Ator & Griffiths, 2003; Balster & Bigelow, 2003) and humans (Griffiths, Bigelow, & Ator, 2003).
Outside of the laboratory, people usually are not presented with only one option but rather have to choose how to allocate time, behavior, and resources among several alternatives. The paradigm for evaluating reinforcer efficacy can be extended to two or more choices, and the theoretical base for considering choice among multiple reinforcers is behavioral choice theory (Vuchinich & Tucker, 1988). The core paradigm for studying choice is to provide access to two or more alternatives and to vary the amount of work (schedules of reinforcement) required to gain access to each of the alternatives. This allows for the determination of the relative reinforcing value of the alternatives. In the concurrent schedule paradigm, the subject needs to choose which alternative to work for. If the schedules of reinforcement are the same for both alternatives, then the pattern of responses will most likely reflect the reinforcing value of each alternative. Choice responding, which is often referred to as preference, depends on the reinforcing value of the alternatives and constraints on the alternatives. For example, if someone has equal access to two types of food in the home, and both are ready to eat and require the same behavioral cost to prepare, then they are likely to choose the meal that they find most reinforcing. However, if the behavioral cost of the preferred meal is much higher than that of the less-preferred meal, and it requires a lot of preparation and perhaps leaving the house to obtain new ingredients, the person may shift preference from the more-preferred to less-preferred meal. If the behavioral cost of an alternative is increased, then someone will shift choice from preferred to less-preferred foods (Lappalainen & Epstein, 1990).
One way to define how reinforcing an alternative is in relationship to a second alternative is to establish baseline responding for both alternatives and then place constraints on access to the more reinforcing alternative while the behavioral cost of responding for the least-preferred alternative stays the same. In this paradigm, there will be a greater rate of responding for the more-preferred alternative when the behavioral costs are equal, but as the behavioral cost for the more-preferred alternative increases relative to the cost for the less-preferred alternative, the responses should indicate a shift from responding for the more- to the less-preferred alternative. The point at which the work shifts from the most-preferred to least-preferred alternative defines the crossover or switch point, which provides an estimate of the relative reinforcing value of that commodity. Another approach is to increase the behavioral cost for each alternative separately as that alternative is earned. In this paradigm, the behavioral costs for each alternative increase independently on the basis of responding for each alternative. As described by Bickel (Bickel et al., 2000), increasing the behavioral cost can shift the pattern of responding. For example, there may be no differences in choice between two alternatives when the behavioral costs to obtain them are the same, but definite preferences for one of the alternatives may be observed when the behavioral cost increases. Alternatively, someone may have a relative preference for one alternative when the cost to obtain the alternatives is low, but as the behavioral cost increases, the preferences may shift to the alternative that was initially least preferred (Bickel et al., 2000).
Comparing the reinforcing value of alternative reinforcers with study-relative reinforcing value is a time-consuming process. An alternative method for determining relative reinforcing value may be to use a questionnaire. Griffiths and colleagues (Griffiths, Rush, & Puhala, 1996) developed a questionnaire to assess the relative reinforcing value of nicotine, which has been adapted for food by Goldfield and colleagues (Goldfield, Epstein, Davidson, & Saad, 2005). Questionnaires have been used in previous research to determine a food reinforcement phenotype that interacted with dopamine genotypes to predict food intake (Epstein et al., 2004b). The use of a questionnaire to get an estimate of relative reinforcing value provides the opportunity to test a wider number of alternatives that can then be further studied with more precise behavioral measures in laboratory settings.
The relationship between responding and response requirements as defined by the schedule of reinforcement can be considered by constructing demand curves. Demand curves provide an indication of the proportional change in responding as a function of proportional change in behavioral requirements to gain access to food (Figure 1). Figure 1, adapted from a theoretical analysis of demand curves and reinforcer efficacy in behavioral pharmacology (Bickel et al., 2000), presents rates of responding and energy consumed as a function of the amount of work required to earn a reinforcer, which is specified at fixed ratio (FR) schedules of FR 1, FR 10, through FR 10,000. An FR 10,000 schedule requires 10,000 responses to gain access to a reinforcer, which is a higher behavioral cost than an FR 1,000 schedule, which requires 1,000 responses to gain access to the reinforcer, and so on. As the figure shows, as behavioral cost increases from FR 1,000 to FR 10,000, responses to obtain food decrease, and so does energy consumption. This relationship can be considered as an indication of demand elasticity. Elasticity is a function of behavioral cost such that from an FR of 1 to an FR of 1,000 the curve is relatively inelastic, that is, subjects increase their responding linearly to maintain a relatively constant energy intake. However, the relationship between responding and food is elastic for FRs greater than 1,000. These shifts in the pattern of responding as a function of changes in the behavioral cost to obtain the reinforcer provide a strong rationale that reinforcer efficacy cannot be established on the basis of comparisons at one response requirement. Because preference depends in part on the behavioral cost, evaluation of choice on reinforcer efficacy at one behavioral cost provides only a very limited view of absolute or relative reinforcing values of behaviors. Just as the effects of a drug cannot be determined by studying one dose, reinforcer effectiveness needs to be tested across multiple response requirements.
Figure 1.
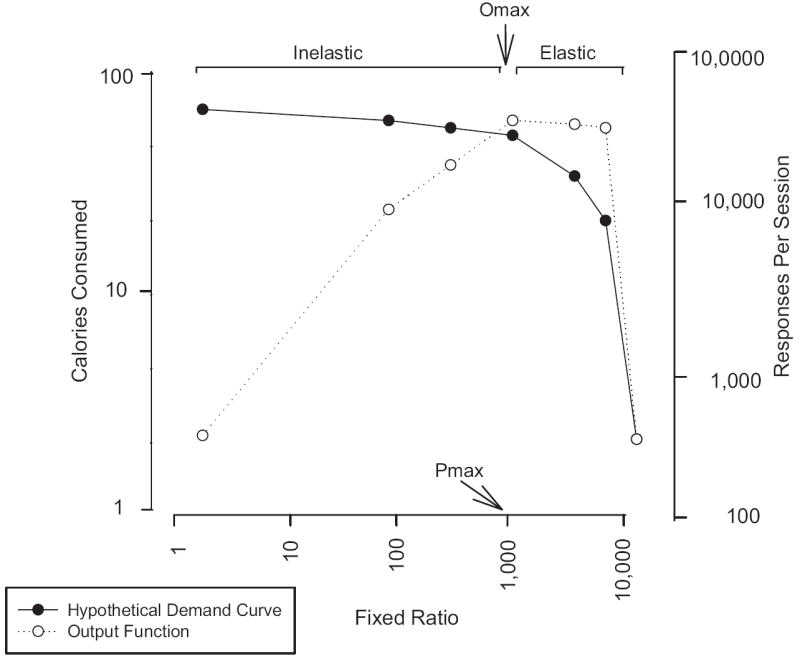
Hypothetical demand curve for food. The Omax represents the maximal amount of responding for food, and the Pmax the behavioral price at which the greatest amount of responding is observed. As the behavioral cost to obtain food increases, responding increases up to an FR 1,000, and the relationship between behavioral cost and consumption is inelastic. After behavioral costs of FR 1,000 or greater, response rate decreases, and the relationship is elastic. The reduction in responding may be due to a combination of reinforcer satiation and the behavioral cost of obtaining the food. Adapted from a figure describing demand curves for drug reinforcers by Bickel and colleagues (Bickel et al., 2000).
One of the most relevant aspects of the demand curve for application to eating is the cause of the shift from elastic to inelastic consumption, or a shift from increasing responding for food to reducing responding for food. The change in the shape of the curve is likely due to a combination of satiation of the reinforcer and demands placed on the subject by the increased work required by the schedule. The use of the term satiation in reinforcement theory refers to the point at which subjects have consumed enough of the reinforcer so that they will not engage in any more behavior to obtain it. We return to other uses of the term satiation in a later section.
Absolute Versus Relative Reinforcing Value
Food reinforcement can be measured with either absolute or relative reinforcing value, or whether only one or multiple options are available. The patterns of responding in single versus concurrent schedules may be similar, and a behavior that is very reinforcing in one paradigm may be determined to be very reinforcing in the other paradigm (Bickel et al., 2000). Whereas this is true for many comparisons, there may be important exceptions that warrant use of choice paradigms. One of the basic assumptions of choice paradigms is that choice depends in part on the alternative that is available (Bickel, Madden, & Petry, 1998). Thus, if the alternative has low reinforcing value, then there may be few differences between absolute and relative reinforcing value. However, if the alternative is very reinforcing, then the absolute and relative reinforcing values may be quite different. Take for example assessment of the absolute reinforcing value of an apple or the relative reinforcing value of the apple versus broccoli. Because for most people broccoli is not a very reinforcing alternative, the determination of absolute and relative reinforcing value for the apple should be similar. However, consider if chocolate is the alternative to the apple. In this case, the relative reinforcing value of the apple versus chocolate would likely be lower in comparison with the absolute reinforcing value of the apple when studied alone.
In addition, a choice paradigm provides information on relative reinforcer efficacy that is not available when only one reinforcer is studied. There is ecological validity to a choice paradigm because in the real world eating is almost always a choice, and studying food intake when only one behavioral option is available may not provide a realistic appraisal of the reinforcing value of food. In an interesting study on measuring liking of food in laboratory and field settings, investigators showed that the correspondence between ratings was highest when the laboratory setting involved a choice among foods, rather than presenting individual foods, as providing a choice may simulate the natural environment (de Graaf et al., 2005).
Integrating General Reinforcement Theory With Food Reinforcement
Food reinforcement can be considered to be a specialized example of more general reinforcement theory. There are general theories of reinforcement that describe the conditions under which behaviors function as reinforcers and provide a priori determination of what types of activities or stimuli can be used to motivate people to change (Timberlake & Farmer-Dougan, 1991). A priori definition of what types of behaviors or activities can function as reinforcers reduces the circularity in definitions of reinforcers that are based only in terms of their function, which is an important addition to reinforcement theory. A theoretical approach to understanding how something becomes reinforcing is disequilibrium theory, which defines reinforcers in terms of constraints on access to usual responding (Timberlake & Farmer-Dougan, 1991). This is an approach that extends the Premack principle (Premack, 1959), which states that the unconstrained rate of a behavior is an indication of the reinforcing value of that behavior, as high probability responses can be used to reinforce lower probability responses. In simple terms, this means that most people engage in behaviors they find reinforcing and that you can motivate people to perform a low rate, nonreinforcing behavior by scheduling a higher rate, more reinforcing behavior contingent on completing the lower rate behavior. Disequilibrium theory also uses response rates as the basis for reinforcer effectiveness, but disequilibrium theory argues that base rate alone is not sufficient to assess reinforcer efficacy; rather, constraint on access to behavior is critical for something to be reinforcing and for that to motivate behavior. In this account, any behavior can be reinforcing if that behavior is constrained to be reduced below baseline levels. Although reinforcing value is usually conceptualized in relationship to high preference behaviors that people are very motivated to engage in, the concepts of response deprivation and disequilibrium theory bring these constructs to bear on everyday behaviors, such as reading, sewing, painting, and candle making (Bernstein & Ebbesen, 1978).
Response deprivation is a condition that can increase the reinforcing value of a behavior, just as food deprivation is a condition that can increase the reinforcing value of food (H. A. Raynor & Epstein, 2003), which will be discussed later in the review. Similarly, response satiation can reduce the reinforcing value of a behavior, just as food satiation reduces the motivation to consume more food. These analogies argue that at least some of the effects of food deprivation or satiation are examples of more general principles of behavior. Research is needed to determine the commonalities and differences between food-specific effects on motivation because of food deprivation and the more general effects that may be due to response deprivation.
Factors That Influence the Reinforcing Value of Food
Relationships Between Alternatives in a Choice Paradigm: Substitutes and Complements
In a choice situation the reinforcing value of food depends on the reinforcing value of the alternatives as well as the constraints on access to food. For example, adults and children chose to work for the more-preferred food when given a choice between preferred versus less-preferred foods, but as constraints on the preferred food increased, subjects chose to work for the less-preferred food (Lappalainen & Epstein, 1990; J. A Smith & Epstein, 1991). Likewise, when provided a choice between preferred snack foods versus fruits and vegetables or pleasurable sedentary activities, subjects chose snacks but shifted their choice to the alternatives as the work needed to gain access to snacks increased (Goldfield & Epstein, 2002). Thus, increasing the behavioral cost to obtain the preferred commodity reduces responding for that commodity (increased behavioral cost for A reduces consumption of A). In addition, increasing the behavioral cost to obtain the preferred commodity can increase responding for the less-preferred commodity (increased behavioral cost for A increases consumption of B). The alternative commodity is called a substitute. The substitute may not be as reinforcing but may be chosen when the behavioral cost to obtain the preferred commodity becomes too high. For example, assume that someone regularly drinks coffee as a stimulant to wake up in the morning. The person is out of coffee that morning but has access to a supply of breakfast tea. The choice is to get dressed and go out to get coffee, which requires more work, or to drink breakfast tea, which also has caffeine, but is not as preferred, but is more accessible. The difference in the amount of work needed to switch from the usual choice to a new choice provides a way to index how substitutable are different commodities.
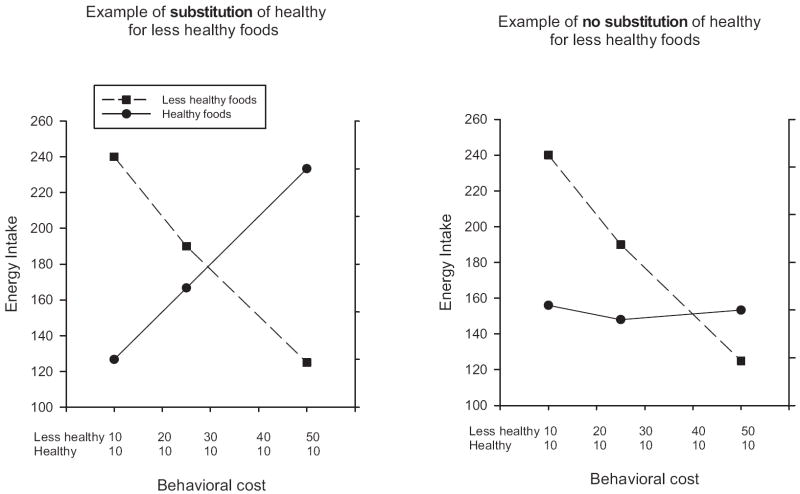
Figure 2 provides graphs of hypothetical youths who substitute or do not substitute healthy food for less healthy food (e.g., potato chips) when the behavioral cost of obtaining the less healthy food is increased and the behavioral cost of obtaining the healthier food (apples, for example) stays the same. The left graph demonstrates substitutions. Consumption of less healthy foods decreases as the behavioral cost of less healthy foods increases, whereas consumption of healthy foods concomitantly increases. In the right graph, the youths also reduce consumption of less healthy foods as behavioral cost increases but do not increase consumption of healthier alternatives. They do not substitute healthier eating as the behavioral cost of less healthy eating increases.
