Abstract
Background
We investigated the association of process of care measures with adverse limb and systemic events in patients with peripheral arterial disease (PAD).
Methods
We conducted a retrospective cohort study of patients with PAD, as defined by an ankle-brachial index (ABI) <0.9. The index date was defined as the date, during 1995 to 1998, when the patient was seen in the Michael E. DeBakey VA Medical Center noninvasive vascular laboratory and found to have PAD. We conducted a chart review for process of care variables starting 3 years before the index date and ending at the time of the first event or the final visit (December 31, 2001), whichever occurred first. We examined the association between PAD process of care measures, including risk factor control, and prescribing of medication, with time of the patient’s first major limb event or death.
Results
Of the 796 patients (mean age, 65 ± 9.9 years), 230 (28.9% experienced an adverse limb event (136 lower-extremity bypass, 94 lower-extremity amputation), and 354 (44.5%) died. Of the patients who died, 247 died without a preceding limb event. Glucose control was protective against death or a limb event with a hazard ratio (HR) of 0.74 (95% confidence limits [CL] 0.60, 0.91, P = 0.004). African Americans were at 2.8 (95% CL 1.7, 4.5) times the risk of Whites or Hispanics for an adverse limb event. However, this risk was no longer significant if their glucose was controlled. For process measures, the dispensing of PAD specific medication (HR 1.4, 95% CL 1.1, 1.7) was associated an increased risk for an adverse outcome.
Conclusions
Our data suggest that glucose control is key to reducing the risk for adverse outcomes, particularly limb events in African Americans. Certain process of care measures, as markers of disease severity and disease management, are associated with poor outcomes in patients with PAD. Further work is needed to determine the role of early disease intervention to reduce poor outcomes in patients with PAD.
KEY WORDS: peripheral arterial disease, process of care, glucose control, surgery, atherosclerosis risk factors
BACKGROUND
Peripheral arterial disease (PAD) is an atherosclerotic syndrome for which prevalence increases with age older than 60 years and exposure to modifiable atherosclerosis risk factors.1–3 Process of care is an all-inclusive term for the services that are provided to or for patients or by patients themselves based on medical advice.4 The primary goals in the management of individuals with PAD are to reduce systemic ischemic events and limb events (i.e., progressive ischemia that requires lower extremity surgical bypass or amputation).5 Lower extremity bypass surgery, even when successful, is associated with a 30-day mortality of 2% to 8%, 5-year graft failure rates of 20% to 90%, and 5-year risk of amputation that remains as high as 20%.6 Leg amputation is associated with an impairment of functional status, reduced quality of life, and high mortality.7–11 Thus, although most prior PAD specific clinical trials have evaluated interventions to decrease systemic ischemic events, or to improve claudication, the role of process of care to reduce limb events has received much less attention. Therefore, we sought to determine the impact of process of care on ischemic limb outcomes and death in patients with PAD.
METHODS
Study Population
Sequential patients evaluated at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) noninvasive vascular laboratory from 1995 to 1998 with a diagnosis of PAD based on an ankle-brachial index (ABI) of <0.90 were included in this study. Individuals were stratified into tertiles of PAD severity based on ABI values: mild (0.7–0.89), moderate (0.41–0.69), or severe (≤0.40). Patients who had undergone a prior lower extremity vascular surgical revascularization procedure or transmetatarsal, (below-the-knee, or above-the-knee) amputation were excluded. The Baylor College of Medicine Institutional Review Board approved this study.
Based on prior information highlighting meaningful intervals for atherosclerotic risk factor control (12), we chose 3 years before diagnosis of PAD (index date) as the starting point for observing risk factors and their modification (time 0). All patients were followed until their first limb event (bypass surgery or amputation), death, or the end of the observation period (December 31, 2001). Thus, our retrospective cohort involved patient record abstractions starting as early as January 1, 1992 (time 0), and ending at the time of the first adverse limb event, death, or December 31, 2001. The average period of post index visit follow-up was 16.3 months (range 0–77 months).
Data Source
We used medical records from Veterans Integrated Systems Technology Architecture (VISTA) to identify our cohort and its outcomes. Medical charts were reviewed from time 0 to the end of follow-up. Data on a veteran’s death were abstracted from the medical record when available and from the VA administrative files, the Beneficiary Identification and Records Locator Subsystem (BIRLS) Death File and the patient treatment file (PTF).
