Abstract
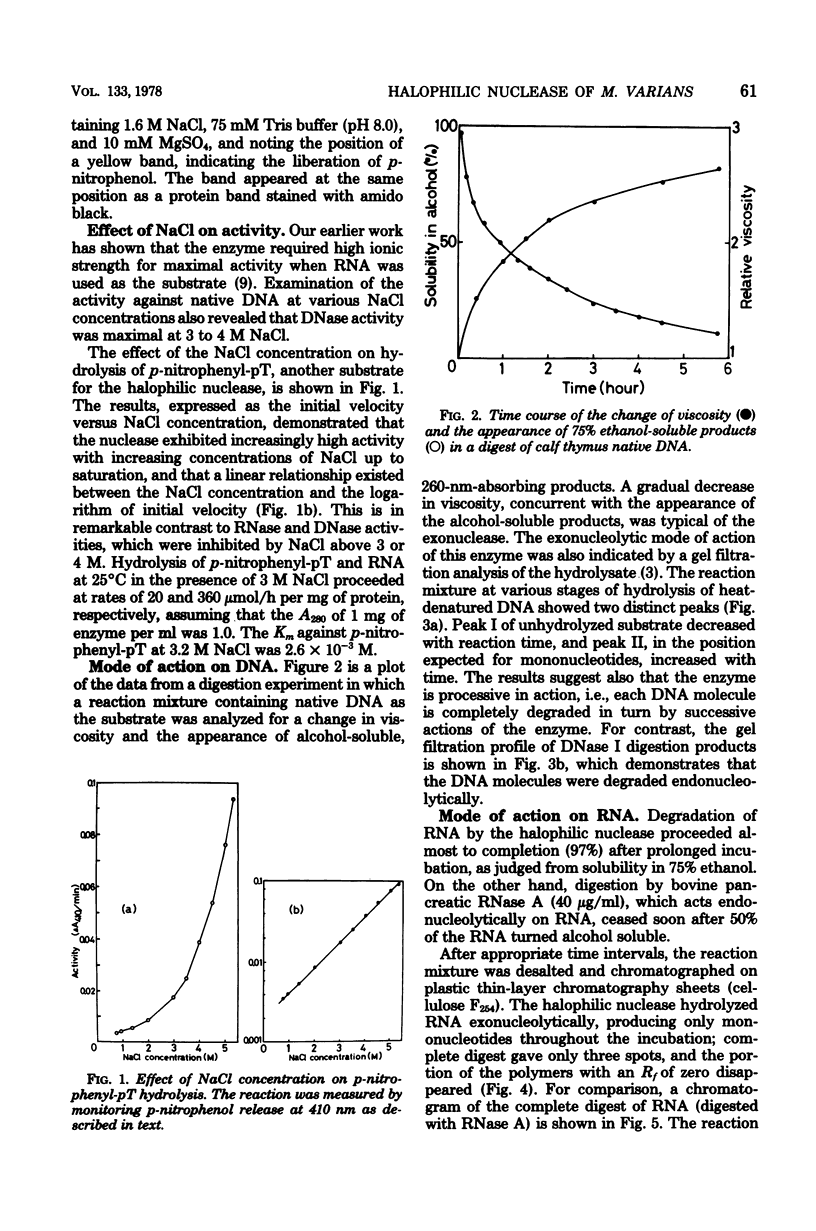
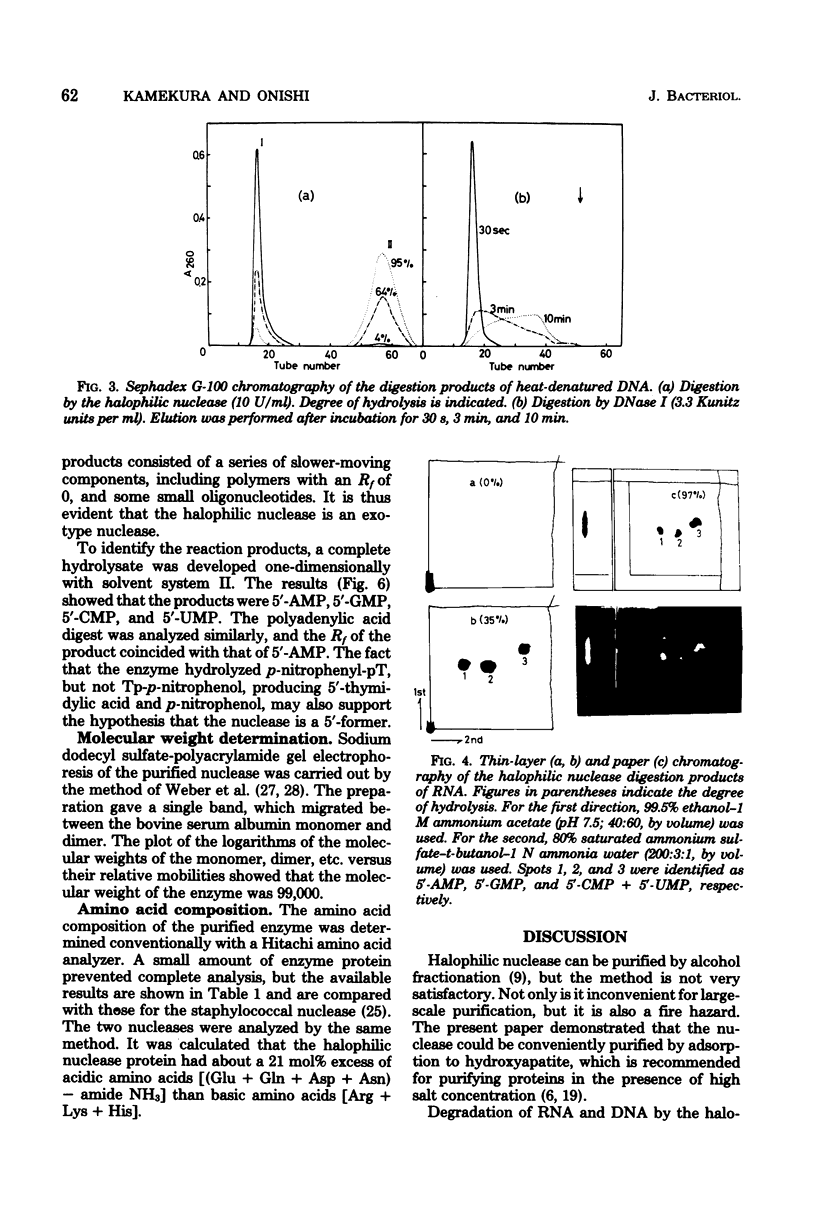
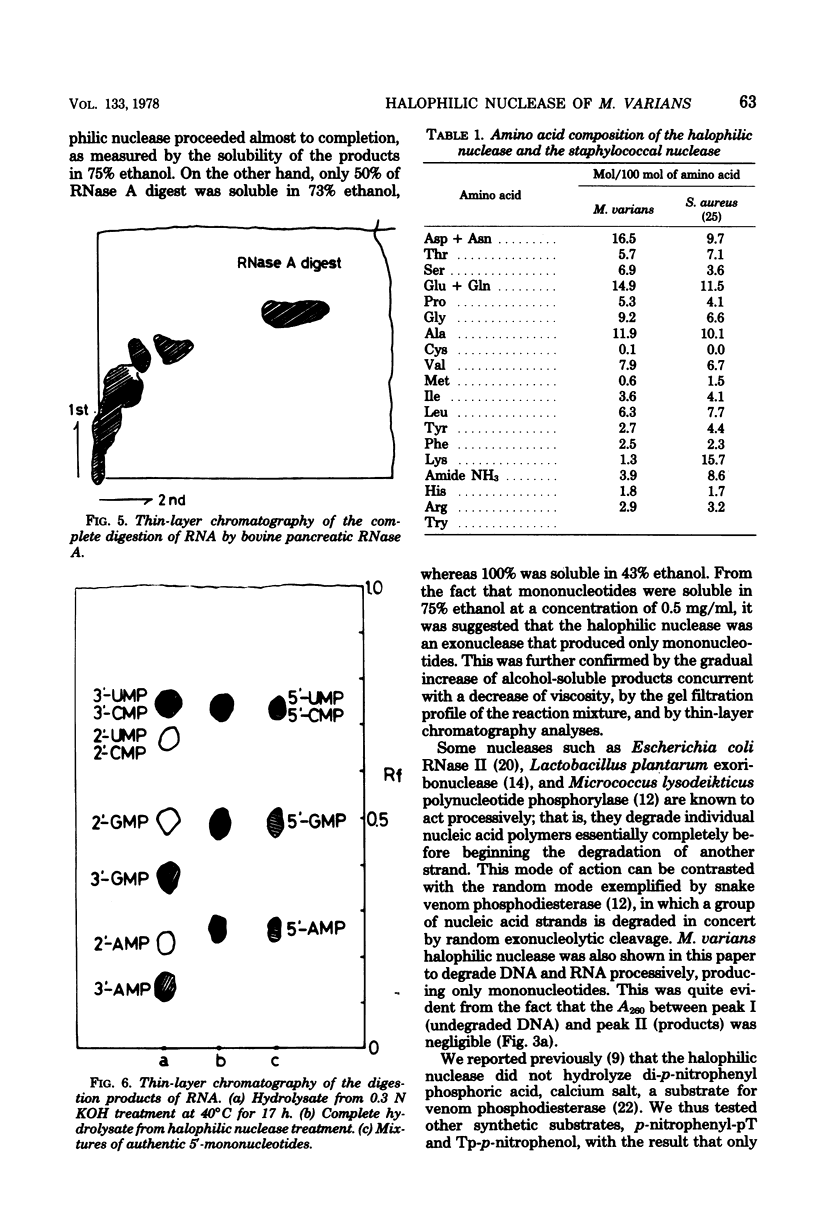
The halophilic nuclease of Micrococcus varians ATCC 21971 hydrolyzed thymidine 5'-monophospho-p-nitrophenyl ester at a rate that increased with the NaCl concentration up to saturation. The nuclease attacked RNA and DNA exonucleolytically and processively, producing 5'-mononucleotides. The molecular weight of the enzyme as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was 99,000, approximately the same as that previously determined for the native enzyme. Examination of amino acid composition showed that acidic amino acids were in high excess over basic amino acids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAXTER R. M. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can J Microbiol. 1959 Feb;5(1):47–57. doi: 10.1139/m59-006. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley S. T. Composition of ribosomes of an extremely halophilic bacterium. J Mol Biol. 1966 Feb;15(2):420–427. doi: 10.1016/s0022-2836(66)80117-1. [DOI] [PubMed] [Google Scholar]
- Birnboim H. The use of gel filtration to distinguish between endonucleolytic and exonucleolytic types of degradation. Biochim Biophys Acta. 1966 Apr 18;119(1):198–200. doi: 10.1016/0005-2787(66)90052-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dundas I. E. Purification of ornithine carbamoyltransferase from Halobacterium salinarium. Eur J Biochem. 