Figure 2.
Hypothetical examples of youths who substitute healthy foods for less healthy foods (left graph) or do not substitute healthy foods for less healthy foods (right graph) when the behavioral cost of obtaining the less healthy food is increased.
Responding for alternatives in a choice methodology can be related in another way. When responding to gain access to a behavior decreases, responding for a different, related behavior may also decrease (increased cost for A reduces consumption of B). This is evidence for a complementary relationship. For example, food intake is often associated with television watching, and reducing television watching can reduce food intake (Epstein, Roemmich, Paluch, & Raynor, 2005a). Thus, food intake can be reduced by increasing the behavioral cost to obtain the food, providing reinforcing alternatives to eating, or reducing access to variables that are reliably associated with eating.
Food Deprivation or Restriction
Recent experience with a reinforcer will alter reinforcing effectiveness. Deprivation can increase reinforcer effectiveness and the motivation to consume the reinforcer (H. A. Raynor & Epstein, 2003). This occurs after as little as 4 hours, when the person is expecting the next meal, and prefeeding prior to a meal reduces the reinforcing value of that meal (H. A. Raynor & Epstein, 2003). Reinforcer sensitivity theory (Reiss & Havercamp, 1996) argues that individual differences in reinforcement should not be tested under deprivation conditions because deprivation increases reinforcer effectiveness for everyone. Reinforcer effectiveness should rather be tested when the person is not deprived of the reinforcer but remains motivated to obtain it. This suggests that in studies of individual differences in food reinforcer effectiveness, deprivation should be limited. One way to do that is to preload, or provide food prior to beginning the experimental trials, to remove responding due to hunger to provide a better indication of the influence of reinforcement effects on responding. This is the strategy that was used in research on the influence of interaction of food reinforcement and dopamine genotypes as markers of dopaminergic activity in smokers (Epstein et al., 2004b).
In addition to the acute effects of reinforcer deprivation, there may also be long-term effects of food deprivation on reinforcer effectiveness. For example, Carr and associates have shown that food deprivation can increase bar pressing of rats to gain access to electrical stimulation in the lateral hypothalamus (Carr, 1996). This increased responding has been described as sensitization to reward effects. Sensitization is a term often used to define increases in drug reinforcement for the same drug dose over time. If repeated or chronic food deprivation can also sensitize food reinforcer effects, then repeated deprivation that occurs with very restrictive diets may increase the reinforcing value of food over time.
A construct that may be related to deprivation is dietary restraint. People who evidence dietary restraint inhibit eating in situations in which they may want to eat (Lowe, 1993). There is an important conceptual difference between deprivation and restraint in that many people with dietary restraint are not in fact reducing their energy intake (Lowe, 1993). It is also possible that dietary restraint, although not always associated with negative energy balance and weight loss, does involve intermittent periods of food deprivation followed by periods of usual eating or overeating that result in energy homeostasis and weight maintenance or gain (Lowe, 1993). Several studies have now shown that girls and adolescents who self-reported dieting are heavier later (Field et al., 2003; Stice, Cameron, Killen, Hayward, & Taylor, 1999; Stice, Presnell, Shaw, & Rohde, 2005). If dietary restraint does involve intermittent periods of brief food deprivation, or deprivation of specific foods, these girls may paradoxically be increasing the reinforcing value of these foods. There are of course a number of other potential explanations for this effect. For example, the girls who restrict energy intake may be those girls who recognize that they are gaining weight and restrict energy intake as a protective strategy, so the weight problem may be the cause rather than the result of the restriction. Research is needed to assess whether dietary restraint, characterized by not consuming as much food as one may want, involves brief periods of energy restriction, which may increase the reinforcing value of food.
The effects of dieting on craving have been studied. Craving is related to the conditioned incentive value of a reward (Robinson & Berridge, 1993). A series of studies on dieting subjects, who would be expected to be deprived of both total energy intake as well as specific foods, showed a reduction in food cravings (Harvey, Wing, & Mullen, 1993; Martin, O’Neil, & Pawlow, 2006), with greater reduction for those who eliminated more of these foods from their diet (Martin et al., 2006). One possible explanation for a reduction in cravings during dieting is based on the idea that craving is in part a conditioned response, and removing the conditioned stimuli could reduce craving. Programs that remove the opportunity for presentation of cues related to restricted foods would reduce cravings for these foods. Craving and incentive salience are theoretically distinct constructs from reinforcing value (Berridge & Robinson, 2003), and it is important to understand conditions that are associated with similar versus distinct patterns of change in these constructs.
Reinforcer Satiation
Recent experience with a reinforcer can reduce the motivation to obtain that reinforcer, which is called reinforcer satiation. This general principle applies to food as well because preloading may reduce energy intake in comparison with food deprivation (Shide, Caballerro, Reidelberger, & Rolls, 1995; Spiegel, Shrager, & Stellar, 1989). On the basis of analysis of demand curves (Bickel et al., 2000), responding to obtain a reinforcer might show an increase in responding as the session is initiated, with a reduction in rate of responding over time until reinforcer satiation, when there is reduced responding to obtain food. The observation of shifts in motivation to eat is mirrored by data on eating rate, which show a greater eating rate in the beginning of a meal followed by a reduced eating rate over time (Bellisle, Lucas, Amrani, & LeMagnen, 1984; Spiegel et al., 1989).
The shifts in response rate depend on the types and variety of food that are available, not only on cessation of responding from reduction of energy depletion. A consistent body of research has shown that animals (McSweeney, Hinson, & Cannon, 1996; McSweeney & Swindell, 1999) and humans (Epstein, Saad, et al., 2003; Temple, Kent, et al., 2006) will reduce responding if the same food is presented over time but will reinstate instrumental responding for food when a new food is presented. This suggests that reinforcer satiation does not depend only on shifts in energy stores.
It is relevant to consider that ingestive behavior researchers also use the term satiation. In eating behavior studies, satiation usually refers to the termination of eating, and the amount of food consumed is used as an objective measure of satiation. There may be situations in which the findings in experiments used to study satiation are related to changes in reinforcer effectiveness. For example, in studies in which preference for two foods is studied and the amount consumed is used as the measure of preference, shifts in responding for the two foods may involve reinforcement mechanisms. However, there are also many variables in ingestive behavior research that are not likely to be related to reinforcement. For example, shifts in energy intake as a function of energy density and food volume may be examples of dietary factors that alter food intake through mechanisms other than food reinforcement (B. J. Rolls et al., 1998; B. J. Rolls, Roe, & Meengs, 2006).
Macronutrient Composition
Although the construct of food reinforcement is often used to describe the motivation to eat, it probably is more appropriate to consider the reinforcing value of particular types of food rather than that of food in general. Someone who finds apple pie reinforcing may not work hard for broccoli. It is possible for someone to work hard for several different types of foods, and it is likely that there are particular classes of foods that the person would not be very motivated to obtain. There has been extensive research on how macronutrient composition of food may influence food intake (B. J. Rolls, 1995), and some of these effects may be related to food reinforcement. People usually select foods because they taste good, not on the basis of whether they are primarily constituted of carbohydrates, proteins, or fats (Levine, Kotz, & Gosnell, 2003). Foods that taste good are often high in sugars and/or fat (e.g., ice cream; Drewnowski & Greenwood, 1983). Obesity has been associated with a craving for foods containing mixtures of fats and sugars (Drewnowski, Krahn, Demitrack, Nairn, & Gosnell, 1992; Drewnowski, Kurth, Holden-Wiltse, & Saari, 1992).
Sugar preference may be established early in development (Desor, Maller, & Turner, 1973), as sugar can calm stressed infants (Blass & Watt, 1999; B. A. Smith & Blass, 1996), and suckling is motivated in part in infant rats by sugar (Blass, 1990). Sugar reinforcement may be primary because animals prefer sugar to water in their initial exposure (Ackerman, Albert, Shindledecker, Gayle, & Smith, 1992). Research suggests that the opioid system is in part responsible for the pleasantness of sweet taste (Ackerman et al., 1992; Bertino, Beauchamp, & Engelman, 1991) and in part for food reward in general (Kelley et al., 2002). Animals are motivated to work for sugar reward (Sclafani & Ackroff, 2003), and sugar has effects similar to some drugs of abuse, as intermittent bingeing on sucrose and drugs of abuse repeatedly increases extracellular dopamine in the nucleus accumbens shell (Rada, Avena, & Hoebel, 2005).
Dietary fats may also influence the reinforcing value of food. Infant rats respond for dietary fat (Ackerman et al., 1992), and rats prefer petroleum or mineral oil, which have no calories, as well as lard or Crisco-blended lab chow, suggesting that at least some of the effects of fat as a reinforcer may not depend on energy (Ackroff, Vigorito, & Sclafani, 1990; Hamilton, 1964). Freed and Green (1998) explored the relative reinforcing value of corn oil versus mineral oil and of sucrose or saccharin solutions versus plain water in rats. The demand and consumption for corn oil depended on the changes in response requirements (reinforcement schedule) for corn oil as well as availability of alternatives. When the behavioral cost to obtain mineral oil, sucrose, or saccharin alternatives was lower than the behavioral cost of corn oil, then the rats responded for the alternatives. However, plain water did not serve as a substitute for corn oil. These results do not suggest that there is a basic need for dietary fat that is defended as the behavioral cost increases but that dietary fat is a reinforcer that can be substituted for by other substances differing in energy and in taste.
To our knowledge, there is no research on the specific reinforcing value of dietary protein. This research may be useful in shedding light on the way in which protein influences dietary intake because dietary protein has a major influence in satiety (Vandewater & Vickers, 1996).
Food Variety
A consistent body of research has shown that people consume more energy when given a variety of food than when given the same food (H. A. Raynor & Epstein, 2001; B. J. Rolls et al., 1981), even when variety involves small differences in food characteristics, such as differently shaped pasta or different flavors of yogurt (B. J. Rolls, Rowe, & Rolls, 1982). Several studies have demonstrated that meal variety increases energy intake (H. A. Raynor & Epstein, 2001). For example, subjects receiving a four-course meal consisting of different foods in each course consumed 60% more calories than did subjects receiving the same food in each course (B. J. Rolls, van Duijvenvoorde, & Rolls, 1984). Even when the variation in the food was more subtle, such as different-flavored cream cheese in sandwiches, subjects consumed more in the variety group than in the group receiving the same-flavored sandwich (B. J. Rolls, Rolls, & Rowe, 1982). Studies have also investigated the influence of variety when all foods are served in a single course and the same phenomenon is observed, even when the total energy density presented is held constant (Pliner, Polivy, Herman, & Zakalusny, 1980; Spiegel & Stellar, 1990).
A propensity to seek out and respond to food variety may ensure a balanced nutrient intake (H. A. Raynor & Epstein, 2001). However, in the current obesiogenic environment, dietary variety may be contributing to the growing obesity epidemic (McCrory et al., 1999; H. A. Raynor & Epstein, 2001). Consumption of a variety of high energy density foods (energy density = kj/g), coupled with low intake of fruits and vegetables and a lack of physical activity, leads to maintenance of positive energy balance and, possibly, to obesity (McCrory et al., 1999; H. A. Raynor & Epstein, 2001). McCrory and colleagues (McCrory et al., 1999) reported that there was a positive relationship between body mass index (BMI) and variety of sweets, snacks, carbohydrates, entrees, and condiments consumed as well as a negative relationship between BMI and consumption of a variety of vegetables (excluding potatoes) in weight-stable adults who accurately reported food intake for 6 months. Likewise, those who lost the most weight and maintained the greatest degree of weight loss in an obesity treatment program consumed the smallest variety of high-fat foods, oils, and sweets and the largest variety of low-fat breads and vegetables (H. A. Raynor, Jeffery, Tate, & Wing, 2004).
Variety of foods also increases responding for food in comparison with presentation of one food in adults (Myers & Epstein, 2002) and children (Temple, Giacomelli, Roemmich, & Epstein, in press). Similarly, introducing a new food after responding for that food has decreased is associated with an increase in responding to obtain the new food (Epstein, Saad, et al., 2003). Introducing a variety of foods, or new foods after reinforcer satiation, may maintain the motivation to eat.
Food variety can be conceptualized more broadly than the foods that are available within a meal in terms of foods available in cafeterias, stores, or in the food supply. The increase in food variety is greater for higher energy dense snack foods than for healthier alternatives, such as fruits and vegetables (McCrory et al., 1999). A potentially important public health and policy direction might be to limit the varieties of less healthy alternatives in schools to promote healthier eating and perhaps to encourage development of new varieties of forms of healthier foods, which may provide more alternatives to people when they are making the choice of what types of foods to eat as well as potentially increasing intake of healthier alternatives.
Stimulus Control and Conditioned Reinforcing Value of Food
When a behavior is consistently reinforced in the presence of a unique stimulus, that stimulus begins to influence the rate of the behavior. This is called stimulus control. There are many examples of how environmental stimuli can influence eating. For example, when a unique environmental stimulus is reliably paired with eating, then presentation of that stimulus will initiate eating in sated rats (Weingarten, 1983). Similarly, time of day can serve to initiate eating, and humans eat at regular intervals that are defined by time rather than by nutrient depletion (Schachter, 1968).
In addition to the association of neutral external stimuli on motivated responding for food, stimulus characteristics of food, such as smell or sight of food, may alter motivated responding (E. T. Rolls, 1997). Small portions of food may prime an increase in motivated responding for food similar to low doses of drugs priming increases in drug self-administration (de Wit, 1996). The effect of priming is another example of how changes in food reinforcement cannot be explained by shifts in energy depletion, because an energy depletion model would argue that providing a priming amount of food would reduce, rather than increase, subsequent food intake.
There may be individual differences in response to stimulus control of eating that are important in the development of obesity. For example, obese children are more responsive to olfactory cues than are leaner youths (Jansen et al., 2003). Responsivity to food cues could lead to an increased motivation to eat and, thus, to positive energy balance and obesity. In addition, the construct of eating in the absence of hunger may be related to cue control of eating. In this paradigm, youths are allowed to eat to fullness and then presented with additional food. Individual differences in eating in the absence of hunger have been related to pediatric obesity (Fisher & Birch, 2002).