Primary Outcome
The primary outcome was time to lower extremity bypass surgery, major amputation, or death. Lower extremity bypass surgeries included aortoiliac or aortobifemoral, femoropopliteal above or below the knee, and femorotibial vascular bypass. Lower extremity amputations included below-the-knee and above-the-knee major procedures (transmetatarsal or toe amputations were not included). This study did not evaluate lower extremity endovascular procedures as a component of the primary outcome, as these procedures were not available during our study period. Secondary outcomes comprised time to death and time to bypass or amputation.
Independent Variables
In addition to basic patient demographic and health measures comprising age, race, prior history of CAD, and ABI-based PAD severity, our independent variables comprised actual risk factor control (smoking cessation, glycemic control in diabetes, hypertension control, lipid control in dyslipidemia). We also tracked cohort members’ serum creatinine, exposure to antiplatelet therapy, and exposure to pharmacological therapy for each of the 4 risk factors. This latter set of medications included use of ace inhibitors, beta blockers, calcium channel blockers, diuretics, diabetes medications, lipid controlling medications, other antihypertensive medications, and smoking cessation medications. We identified the dates on which a patient was on a given class of medications using the prescription fill date and the days supply of medication from the pharmacy record.
Data Collection
Trained nurse chart abstractors who were blinded to the study hypotheses completed the chart reviews, using standardized data extraction forms developed by study investigators. For each chart review, nurses captured information on the patient’s age, dates of clinic visits, reasons for clinic visits, symptoms, physical examination findings (e.g., vital signs), diagnoses, and risk factor modification.
VISTA data served as the source of information on race/ethnicity and laboratory results relevant to atherosclerotic risk factors (e.g., glucose, total cholesterol, creatinine), vascular laboratory reports, radiology reports (i.e., imaging of lower limb blood flow), and pharmacy data.
Risk Factor Control
We determined effective control of a risk factor based on documentation from charted vital signs or laboratory data. We defined control of each risk factor according to national guidelines, including those of the National Cholesterol Education Panel; Joint National Committee on Detection, Evaluation, and Management of Hypertension; American Diabetes Association; and American Cancer Society.12–16 Low-density lipoprotein (LDL) cholesterol was considered under control if the value was 100 mg/dL or less. Smoking was considered to be out of control if the patient was referred to a smoking cessation clinic, recommended to be on a nicotine gum or nicotine patch, was prescribed other smoking medications, or advised not to smoke in addition to whether it was indicated that the patient was currently smoking or refused to quit smoking. A glycosylated hemoglobin value (Hgb A1c) above 7 or, in the absence of a Hgb A1c measure, a fasting glucose greater than 140 indicated that glucose was not under control. A systolic blood pressure greater than 140 or a diastolic blood pressure greater than 90 indicated that blood pressure was not under control.
Statistical Analysis
We assessed potential associations between control of these risk factors with the time to critical ischemic limb outcome (vascular surgery or amputation) or death. We also examined potential associations between control of these risk factors and time to death and time to bypass or major amputation.
We used multivariate Cox survival models to identify risk factors for the primary outcome (lower extremity bypass surgery, amputation, or death). The dependent variable was the length of time from vascular laboratory visit to event, measured in days. Potential explanatory variables in the Cox model comprised age, race, prior history of CAD, ABI-based PAD severity, the time-varying measures of risk factor control (smoking cessation, glycemic control, hypertension control, and lipid control), serum creatinine, pharmacological therapy for each of the 4 risk factors, and antiplatelet therapy. We also considered potential interactions between race and the risk factor control measures. We used stepwise selection to identify significant variables, allowing variables with p < 0.25 to enter the models and variables with p < 0.15 to stay. Similar approaches were used to assess risk factors for the secondary outcomes.
The initial value for the time-dependent covariate was based on the information available in the patient chart on the index date. If a patient did not have the value of a variable recorded on the index date, we used the most recent value before the index. The value of a variable could change at every time within the patient’s record that new information about that variable was available.
For illustrative purposes we present Kaplan–Meier survival analysis curves for time to the first event stratified by disease severity (i.e., level of the ABI; Fig. 1) and separate curves stratified by race (Fig. 2), as race and severity were identified as relevant predictors for each of the outcomes.
Figure 1.
