Abstract
BACKGROUND
Dementia screening is currently recommended only for symptomatic patients.
OBJECTIVE
To evaluate memory complaints, a mental status test, and several cognitive tests as dementia screens in primary care.
DESIGN
Cross-sectional clinical epidemiologic study.
PARTICIPANTS
Three hundred thirty-nine comprehensively assessed, primary care patients aged ≥65 years.
MEASUREMENTS
Memory complaints were abstracted from chart review. Scores on Mini-Mental State Examination (MMSE) and domain-specific cognitive testing were compared to a dementia diagnosis based on Clinical Dementia Rating score ≥ 1, and areas under the receiver operating characteristic curves (AUC) were calculated. Classification and regression tree analyses were performed on memory complaints and tests with the highest AUCs.
RESULTS
Of 33 patients with dementia, only 5 had documented memory complaints. In 25 patients with documented memory complaints, no cognitive tests further improved identification of the 5 with dementia. In 28 patients with dementia but without memory complaints, an MMSE score < 20 identified 8 cases; among those with MMSE scores 20–21, a visual memory test identified a further 11 cases. Further cognitive testing could not detect 9 dementia cases without memory complaints and with MMSE scores ≥ 22.
CONCLUSIONS
In older primary care patients with memory complaints, cognitive screening does not help identify those who require further examination for dementia. Most patients with dementia do not report memory complaints. In these asymptomatic individuals, general mental status testing, supplemented by a memory test when the mental status score is equivocal, will identify lower-scoring patients who need dementia assessment. However, high-scoring asymptomatic dementia cases will remain undetected.
KEY WORDS: geriatrics, older adults, mental status, cognitive screening
INTRODUCTION
Cognitive impairment and dementia pose growing health care burdens among older adults.1 Patients with dementia often lack awareness of their cognitive impairments2 or may not report symptoms to their physicians. Family members, when available, may report memory and other cognitive losses to physicians. Astute clinicians themselves may observe cognitive impairment in their patients. Early identification is potentially useful for etiologic diagnosis, education of patients and families,3 and appropriate treatment planning.4,5 However, detection by primary care physicians remains low.6,7
Evidence-based standards for clinical practice require that asymptomatic individuals be screened for conditions such as breast cancer and hyperlipidemia,8,9 but screening for cognitive impairment or dementia remains controversial. Citing lack of evidence, current U.S. Preventive Services Task Force (USPSTF) guidelines argue neither for nor against screening asymptomatic adults for dementia.10 Evaluation of cognitive function is recommended by the USPSTF and others only if an impairment or deterioration in cognitive function is suspected,4,10–12 although this recommendation has been challenged.3,5,13–15 Besides lack of an empirical evidence base, barriers to screening include time and cost, lack of readily acceptable screening measures, lack of effective treatments, risk of distress and other adverse consequences in patients, and opposition to any routine mental health screening.6,16–21 Our purpose in this article is not to reiterate these arguments but to contribute data on the relative accuracy of different cognitive measures for the detection of dementia, in older primary care patients, in the presence and absence of self-reported symptoms.
We have previously reported that only a minority of medical records of elderly primary care patients reflected reports of cognitive problems by patients, families, or physicians.7 We now report a comparison of several cognitive tests on their ability to correctly classify older primary care patients both with self-reported memory complaints, referred to hereafter as “symptomatic,” and without such complaints. Earlier studies on screening have focused on both general mental status tests14,22,23 and domain-specific cognitive tests.24,25 To our knowledge, previous studies have not examined the relative utility of these tests for the detection of dementia in relation to self-awareness, reflected in subjective memory complaints.