Because food is a primary reinforcer, activities associated with food may derive secondary reinforcing value. For example, if youths regularly eat in association with television watching, and eating is reinforcing for these youths, then television watching can become a conditioned reinforcer. This represents a powerful way in which a response class of behaviors associated with eating can become reinforcing and can motivate further behavior. The greater the association between food and activities associated with eating, the stronger the conditioned reinforcing value of these behaviors (Mazur, 1995). When youths are in situations in which they cannot eat and have to choose alternative activities, conditioned reinforcing activities that have been associated with eating may take precedence over activities that are incompatible with, and have not been paired with, eating. This may stimulate further eating when food is available. Pairing of environments with eating may represent an analogue to conditioned place preference in animal studies. Animals reliably prefer to allocate time to environments that have been associated with drug delivery (Bardo & Bevins, 2000), and conditioned place preference is used to assess motivational value of drugs (Meririnne, Kankaanpaa, & Seppala, 2001). Similarly, people who find food reinforcing may prefer to spend time in environments associated with food, which may then stimulate further eating when food is available.
Depression and the Reinforcing Value of Food
There are many psychological factors that may influence eating (Greeno & Wing, 1994; Laitinen, Ek, & Sovio, 2002; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004), and some of these may in part be mediated by changes in reinforcing value of food. It is interesting to note that basic research in both animals and humans (Willner et al., 1998) has shown that subjects find sweet foods to be more reinforcing after depressive mood induction. There are wide individual differences in the influence of depression on body weight, with some people gaining weight while depressed and others losing weight (Barefoot et al., 1998). One reliable individual difference between who may eat more or less is a history of weight fluctuation or obesity. Individuals with high levels of depressive symptoms with a higher baseline BMI show greater weight gain over time than do those with lower BMI levels (Barefoot et al., 1998). Likewise, heavier individuals show less reduction in appetite with major depression than do those who weighed less (Berlin & Lavergne, 2003). Obesity has been associated with depressive moods (Lee, Kim, Beck, Lee, & Oh, 2005). Additional research is needed to test whether depressive mood influences a variety of foods, in addition to the reinforcing value of sweet foods and whether the reinforcing value of food is increased under other psychological states that increase food intake, such as stress (Greeno & Wing, 1994; Roemmich, Wright, & Epstein, 2002).
Validity and Reliability of Food Reinforcement
Reinforcement has been an important area of research, with a long history of relevance to basic and applied behavioral science as well as an intense area of study in neuroscience (Salamone & Correa, 2002). It is interesting that the same basic paradigms can be used to study reinforcer efficacy in animals and humans (Ator & Griffiths, 2003; Griffiths et al., 2003). The majority of research on reinforcement is laboratory research to study reinforcement processes and variables that influence and mediate reinforcement. Outside of the laboratory in the natural environment, people do not respond on an operant task to gain access to food, though there are many aspects of food procurement, preparation, and consumption varying in their behavioral costs that may be related to food reinforcement. For example, foods may be stored in your home and easy to access or may have to be purchased outside of the home and are thus less accessible. Foods may be prepared or easy to eat versus in their raw form and much less accessible. A good example of this is snack food. High energy dense snack foods generally come ready to eat, whereas healthier, lower energy dense snack foods often require some preparation. Some fruits and vegetables require preparation to clean, cut, and make them into appropriate portions sizes, though some important advances in convenience are fruit and vegetable snacks such as baby carrots or prepared celery sticks. Given the differences between the laboratory and extralaboratory contexts, it is worthwhile to consider the validity of laboratory measures of food reinforcement.
There are three ways in which the validity of food reinforcement has been studied. First, the relationship between food earned and food consumed within a food reinforcement session has been studied. In laboratory measures of food reinforcement, subjects can consume the food they earned, and thus it would be expected that there would be a relationship between the amount and types of food earned and the amount of food consumed in that session. Research has shown that when the reinforcing value of food is increased, the amount of food consumed and energy intake also increases (Temple, Giacomelli, et al., in press). Second, it would be expected that subjects high in food reinforcement would consume more food and energy in a separate ad-lib eating task, which has been demonstrated (Epstein et al., in press; Epstein et al., 2004a). Third, if elevated food reinforcement is associated with increased energy intake, then it would be expected that obese persons, who are in positive energy balance because of higher energy intake than that of leaner peers, would respond more for food in laboratory reinforcement tasks than would leaner subjects. This relationship has been observed (Johnson, 1974; Saelens & Epstein, 1996).
An extension of reinforcement theory is to consider reinforcers as commodities and behavioral cost as equivalent to price by using economic principles (Staddon & Ettenger, 1989). A basic principle of economics is that increasing the price of a commodity reduces purchases, just as increasing the behavioral cost of obtaining a reinforcer reduces consumption (Bickel et al., 2000). Consistent with this notion, we have hypothesized that willingness to pay more for a commodity is related to reinforcing value of that commodity (Epstein, Dearing, Paluch, Roemmich, & Cho, in press). Additional implications of an economic analysis of reinforcement are discussed later in this review.
In many animal laboratory studies, animals are studied over repeated sessions until a steady state of responding is observed, which ensures that the effects observed in each condition are reproducible. In many human laboratory studies, particularly those in which individual differences are studied, only one measure of reinforcing value is obtained, and it is important to assess in these studies whether patterns of responding are reliable over days. To provide initial data on the test–retest reliability of reinforcing value of food, keeping the conditions of measurement constant, we retested 20 subjects who participated in a study on the interaction of food reinforcement and dopamine genotypes on eating and found a correlation of .80 between the switch point in the two sessions (Epstein et al., in press).
Food Reinforcement and Obesity
Obesity is a disorder of positive energy balance, in which energy intake exceeds energy expenditure. It is possible that obese persons are more motivated to eat than are nonobese persons, and research shows that obese persons find food more reinforcing than do nonobese persons (Johnson, 1974; Saelens & Epstein, 1996). In addition, when pleasurable activities are considered for obese and nonobese persons, eating is at the top of the list for the obese (Doell & Hawkins, 1982; Jacobs & Wagner, 1984; Westenhoefer & Pudel, 1993). Not only is the reinforcing value of food greater for obese than nonobese subjects, but craving for food may be greater, which may promote greater food seeking (Gendall, Joyce, Sullivan, & Bulik, 1998).
In addition to the influence of individual differences in food reinforcement in the development of obesity, food reinforcement may play a role in challenges associated with weight loss. One of the major problems in obesity research is the difficulty in long-term maintenance of weight loss (Jeffery et al., 2000). This may be due in part to the fact that reducing energy to produce negative energy balance and weight loss necessarily involves some degree of food deprivation, which may increase the reinforcing value of food (H. A. Raynor & Epstein, 2003) and energy intake (Telch & Agras, 1996). This creates a tension for obese persons, because the longer they are in negative energy balance and the more weight they lose, the more food reinforcement may increase and the more motivated they may be to eat.
The energy depletion model of eating suggests that people eat because they are hungry, and eating should reduce hunger and thus the amount of food subsequently consumed. Preloads should reduce eating in a meal, and this pattern is often shown in nonobese, nondieting subjects (Spiegel et al., 1989). Obese subjects, however, often increase eating and energy intake after a preload or a first-course appetizer (Jansen et al., 2003; Spiegel et al., 1989). This effect may be due in part to the fact that obese individuals often restrict energy intake, and it is the food restriction that enhances the effect of the preload (Lowe, Foster, Kerzhnerman, Swain, & Wadden, 2001; McCann, Perri, Nezu, & Lowe, 1992). The observation that consumption of a preload or a small amount of food increases energy intake in a person who is food deprived may be a similar phenomenon to priming, in which presenting a small amount of a drug increases drug self-administration (de Wit, 1996). Priming effects do not necessarily depend on food deprivation or restriction, as priming subjects with a palatable food can increase eating even in satiated subjects (Cornell, Rodin, & Weingarten, 1989).
Dopamine and the Neurobiology of Food Reinforcement
Given the importance of food for survival, it is not surprising that there are multiple pathways and physiological systems working to ensure that adequate energy intake is achieved. The regulation of food intake or appetite has both central and peripheral influences (Ahima & Osei, 2001; Schwartz, Woods, Porte, Seeley, & Baskin, 2000) and is responsive to sensory information related to the sight and smell of food, previous feeding experiences, satiety signals elicited by ingestion, and hormonal signals related to energy balance (Ahima & Osei, 2001; Schwartz et al., 2000). Imaging studies show a comprehensive neuronal network controlling human feeding behavior that includes parts of the cortex, hypothalamus, thalamus, and the limbic system (Gautier et al., 2000; Tataranni et al., 1999). This network connects the regions of the brain that control the perception of sensations and flavors (the cortex); that influence the reinforcing value of food (the limbic system); and that influence appetite, body weight, and energy balance (the thalamus and hypothalamus). The goal of this section is not to provide a complete review of the neurobiology of food reinforcement but to focus on one factor that may be related to food reinforcement, dopaminergic activity, and illustrate how dopaminergic activity may be related to factors that influence food reinforcement.
Dopamine is generally considered to be a primary neurotransmitter involved in food reinforcement (Wise, 2006), though the mechanisms for this effect are debated (Salamone & Correa, 2002). Dopaminergic neurons in the substantia nigra and ventral tegmental area project to the caudate putamen and nucleus accumbens, where they modulate movement and reward (Koob, 1999; Schultz, 1998). Other projections from the nucleus accumbens to the lateral hypothalamus in part regulate feeding (Maldonado-Irizarry, Swanson, & Kelley, 1995). The process whereby food is or becomes reinforcing depends, in part, on the release, transport (reuptake), and receptor binding of synaptic dopamine (Wise, 2006). The levels of extracellular dopamine in the brain depend on two processes: tonic dopamine release (Schultz, 1998; Stricker & Zigmond, 1984) and dopamine release in response to a stimulus (phasic dopamine release; Grace, 2000).
There are a number of ways in which understanding the neurobiology of eating may elucidate mechanisms for some of the behavioral phenomena that have been discussed. Eating elicits dopamine release, which may be related to appetitive aspects of food seeking (Berridge, 1996). Food deprivation reduces messenger RNA for the dopamine transporter and reduces extracellular dopamine (Patterson et al., 1998; Pothos, Hernandez, & Hoebel, 1995), which may stimulate the increase in reinforcing value of food after food deprivation. The dopamine release observed with consuming food and then with stimuli associated with food may be in part the mechanism for two behavioral constructs discussed in the previous section: stimulus control of eating and conditioned reinforcement value for stimuli associated with eating. It is possible that conditioned increases in dopaminergic activity that are elicited by stimuli associated with eating play a role in craving for food (Berridge, 1996). Initially, dopamine is released when food is consumed (Hoebel, Hernandez, Schwartz, Mark, & Hunter, 1989), and its activity in the nucleus accumbens may relate not only to consumption but also to rewarding properties of food (Hernandez & Hoebel, 1988). Over repeated pairings of an environmental stimulus with eating, dopamine is released after presentation of a cue paired with food in anticipation of eating. Dopamine then binds to dopamine D2 receptors in the nucleus accumbens, perhaps initiating food-seeking behaviors (Kiyatkin & Gratton, 1994). Positron emission tomography studies in humans have shown that brain dopamine increases both in anticipation of and during the ingestion of food (Small, Jones-Gotman, & Dagher, 2003; Volkow et al., 2003). The influence of food variety on increased motivation to eat may also be regulated in part by dopamine. Research suggests that brain dopamine activity increases in response to novel stimuli (Salamone, Correa, Mingote, & Weber, 2005), which could elicit the motivation to consume new foods and increase energy intake when a variety of foods rather than just one food is available.
An interesting aspect of research on behavioral choice and food reinforcement is that dopamine may be involved in the demand elasticity for food (Salamone & Correa, 2002). Salamone (Salamone & Correa, 2002) has used a behavioral choice paradigm in which rats can choose to work for very palatable food or have free access to chow. The rats will normally choose to engage in work to gain access to favorite foods, but dopamine depletion shifts choice to the freely available chow. In many conditions, depletions will have a greater effect on higher ratio schedules. This has led Salamone to conclude that dopamine is involved in the amount of instrumental behavior that is performed to gain access to food rewards (Salamone & Correa, 2002). Thus, relative reinforcing value and behavioral choice paradigms may provide unique information about how dopamine influences behavior that cannot be determined by studying reinforcing value with single schedules of reinforcement in the absence of choice.
Obese Versus Lean: Altered Dopaminergic Activity
Differences in neuronal activity in response to food and satiation in several brain regions of humans have been identified in obese female subjects compared with lean female subjects (Karhunen, Lappalainen, Vanninen, Kuikka, & Uusitupa, 1997) and male subjects (Gautier et al., 2000). For example, obese subjects have significantly higher metabolic activity in the parietal somatosensory cortex, where sensation to the mouth, lips, and tongue is located (Wang et al., 2002). Enhanced activity in the cortical regions involved with sensory processing of food in the obese could make them more sensitive to the reinforcing properties of food related to palatability and could be one of the variables contributing to their excess food consumption.
There is an extensive animal literature that supports the relationship between obesity and altered dopamine metabolism. Obese rats release more dopamine during eating than do their lean counterparts (Yang & Meguid, 1995). Dopamine may also inhibit feeding through its influence on other central mediators of appetite. In the hyperphagic obese leptin-deficient mouse model, increasing central dopaminergic tone with dopamine agonists eliminates the hyperphagia via inhibition of Neuropeptide Y, one of the most potent orexigenic (appetite stimulating) agents known (Bina & Cincotta, 2000). Central dopamine activity may also be involved in the appetite suppression associated with Interleukin-1 alpha-associated anorexia (Yang et al., 1999) and with the central effects of cholecystokinin (Chung et al., 1994).
Deficiencies in dopaminergic receptors may also play a role in the development of obesity. For example, obese Zucker rats have fewer dopamine D2 receptors and reduced hypothalamic dopamine activity when fasting but release more dopamine when eating and do not stop eating in response to the peripheral administration of insulin and glucose when compared with the same in lean Zucker rats (Orosco, Rouch, & Nicolaidis, 1996). Obese male Sprague-Dawley rats have reduced dopamine turnover in the hypothalamus compared with that of the diet-resistant strain before they become obese (Levin & Dunn-Meynell, 1997), and develop the obese phenotype only when given access to a palatable high energy diet (Levin & Dunn-Meynell, 2002). Dopamine D2 receptor blockade causes obese but not lean rats to overeat (Fetissov, Meguid, Sato, & Zhang, 2002; Orosco, Gerozissis, Rouch, Meile, & Nicolaidis, 1995). Because a chronic change in receptor expression is a prerequisite for behavioral sensitization (Kuczenski & Segal, 1999), these data suggest that the blockage of an already low D2 receptor level may sensitize obese rats to food.