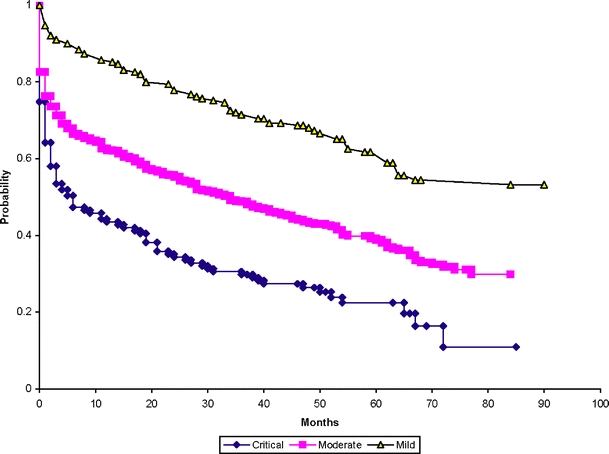
Survival function estimates for adverse limb outcome or death by disease severity.
Figure 2.
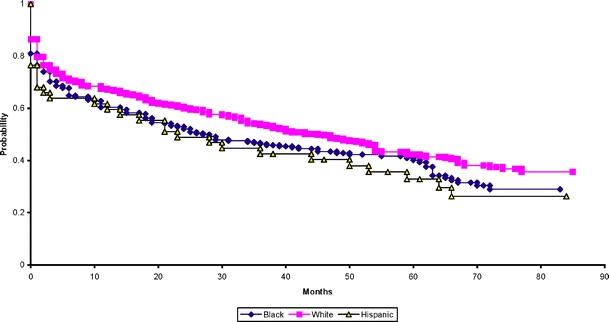
Survival function estimates for adverse limb outcome or death by race.
RESULTS
A total of 816 patients met the criteria for inclusion in this study; 796 (97.5%) had notes or laboratory data that could be used to assess their process of care. Mean age was 64.7 ± 9.9 years; 99% were men; 30.4% were African American; 5.9% were Latino; and 507 (63.7%) were White or of unknown race (see Table 1). As defined by the ABI level, the prevalence of critically severe PAD was 16.5%, of moderately severe was 59.8%, and of mildly severe was 23.7%. Within the cohort, 136 (17.1%) and 94 (11.8%) patients, respectively, had a lower extremity bypass operation or amputation as their first event; 247 (31%) patients died without a limb event; and the total number of deaths was 354 (44.5%). Of the patients who died, 104 (29.4%) were dead within 1 year of the index date. As captured anytime during the observation period, a total of 509 (63.9%) were smokers, 488 (61.3%) had diabetes mellitus, 439 (55.2%) had elevated LDL, and 697 (87.6%) had hypertension.
Table 1.
Baseline Characteristics of the Cohort
| Variable | Total cohort N = 796 | Patients without any event N = 319 | Patients who underwent lower extremity bypass surgery N = 136 | Patients who underwent a lower extremity amputation N = 94 | Patients who died without a limb event N = 247 |
|---|---|---|---|---|---|
| Mean age (SD) | 64.7 (9.9) | 62.9 (10.1) | 62.3 (9.8) | 66.4 (10.0) | 67.7 (8.7) |
| Age <55 y | 145 (18.2%) | 75 (23.5%) | 32 (23.5%) | 13 (13.8%) | 25 (10.1%) |
| Age 55–74 y | 533 (67.0%) | 208 (65.2%) | 94 (69.1%) | 61 (64.9%) | 170 (68.8%) |
| Age 75 y and older | 118 (14.8%) | 36 (11.3%) | 10 (7.4%) | 20 (21.3%) | 52 (21.1%) |
| Sex (male) | 788 (99.0%) | 318 (99.7%) | 134 (98.5%) | 94 (100%) | 242 (98.0%) |
| Race/Ethnicity: | |||||
| African American | 242 (30.4%) | 89 (27.9%) | 41 (30.1%) | 44 (46.8%) | 68 (27.5%) |
| Latino | 47 (5.9%) | 14 (4.4%) | 4 (2.9%) | 11 (11.7%) | 18 (7.3%) |
| White/Other/Unknown | 507 (63.7%) | 216 (67.7%) | 91 (66.9%) | 39 (41.5%) | 161 (65.2%) |
| ABI | |||||
| Critical ≤0.40 | 131 (16.5%) | 29 (9.1%) | 37 (27.2%) | 29 (30.9%) | 36 (14.6%) |
| Moderate 0.41 to 0.69 | 476 (59.8%) | 177 (55.5%) | 92 (67.6%) | 55 (58.5%) | 152 (61.5%) |
| Mild 0.70–0.89 | 189 (23.7%) | 113 (35.4%) | 7 (5.1%) | 10 (10.6%) | 59 (23.9%) |
| Creatinine levels | |||||
| <1.5 | 644 (80.9%) | 271 (85.0%) | 121 (89.0%) | 69 (73.4%) | 183 (74.1%) |
| 1.5–2.0 | 86 (10.8%) | 30 (9.4%) | 11 (8.1%) | 10 (10.6%) | 35 (14.2%) |
| 2.1 or higher | 66 (8.3%) | 18 (5.6%) | 4 (2.9%) | 15 (16.0%) | 29 (11.7%) |
| Atherosclerotic risk factors: | |||||
| Diabetes mellitus | 488 (61.3%) | 185 (58%) | 62 (45.6%) | 72 (76.6%) | 169 (68.4%) |
| Hypertension | 697 (87.6%) | 293 (91.8%) | 102 (75%) | 79 (84%) | 220 (89.1%) |
| Hyperlipidemia | 439 (55.2%) | 216 (67.7%) | 67 (49.3%) | 35 (37.2%) | 21 (49%) |