METHODS
Subjects
The Steel Valley Seniors Survey was a clinical epidemiologic study of dementia in primary care patients aged 65 years and older in a small-town community in southwestern Pennsylvania. Study procedures were approved by the University of Pittsburgh Institutional Review Board. From 1999 to 2001, participants were recruited from the offices of 15 physicians who provided care to older adults and agreed to provide access to patients and to medical records of consenting patients.7
In the physician’s office, written informed consent was obtained, demographic information was collected, and a general mental status test, the Mini-Mental State Examination (MMSE), was administered. The MMSE briefly assesses global cognitive function with scores ranging from 0 to 30; lower scores indicate worse performance.26 A trained research nurse used a standardized chart abstraction protocol to review the medical record over the preceding 5 years for several variables including documentation of memory complaints.7
All participants who scored ≤24 on the MMSE, and a randomly selected comparison group of participants who scored ≥25, were offered a comprehensive assessment at home. The assessment included a cognitive test battery, brief medical history, physical and neurologic examination, depression symptom screen, questionnaire-based assessment of function, and detailed medication review, among other items.27
Memory Complaint
Participants were designated as “symptomatic” if self-reported memory complaints were documented in their charts. Reports by family members, if any, were excluded because many participants were unaccompanied by relatives when they visited their physicians. Details and examples have been reported previously.27
Cognitive Test Battery
The test battery included several simple tasks tapping a wide range of cognitive domains known to be affected in dementing disorders.
Verbal Memory The Hopkins Verbal Learning Test (HVLT) is a 12-item word list learning test with three learning trials, a 20-minute delayed recall, and forced choice recognition.28
Visual Memory The modified Rey–Osterrieth Complex Figure (Rey Figure) tests immediate and delayed visual memory, visuospatial skills, and constructional praxis,29 (initiating and completing new motor tasks that involve spatial organization, usually tested by ability to copy line diagrams).
Speed and Executive Functioning Trailmaking Tests A and B measure attention, processing speed, and visual search. Trailmaking B also measures executive functions including set-shifting and mental flexibility.30 We report Trailmaking scores as number of correct connections divided by time (seconds) to complete, normalize the distribution, and make higher scores represent better performance.
Verbal Fluency This tests word generation in a 60-second period, including initial letter fluency (P and S) and category fluency (animals).31
Constructional Praxis The Clock Drawing Test involves constructional praxis with elements of executive functioning (planning, motor sequencing, selective attention).32
Depressive Dymptom Screening A modified version of the Center for Epidemiological Studies Depression Scale33 was administered. Our modification, the mCES-D34 includes all original 20 items scored as present/absent.
Assessment of Dementia
The Clinical Dementia Rating (CDR) scale stages dementia based on cognition-related daily functioning derived from the entire comprehensive assessment except for the cognitive tests.35 Six domains of functional status are assessed, including memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. A standard algorithm is used to generate a summary score35,36 ranging from 0 (no dementia) through 0.5, 1, 2, and 3, reflecting questionable, mild, moderate, and severe dementia. A CDR rating ≥ 1 was treated as a diagnosis of dementia.
Statistical Analysis
Distributions are reported as percentages for categoric variables and as means, standard deviations (SD), and medians for continuous variables.
Two approaches were used to examine the classificatory properties of the cognitive tests.
Receiver Operational Characteristics (ROC) Method
For each cognitive test, ROC curves were generated by plotting sensitivity against 1-specificity for dementia at all possible cut points. Tests were rank ordered according to areas under the curves (AUC) and assessed for significant differences in AUC between pairs of tests. ROC curves and AUC were obtained using STATA 9.0.37
Classification and Regression Tree (CART) Method
CART analyses were performed on the cognitive tests with the highest AUCs that were not statistically significantly different from one another. Based on recursive partitioning,38,39 CART involves the segregation of different values of predictors through a decision tree composed of progressive binary splits. The optimal split is selected based on the impurity criterion (the reduction in the residual sum of squares because of a binary split of the data at that tree node). The validity of the final model was assessed using a modified version of the Gini index.39,40
The CART analyses were used to identify the cognitive tests, which would provide the greatest accuracy in classifying subjects as to the presence of dementia (CDR ≥ 1 vs CDR < 1). We specified the model based on clinically plausible steps rather than purely statistical model selection. Thus, the first variable in the CART was the presence/absence of a memory complaint, followed by the MMSE as the next step. The domain-specific cognitive tests were then introduced together to identify the tests and cut points, which would provide the best classification beyond the MMSE. The CART procedure automatically selects the best cut points (splits) for achieving the best possible classification. In post hoc analyses, a tree was generated introducing depressive symptoms (mCES-D score) into the models before the MMSE or other cognitive tests.
A tenfold cross-validation was used to assess the predictive ability of the tree model. The numbers of demented and non-demented individuals at each of the terminal nodes in the CART tree were used to calculate the sensitivity, specificity, positive predictictive value (PPV), and negative predictive value (NPV) at each split. OPen-source statistical software, R version 2.3.1, was used.