Common Neurobiological Substrates for Obesity and Drug Addiction
Recent research suggests that common neurobiological substrates underlie obesity and drug addiction (Volkow & Wise, 2005). Similar to drug use, ingestive behavior may be unrelated to homeostatic need, that is, driven not by the need to maintain energy balance but by cognitive and environmental factors that are not compensated for by the body’s internal feedback system to maintain stable body weight (Berthoud, 2004), and individual differences in the responsiveness of brain dopamine to this “nonregulatory ingestive behavior” have been identified in animals (Mittleman, Castaneda, Robinson, & Valenstein, 1986) and humans (B. J. Rolls, 2003). There are other parallels between hyperphagia and drug addiction. One interesting phenomenon that may suggest common pathways for food consumption and drug use is that chronic food restriction increases food reinforcement (H. A. Raynor & Epstein, 2003) and also increases the self-administration and motor-activating effects of abused drugs (Carr, 2002; Carroll & Meisch, 1984; Pothos, Creese, & Hoebel, 1995), along with improving dopamine receptor function (Wilson, Nomikos, Collu, & Fibiger, 1995). In addition, an animal’s level of sucrose preference can predict its desire to self-administer cocaine (Levine et al., 2003), and sweets will reduce cocaine’s reinforcing value (Comer, Lac, Wyvell, & Carroll, 1996), suggesting a relation between sweet taste and drug reward. Both obese rats and chronic drug users have low basal dopamine levels (Hamdi, Porter, & Prasad, 1992), experience periodic exaggerated dopamine release associated with either food (Fetissov et al., 2002) or drug intake (Worsley et al., 2000), and have reduced dopamine D2 receptor and increased D1 receptor expression (Fetissov et al., 2002). A number of addictive behaviors (alcoholism; cocaine, heroin, marijuana, and nicotine use; and glucose bingeing) have been associated with low expression or dysfunction of D2 receptors (Comings & Blum, 2000). Thus, reduced expression of D2 receptors may represent a common pathway in obesity and drug addiction that elicits behavior to release dopamine into areas of the brain that “crave” dopamine.
There are many similarities between food and drugs; there also may be important differences (Bassareo et al., 2003; Kelley & Berridge, 2002; Parkinson, Fudge, Hurd, Pennartz, & Peoples, 2000; Salamone & Correa, 2002). For example, whereas food and drugs of abuse both elicit dopamine release in the shell of the nucleus accumbens, habituation (reduced response with repeated exposure) occurs with food (specific to the food ingested) that is responsive to the motivational state of the animal (i.e., food restricted or not; Bassareo & Di Chiara, 1999). Such habituation is not always seen with drug use (Bassareo et al., 2003). Research is needed to examine the similarities and differences between eating and drug self-administration to understand how best to apply research from drug addiction to eating and obesity.
This brief overview of dopamine and food reinforcement focuses on dopamine as an example of how a more basic understanding of the neurobiology of reinforcement may provide a better understanding of mechanisms that influence food reinforcement. Although understanding the role of dopamine in reinforcement is a major issue in contemporary neuroscience (Salamone & Correa, 2002), new research may provide a better understanding of the role of dopamine in food reinforcement (Salamone & Correa, 2002) and may identify other neurobiological variables that influence food reinforcement in addition to, or interacting with, dopamine.
Behavioral Genetics of Food Reinforcement
If dopamine is involved in food reinforcement, then individual differences in dopaminergic activity that are related to dopamine genotypes may predict factors related to dopaminergic activity, food reinforcement, and obesity. Most of the focus on dopamine and eating involves the D2 receptor and the DRD2 gene. Both the D1 and D2 dopamine receptors act synergistically to inhibit feeding (Terry, Gilbert, & Cooper, 1995). It is the DRD2 gene, however, that appears to function primarily as a reinforcement or reward gene (Noble, 2003) having been associated with feeding and addictive behaviors (Baptista, 1999).
The DRD2 gene has three allelic variants (A1/A1, A1/A2, and A2/A2). Individuals with genotypes containing either one or two copies of the A1 allele have fewer D2 receptors than have those lacking an A1 allele and so are associated with reduced brain dopamine signaling (Jonsson et al., 1999; Pohjalainen et al., 1998; Ritchie & Noble, 2003). In genetically homogeneous populations, such as the Pima Indians, a missense substitution in the DRD2 gene has been associated with reduced resting energy expenditure (Tataranni et al., 2001), greater BMI, and type II diabetes (Jenkinson et al., 2000). Epidemiological studies in genetically heterogeneous human populations have demonstrated a higher prevalence of the A1 DRD2 allele in obese individuals (~50%) compared with lean individuals (~30%; Blum et al., 1996; Comings et al., 1993; Comings, Gade, MacMurray, Muhleman, & Peters, 1996; Noble et al., 1994). Low D2 receptor availability in the presence of the DRD2 A1 allele likely makes subjects less sensitive to stimulation of dopamine-regulated reward circuits (Blum et al., 2000), and so they may seek powerful reinforcers to overcome their “dopamine deficit,” increasing the risk not only for obesity but also for other related addictive behaviors (Blum et al., 1996). The DRD2 A1 allele may reduce the efficiency of the dopaminergic system, rewarding behaviors that increase brain dopamine levels (Volkow, Fowler, & Wang, 2002), such as food ingestion. Consistent with this hypothesis, Wang et al. (2001) have shown that D2 receptors are reduced in the striatum of the brains of very obese (BMI > 40) humans in proportion to their BMI. They argued that persons with fewer D2 receptors seek powerful reinforcers to overcome their “dopamine deficit,” increasing the risk of those with fewer receptors for obesity as well as for drug abuse (Wang et al., 2001). They and others (Baumann et al., 2000) have suggested that raising brain dopamine levels may be a treatment for obesity.
The SLC6A3 gene codes for the dopamine transporter (DAT-1), a protein that controls the levels of dopamine in the synapse by rapidly carrying the neurotransmitter back into nerve terminals after its release, limiting the level and duration of dopamine receptor activation (Bannon et al., 1992). Synaptic dopamine increases when, for example, cocaine inhibits the reuptake of dopamine by binding to the DAT-1 or when the dopamine transporters have been eliminated by knockout of the SLC6A3 gene (Leshner, 1996). The majority of studies examining polymorphisms of the SLC6A3 gene have focused on the variable number of tandem repeats at the 3′UTR of the gene with reported evidence for association with the 10R allele (Need, Ahmadi, Spector, & Goldstein, 2006). This allele may be associated with increased dopamine transporter density (Kirley et al., 2002) and transport (Swanson et al., 2000) and therefore to lower levels of postsynaptic dopamine (Heinz et al., 2000) when compared with the 9R allele, although this is not consistent across all studies (Jacobsen et al., 2000; Martinez et al., 2001). Recent data suggest that DAT-1 may have a role in the development or perpetuation of obesity. Elevated levels of DAT-1 have been found in obese rats (Figlewicz et al., 1998), and food deprivation (Patterson et al., 1998), food restriction (Bello, Sweigart, Lakoski, Norgren, & Hajnal, 2003), and the administration of insulin (Figlewicz, Szot, Chavez, Woods, & Veith, 1994) to the brains of rats increases DAT-1 messenger RNA and alters DAT-1 function (Baskin et al., 1999; Figlewicz, 2003a, 2003b). The up-regulation of DAT-1 during food restriction in rats is similar to the neuroadaptation in the mesolimbic dopamine circuitry resulting from drugs of abuse and is associated with increased intake of sucrose, a palatable food (Bello et al., 2003). This mechanism may have relevance to pathological human feeding behaviors such as restrictive eating disorders, bingeing, and hyperphagia.
One application of the knowledge about genotypes related to dopaminergic activity would be to use the genotype as a marker of neurotransmitter activity. For example, if the 10R allele of the SLC6A3 gene is associated with higher density of DAT-1 protein, then individuals with this allele would presumably have less dopamine available in the synapse. Similarly, if the A1 allele of the dopamine D2 receptor gene is related to fewer D2 receptors, then individuals with this allele would have fewer D2 receptors and lower dopaminergic activity. Therefore, these genotypes may be a marker of dopamine neurotransmission. This provides for the opportunity to indirectly assess relationships between dopaminergic activity and eating. Currently, we are not able to assess dynamic changes in dopaminergic activity in human brains. Some ways to study the neurobiology of eating include using positron emission tomography scans to examine dopamine receptor binding (Elsinga, Hatano, & Ishiwata, 2006), functional magnetic resonance imaging studies to examine regional blood flow and activation of specific brain regions (Holsen et al., 2005), and postmortem brain analyses to examine dopamine receptor density (Piggott et al., 1999). The use of genotypes as markers of dopaminergic activity may provide a useful tool to better understand how dopaminergic activity is related to eating in behaviorally active contexts.
As an example of this strategy, we used the DRD2 and SLC6A3 genotypes as markers of dopaminergic activity in combination with a food reinforcement phenotype that has been examined in smokers. The food reinforcement phenotype was defined by using a relative reinforcing value questionnaire that asked how hard subjects would work for food or for money. Food reinforcement had a main effect on energy intake as those high in food reinforcement consumed more food than did those low in food reinforcement. High food reinforcement interacted with both the SLC6A3 10R/10R and the DRD2 Taq 1 A1 genotypes (A1/A1 + A1/A2) to increase energy intake beyond high food reinforcement with other genotypes (Epstein et al., 2004b). This study suggests there is an interaction of dopamine genotypes with food reinforcement that influences energy intake. The genotypes provide a framework for how two aspects of dopaminergic activity, the number of dopamine D2 receptors and the density of dopamine transporters, may relate to food reinforcement, energy intake, and obesity.
A behavioral genetic approach to eating may provide insights into individual differences that affect the substitution of alternatives to food. There may be genetic differences that facilitate choice of nonfood alternatives when a person has the choice to eat or engage in alternative behaviors. Likewise, there may be individual differences in how motivating are different types of reinforcers, ranging from food to pharmacological reinforcers to physical activity. The expression of a behavioral phenotype, such as high food reinforcement or response to food deprivation, may result from differences in gene expression based on the interaction of genes with biological, nutritional, or behavioral factors (Martin-Gronert & Ozanne, 2005). For example, in both animals (de Fourmestraux et al., 2004) and humans (Pietilainen et al., 2004) with identical genes there are differences in phenotypes that result from interactions between the genes and the environment. Epigenetic effects on dopamine gene expression may provide insight into how dopamine genotypes interact with experiences that shape food reinforcement to influence excess energy intake and obesity.
There are many ways in which genetic predispositions may interact with behavioral and environmental factors to influence food reinforcement. The above discussion is just a beginning in the attempt to understand how genes may influence food reinforcement. Studying different genotypes and developing new ways to study how genetics can influence behavior may yield important insights into food reinforcement.
Behavioral and Pharmacological Methods to Modify Food Reinforcement and Obesity
Behavioral Methods
There are several approaches that may be relevant for improving obesity treatment that are based on factors that influence food reinforcement. Obesity treatment generally involves decreasing food, which obese persons find very reinforcing (Saelens & Epstein, 1996), and increasing physical activity, which is not very reinforcing for obese persons (Epstein, Smith, Vara, & Rodefer, 1991). One option would be to identify nonfood alternatives that maintain the overall level of perceived positive reinforcement but shift the source of reinforcers from food to nonfood alternatives. This approach has proven to be very successful for drug abuse (Higgins, Alessi, & Dantona, 2002) and may be generalized to obesity. In drug abuse treatment, former addicts are reinforced for abstinence by using nondrug reinforcers. Laboratory research has shown that nonfood alternatives can substitute for highly reinforcing foods (Goldfield & Epstein, 2002), but clinical and field research has not been successful in developing programs that enhance eating change or weight loss by using nonfood alternatives (Epstein, Roemmich, Stein, Paluch, & Kilanowski, 2005).
Variety increases energy intake and the reinforcing value of food. It may be advisable for people who are attempting to lose weight to reduce the variety of high energy dense, low nutrient dense foods they store in their homes and eat (H. A. Raynor, Jeffery, Phelan, Hill, & Wing, 2005; H. A. Raynor et al., 2004). The ensuing reduction in the reinforcing value of food could result in a decrease in the motivation to eat and lower energy intake. Food craving and stimulus control of eating become conditioned to cues associated with eating (Jansen et al., 2003; Weingarten, 1983). People who are reducing energy intake should limit the places in which they eat, to narrow stimulus control to the dining room for example, and should not engage in alternative behaviors when eating, to reduce the possibility that other behaviors set the occasion for eating.
Pharmacological Treatments to Increase Brain Dopamine Levels in the Treatment of Obesity
If the desire to consume more food and high levels of food reinforcement is based in part on low levels of brain dopamine, then drugs that increase brain dopamine may reduce food reinforcement and reduce energy intake. One way to increase brain dopamine is to inhibit dopamine reuptake. Methylphenidate, a DAT inhibitor used in the treatment of childhood and adult attention-deficit/hyperactivity disorder (Wilens, Biederman, Spencer, & Prince, 1995), increases brain synaptic dopamine (Volkow et al., 2001). A commonly reported side effect of methylphenidate is anorexia (Spencer et al., 1995). Chronic administration of methylphenidate reduces the responsiveness to drugs of abuse (Carlezon, Mague, & Andersen, 2003) and natural rewards such as sucrose (Comings & Blum, 2000). Weight loss has been reported in children given methylphenidate with largest reductions for the heaviest children (Schertz, Adesman, Alfieri, & Bienkowski, 1996). In adults, methylphenidate significantly reduced energy intake over one meal by 33% in obese male subjects compared with placebo (Leddy et al., 2004). In combination with diet and exercise counseling, buproprion, another DAT inhibitor, was effective in reducing weight in obese men and women over 26 weeks (Jain et al., 2002) to 48 weeks (Anderson et al., 2002) when compared with placebo.
Another way to enhance central dopamine function is to use a dopamine receptor agonist. In animal experiments, bromocriptine, a dopamine D2 receptor agonist, reduced hyperinsulinemia and body fat stores (Cincotta, MacEachern, & Meier, 1993), purportedly by resetting hypothalamic circadian rhythms under dopaminergic control (that are altered in obesity; Cincotta et al., 1993), and other dopamine agonists such as mesulergine will reduce food intake in rats (Giannakopoulos, Galanopoulou, Daifotis, & Couvaris, 1998). In obese humans, bromocriptine improved fasting and postprandial glucose, free fatty acid, and triglyceride levels (Kamath et al., 1997); reduced body fat stores (Meier, Cincotta, & Lovell, 1992); and improved glycemic control and glucose tolerance in type II diabetics (Meier et al., 1992). In overweight and obese patients with prolactinomas, increasing dopaminergic tone with bromocriptine significantly reduced serum leptin levels and BMI over 6–24 months, without a diet or exercise prescription (Doknic et al., 2002).