| Current smoking | 509 (63.9%) | 241 (75.5%) | 85 (62.5%) | 37 (39.4%) | 146 (59.1%) |
Time to Limb Event or Death
Within our multivariate modeling to assess risk factors for time to limb event or death (Table 2), variables that were significantly associated with an increased risk for any event included moderately severe disease (HR 1.9, 95% CL 1.5, 2.5), critically severe disease (HR 3.1, 95% CL 2.3, 4.2). In addition, serum creatinine 1.5 to 2.0 mg/dL (HR 1.3, 95% CL 1.0, 1.8, p = .064) and serum creatinine greater than 2.0 mg/dL (HR 3.1, 95% CL 2.4, 4.1) were both found to be associated with time to event. Blood pressure control was associated with an increased risk of an event (HR 1.4, 95% CL 1.2, 1.7). Additional risk factors associated with an increased risk for a limb event or death were the use of PAD specific medication, ace inhibitors, and diuretics. In contrast, a history of coronary artery disease (HR 0.64, 95% CL 0.53, 0.78) and glucose control (HR 0.74, 95% CL 0.63, 0.91) were associated with a lower risk for a limb event or death.
Table 2.
Limb Event or Death
| Variable | P value | Hazard ratio | 95% Confidence limits |
|---|---|---|---|
| Moderate ABI 0.41–0.69 | <.0001 | 1.94 | 1.51, 2.50 |
| Critical ABI ≤0.40* | <.0001 | 3.08 | 2.28, 4.16 |
| History of coronary artery disease | <.0001 | 0.64 | 0.53, 0.78 |
| Current glucose control | .0044 | 0.74 | 0.60, 0.91 |
| Current blood pressure control | .0004 | 1.42 | 1.17, 1.73 |
| Serum creatinine 1.5 to 2.0 | .0643 | 1.35 | 0.98, 1.85 |
| Serum creatinine 2.1 or higher | <.0001 | 3.14 | 2.44, 4.05 |
| Use of PAD specific medications | .0013 | 1.39 | 1.14, 1.69 |
| Use of Ace inhibitors | .0431 | 1.25 | 1.01, 1.56 |
| Use of diuretics | .0033 | 1.37 | 1.11, 1.70 |
| Use of lipid lowering medication | .0812 | 0.80 | 0.63, 1.03 |
The outcome of limb event or death includes any of the 3 outcomes of lower extremity bypass surgery, lower extremity amputation, or death. The reference group for the serum creatinine comparisons is a value of <1.5.
*The reference group is mild ABI (0.7–0.89).
Limb Events Combined
Within the multivariate modeling of time to a limb event (Table 3), the identified risk factors included moderate ABI disease severity (HR 4.0, 95% CL 2.4, 6.6), and critical ABI disease severity (HR 7.7, 95% CL 4.5, 13.2). Patients with a history of coronary artery disease were less likely to receive lower extremity bypass surgery or lower extremity amputation (HR 0.51, 95% CL 0.40, 0.67). A significant interaction between African-American ethnicity and glucose control (p = .003) was indentified where glucose control was associated with a 20% reduction in risk (HR 0.81, 95% CI .56, 1.16) for Whites and Hispanics, whereas for African Americans glucose control was associated with a 67% reduction in risk (HR 0.33, 95% CI 0.21, 0.52, p value <.0001). African Americans with out-of-control glucose are at increased risk (HR 2.8, 95% CI 1.7, 4.5), but African Americans with glucose under control do not have a significantly increased risk (HR 1.1, 95% CI 0.8, 1.6, p = .462). Table 4
Table 3.