RESULTS
Sample Selection and Size
Of 1,107 primary care patients aged 65+ years screened with the MMSE in the physicians’ offices, the comprehensive assessment was offered to 642 participants: 343 with MMSE scores ≤ 24 and a comparison group of 299 randomly selected from those with scores ≥ 25. Three of the 642 (0.5%) moved away, 3 (0.5%) died before the home visit, and 358 (55.8%) underwent the comprehensive assessments.
Demographic Characteristics
Among the 642 participants selected for comprehensive assessment, those who did and did not undergo the assessment were similar (P > .05) with regard to age (77.5 vs 77.7 years), sex, (68.7% vs 64.0% women), education (66.8% vs 63.3% with ≥12 years), and race (92.5% vs 93.5% white). However, those assessed had a marginally higher mean (SD) MMSE score than those who were not [24.5 (SD 3.4) vs 24.0 (SD 3.2), P = .047].
Prevalence of Memory Complaint and Dementia
Of the 358 assessed individuals, 26 (7.3%) had memory complaints documented in their charts, and 6 (23.1%) of those with memory complaints had dementia (CDR ≥ 1). Dementia was also present in 41 (12.4%) of 332 participants without memory complaints.
Complete cognitive data were available on 339 participants, 25 (7.4%) of whose charts revealed memory complaints, and 33 (9.7%) of whom had dementia (CDR ≥ 1).
Among 19 individuals who did not complete all the cognitive tests, 14 had dementia; they were included in the AUC calculations for the tests that they completed but not in the CART analyses.
Screening Properties of Cognitive Tests
Areas Under the ROC Curve The MMSE had the highest AUC at 0.9121, followed by the HVLT immediate and delayed recall (Table 1). However, the AUCs were not significantly different among the MMSE, HVLT immediate and delayed recall, Rey Figure immediate and delayed recall, and Trailmaking B. These tests, as a group, had significantly higher AUCs than the Rey Figure copy, Trailmaking A, Clock Drawing, and verbal fluency tests.
Table 1.
Comparison of Areas Under the Receiver Operating Characteristics Curve (AUC)* for Cognitive Tests
| Tests | Number | AUC* | Significance level (P values) for pairwise comparisons of areas under the curve (AUC) between cognitive measures | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | HVLT immediate recall | Rey Figure delayed recall | Rey Figure immediate recall | HVLT delayed recall | Trailmaking B | Verbal fluency category | Rey Figure copy | Clock Drawing | Verbal fluency letter | Trail-making A | |||
| 0.912 | 0.906 | 0.896 | 0.887 | 0.881 | 0.868 | 0.808 | 0.798 | 0.793 | 0.787 | 0.764 | |||
| MMSE† | 358 | 0.912 | 0.72 | 0.63 | 0.29 | 0.08 | 0.47 | 0.001 | <0.001 | 0.001 | <0.001 | <0.001 | |
| HVLT†immediate recall | 357 | 0.906 | 0.70 | 0.44 | 0.22 | 0.57 | 0.001 | 0.002 | 0.004 | <0.001 | 0.001 | ||
| Rey Figure delayed recall† | 352 | 0.896 | 0.47 | 0.27 | 0.93 | 0.02 | 0.01 | 0.001 | 0.01 | 0.01 | |||
| Rey Figure Immediate recall† | 355 | 0.887 | 0.65 | 0.82 | 0.03 | 0.003 | 0.004 | 0.01 | 0.03 | ||||
| bHVLT delayed recall | 357 | 0.881 | 0.60 | 0.02 | 0.046 | 0.02 | 0.03 | 0.02 | |||||
| Trailmaking B† | 341 | 0.868 | 0.01 | 0.001 | 0.02 | 0.003 | <0.001 | ||||||
| Verbal Fluency category | 353 | 0.808 | 0.77 | 0.52 | 0.57 | 0.36 | |||||||
| Rey Figure copy | 355 | 0.798 | 0.94 | 0.68 | 0.63 | ||||||||
| Clock drawing | 355 | 0.793 | 0.94 | 0.64 | |||||||||
| Verbal Fluency initial letters | 357 | 0.787 | 0.97 | ||||||||||
| Trailmaking A | 351 | 0.764 | |||||||||||
*Area under the curve, referring to receiver operating characteristic curves for each test in relation to dementia (CDR ≥ 1)
†These tests are not significantly different in AUC from one another.