Longer term studies will have to evaluate whether more sustained changes in brain dopamine activity are effective and safe because in animal studies the chronic administration of amphetamines (which raise brain dopamine levels) induces a brief period of anorexia followed by hyperphagia and chronic obesity, accompanied by long-term depletion of dopamine in the striatum and of norepinephrine in the hypothalamus (Hernandez, Parada, & Hoebel, 1983). An additional consideration in using pharmacological agents that modify brain dopamine levels is the abuse potential for some of these drugs. The reinforcing and subjective effects of methylphenidate are similar to amphetamine in non-drug-abusing humans (Rush, Essman, Simpson, & Baker, 2001). In addition, there is evidence that some adolescents use methylphenidate for the psychoactive effects (Musser et al., 1998). Other dopamine agonists such as bromocriptine, a specific D2 receptor agonist, may have less abuse potential, but also may not be as effective in reducing eating (Inoue et al., 1997). If there are common pathways that influence eating and other behaviors (Kelley & Berridge, 2002), an alternative to the use of pharmacological strategies is to identify nonpharmacological agents that increase brain dopamine levels, such as exercise (Ingram, 2000) or other natural rewards (Kelley & Berridge, 2002).
Combining Behavioral Interventions Plus Pharmacological Manipulations of Dopamine
There are innovative studies that have combined behavioral and pharmacological interventions (Wadden, Berkowitz, Sarwer, Prus-Wisniewski, & Steinberg, 2001; Wadden et al., 2005), but these approaches have not focused the behavioral and pharmacological interventions to alter the reinforcing value of food or the motivation to eat. Novel treatments for obesity could target increasing the motivation for low energy dense, high nutrient dense foods or for alternative reinforcers to energy dense foods by pairing these stimuli with dopamine agonists to increase their motivational salience. Such an approach might motivate people to sustain behavior change to maintain reduced weight over the long term. Similarly, behavioral interventions could be designed to focus on enhancing the changes in motivation to eat specific foods that could result from pharmacological interventions. For example, it may be easier to build a repertoire of alternatives to food if the reinforcing value of food is reduced by pharmacological intervention.
Fulton, Woodside, and Shizgal (2000) provided experimental evidence that it may be possible simultaneously to reduce the reinforcing value of food and increase the reinforcing value of nonfood alternatives. By using an animal model, Fulton and colleagues showed that sensitivity to electrical stimulation in the lateral hypothalamus was reduced by leptin, as indicated by a reduction in the rate of responding for lateral hypothalamic stimulation, whereas leptin increased sensitivity for responding to obtain electrical stimulation in adjacent, and potentially non-food-reinforcement-related, areas. As noted by Fulton, the ideal treatment would be one that reduced the motivation to obtain food while increasing the motivation for non-food-related behaviors.
The effectiveness of behaviorally or pharmacologically altering brain dopamine activity may depend on individual dopaminergic tone during specific contexts (Volkow, Wang, et al., 2002). Methylphenidate’s effects are context dependent: It amplifies stimuli-induced dopamine increases when administered with a salient stimulus (for example, visual display of food in food-deprived subjects) when compared with a neutral stimulus (Volkow, Wang et al., 2002) and enhances the motivational saliency of a previously unmotivating or unexciting task (Volkow et al., 2004). A very exciting opportunity would be to associate behaviors with dopamine increases with the goal of increasing the motivation to engage in these behaviors.
Energy Balance and Activity Reinforcement
Obesity is a result of a positive energy balance, with energy intake exceeding energy expenditure. A complete analysis of how reinforcement processes may relate to obesity requires considering the role of energy expenditure and physical activity in the energy balance equation. There is a considerable body of research on reinforcing value of physical activity (reviewed in Epstein & Saelens, 2000). For example, given the choice of sedentary or active alternatives, obese youths find physical activity less reinforcing than do leaner youths (Epstein et al., 1991). For youths, increasing the behavioral cost of gaining access to sedentary behaviors results in a reduction in sedentary behaviors and a switch to being more active (Epstein, Saelens, Myers, & Vito, 1997; Epstein, Saelens, & O’Brien, 1995; Epstein et al., 1991) and for adults (D. A. Raynor, Coleman, & Epstein, 1998; Vara & Epstein, 1993). For example, if youths have a choice between equally accessible sedentary or active alternatives, and being sedentary is more reinforcing, they will choose to be sedentary. However if access to specific sedentary behaviors is reduced, then the youth has to choose how to reallocate the increased availability of leisure time.
Substitution of physical activity for sedentary behaviors is observed when reduction in access to one sedentary behavior results in increases in physically active behaviors. For example, moving a counsel video game system to an unheated, unfinished basement may reduce video game playing while increasing physical activity in the sunlight. In this case, physical activity is a substitute for playing video games. Similarly, inclement weather may reduce running or bicycling outside yet increase treadmill running or using a cycle ergometer or bicycle trainer indoors. Being active can substitute for sedentary behaviors in the laboratory (Epstein et al., 1995, 1991; D. A. Raynor et al., 1998), and even very small differences in accessibility of sedentary behaviors can have marked effects on physical activity (D. A. Raynor et al., 1998). Not all youths substitute being active for being sedentary (Epstein, Roemmich, Paluch, & Raynor, 2005b), so understanding the mechanisms for substitution has great theoretical importance for determining individual differences in substitution of active behaviors for sedentary behaviors. Substitution of physical activity for sedentary behaviors depends in part on individual differences such as child gender and child weight status (Epstein, Roemmich, Paluch, & Raynor, 2005b). In addition, the macro environment influences the substitution of physically active behaviors for sedentary behaviors (Epstein, Roemmich, Paluch, & Raynor, 2005b).
Dopamine is also involved in physical activity (Ingram, 2000). Dopamine agonists increase (Scislowski et al., 1999), whereas dopamine antagonists reduce (Fujiwara, 1992) physical activity. Disorders that involve destruction of dopamine neurons are associated with substantial reductions in physical activity in humans (Comings et al., 1991), whereas exercise can increase dopamine levels (de Castro & Duncan, 1985). Dopamine genotypes may be related to differences in activity levels (Perusse, Tremblay, Leblanc, & Bouchard, 1989). The absence of DRD2 produces profound bradykinesia and hypothermia in mice (Baik et al., 1995). In humans, a mutation of the DRD2 gene that impairs D2 receptor function is associated with lower total and 24-hour resting energy expenditure and greater BMI in Pima Indians (Tataranni et al., 2001). Polymorphisms of the DRD2 gene have been associated with reported yearly physical activity levels in white female subjects (Simonen et al., 2003). Given that patterns of dopamine release can change in association with familiarity or repeated exposure (e.g., training) as well as with individual experience (Schultz, 2002; Wise, 2002), increasing physical activity in the obese may improve central dopamine function and serve to reinforce liking of regular physical activity (Meeusen et al., 1997). As with the study of eating, using dopamine genotypes as markers for dopaminergic activity may provide insight into how dopamine is related to physical activity.
Alternative Theoretical Approaches to Food Reinforcement
There are a wide variety of alternative explanations beyond food reinforcement as to why people eat. Taste, culture, class, and experience can influence eating, but a thorough review of these factors is not central to understanding the role of food reinforcement and is beyond the scope of this article. There are two alternative theoretical models, however, that are also designed to understand how food motivates food-seeking behavior and eating and should be considered in a discussion of food reinforcement. These models will be briefly described.
Incentive Salience Models of Ingestive Behavior
Incentive salience models of motivated behavior such as eating or drug use separate the process of reward into two components: wanting and liking (Berridge & Robinson, 1998). Liking refers to the psychological processes that produce pleasure, hedonics, or liking of food. Liking thus refers to subjective characteristics associated with food. Wanting refers to the psychological process that instigates goal-directed behavior, attraction to an incentive stimulus, and consumption of the goal object. Wanting is characterized by the process often referred to as craving, or the motivation to obtain food even when it is not available (Berridge, 1996). The separation of wanting and liking is important to distinguish factors that motivate people to eat.
One important aspect of incentive salience theory is developing a better understanding of the neurobiological substrates for liking and wanting. These processes can be distinguished behaviorally and may be influenced by different neurobiological systems, with the opiate system being more important for liking and the dopaminergic system more important for wanting (Robinson & Berridge, 2000). Conditioned, or cue-triggered, wanting is measured by using conditioned incentive paradigms (Wyvell & Berridge, 2000). In an interesting discussion of ways to conceptualize reward, Berridge and Robinson (2003) differentiated incentive salience models from instrumental conditioning models. They argued that reward includes learning, affect, or emotion, as well as motivation components. In their model, instrumental conditioning is an example of learning, whereas incentive salience is an example of motivation. They recognize that dopamine can change instrumental performance but does not influence liking (Berridge & Robinson, 2003). They also indicated that incentive salience does not mediate the influence of the reinforcement contingency in reinforcement. Berridge (2004) has followed that article with a perspective on motivation concepts in neuroscience that places incentive salience within the history of motivation. Salamone and Correa (2002) have argued that wanting can be divided into the “appetite to consume” and the “working to obtain,” which serves as the basis for his contention that dopamine depletions, and by analogy dopaminergic activity, primarily influence instrumental performance to obtain reinforcers. As noted previously, Salamone has used choice experiments to elucidate the influence of dopamine depletions on instrumental performance, which may lead to a greater appreciation of behavioral choice approaches for studying motivation and reinforcement (Salamone & Correa, 2002). Given the contemporary importance of incentive salience theory, research may sharpen the ways in which wanting and reinforcing value are related, extending the theorizing of Salamone (Salamone & Correa, 2002).
The distinction between wanting and liking also may be relevant for differences between liking and the reinforcing value of food. The motivation to obtain food may be a more powerful determinant of eating than is liking or hedonic value of food. Not surprisingly, the reinforcing value and liking value of food may be independent processes, and increasing the reinforcing value of food in humans via food deprivation may not alter the liking of selected foods (Epstein, Truesdale, Wojcik, Paluch, & Raynor, 2003). In addition, the reinforcing value of food is a better predictor of food intake than are food hedonics in adult smokers (Epstein et al., 2004a).
Habituation Theory and Food Reinforcement
Habituation refers to a decrease in responding after repeated presentations of a stimulus. The application of habituation theory to ingestive behavior has been consistently supported by research showing that a variety of responses related to ingestive behaviors, and eating itself, are reduced with repeated presentation of visual, olfactory, or gustatory cues (Epstein, Rodefer, Wisniewski, & Caggiula, 1992; Epstein, Saad, Giacomelli, & Roemmich, 2005; Epstein, Saad, et al., 2003; Wisniewski, Epstein, & Caggiula, 1992; Wisniewski, Epstein, Marcus, & Kaye, 1997). Paradigms that test habituation assess whether the reductions are stimulus specific, whether response strength can be reinstated by presenting a new stimulus, and whether the reductions can be prevented by altering stimuli during presentations of repeated food cues (Epstein et al., 1992; Epstein, Saad, et al., 2005; Epstein, Saad, et al., 2003; Wisniewski et al., 1992, 1997). Increased intake with food variety may be due in part to dishabituation or the failure to habituate when a variety of foods is presented (H. A. Raynor & Epstein, 2001).
Theoretical advances by McSweeney and colleagues (McSweeney et al., 1996; McSweeney & Roll, 1998; McSweeney & Swindell, 1999) have shown that instrumental behavior can also habituate. For example, responses engaged in to obtain food usually are high in the beginning of an experimental session but decrease within the session. Providing a new food reinstates responding, consistent with a habituation model of instrumental behavior (Myers & Epstein, 2002) and inconsistent with energy depletion or homeostatic models of eating. The usual mechanism thought to inhibit further eating is satiation, which McSweeney argued cannot provide a complete explanation of why people stop eating (McSweeney & Roll, 1998).
Research Gaps and Ideas for Research
There are many gaps in research on food reinforcement. Ideas for research have been presented throughout this review, but several areas for future research on food reinforcement are highlighted.
Naturalistic Analogues to Laboratory Measures of Food Reinforcement
Laboratory methods for assessing food reinforcement provide a flexible, objective way to assess food reinforcement. The construct of reinforcement may not be limited to laboratory measures but may be readily generalized to a variety of naturalistic measures. There are natural differences in access to foods based on food purchases and storage, convenience, and amount of preparation required. The reinforcing value of a processed, portion-controlled, and prepared high energy dense snack food may be more reinforcing than that of a package of raw vegetables that requires washing, cutting up, and creating servings (consider a bag of potato chips versus a bag of whole carrots), but the situation may be very different if you compare a bag of potato chips versus a portion-controlled package of baby carrots served with a nonfat ranch dressing. Similarly, a full-fat hamburger may be preferred when compared with a turkey burger, but what about the comparison of a cooked turkey burger on a bun with appropriate condiments versus a frozen hamburger that has to be cooked?
Reinforcer efficacy in the natural environment can be quantified by using token economies, or systems in which points or tokens are earned and then exchanged for reinforcers. It would be possible to vary the amount of work required to earn tokens, and stronger reinforcers should support more work. The amount of work can be conceptualized in different ways. For example, if the basic unit of work was conceptualized as an office task, such as folding letters, or piece work, such as sewing a garment, then work could be conceptualized as the number of units of work completed. It is also possible to conceptualize the amount of work in relationship to the difficulty of the task, with the hypothesis that more reinforcing alternatives would support effort for more challenging tasks. Work can also be defined in terms of the amount of physical work completed, such that more reinforcing alternatives would support more work as defined by watts on a calibrated bicycle ergometer.
An analogue for response effort may be price, and behavioral cost to obtain a reinforcer may influence response rates similarly to how price influences purchases of a commodity. For example, as price increases, purchases of a product decrease, similar to the relationship between increases in behavioral cost and decreases in response rate and consumption. Likewise, increasing purchases of a product when the price of that product stays the same but the price of an alternative product increases is evidence of substitution (or cross–price elasticity), similar to the increases in responding for access to a behavior when the behavioral cost of that behavior is stable but the behavioral cost of the alternative is increased (Goldfield & Epstein, 2002). The theoretical analysis of the relationship between behavioral cost and response rate in terms of demand functions presented in the earlier section on reinforcing value is based on economic principles. Bickel and Madden (1999) suggest choice of responding when presented with concurrent alternatives provides similar information to demand curves observed when single alternatives are studied. A consistent body of research has shown that manipulating price is a reliable method for modifying food purchases (French et al., 2001; French, Jeffery, Story, Hannan, & Snyder, 1997; French, Story, et al., 1997; Horgen & Brownell, 2002). Changes in price could be effective in reducing energy consumption even if obese people found food more reinforcing and were more motivated to purchase unhealthy foods. It would be important to complement changes in prices of less healthy alternatives with incentives to purchase healthier foods in combination with educational and media programs educating consumers about what types of foods to purchase.