Major Limb Event (Lower Extremity Bypass Surgery or Lower Extremity Amputation)
| Variable | P value | Hazard ratio | 95% Confidence limits |
|---|---|---|---|
| Moderate ABI 0.41–0.69* | <.0001 | 4.02 | 2.43, 6.65 |
| Critical ABI ≤0.40 | <.0001 | 7.69 | 4.49, 13.17 |
| History of coronary artery disease | <.0001 | 0.51 | 0.40, 0.67 |
| African-American ethnicity | <.0001 | 2.75 | 1.70, 4.45 |
| Current glucose control | .2458 | 0.81 | 0.56, 1.16 |
| Current glucose control in African Americans | <.0001 | 0.33 | 0.21, 0.52 |
The outcome of limb events is limited to the 2 limb outcomes censoring at death. ABI refers to ankle-brachial index.
*The reference group is mild ABI (0.7–0.89); reference group for African-American race, white/other/unknown.
Table 4.
Mortality
| Variable | P value | Hazard ratio | 95% Confidence limits |
|---|---|---|---|
| Moderate ABI 0.41–0.69* | .0234 | 1.38 | 1.05, 1.83 |
| Critical ABI ≤0.40 | <.0001 | 2.03 | 1.44, 2.86 |
| Age 55 to 74 years | .1391 | 1.29 | 0.92, 1.80 |
| Age 75 years or older | .0003 | 2.04 | 1.38, 3.00 |
| Current LDL cholesterol control | .0020 | 1.42 | 1.14, 1.77 |
| Current blood pressure control | <.0001 | 1.74 | 1.38, 2.19 |
| Serum creatinine 1.5–2.0** | .0786 | 1.36 | 0.97, 1.92 |
| Serum creatinine 2.0 or higher** | <.0001 | 4.49 | 3.45, 5.84 |
| Use of PAD specific medications | .0041 | 1.41 | 1.11, 1.78 |
| Use of medication for diabetes mellitus | .0401 | 1.29 | 1.01, 1.63 |
| Use of Ace Inhibitors | .0694 | 1.26 | 0.98, 1.61 |
| Use of diuretics | <.0001 | 1.66 | 1.30, 2.12 |
| Use of lipid lowering medication | .0629 | 0.78 | 0.59, 1.01 |
The outcome of death includes death with or without a preceding limb event. ABI refers to ankle-brachial index.
*The reference group is mild ABI (0.7–0.89).
**The reference group for the serum creatinine comparisons is a value of <1.5.
Mortality
Risk factors associated with an increased risk for mortality included age 75 years or older (HR 2.0, 95% CL 1.4, 3.0), moderately severe disease (HR 1.4, 95% CL 1.0, 1.8), critically severe disease (HR 2.0, 95% CL 1.4, 2.8), and serum creatinine greater than 2.0 (HR 4.5, 95% CL 3.4, 5.8). The process variables of lipid control (HR 1.4, 95% CL 1.1, 1.8) and blood pressure control (HR 1.7, 95% CL 1.4, 2.2) were both associated with increased risk of death. Additional factors associated with mortality included the use of PAD-specific medication, diuretics, and medications to control diabetes mellitus. Of these 3 processes of care measures, the use of diuretics was associated with a greatest risk for mortality (HR 1.7, 95% CL 1.3, 2.1).
DISCUSSION
These data demonstrate that sociodemographics, disease severity, and renal dysfunction are associated with an increased risk for critical limb and systemic events. Our survival analyses indicate that critical outcomes are more likely to occur with lack of glucose control. Additional process measures that were associated with adverse outcomes included the use of diuretics, ace inhibitors, glucose controlling agents, and PAD-specific medications. To our knowledge, this is the first study to find associations between process of care factors and adverse outcomes in patients with PAD.