1970 Oct;16(2):393–398. doi: 10.1111/j.1432-1033.1970.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- FELIX F., POTTER J. L., LASKOWSKI M. Action of venom phosphodiesterase on deoxyribooligonucleotides carrying a monoesterified phosphate on carbon 3'. J Biol Chem. 1960 Apr;235:1150–1154. [PubMed] [Google Scholar]
- Hubbard J. S., Miller A. B. Reversible inactivation of the isocitrate dehydrogenase from an obligate halophile: changes in the secondary structure. Arch Biochem Biophys. 1972 Jan;148(1):318–319. doi: 10.1016/0003-9861(72)90147-6. [DOI] [PubMed] [Google Scholar]
- Kamekura M., Onishi H. Effect of magnesium and some nutrients on the growth and nuclease formation of a moderate halophile, Micrococcus varians var. halophilus. Can J Microbiol. 1976 Oct;22(10):1567–1576. doi: 10.1139/m76-229. [DOI] [PubMed] [Google Scholar]
- Kamekura M., Onishi H. Halophilic nuclease from a moderately halophilic Micrococcus varians. J Bacteriol. 1974 Aug;119(2):339–344. doi: 10.1128/jb.119.2.339-344.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M., Sakaguchi K. Chemical and physical properties of peptidoglutaminase I and II from Bacillus circulans. Biochim Biophys Acta. 1976 Mar 18;427(1):285–294. doi: 10.1016/0005-2795(76)90304-4. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Singer M. F. The processive degradation of individual polyribonucleotide chains. II. Micrococcus lysodeikticus polynucleotide phosphorylase. J Biol Chem. 1968 Mar 10;243(5):923–927. [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D. M., Singer M. F. The processive degradation of individual polyribonucleotide chains. 3. Lactobacillus plantarum exoribonuclease. J Biol Chem. 1968 Dec 10;243(23):6161–6166. [PubMed] [Google Scholar]
- Louis B. G., Fitt P. S. Isolation and properties of highly purified Halobacterium cutirubrum deoxyribonucleic acid-dependent ribonucleic acid polymerase. Biochem J. 1972 Mar;127(1):69–80. doi: 10.1042/bj1270069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis B. G., Fitt P. S. Purification and properties of the ribonucleic acid-dependent ribonucleic acid polymerase from Halobacterium cutirubrum. Biochem J. 1972 Jul;128(4):755–762. doi: 10.1042/bj1280755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. L., Wicken A. J., Brown A. D. The outer layer of the cell envelope of Halobacterium halobium. Can J Biochem. 1969 Jan;47(1):71–74. doi: 10.1139/o69-013. [DOI] [PubMed] [Google Scholar]
- McWethy S. J., Hartman P. A. Purification and some properties of an extracellular alpha-amylase from Bacteroides amylophilus. J Bacteriol. 1977 Mar;129(3):1537–1544. doi: 10.1128/jb.129.3.1537-1544.