Although there are similarities between price and behavioral cost, to our knowledge there have been no direct comparisons of how changes in behavioral cost might be related to changes in price. Research should compare if changes in behavioral cost and changes in price are associated with similar changes in behavior by using laboratory analogues of price changes (Epstein, Dearing, Handley, Roemmich, & Paluch, 2006; Epstein, Handley, et al., 2006) as well as naturalistic experiments that manipulate price and observe purchases and consumption (French et al., 2001; French, Jeffery, et al., 1997; French, Story, et al., 1997; Horgen & Brownell, 2002). Because changes in price, either by reducing the relative price of healthier alternatives or increasing the price of less healthy alternatives, could provide policy implications for improving eating and health, these studies could represent an important bridge between basic laboratory research and public policy. Bickel and colleagues have discussed the use of these behavioral paradigms to examine public policy implications for drug abuse (Bickel & DeGrandpre, 1995, 1996).
Behavioral Choice and Eating
One common goal in eating and obesity research is to shift preference from less healthy, highly reinforcing food to healthier, but perhaps less reinforcing, food. Many people attempt to shift consumption from less healthy to healthier foods to lose weight, reduce cholesterol, and so on. One of the biggest challenges for these behavior changes is that there is substantial relapse in these behavior changes (Jeffery et al., 2000), which suggests that regular consumption of these healthier foods does not result in these foods becoming very reinforcing in the context of less healthy alternatives. Research is needed to study how to increase the reinforcing value of these healthier alternatives. For example, it would be ideal if procedures could be developed to increase the motivation of people to consume fruits and vegetables. If an increase in the reinforcing value of these healthier alternatives cannot be achieved, research is needed to evaluate how much constraint on access to the less healthy, more-preferred alternatives is required before people shift choice on a long-term basis to healthier foods.
An attractive alternative to healthier foods as an option for behavior change is to identify or develop behavioral, nonfood alternatives or substitutes to food reinforcement. The development of nonfood alternatives may directly apply to situations in which excess energy intake is consumed in snacks and a behavioral goal is snack reduction. An alternative goal may be to reduce the time spent in meal consumption by providing attractive alternatives that reduce the motivation to consume excess energy. Many people have the experiences of being engaged in interesting or motivated activities that cause missing meals or of only allocating sufficient time to the meal to consume needed energy but not allocating enough time to eating to result in excess energy consumption. Although initial attempts to develop alternatives to eating have not resulted in reductions in energy intake or body weight (Epstein, Roemmich, Stein, Paluch, & Kilanowski, 2005), more research is needed to assess other types of alternatives, or new ways to frame the choice situations.
There may be individual differences in who substitutes healthier foods for less healthy foods or who substitutes alternative activities for less healthy foods. These could be behavioral differences based on experience or the history of eating or could be genetic differences that are related to the reinforcing value of food. Identifying individual differences in food reinforcement could help identify who may benefit most from specific interventions that focus on substitution of healthier foods, such as fruits and vegetables, for less healthy alternatives (Goldfield & Epstein, 2002).
Parameters of Food Deprivation/Satiation and Reinforcing Value
One of the most powerful ways to increase the reinforcing value of food is food deprivation. This is important because many approaches to changing eating behavior require or are associated with reductions in energy intake. Research is needed to identify the relationship between food deprivation and changes in food reinforcement.
One hypothesis is that food reinforcement increases when energy depletion occurs, and there is a dose–response relationship between the degree of deprivation and the increase in reinforcing value. Parametric work is needed to assess how food deprivation is related to changes in reinforcing value and if there a window in which energy can be restricted without increasing reinforcing value. For example, is an energy restriction of 50 calories, 100 calories, or 250 calories enough to increase the motivation to eat? Although a reduction of 250 calories per day may represent small behavior changes, this could lead to a weight loss of one-half pound per week, or 26 pounds for the year, which would represent substantial weight change. Research does suggest that more moderate changes that are maintained can result in better long-term weight loss than can traditional dietary approaches (Sbrocco, Nedegaard, Stone, & Lewis, 1999).
It is also possible that it is not the energy deficit that causes the increase in reinforcing value but rather the deprivation of specific foods. It would be interesting to assess changes in reinforcing value over time in conditions that reduced energy intake in part by removing unhealthy often-preferred alternatives versus a condition that reduced energy intake but ensured that the person had access to usual preferred foods, though the portion sizes of these foods would be controlled.
Satiation is the opposite of deprivation, and the reinforcing value decreases as food is consumed until reinforcer satiation is reached. It would be interesting to assess the dose–response effect for satiation to determine whether regular consumption of small portions of a favorite food could result in a reduction in reinforcing value of that food, which may make it easier to reduce energy intake without increasing food deprivation. It may be possible to consume small portions of a favorite food within an energy-restricted diet if consuming that favorite food reduces the motivation to overeat and be in positive energy balance.
Determinants of Food Reinforcement
Research is needed to identify the determinants of food reinforcement. This includes the characteristics of foods as well as differences in experience and learning history. Is reinforcing value related to macronutrients of food, or to sensory characteristics of food, such as taste or smell? There is a very large research base on how these factors relate to eating energy/macronutrient intake but limited research that generalizes this to the mechanism of food reinforcement. It would be important to know whether there are individual differences in food reinforcement, as some people may just be motivated to eat, whereas others are motivated to eat only specific foods.
Research is needed on experience or learning history factors that could influence food reinforcement. Food may become more reinforcing on the basis of pairing eating and specific foods with very pleasurable activities. For example, it is common to eat in social situations (de Castro, 1994), which may facilitate eating, and the pairing of eating with a pleasurable situation may increase the reinforcing value of food in that situation. The use of food as a reward is common within families (Baughcum, Burklow, Deeks, Powers, & Whitaker, 1998; Sherry et al., 2004), and there may be situations in which parents or other adults use food as rewards in a positive context (Birch, Zimmerman, & Hind, 1980) that can increase the reinforcing value of food. If reinforcing value is central to the motivation to eat and is a factor in obesity, then developing a better understanding of these factors may be important for the prevention or treatment of obesity.
Summary
This article was designed to provide an overview of how food reinforcement and behavioral theories of choice can be used to understand eating and energy intake. One of the unique aspects of behavioral theories of choice is the way in which they can be used to understand factors that influence eating at multiple levels. These levels range from molecular genetics to the neurobiology of food reinforcement through how to treat and prevent obesity.
An important distinction between food reinforcement and more traditional approaches to eating is the potential for understanding nonregulatory ingestive behavior as opposed to eating that is maintained by nutrient or energy depletion or homeostatic need. It is likely that both of these areas of research are important in understanding factors that regulate intake. A focus on factors that influence eating outside of homeostatic need provides the opportunity to examine how nonfood reinforcers or behaviors influence eating as well as how factors such as accessibility and behavioral cost can shift consumption.
In a field that is potentially so broad, there is the need for research at each level of analysis, including research that focuses on behavioral conditions that influence food reinforcement as well as the biological basis for food reinforcement. There is much to be learned about how food reinforcement develops, what maintains food reinforcement, and how behavioral or pharmacological interventions modify food reinforcement. Perhaps the most informative research will be research that extends to multiple levels, that is, that combines the best behavioral and neurobiological approaches to better understand variables that influence the reinforcing value of food and the motivation to eat. Likewise, developing a better understanding of behavioral factors that influence food reinforcement and food intake may be important to improving the effectiveness of public health efforts to reduce the number of people with obesity and comorbid medical conditions associated with obesity.
Acknowledgments
The development of this article was funded in part by National Institute of Child Health and Human Development Grants HD 39792 and HD 44725 to Leonard H. Epstein. We thank Tony Caggiula and Ken Perkins, who helped develop the initial research program on food reinforcement at the University of Pittsburgh.
Contributor Information
Leonard H. Epstein, Department of Pediatrics, University at Buffalo School of Medicine and Biomedical Sciences
John J. Leddy, Department of Orthopedics, University at Buffalo School of Medicine and Biomedical Sciences
Jennifer L. Temple, Department of Pediatrics, University at Buffalo School of Medicine and Biomedical Sciences
Myles S. Faith, Department of Psychiatry, University of Pennsylvania School of Medicine
References
- Ackerman SH, Albert M, Shindledecker RD, Gayle C, Smith GP. Intake of different concentrations of sucrose and corn oil in preweanling rats. American Journal of Physiology. 1992;262:R624–627. doi: 10.1152/ajpregu.1992.262.4.R624. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: The response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15:171–188. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Osei SY. Molecular regulation of eating behavior: New insights and prospects for therapeutic strategies. Trends in Molecular Medicine. 2001;7:205–213. doi: 10.1016/s1471-4914(01)01989-x. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: A 48-week double-blind, placebo-controlled trial. Obesity Research. 2002;10:633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug and Alcohol Dependence. 2003;70:S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995 October 5;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug and Alcohol Dependence. 2003;70:S13–40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G. Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:7095–7099. doi: 10.1073/pnas.89.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista T. Body weight gain induced by antipsychotic drugs: Mechanisms and management. Acta Psychiatrica Scandinavica. 1999;100:3–16. doi: 10.1111/j.1600-0447.1999.tb10908.x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berlin) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Heitmann BL, Helms MJ, Williams RB, Surwit RS, Siegler IC. Symptoms of depression and changes in body weight from adolescence to mid-life. International Journal of Obesity and Related Metabolic Disorders. 1998;22:688–694. doi: 10.1038/sj.ijo.0800647. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: Dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Research. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Aresu M, Aste A, Ariu T, Di Chiara G. Differential adaptive properties of accumbens shell dopamine responses to ethanol as a drug and as a motivational stimulus. European Journal of Neuroscience. 2003;17:1465–1472. doi: 10.1046/j.1460-9568.2003.02556.x. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Baughcum AE, Burklow KA, Deeks CM, Powers SW, Whitaker RC. Maternal feeding practices and childhood obesity: A focus group study of low-income mothers. Archives of Pediatrics and Adolescent Medicine. 1998;152:1010–1014. doi: 10.1001/archpedi.152.10.1010. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: Therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Bellisle F, Lucas F, Amrani R, LeMagnen J. Deprivation, palatability and the micro-structure of meals in human subjects. Appetite. 1984;5:85–94. doi: 10.1016/s0195-6663(84)80027-6. [DOI] [PubMed] [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2003;284:R1260–1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Berlin I, Lavergne F. Relationship between body-mass index and depressive symptoms in patients with major depression. European Psychiatry. 2003;18:85–88. doi: 10.1016/s0924-9338(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Bernstein DJ, Ebbesen EB. Reinforcement and substitution in humans: A multiple-response analysis. Journal of the Experimental Analysis of Behavior. 1978;30:243–253. doi: 10.1901/jeab.1978.30-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: Brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology & Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research/Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiology & Behavior. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Bertino M, Beauchamp GK, Engelman K. Naltrexone, an opioid blocker, alters taste perception and nutrient intake in humans. American Journal of Physiology. 1991;261:R59–63. doi: 10.1152/ajpregu.1991.261.1.R59. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ. Modeling drug abuse policy in the behavioral economics laboratory. In: Green L, Kagel JH, editors. Advances in behavioral economics. Vol. 3. New York: Ablex; 1995. pp. 69–95. [Google Scholar]
- Bickel WK, DeGrandpre RJ. Basic psychological science speaks to drug policy: Drug cost and competing reinforcement. In: DeGrandpre WBR, editor. Psychological perspectives on the control, prevention and treatment of illicit drug use. New York: Plenum; 1996. pp. 31–52. [Google Scholar]
- Bickel WK, Madden GJ. A comparison of measures of relative reinforcing efficacy and behavioral economics: Cigarettes and money in smokers. Behavioral Pharmacology. 1999;10:627–637. doi: 10.1097/00008877-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ, Petry NM. The price of change: The behavioral economics of drug dependence. Behavior Therapy. 1998;29:545–565. [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology. 2000;71:68–78. doi: 10.1159/000054522. [DOI] [PubMed] [Google Scholar]
- Birch LL, Zimmerman SI, Hind H. The influence of social affective context on the formation of children’s food preferences. Child Development. 1980;51:856–861. [Google Scholar]
- Blass EM. Suckling: Determinants, changes, mechanisms, and lasting impressions. Developmental Psychology. 1990;26:520–533. [Google Scholar]
- Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborns. Pain. 1999;83:611–623. doi: 10.1016/S0304-3959(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32(Suppl i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Wood RC, Gill J, Li C, Chen TJ, et al. Increased prevalence of the Taq I A1 allelle of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: A preliminary report. Pharmacogenetics. 1996;6:297–305. doi: 10.1097/00008571-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: A contemporary synthesis. Sunderland, MA: Sinauer Associates Inc; 2007. [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biological Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochemical Research. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: Behavioral evidence and underlying mechanisms. Physiology & Behavior. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Advances in Behavioral Pharmacology. 1984;4:47–88. [Google Scholar]
- Chung J, Kim Y, Yim S, Park S, Ko K, Jung J. Immunohistochemical and biochemical studies on dopamine content in rat brain during cholecystokinin-induced suppression of feeding. Korean Journal of Pharmacology. 1994;30:39–48. [Google Scholar]
- Cincotta AH, MacEachern TA, Meier AH. Bromocriptine redirects metabolism and prevents seasonal onset of obese hyperinsulinemic state in Syrian hamsters. American Journal of Physiology. 1993;264:E285–293. doi: 10.1152/ajpendo.1993.264.2.E285. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on I.V. cocaine self-administration in rats maintained under FR schedules. Psychopharmacology (Berlin) 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. Journal of the American Medical Association. 1991;266:1793–1800. [PubMed] [Google Scholar]