African-American race, older age, disease severity (per the ankle-brachial index), and renal insufficiency are known risk factors for poor outcomes in patients with PAD. We know from prior work that, in comparison to Whites, African-American persons with a diagnosis of PAD are more likely to undergo a lower limb amputation versus a lower limb bypass operation.17 Our current findings further support the association of race with poor outcomes in PAD. Interestingly, our findings suggest that for African Americans whose glucose levels are controlled, their risk for an adverse limb event is not significantly different than the risk for a White or Hispanic.
The ankle-brachial index (ABI) is a marker for PAD severity and a well-known predictor of both limb and systemic outcomes. Lower levels of the ABI have been linked to increased mortality,18 nonfatal cardiovascular events,19 and reduced walking ability.20,21 Among illnesses that may coexist with PAD, end-stage renal disease is common. The presence of both PAD and end-stage renal disease is associated with an increased risk for cardiovascular-related mortality, morbidity, hospitalization, and poor quality of life.22 Renal insufficiency, as measured using serum creatinine, was a risk factor for both mortality and adverse surgical limb outcomes within our study. The role of early intervention for PAD specific to persons with renal insufficiency warrants further study.
Process measures that were associated with adverse events in PAD included glucose control and the use of glucose-lowering agents. We found an association between glucose control and the risk for an adverse surgical limb event in patients with PAD. We observed that patients with glucose control were less likely to undergo lower extremity bypass surgery or a lower extremity amputation. Patients with diabetes mellitus are known to be at increased risk for PAD progression, particularly development of critical limb ischemia and subsequent limb loss.23 Also, prior investigations have demonstrated that veterans with diabetes are more likely to undergo lower limb amputation.17 Our cohort study offers an association of the role of glycemic control to reduce the need for lower extremity surgery. Further prospective investigation will be required to verify that glycemic interventions offer risk reduction in patients with PAD.
Diabetes mellitus is managed with diet, oral agents, and/or insulin. In persons with type 2 diabetes mellitus, who comprise the majority of veterans with PAD,17 the need for either insulin or oral agents instead of diet control is a marker for more advanced disease. Not surprisingly within our study, persons with diabetes who required oral agents or insulin were more likely to have an adverse outcome. As a process measure, the use of medications to control glucose likely reflects a sicker patient who is more likely to experience an adverse event.
Additional process measures that were associated with adverse events in PAD included the use of PAD specific medication and diuretics. Within our study, PAD-specific medications were limited to cilostazol and pentoxyphylline—these agents are specific for the treatment of leg pain in PAD. With antiplatelet and vasodilator properties, cilostazol is indicated for the treatment of intermittent claudication.24,25 Cilostazol inhibits vascular smooth muscle proliferation and causes vasodilatation. Cilostazol improves both pain-free and maximal treadmill walking distance. A drug that was available before cilostazol and which is now used much less often is pentoxyifylline. This lower use of pentoxyphylline is a result of several reports, indicating that it was not substantially better than placebo in improving pain-free or absolute walking distances in patients with PAD.26–31 The association of the use of these medications with an increased likelihood of adverse events likely reflects an association between disease severity and outcomes beyond that captured by the ABI. Persons with PAD and leg symptoms have advanced atherosclerosis with reduced flow to the lower limbs.32 Efforts are needed to determine the role of aggressive management of persons with PAD and leg symptoms to reduce mortality and the need for limb surgery. Similarly, persons with PAD who received diuretics were more likely to die or have an adverse limb event. As diuretics are used in persons with hypertension, chronic heart failure, and/or lower limb edema, this process measure is also likely a marker for a sicker patient.
In contrast to the above findings, a history of coronary artery disease (CAD) was associated with a lower risk for death or an adverse limb event. Although this finding may seem counterintuitive, the apparent protective nature of this variable has been addressed in prior work. In a national physician survey to ascertain reported practice behavior based on case scenarios, physicians believed that risk factor treatment was more important for CAD than for PAD.33 This study found strong correlations between physicians’ report of the importance of risk factor interventions and their current practice behavior. Similarly, Banta et al34 reported that persons with PAD were more likely to receive lipid lowering medication and achieve goal LDL if they had a history of CAD. Within our study of veterans with PAD, a history of CAD may have led to a more aggressive treatment of atherosclerotic risk factors, which would have reduced the risk for adverse events.