CART
The CART model (Fig. 1) and the sensitivity, specificity, PPV, and NPV of the final tree are based on test cut points selected by the CART procedure to maximize correct classification.
Figure 1.
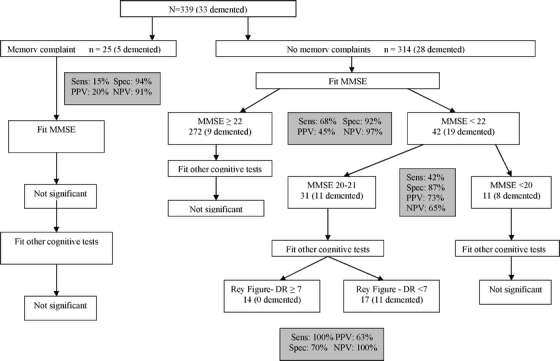
Outcomes of CART analyses for the presence/absence of memory complaints in participants with complete cognitive data. Sens = sensitivity; spec = specificity; PPV = positive predictive value; NPV = negative predictive value; dr = delayed recall.
Among 25 participants with memory complaints and complete cognitive data, of whom 5 (20%) had dementia, neither the MMSE nor any of the domain-specific cognitive tests further improved their classification into subgroups with and without dementia. Because this was a small subsample, lack of power is a potential explanation.
Among 314 participants without memory complaints, 28 (8.9%) had dementia. CART revealed that 8 of these dementia cases had MMSE scores < 20, 11 had MMSE scores 20–21, and 9 had MMSE scores ≥ 22. The 11 dementia cases with mid-range MMSE scores 20–21 were all detectable by a score < 7 on the visual memory test (Rey Figure delayed recall). The algorithm failed to detect 9 patients with dementia but without memory complaints and with MMSE scores ≥ 22.
As noted, 19 individuals who did not complete all the cognitive tests were excluded from the CART analyses. Of these, 14 had dementia, and only one had a documented memory complaint. Their MMSE scores ranged from 10 to 21, with a mean (SD) of 16.6 (3. 4), suggesting they would also have been detected by the MMSE cut points suggested by CART analysis.
In post hoc analyses, depressive symptoms (mCES-D) score was introduced into the tree but did not alter the previous classification. Eleven participants with incomplete mCES-D data were excluded from these post hoc models.
DISCUSSION
In a sample of older primary care patients, we used two complementary statistical approaches to examine the relative abilities of self-reported memory complaints and several cognitive measures to classify individuals according to their probability of having dementia. This classification is not equivalent to a diagnosis of dementia but rather identifies the subgroup of individuals for whom a diagnostic assessment for dementia might be warranted. Based on receiver operating characteristics, the general mental status test (MMSE) had the best classificatory ability although not significantly higher than tests of verbal memory, visual memory, speed/executive functioning. In CART analyses, among participants with memory complaints, cognitive testing did not further improve classification. In those without memory complaints, the MMSE was an effective first-pass screen, supplemented by the visual memory test among those with equivocal MMSE scores. Several participants with dementia did not complain of memory loss and could not complete the entire cognitive assessment, but did, in fact, obtain low MMSE scores; they would also have been detected using the MMSE. Approximately 3% of participants without memory complaints, and with higher scores on the MMSE, also had dementia, which would remain undetected by these steps. Depression can be associated with negative self-assessment including memory complaints,41 but did not alter the outcome of the tree analyses.
Generally, the term case finding describes the selective assessment of individuals with symptoms or risk factors, whereas the term screening refers to assessment of unselected individuals.42 Case finding requires a lower threshold of evidence for assessment because the individuals have already identified themselves as symptomatic or otherwise at risk. Our data suggest that case finding in patients with symptoms of memory loss should proceed directly to clinical assessment without further testing. However, focused cognitive screening can improve classification in those without memory complaints, identifying a smaller subgroup for clinical assessment. In a sense, the memory complaint itself functions as a positive “screen,” with high specificity albeit low sensitivity because most participants with dementia had no memory complaints.