- Comings DE, Flanagan SD, Dietz G, Muhleman D, Knell E, Gysin R. The dopamine D2 receptor (DRD2) as a major gene in obesity and height. Biochemical Medicine and Metabolic Biology. 1993;50:176–185. doi: 10.1006/bmmb.1993.1059. [DOI] [PubMed] [Google Scholar]
- Comings DE, Gade R, MacMurray JP, Muhleman D, Peters WR. Genetic variants of the human obesity (OB) gene: Association with body mass index in young women, psychiatric symptoms, and interaction with the dopamine D2 receptor (DRD2) gene. Molecular Psychiatry. 1996;1:325–335. [PubMed] [Google Scholar]
- Cornell CE, Rodin J, Weingarten HP. Stimulus-induced eating when satiated. Physiology & Behavior. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- de Castro JM. Family and friends produce greater social facilitation of food intake than other companions. Physiology & Behavior. 1994;56:445–455. doi: 10.1016/0031-9384(94)90286-0. [DOI] [PubMed] [Google Scholar]
- de Castro JM, Duncan G. Operantly conditioned running: Effects on brain catecholamine concentrations and receptor densities in the rat. Pharmacology, Biochemistry and Behavior. 1985;23:495–500. doi: 10.1016/0091-3057(85)90407-1. [DOI] [PubMed] [Google Scholar]
- de Fourmestraux V, Neubauer H, Poussin C, Farmer P, Falquet L, Burcelin R, et al. Transcript profiling suggests that differential metabolic adaptation of mice to a high fat diet is associated with changes in liver to muscle lipid fluxes. Journal of Biological Chemistry. 2004;279:50743–50753. doi: 10.1074/jbc.M408014200. [DOI] [PubMed] [Google Scholar]
- de Graaf C, Cardello AV, Matthew Kramer F, Lesher LL, Meiselman HL, Schutz HG. A comparison between liking ratings obtained under laboratory and field conditions: The role of choice. Appetite. 2005;44:15–22. doi: 10.1016/j.appet.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Desor JA, Maller O, Turner RE. Taste and acceptance of sugars by human infants. Journal of Comparative and Physiological Psychology. 1973;84:496–501. doi: 10.1037/h0034906. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4:5–10. [Google Scholar]
- Doell SR, Hawkins RC. Pleasures and pounds: An exploratory study. Addictive Behaviors. 1982;7:65–69. doi: 10.1016/0306-4603(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, et al. Dopaminergic tone and obesity: An insight from prolactinomas treated with bromocriptine. European Journal of Endocrinology. 2002;147:77–84. doi: 10.1530/eje.0.1470077. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Greenwood MRC. Cream and sugar: Human preferences for high-fat foods. Physiology & Behavior. 1983;30:629–633. doi: 10.1016/0031-9384(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: Evidence for opioid involvement. Physiology & Behavior. 1992;51:371–379. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: Carbohydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Elsinga PH, Hatano K, Ishiwata K. PET tracers for imaging of the dopaminergic system. Current Medicinal Chemistry. 2006;13:2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Handley EA, Roemmich JN, Paluch RA. Relationship of mother and child food purchases as a function of price: A pilot study. Appetite. 2006;47:115–118. doi: 10.1016/j.appet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Paluch RA, Roemmich JN, Cho D. Price and maternal obesity influence purchases of low and high energy dense foods. American Journal of Clinical Nutrition. doi: 10.1093/ajcn/86.4.914. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Handley EA, Dearing KK, Cho DD, Roemmich JN, Paluch RA, et al. Purchases of food in youth: Influence of price and income. Psychological Science. 2006;17:82–89. doi: 10.1111/j.1467-9280.2005.01668.x. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Rodefer JS, Wisniewski L, Caggiula AR. Habituation and dishabituation of human salivary response. Physiology & Behavior. 1992;51:945–950. doi: 10.1016/0031-9384(92)90075-d. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Paluch RA, Raynor HA. The influence of changes in sedentary behavior on energy and macronutrient intake in youth. American Journal of Clinical Nutrition. 2005a;81:361–366. doi: 10.1093/ajcn.81.2.361. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Physical activity as a substitute for sedentary behavior in youth. Annals of Behavioral Medicine. 2005b;29:200–209. doi: 10.1207/s15324796abm2903_6. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Stein RI, Paluch RA, Kilanowski CK. The challenge of identifying behavioral alternatives to food: Clinic and field studies. Annals of Behavioral Medicine. 2005;30:201–209. doi: 10.1207/s15324796abm3003_4. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saad FG, Giacomelli AM, Roemmich JN. Effects of allocation of attention on habituation to olfactory and visual food stimuli in children. Physiology & Behavior. 2005;84:313–319. doi: 10.1016/j.physbeh.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saad FG, Handley EA, Roemmich JN, Hawk LW, McSweeney FK. Habituation of salivation and motivated responding for food in children. Appetite. 2003;41:283–289. doi: 10.1016/s0195-6663(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saelens BE. Behavioral economics of obesity: Food intake and energy expenditure. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavioral economics. Mahwah, NJ: Erlbaum; 2000. pp. 293–311. [Google Scholar]
- Epstein LH, Saelens BE, Myers MD, Vito D. Effects of decreasing sedentary behaviors on activity choice in obese children. Health Psychology. 1997;16:107–113. doi: 10.1037//0278-6133.16.2.107. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saelens BE, O’Brien JG. Effects of reinforcing increases in active behavior versus decreases in sedentary behavior for obese children. International Journal of Behavioral Medicine. 1995;2:41–50. doi: 10.1207/s15327558ijbm0201_4. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Smith JA, Vara LS, Rodefer JS. Behavioral economic analysis of activity choice in obese children. Health Psychology. 1991;10:311–316. doi: 10.1037//0278-6133.10.5.311. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype and energy intake in obese and non-obese humans. Behavioral Neuroscience. doi: 10.1037/0735-7044.121.5.877. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiology & Behavior. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jaroni JL, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiology & Behavior. 2004a;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jaroni JL et al. The relationship between food reinforcement and dopamine genotypes on food intake in smokers. American Journal of Clinical Nutrition. 2004b;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2002;283:R905–910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Field AE, Austin SB, Taylor CB, Malspeis S, Rosner B, Rockett HR, et al. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics. 2003;112:900–906. doi: 10.1542/peds.112.4.900. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP. Adiposity signals and food reward: Expanding the CNS roles of insulin and leptin. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2003a;284:R882–892. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP. Insulin, food intake, and reward. Seminars in Clinical Neuropsychiatry. 2003b;8:82–93. doi: 10.1053/scnp.2003.50012. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Research Bulletin. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Research. 1994;644:331–334. doi: 10.1016/0006-8993(94)91698-5. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. American Journal of Clinical Nutrition. 2002;76:226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed DE, Green L. A behavioral economic analysis of fat appetite in rats. Appetite. 1998;31:333–349. doi: 10.1006/appe.1998.0170. [DOI] [PubMed] [Google Scholar]
- French SA, Jeffery RW, Story M, Breitlow KK, Baxter JS, Hannan P, et al. Pricing and promotion effects on low-fat vending snack purchases: The CHIPS Study. American Journal of Public Health. 2001;91:112–117. doi: 10.2105/ajph.91.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Jeffery RW, Story M, Hannan P, Snyder MP. A pricing strategy to promote low-fat snack choices through vending machines. American Journal of Public Health. 1997;87:849–851. doi: 10.2105/ajph.87.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Story M, Jeffery RW, Snyder P, Eisenberg M, Sidebottom A, et al. Pricing strategy to promote fruit and vegetable purchase in high school cafeterias. Journal of the American Dietetic Association. 1997;97:1008–1010. doi: 10.1016/S0002-8223(97)00242-3. [DOI] [PubMed] [Google Scholar]
- Fujiwara H. Comparative studies of sulpiride and classical neuroleptics on induction of catalepsy, locomotor activity, and brain dopamine metabolism in mice. Pharmacology Biochemistry and Behavior. 1992;41:301–308. doi: 10.1016/0091-3057(92)90102-l. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000 January 7;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- Gendall KA, Joyce PR, Sullivan PF, Bulik CM. Food cravers: Characteristics of those who binge. International Journal of Eating Disorders. 1998;23:353–360. doi: 10.1002/(sici)1098-108x(199805)23:4<353::aid-eat2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos G, Galanopoulou P, Daifotis Z, Couvaris C. Effects of mesulergine treatment on diet selection, brain serotonin (5-HT) and dopamine (DA) turnover in free feeding rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1998;22:803–813. doi: 10.1016/s0278-5846(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH. Can fruits and vegetables and activities substitute for snack foods? Health Psychology. 2002;21:299–303. [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH, Davidson M, Saad FG. Validation of a multiple choice questionnaire measure of the relative reinforcing value of food. Eating Behaviors. 2005;6:283–292. doi: 10.1016/j.eatbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(supp 2):s119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug and Alcohol Dependence. 2003;70:S41–54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Experimental and Clinical Psychopharmacology. 1996;4:97–106. [Google Scholar]
- Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: Changes during aging. Brain Research. 1992;589:338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- Hamilton CL. Rats’ preference for high fat diets. Journal of Comparative and Physiological Psychology. 1964;58:459–460. doi: 10.1037/h0047142. [DOI] [PubMed] [Google Scholar]
- Harvey J, Wing RR, Mullen M. Effects on food cravings of a very low calorie diet or a balanced, low calorie diet. Appetite. 1993;21:105–115. doi: 10.1016/0195-6663(93)90003-3. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sciences. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Parada M, Hoebel BG. Amphetamine-induced hyperphagia and obesity caused by intraventricular or lateral hypothalamic injections in rats. Journal of Pharmacology & Experimental Therapeutics. 1983;227:524–530. [PubMed] [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives: A substance abuse treatment innovation. Addictive Behaviors. 2002;27:887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior: Theoretical and clinical implications. Annals of the New York Academy of Sciences. 1989;575:171–193. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, et al. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgen KB, Brownell KD. Comparison of price change and health message interventions in promoting healthy food choices. Health Psychology. 2002;21:505–512. doi: 10.1037//0278-6133.21.5.505. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Bauman RA. The behavioral analysis of demand. In: Green L, Kagel JH, editors. Advances in behavioral economics. Vol. 1. Norwood, NJ: Ablex; 1987. pp. 117–165. [Google Scholar]
- Ingram DK. Age-related decline in physical activity: Generalization to nonhumans. Medicine & Science in Sports & Exercise. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kiriike N, Kurioka M, Fujisaki Y, Iwasaki S, Yamagami S. Bromocriptine enhances feeding behavior without changing dopamine metabolism. Pharmacology, Biochemistry and Behavior. 1997;58:183–188. doi: 10.1016/s0091-3057(97)00004-x. [DOI] [PubMed] [Google Scholar]
- Jacobs SB, Wagner MK. Obese and nonobese individuals: Behavioral and personality characteristics. Addictive Behaviors. 1984;9:223–226. doi: 10.1016/0306-4603(84)90062-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, et al. Prediction of dopamine transporter binding availability by genotype: A preliminary report. American Journal of Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Jain AK, Kaplan RA, Gadde KM, Wadden TA, Allison DB, Brewer ER, et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obesity Research. 2002;10:1049–1056. doi: 10.1038/oby.2002.142. [DOI] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boob B, Mulkens S. Overweight children overeat after exposure to food cues. Eating Behaviors. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: Current status. Health Psychology. 2000;19(Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Jenkinson CP, Hanson R, Cray K, Wiedrich C, Knowler WC, Bogardus C, et al. Association of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA with obesity or type 2 diabetes mellitus in Pima Indians. International Journal of Obesity and Related Metabolic Disorders. 2000;24:1233–1238. doi: 10.1038/sj.ijo.0801381. [DOI] [PubMed] [Google Scholar]
- Johnson WG. Effect of cue prominence and subject weight on human food-directed performance. Journal of Personality and Social Psychology. 1974;29:843–848. doi: 10.1037/h0036390. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kamath V, Jones CN, Yip JC, Varasteh BB, Cincotta AH, Reaven GM, et al. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care. 1997;20:1697–1701. doi: 10.2337/diacare.20.11.1697. [DOI] [PubMed] [Google Scholar]
- Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain. 1997;120:1675–1684. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- Karoly AJ, Winger G, Ikomi F, Woods JH. The reinforcing property of ethanol in the rhesus monkey: II. Some variables related to the maintenance of intravenous ethanol-reinforced responding. Psychopharmacology (Berlin) 1978;58:19–25. doi: 10.1007/BF00426785. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology & Behavior. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, et al. Dopaminergic system genes in ADHD: Toward a biological hypothesis. Neuropsychopharmacology. 2002;27:607–619. doi: 10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Research. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Annals of the New York Academy of Sciences. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response. Journal of Pharmacology & Experimental Therapeutics. 1999;288:699–709. [PubMed] [Google Scholar]
- Laitinen J, Ek E, Sovio U. Stress-related eating and drinking behavior and body mass index and predictors of this behavior. Preventive Medicine. 2002;34:29–39. doi: 10.1006/pmed.2001.0948. [DOI] [PubMed] [Google Scholar]
- Lappalainen R, Epstein LH. A behavioral economics analysis of food choice in humans. Appetite. 1990;14:81–93. doi: 10.1016/0195-6663(90)90002-p. [DOI] [PubMed] [Google Scholar]
- Leddy JJ, Epstein LH, Jaroni JL, Roemmich JN, Paluch RA, Goldfield GS, et al. The influence of methylphenidate on eating in obese men. Obesity Research. 2004;12:224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- Lee ES, Kim YH, Beck SH, Lee S, Oh SW. Depressive mood and abdominal fat distribution in overweight premenopausal women. Obesity Research. 2005;13:320–325. doi: 10.1038/oby.2005.43. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Molecular mechanisms of cocaine addiction. New England Journal of Medicine. 1996;335:128–129. doi: 10.1056/NEJM199607113350211. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Dysregulation of arcuate nucleus preproneuropeptide Y mRNA in diet-induced obese rats. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 1997;272:R1365–1370. doi: 10.1152/ajpregu.1997.272.5.R1365. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2002;282:R46–54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars and fats: The neurobiology of preference. Journal of Nutrition. 2003;133:831S–834S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- Lowe MR. The effects of dieting on eating behavior: A three-factor model. Psychological Bulletin. 1993;114:100–121. doi: 10.1037/0033-2909.114.1.100. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Foster GD, Kerzhnerman I, Swain RM, Wadden TA. Restrictive dieting vs. “undieting” effects on eating regulation in obese clinic attenders. Addictive Behaviors. 2001;26:253–266. doi: 10.1016/s0306-4603(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. Journal of Neuroscience. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, O’Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity (Silver Spring) 2006;14:115–121. doi: 10.1038/oby.2006.14. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, et al. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24:553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- Martin-Gronert MS, Ozanne SE. Programming of appetite and type 2 diabetes. Early Human Development. 2005;81:981–988. doi: 10.1016/j.earlhumdev.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Mazur JE. Conditioned reinforcement and choice with delayed and uncertain primary reinforcers. Journal of the Experimental Analysis of Behavior. 1995;63:139–150. doi: 10.1901/jeab.1995.63-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KL, Perri MG, Nezu AM, Lowe MR. An investigation of counterregulatory eating in obese clinic attenders. International Journal of Eating Disorders. 1992;12:161–169. [Google Scholar]