An additional finding within our study was the increased risk for adverse outcomes in persons whose blood pressure was controlled. Our blood pressure variable presents unexpected results. We know that blood pressure control is important to reduce cardiovascular events.12 We believe that our findings reflect some important unmeasured confounders or a potential bias in the approach taken as discussed in the limitations section. Similarly, LDL control was associated with an increased risk for death. Patients with PAD warrant aggressive lipid control.13 For a patient with PAD, as the disease progresses, their lipids may be well controlled with the use of medication, but their underlying medical status continues to place them at an increased risk for mortality.
Limitations
First, we note that we present findings from a retrospective cohort. Our results reflect the care received, which is influenced by the complexities of patient comorbidities. Whereas the presence of a risk factor may reflect the burden of disease, the same risk factor may also be a marker of how care is delivered. For example, patients with coronary artery disease are not low-risk patients, but when a physician knows that a patient has coronary artery disease, he/she may provide more aggressive care.33 Although we do not have causal association within our findings, our findings are hypotheses generating. Second, our process measure for the use of a given medication was based on dispensing and refill patterns. We do not have data on patient adherence. Third, we note that we could not evaluate other potentially important covariables in this retrospective study design, such as access to other non-VA medical care and family or social support, which could have contributed to our observed outcomes. Finally, as discussed by Hernan, Brumback, and Robins,35 as the risk factor control and pharmaceutical measures may affect one another over time, the hazard ratios presented above may be biased estimates of any causal associations between risk factor control and the outcomes examined. Methods exist to examine causal effects in such situations, but these methods can be difficult to apply. In applying the marginal structural model methods outlined by Hernan et al, the estimated weights central to the method were so variable and skewed that 70% to 90% of the observed follow up was essentially dropped from the analysis, leading us to view the application of this method to these data with skepticism.
CONCLUSION
Our data highlight those process measures that are markers for poor outcomes in persons with PAD. PAD interventions early in the disease process and before the need for aggressive process of care may positively impact the risk for death, bypass surgery, or amputation in patients with PAD. This hypothesis could best be evaluated in a prospective clinical trial comparing standard versus aggressive care in patients with PAD.
Acknowledgments
This work was supported by an Investigator-Initiated Grant (#01-180-1; Principal Investigator, Dr. Tracie C. Collins) from the Department of Veterans Affairs, Health Services Research and Development, along with resources from the Houston Center for Quality of Care and Utilization Studies, Michael E. DeBakey Veterans Affairs Medical Center. Dr. Collins was a recipient of the Department of Veterans Affairs Advanced Clinical Research Career Development Award. Dr. Beyth was a recipient of the Department of Veterans Affairs HSR&D Advanced Research Career Development Award during data collection for this work. We acknowledge and are very grateful for the excellent study support by Patricia N. Krueger, Meei Ku-Goto, and Diana Urbauer.
Conflicts of Interest The following were reported by the authors: Dr. Collins is a consultant (Data and Safety Monitoring Committee) for Synteract. Dr. Hirsch has grants from AstraZeneca, Biomedix, Bristol-Myers Squibb-Synthelabo, Omron, PreMD, and SonoSite. He is a consultant for Pfizer. Dr. Bush is a consultant for Guidant, Abbott and Endologix.
Footnotes
This work was conducted by Dr. Collins while at the Houston Center for Quality of Care and Utilization Studies, Michael E. DeBakey VA Medical Center, and Section of Health Services Research, Baylor College of Medicine, Houston, Texas.
REFERENCES
- 1.Collins T, Petersen N, Suarez-Almazor M, Ashton C. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163:1469–74. [DOI] [PubMed]
- 2.Hirsch A, Criqui M, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. [DOI] [PubMed]
- 3.Newman A, Sutton-Tyrrell K, Kuller L. Lower-extremity arterial disease in older hypertensive adults. Arterioscler Thromb. 1993;13(4):555–62. [DOI] [PubMed]
- 4.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44:166–206. [PubMed]
- 5.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. [DOI] [PubMed]
- 6.Morise AP, McDowell DE, Savrin RA, et al. The prediction of cardiac risk in patients undergoing vascular surgery. Am J Med Sci. 1987;293(3):150–8. [DOI] [PubMed]
- 7.Cheng SW, Ting AC, Lau H, Wong J. Survival in patients with chronic lower extremity ischemia: a risk factor analysis. Ann Vasc Surg. 2000;14(2):158–65. [DOI] [PubMed]
- 8.Harness N, Pinzur MS. Health related quality of life in patients with dysvascular transtibial amputation. Clin Orthop Relat Res. 2001;(383):204–7. [DOI] [PubMed]
- 9.Mackey WC, McCullough JL, Conlon TP, et al. The costs of surgery for limb-threatening ischemia. Surgery. 1986;99:26–35. [PubMed]
- 10.Raviola C, Nichter L, Baker J, Busuttil R, Machleder H, Moore W. Cost of treating advanced leg ischemia: bpass graft vs. primary amputation. Arch Surg. 1988;123:495–6. [DOI] [PubMed]
- 11.Weiss GN, Gorton TA, Read RC, Neal LA. Outcomes of lower extremity amputations. J Am Geriatr Soc. 1990;38(8):877–83. [DOI] [PubMed]
- 12.National Institutes of Health: National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 1997;157–46.