Approaches previously suggested for dementia detection include waiting for spontaneous complaints from patients and/or families, routinely asking informants about changes in patients’ cognitive functioning,43,44 screening with general mental status tests,14,22,23 and screening with specific memory tests24,25 Whereas families may validly report the patient’s memory problems,43–46 they may also underreport patients’ difficulties,20,47 and not all individuals have reliable informants.
In our sample, the MMSE was of considerable value in the detection of dementia. The MMSE is both widely used and widely criticized in the research literature22,24; its superior performance in the current context is understandable by its relatively global scope. Much of the literature on cognitive screening is focused on general mental status tests. In studies of the MMSE using a cut point of 23/24, sensitivity ranged from 21 to 100%, whereas specificity ranged from 46 to 100%22; the wide range reflects the range of dementia severity in these studies. Other global screening tests, such as the Mini-Cog, with a sensitivity of 76% and specificity of 89%,14 and the 7-Minute Screen, with a sensitivity of 92% and specificity of 96%,23 have been recommended for their brevity. Tests of specific cognitive domains have been examined less frequently. In different studies, the MMSE had significantly higher AUC than the Trailmaking Tests and Clock Drawing24 and performed better than verbal fluency, Trailmaking Test, and Word List Learning at discriminating between individuals with and without dementia.25 In our study, the equally good performance of the memory and executive functioning tests is most likely explained by the weighting toward memory in current definitions of dementia, reflected in the CDR.35 Our data suggest a complementary role for a specific memory test if used only when scores on the general mental status test are equivocal; we believe this to be a novel finding and a practical suggestion.
The predictive value of a screening test depends in part on the prevalence of the disorder in a given population. The prevalence of dementia is relatively high in specialty settings such as geriatrics or memory disorders clinics,47,48 relatively low in epidemiologic surveys,49 and possibly intermediate in primary care. Because the majority of older adults receive their health services solely in primary care, these are the settings in which the utility of cognitive screening most needs to be determined.21,50
Our cognitive testing was performed by nurses with geriatric experience and stringent, systematic training and supervision in these specific assessments. In the typical primary care setting, this degree of training is neither available nor necessarily appropriate. Although these tests are simple, it is critical that clinicians who use them be adequately trained in their administration, scoring, interpretation, and limitations. They should not be portrayed as suitable for use by untrained personnel or as “do-it-yourself Alzheimer’s tests.”
A potential limitation is the relatively large proportion of participants who refused detailed assessment and might, thus, have introduced response bias51; however, they were similar to those who were assessed with regard to demographics and only marginally different in MMSE score. We were limited to chart review in determining whether patients had reported memory problems to their physicians, and the relatively small number with documented memory complaints may have reduced power for CART analyses in their subgroup. Our specific results may not generalize exactly beyond our sample; that is, in another sample, CART might reveal a different MMSE cut point to be ideal, and a different memory (delayed recall) test may provide equal or better classification. With a larger sample, we may have found that additional tests improved classification further; however, the practical likelihood seems low that a primary care assessment can include more than two cognitive tests.
This study contributes data on how different screening measures and memory complaints may be useful in detecting dementia, but the detection of dementia is not an end in itself. When and whether dementia should be detected in asymptomatic patients will depend on the potential costs, harms, and benefits of detecting dementia,50 along with relevant ethical considerations in different populations. A comprehensive discussion of harms and benefits is beyond the scope of this article, but key issues include the burden on physicians of providing detailed assessments to screen positive patients, the relative clinical and public health impact of false positive diagnoses versus leaving cognitive impairment and dementia undetected, and of early detection of an untreatable disorder in an asymptomatic patient. However, all cognitive impairment is not dementia, and not all dementia represents irreversible neurodegeneration; early detection and diagnosis may provide providers and patients with opportunities to intervene appropriately and limit excess disability through secondary and tertiary prevention.
Acknowledgments
We wish to thank Joni Vander Bilt and Eric Rodriguez for their comments and suggestions. Sources of funding include R01 AG16705 from NIA, K24 AG022035-03 from NIA, K25 059928 from NIDDK, and T32 AG021885 from NIA.