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg P., von Hofsten B. Chromatography of a halophilic enzyme on hydroxylapatite in 3.4 M sodium chloride. Biochim Biophys Acta. 1970 Oct 14;220(1):132–133. doi: 10.1016/0005-2744(70)90239-1. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Studies on polynucleotides. III. Enzymic degradation; substrate specificity and properties of snake venom phosphodiesterase. J Biol Chem. 1959 Aug;234(8):2105–2113. [PubMed] [Google Scholar]
- Reistad R. On the composition and nature of the bulk protein of extremely halophilic bacteria. Arch Mikrobiol. 1970;71(4):353–360. doi: 10.1007/BF00417131. [DOI] [PubMed] [Google Scholar]
- Steensland H., Larsen H. A study of the cell envelope of the halobacteria. J Gen Microbiol. 1969 Mar;55(3):325–336. doi: 10.1099/00221287-55-3-325. [DOI] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. I. Linear order of the fragments produced by cleavage with cyanogen bromide. J Biol Chem. 1966 Oct 10;241(19):4366–4385. [PubMed] [Google Scholar]
- Tunis M. J., Hearst J. E. Optical rotatory dispersion of DNA in concentrated salt solutions. Biopolymers. 1968;6(8):1218–1223. doi: 10.1002/bip.1968.360060816. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B. Precipitation of polynucleotides from acidic solution. Effects of base composition and ionic strength. Biochemistry. 1973 Jul 17;12(15):2916–2925. doi: 10.1021/bi00739a023. [DOI] [PubMed] [Google Scholar]