- 13.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285(19):2486–97. [DOI] [PubMed]
- 14.American Diabetes Association Consensus Statement: Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–41. [DOI] [PubMed]
- 15.McAlister AL, Rabius V, Geiger A, Glynn TJ, Huang P, Todd R. Telephone assistance for smoking cessation: one year cost effectiveness estimations. Tob Control. 2004;13(1):85–6. [DOI] [PMC free article] [PubMed]
- 16.Stevenson RB. American Cancer Society and smoking cessation. J Am Dent Assoc. 1989;118(3):274. [DOI] [PubMed]
- 17.Collins T, Johnson M, Henderson W, Khuri S, Daley J. Lower extremity non-traumatic amputation among veterans with peripheral arterial disease: is race an independent factor. Med Care. 2002;40(Suppl):I-106–16. [DOI] [PubMed]
- 18.Criqui M, Langer R, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. [DOI] [PubMed]
- 19.Leng G, Fowkes F, Lee A, Dunbar J, Housley E, Ruckley C. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. Br Med J. 1996;313:1440–4. [DOI] [PMC free article] [PubMed]
- 20.McDermott M, Criqui M, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–71. [DOI] [PubMed]
- 21.McDermott M, Greenland P, Liu K, et al. The ankle-brachial index is associated with leg function and physical activity: The Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–83. [DOI] [PubMed]
- 22.Rajagopalan S, Dellegrottaglie S, Furniss AL, et al. Peripheral arterial disease in patients with end-stage renal disease: observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation. 2006;114:1914–22. [DOI] [PubMed]
- 23.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Concensus (TASC). J Vasc Surg. 2000;31(1 Pt 2):S1–296. [PubMed]
- 24.Dawson DL, Cutler BS, Meissner MH, Strandness DE, Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–86. [DOI] [PubMed]
- 25.Money SR, Herd JA, Isaacsohn JL, et al. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg. 1998;27:267–74. [DOI] [PubMed]
- 26.Ernst E. Pentoxifylline for intermittent claudication: a critical review. Angiology. 1994;45:339–45. [DOI] [PubMed]
- 27.Girolami B, Bernardi E, Prins MH, et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta-analysis. Arch Intern Med. 1999;159:337–45. [DOI] [PubMed]
- 28.Hood SC, Moher D, Barber GG. Management of intermittent claudication with pentoxifylline: meta-analysis of randomized controlled trials. CMAJ. 1996;155:1053–59. [PMC free article] [PubMed]
- 29.Lindgarde F, Jelnes R, Bjorkman H, et al. Conservative drug treatment in patients with moderately severe chronic occlusive peripheral arterial disease. Circulation. 1989;80:1549–56. [DOI] [PubMed]
- 30.Porter JM, Cutler BS, Lee BY, et al. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am Heart J. 1982;104:66–72. [DOI] [PubMed]
- 31.Radack K, Wyderski RJ. Conservative management of intermittent claudication. Ann Intern Med. 1990;113(2):135–46. [DOI] [PubMed]
- 32.McDermott M, Mehta S, Liu K, et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14(3):173–81. [DOI] [PMC free article] [PubMed]
- 33.McDermott M, Hahn E, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med. 2002;17(895):904. [DOI] [PMC free article] [PubMed]
- 34.Banta MR, Ma F, Bravata DM, Kirsner RS, Federman DG. Incidence of and factors associated with achieving target lipid levels in patients with peripheral arterial disease. J Gen Intern Med. 2006;21(7):711–4. [DOI] [PMC free article] [PubMed]
- 35.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. [DOI] [PubMed]