Conflict of Interest Dr. Chang, Dr. Saxton, and Dr. Ganguli are recipients of NIH grant funding. Dr. Saxton has been a consultant for Forest Research Institute and has received royalties from Harcourt Press. Dr. Ganguli has received speaker honoraria from the Mayo Clinic, Columbia University, the University of California at San Diego, the University of North Carolina, Servier, the Areces Foundation, and the Karolinska Institutet.
References
- 1.Pressley JC, Trott C, Tang M, Durkin M, Stern Y. Dementia in community-dwelling elderly patients: a comparison of survey data, Medicare claims, cognitive screening, reported symptoms, and activity limitations. J Clin Epidemiol. 2003;56:896–905. [DOI] [PubMed]
- 2.Feher EP, Larrabee GJ, Sudilovsky A, Crook TH 3rd. Memory self-report in Alzheimer’s disease and in age-associated memory impairment. J Geriatr Psychiatry Neurol. 1994;7:58–65. [PubMed]
- 3.Boise L, Camicioli R, Morgan DL, Rose JH, Congleton L. Diagnosing dementia: perspectives of primary care physicians. Gerontologist. 1999;39:457–64. [DOI] [PubMed]
- 4.Doraiswamy PM, Steffens DC, Pitchumoni S, Tabrizi S. Early recognition of Alzheimer’s disease: what is consensual? What is controversial? What is practical? J Clin Psychiatry. 1998;59(supp 13):6–18. [PubMed]
- 5.Lawrence J, Davidoff D, Katt-Lloyd D, Auerbach M, Hennen J. A pilot program of improved methods for community-based screening for dementia. Am J Geriatr Psychiatry. 2001;9:205–11. [PubMed]
- 6.Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–8. [DOI] [PubMed]
- 7.Ganguli M, Rodriguez E, Mulsant B, et al. Detection and management of cognitive impairment in primary care: the Steel Valley Seniors Survey. J Am Geriatr Soc. 2004;52:1668–75. [DOI] [PubMed]
- 8.Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137:347–60. [DOI] [PubMed]
- 9.Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [DOI] [PubMed]
- 10.US Preventive Services Task Force. Screening for dementia: recommendation and rationale. Ann Intern Med. 2003;138:925–26. [DOI] [PubMed]
- 11.Brodaty H, Clarke J, Ganguli M, et al. Screening for cognitive impairment in general practice: toward a consensus. Alzheimer Dis Assoc Disord. 1998;12:1–13. [DOI] [PubMed]
- 12.Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–71. [PubMed]
- 13.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The Mini-Cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021–7. [DOI] [PubMed]
- 14.Borson S, Scalan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53:871–4. [DOI] [PubMed]
- 15.Brodaty H, Low LF, Gibson L, Burns K. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry. 2006;14:391–400. [DOI] [PubMed]
- 16.Brodaty H, Howarth GC, Mant A, Kurrle SE. General practice and dementia. A national survey of Australian GPs. Med J Aust. 1994;160:10–4. [PubMed]
- 17.Boustani M, Watson L, Fultz B, Perkins AJ, Druckenbrod R. Acceptance of dementia screening in continuous care retirement communities: a mailed survey. Int J Geriatr Psychiatry. 2003;18:780–6. [DOI] [PubMed]
- 18.Boustani M, Petersen B, Hanson L, Harris R, Lohr K. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927–37. [DOI] [PubMed]
- 19.Bush C, Kozak J, Elmslie T. Screening for cognitive impairment in the elderly. Can Fam Physician. 1997;43:1763–8. [PMC free article] [PubMed]
- 20.Watson LC, Lewis CL, Fillenbaum GG. Asking family about memory loss: is it helpful? J Gen Intern Med. 2004;20:28–32. [DOI] [PMC free article] [PubMed]
- 21.Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20:572–7. [DOI] [PMC free article] [PubMed]
- 22.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. [DOI] [PubMed]
- 23.Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349–55. [DOI] [PubMed]
- 24.Kilada S, Gamaldo A, Grant EA, Moghekar A, Morris JC, O’Brien RJ. Brief screening tests for the diagnosis of dementia: comparison with the Mini-Mental State Exam. Alzheimer Dis Assoc Disord. 2005;19:8–16. [DOI] [PubMed]
- 25.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–80. [DOI] [PubMed]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–98. [DOI] [PubMed]
- 27.Ganguli M, Du Y, Rodriguez E, et al. Discrepancies in information provided to primary care physicians by patients with and without dementia: the Steel Valley Seniors Survey. Am J Geriatr Psychiatry. 2006;14:446–55. [DOI] [PubMed]
- 28.Benedict RHB. Hopkins Verbal Learning Test—revised: normative data and analysis of inter-form and test–retest reliability. Clin Neuropsychol. 1998;12:43–55.