- McCrory MA, Fuss PJ, McCallum JE, Yao M, Vinken AG, Hays NP, et al. Dietary variety within food groups: Association with energy intake and body fatness in men and women. American Journal of Clinical Nutrition. 1999;69:440–447. doi: 10.1093/ajcn/69.3.440. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Hinson JM, Cannon CB. Sensitization–habituation may occur during operant conditioning. Psychological Bulletin. 1996;120:256–271. [Google Scholar]
- McSweeney FK, Roll JM. Do animals satiate or habituate to repeatedly presented reinforcers? Psychonomic Bulletin & Review. 1998;53:428–442. [Google Scholar]
- McSweeney FK, Swindell S. General-process theories of motivation revisited: The role of habituation. Psychological Bulletin. 1999;125:437–457. [Google Scholar]
- Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, et al. Endurance training effects on neurotransmitter release in rat striatum: An in vivo microdialysis study. Acta Physiologica Scandinavica. 1997;159:335–341. doi: 10.1046/j.1365-201X.1997.00118.x. [DOI] [PubMed] [Google Scholar]
- Meier AH, Cincotta AH, Lovell WC. Timed bromocriptine administration reduces body fat stores in obese subjects and hyperglycemia in type II diabetics. Experientia. 1992;48:248–253. doi: 10.1007/BF01930467. [DOI] [PubMed] [Google Scholar]
- Meririnne E, Kankaanpaa A, Seppala T. Rewarding properties of methylphenidate: Sensitization by prior exposure to the drug and effects of dopamine D1- and D2-receptor antagonists. Journal of Pharmacology & Experimental Therapeutics. 2001;298:539–550. [PubMed] [Google Scholar]
- Mittleman G, Castaneda E, Robinson TE, Valenstein ES. The propensity for nonregulatory ingestive behavior is related to differences in dopamine systems: Behavioral and biochemical evidence. Behavioral Neuroscience. 1986;100:213–220. doi: 10.1037//0735-7044.100.2.213. [DOI] [PubMed] [Google Scholar]
- Musser CJ, Ahmann PA, Theye FW, Mundt P, Broste SK, Mueller-Rizner N. Stimulant use and the potential for abuse in Wisconsin as reported by school administrators and longitudinally followed children. Journal of Developmental and Behavioral Pediatrics. 1998;19:187–192. doi: 10.1097/00004703-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Myers MD, Epstein LH. Habituation of responding for food in humans. Appetite. 2002;38:224–234. doi: 10.1006/appe.2001.0484. [DOI] [PubMed] [Google Scholar]
- Need AC, Ahmadi KR, Spector TD, Goldstein DB. Obesity is associated with genetic variants that alter dopamine availability. Annals of Human Genetics. 2006;70:293–303. doi: 10.1111/j.1529-8817.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. American Journal of Medical Genetics. 2003;116B:103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, et al. D2 dopamine receptor gene and obesity. International Journal of Eating Disorders. 1994;15:205–217. doi: 10.1002/1098-108x(199404)15:3<205::aid-eat2260150303>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Orosco M, Gerozissis K, Rouch C, Meile MJ, Nicolaidis S. Hypothalamic monoamines and insulin in relation to feeding in the genetically obese Zucker rat as revealed by microdialysis. Obesity Research. 1995;3:S655–665. doi: 10.1002/j.1550-8528.1995.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Orosco M, Rouch C, Nicolaidis S. Rostromedial hypothalamic monoamine changes in response to intravenous infusions of insulin and glucose in freely feeding obese Zucker rats: A microdialysis study. Appetite. 1996;26:1–20. doi: 10.1006/appe.1996.0001. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Fudge J, Hurd Y, Pennartz C, Peoples L. Finding motivation at Seabrook Island: The ventral striatum, learning and plasticity. Trends in Neurosciences. 2000;23:383–384. doi: 10.1016/s0166-2236(00)01644-1. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68:11–20. doi: 10.1159/000054345. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Perusse L, Tremblay A, Leblanc C, Bouchard C. Genetic and environmental influences on level of habitual physical activity and exercise participation. American Journal of Epidemiology. 1989;129:1012–1022. doi: 10.1093/oxfordjournals.aje.a115205. [DOI] [PubMed] [Google Scholar]
- Pietilainen KH, Rissanen A, Laamanen M, Lindholm AK, Markkula H, Yki-Jarvinen H, et al. Growth patterns in young adult monozygotic twin pairs discordant and concordant for obesity. Twin Research. 2004;7:421–429. doi: 10.1375/1369052042335368. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, et al. Dopaminergic activities in the human striatum: Rostrocaudal gradients of uptake sites and of D1 and D2 but not of D3 receptor binding or dopamine. Neuroscience. 1999;90:433–445. doi: 10.1016/s0306-4522(98)00465-5. [DOI] [PubMed] [Google Scholar]
- Pliner P, Polivy J, Herman CP, Zakalusny I. Short-term intake of overweight individuals and normal weight dieters and non-dieters with and without choice among a variety of foods. Appetite. 1980;1:203–213. [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. Journal of Neuroscience. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: Implications for a possible neurochemical link between weight loss and drug abuse. Obesity Research. 1995;3:525S–529S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Premack D. Toward empirical behavioral laws: I. Positive reinforcement. Psychological Review. 1959;66:219–233. doi: 10.1037/h0040891. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Raynor DA, Coleman KJ, Epstein LH. Effects of proximity on the choice to be physically active or sedentary. Research Quarterly for Exercise and Sport. 1998;69:99–103. doi: 10.1080/02701367.1998.10607674. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. Dietary variety, energy regulation and obesity. Psychological Bulletin. 2001;127:325–341. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Jeffery RW, Phelan S, Hill JO, Wing RR. Amount of food group variety consumed in the diet and long-term weight loss maintenance. Obesity Research. 2005;13:883–890. doi: 10.1038/oby.2005.102. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Jeffery RW, Tate DF, Wing RR. Relationship between changes in food group variety, dietary intake, and weight during obesity treatment. International Journal of Obesity and Related Metabolic Disorders. 2004;28:813–820. doi: 10.1038/sj.ijo.0802612. [DOI] [PubMed] [Google Scholar]
- Reiss S, Havercamp S. The sensitivity theory of motivation: Implications for psychopathology. Behaviour Research and Therapy. 1996;34:621–632. doi: 10.1016/0005-7967(96)00041-1. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemistry Research. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Wright SM, Epstein LH. Dietary restraint and stress-induced snacking in youth. Obesity Research. 2002;10:1120–1126. doi: 10.1038/oby.2002.152. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. Carbohydrates, fats, and satiety. American Journal of Clinical Nutrition. 1995;61:960S–967S. doi: 10.1093/ajcn/61.4.960S. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. The supersizing of America: Portion size and the obesity epidemic. Nutrition Today. 2003;38:42–53. doi: 10.1097/00017285-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Castellanos VH, Halford JC, Kilara A, Panyam D, Pelkman CL, et al. Volume of food consumed affects satiety in men. American Journal of Clinical Nutrition. 1998;67:1170–1177. doi: 10.1093/ajcn/67.6.1170. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Roe LS, Meengs JS. Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. American Journal of Clinical Nutrition. 2006;83:11–17. doi: 10.1093/ajcn/83.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA. The influence of variety on human food selection and intake. In: Barker LM, editor. The psychobiology of human food selection. Westport, CT: AVI Publishing; 1982. pp. 101–122. [Google Scholar]
- Rolls BJ, Rowe EA, Rolls ET. How sensory properties of foods affect human feeding behavior. Physiology & Behavior. 1982;29:409–417. doi: 10.1016/0031-9384(82)90259-1. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rowe EA, Rolls ET, Kingston B, Megson A, Gunary R. Variety in a meal enhances food intake in man. Physiology & Behavior. 1981;26:215–221. doi: 10.1016/0031-9384(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, van Duijvenvoorde PM, Rolls ET. Pleasantness changes and food intake in a varied four course meal. Appetite. 1984;5:337–348. doi: 10.1016/s0195-6663(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste and olfactory processing in the brain and its relation to the control of eating. Critical Reviews in Neurobiology. 1997;11:263–287. doi: 10.1615/critrevneurobiol.v11.i4.20. [DOI] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. Journal of Clinical Psychopharmacology. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. The reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: Alternative functions of nucleus accumbens dopamine. Current Opinion in Pharmacology. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment promotes continuing weight loss: Preliminary results of a cognitive-behavioral decision-based treatment for obesity. Journal of Consulting and Clinical Psychology. 1999;67:260–266. doi: 10.1037//0022-006x.67.2.260. [DOI] [PubMed] [Google Scholar]
- Schachter S. Obesity and eating: Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968 August 23;161:751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- Schertz M, Adesman AR, Alfieri NE, Bienkowski RS. Predictors of weight loss in children with attention deficit hyperactivity disorder treated with stimulant medication. Pediatrics. 1996;98:763–769. [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000 April 6;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Scislowski PW, Tozzo E, Zhang Y, Phaneuf S, Prevelige R, Cincotta AH. Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists. International Journal of Obesity. 1999;23:425–431. doi: 10.1038/sj.ijo.0800893. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiology & Behavior. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Sherry B, McDivitt J, Birch LL, Cook FH, Sanders S, Prish JL, et al. Attitudes, practices, and concerns about child feeding and child weight status among socioeconomically diverse white, Hispanic, and African-American mothers. Journal of the American Dietetic Association. 2004;104:215–221. doi: 10.1016/j.jada.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Shide DJ, Caballerro B, Reidelberger R, Rolls BJ. Accurate energy compensation for intragastric and oral nutrients in lean males. American Journal of Clinical Nutrition. 1995;61:754–764. doi: 10.1093/ajcn/61.4.754. [DOI] [PubMed] [Google Scholar]
- Simonen RL, Rankinen T, Perusse L, Leon AS, Skinner JS, Wilmore JH, et al. A dopamine D2 receptor gene polymorphism and physical activity in two family studies. Physiology & Behavior. 2003;78:751–757. doi: 10.1016/s0031-9384(03)00084-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Smith BA, Blass EM. Taste-mediated calming in premature, preterm, and full-term human infants. Developmental Psychology. 1996;32:1084–1089. [Google Scholar]
- Smith JA, Epstein LH. Behavioral economic analysis of food choice in obese children. Appetite. 1991;17:91–95. doi: 10.1016/0195-6663(91)90064-y. [DOI] [PubMed] [Google Scholar]
- Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1995;52:434–443. doi: 10.1001/archpsyc.1995.03950180020004. [DOI] [PubMed] [Google Scholar]
- Spiegel TA, Shrager EE, Stellar E. Responses of lean and obese subjects to preloads, deprivation, and palatability. Appetite. 1989;13:45–69. doi: 10.1016/0195-6663(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Spiegel TA, Stellar E. Effects of variety on food intake of underweight, normal-weight and overweight women. Appetite. 1990;15:47–61. doi: 10.1016/0195-6663(90)90099-t. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Ettenger RH. Learning: An introduction to the principles of adaptive behavior. New York: Harcourt Brace and Jovanovitch; 1989. [Google Scholar]
- Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. Journal of Consulting and Clinical Psychology. 1999;67:967–974. doi: 10.1037//0022-006x.67.6.967. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Shaw H, Rohde P. Psychological and behavioral risk factors for obesity onset in adolescent girls: A prospective study. Journal of Consulting and Clinical Psychology. 2005;73:195–202. doi: 10.1037/0022-006X.73.2.195. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Zigmond MJ. Brain catecholamines and the central control of food intake. International Journal of Obesity. 1984;8:S39–50. [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, et al. Dopamine genes and ADHD. Neuroscience and Biobehavioral Reviews. 2000;24:21–25. doi: 10.1016/s0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Baier L, Jenkinson C, Harper I, Del Parigi A, Bogardus C. A Ser311Cys mutation in the human dopamine receptor D2 gene is associated with reduced energy expenditure. Diabetes. 2001;50:901–904. doi: 10.2337/diabetes.50.4.901. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch CF, Agras WS. The effects of short-term food deprivation on caloric intake in eating-disordered subjects. Appetite. 1996;26:221–233. doi: 10.1006/appe.1996.0017. [DOI] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Roemmich JN, Epstein LH. Dietary variety impairs habituation in youth. Health Psychology. doi: 10.1037/0278-6133.27.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Kent KM, Giacomelli AM, Paluch RA, Roemmich JN, Epstein LH. Habituation and recovery of salivation and motivated responding for food in children. Appetite. 2006;46:280–284. doi: 10.1016/j.appet.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P, Gilbert DB, Cooper SJ. Dopamine receptor subtype agonists and feeding behavior. Obesity Research. 1995;3:515S–523S. doi: 10.1002/j.1550-8528.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Farmer-Dougan VA. Reinforcement in applied settings: Figuring out ahead of time what will work. Psychological Bulletin. 1991;110:379–391. doi: 10.1037/0033-2909.110.3.379. [DOI] [PubMed] [Google Scholar]
- Vandewater K, Vickers Z. Higher-protein foods produce greater sensory-specific satiety. Physiology & Behavior. 1996;59:579–583. doi: 10.1016/0031-9384(95)02113-2. [DOI] [PubMed] [Google Scholar]
- Vara LS, Epstein LH. Laboratory assessment of choice between exercise or sedentary behaviors. Research Quarterly for Exercise and Sport. 1993;64:356–360. doi: 10.1080/02701367.1993.10608822. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behavioral Pharmacology. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. Journal of Neuroscience. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. American Journal of Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, et al. Brain dopamine is associated with eating behaviors in humans. International Journal of Eating Disorders. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Contributions from behavioral theories of choice to an analysis of alcohol abuse. Journal of Abnormal Psychology. 1988;97:181–195. doi: 10.1037//0021-843x.97.2.181. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: A randomized trial. Archives of Internal Medicine. 2001;161:218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. New England Journal of Medicine. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Felder C, Fowler JS, Levy AV, Pappas NR, et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. NeuroReport. 2002;13:1151–1155. doi: 10.1097/00001756-200207020-00016. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983 April 22;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Westenhoefer J, Pudel V. Pleasure from food: Importance for food choice and consequences of deliberate restriction. Appetite. 1993;20:246–249. doi: 10.1006/appe.1993.1029. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Prince J. Pharmacotherapy of adult attention deficit/hyperactivity disorder: A review. Journal of Clinical Psychopharmacology. 1995;15:270–279. doi: 10.1097/00004714-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheets S, Davies G, Morgan J, et al. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology. 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: Importance of drive. Journal of Neuroscience. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philosophical Transactions of the Royal Society, Series B: Biological Sciences. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski L, Epstein LH, Caggiula AR. Effect of food change on consumption, hedonics, and salivation. Physiology & Behavior. 1992;52:21–26. doi: 10.1016/0031-9384(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Wisniewski L, Epstein LH, Marcus MD, Kaye W. Differences in salivary habituation to palatable foods in bulimia nervosa patients and controls. Psychosomatic Medicine. 1997;59:427–433. doi: 10.1097/00006842-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Molecular Psychiatry. 2000;5:664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: Enhancement of reward “wanting” without enhanced ’liking’ or response reinforcement. Journal of Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Blaha V, Meguid MM, Laviano A, Oler A, Zadak Z. Interleukin-1 alpha injection into ventromedial hypothalamic nucleus of normal rats depresses food intake and increases release of dopamine and serotonin. Pharmacology Biochemistry and Behavior. 1999;62:61–65. doi: 10.1016/s0091-3057(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Meguid MM. LHA dopaminergic activity in obese and lean Zucker rats. NeuroReport. 1995;6:1191–1194. doi: 10.1097/00001756-199505300-00029. [DOI] [PubMed] [Google Scholar]