- 29.Saxton JA, Becker JT. The Rey–Osterrieth complex figure and dementia. In: Knight JA, Kaplan EF, eds. Handbook of Rey–Osterrieth complex Figure Usage. Psychological Assessment Resources. Odessa, FL.
- 30.Reitan RM. The relation of the Trailmaking Test to organic brain damage. J Consult Psychol. 1955;19:393–4. [DOI] [PubMed]
- 31.Borkowski JG, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1976;5:135–40.
- 32.Freedman M, Leech L, Kaplan E. Clock Drawing: A Neuropsychological Analysis. New York: Oxford University Press; 1994.
- 33.Radloff LS, Teri L. Use of the Center for Epidemiological Studies–Depression Scale with older adults. Clin Gerontol. 1986;5:119–37.
- 34.Ganguli M, Gilby J, Seaberg E, Belle S. Depressive symptoms and associated factors in a rural elderly population: The MoVIES Project. Am J Geriatr Psychiatry. 1995;3:144–60. [DOI] [PubMed]
- 35.Hughes CP, Berg L, Danzinger WL, Coben LA, Martin RL. A new clinical rating scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. [DOI] [PubMed]
- 36.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(suppl 1):173–6. [DOI] [PubMed]
- 37.StaCorp. Stata statistical software: Release 9. College Station, TX: StataCorp LP. 2005.
- 38.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Wadsworth; 1984.
- 39.Ripley BD. Pattern Recognition and Neural Networks. Cambridge: Cambridge University Press; 1996.
- 40.Gini C. “Variabilitá e mutabilita.” 1912. In: Pizetti E, Salvemini T, eds. Reprinted in Memorie di metodologia statistica. Rome: Libreria Eredi Virgilio Veschi; 1955.
- 41.Zandi T. Relationship between subjective memory complaints, objective memory performance, and depression among older adults. Am J Alzheimers Dis Other Dement. 2004;19:353–60. [DOI] [PMC free article] [PubMed]
- 42.Rubenstein LZ, Alessi CA, Josephson KR, Trinidad Hoyl M, Harker JO, Pietruszka FM. A randomized trial of a screening, case finding, and referral system for older veterans in primary care. J Am Geriatr Soc. 2007;55:166–74. [DOI] [PubMed]
- 43.Jorm AF. Assessment of cognitive impairment and dementia using informant reports. Clin Psychol Rev. 1996;16:51–73.
- 44.Mackinnon A, Mulligan R. Combining cognitive testing and informant report to increase accuracy in screening for dementia. Am J Psychiatry. 1998;155:1529–35. [DOI] [PubMed]
- 45.Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724–6. [DOI] [PubMed]
- 46.Monnot M, Brosey M, Ross E. Screening for dementia: family caregiver questionnaires reliably predict dementia. J Am Board Fam Pract. 2005;18:240–56. [DOI] [PubMed]
- 47.Ross GW, Abbott RD, Petrovitch H, et al. Frequency and characteristics of silent dementia among elderly Japanese-American men. The Honolulu–Asia Aging Study. JAMA. 1997;277:800–5. [PubMed]
- 48.Kukull WA, Larson EB, Teri L, Bowen J, McCormick W, Pfanschmidt ML. The Mini-Mental State Examination score and the clinical diagnosis of dementia. J Clin Epidemiol. 1994;47:1061–7. [DOI] [PubMed]
- 49.Cossa FM, Della Sala S, Musicco M, Spinnler H, Ubezio M. The Milan Overall Dementia Assessment and the Mini-Mental State Examination compared: an epidemiological investigation of dementia. Eur J Neurol. 1999;6:289–94. [DOI] [PubMed]
- 50.Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295:2148–57. [DOI] [PubMed]
- 51.Boustani M, Perkins AJ, Fox C, et al. Who refuses the diagnostic assessment for dementia in primary care? Int J Geriatr Psychiatry. 2006;21:556–63. [DOI] [PubMed]


