Abstract
Background
Patient adherence to warfarin may influence anticoagulation control; yet, adherence among warfarin users has not been rigorously studied.
Objective
Our goal was to quantify warfarin adherence over time and to compare electronic medication event monitoring systems (MEMS) cap measurements with both self-report and clinician assessment of patient adherence.
Design
We performed a prospective cohort study of warfarin users at 3 Pennsylvania-based anticoagulation clinics and assessed pill-taking behaviors using MEMS caps, patient reports, and clinician assessments.
Results
Among 145 participants, the mean percent of days of nonadherence by MEMS was 21.8% (standard deviation±21.1%). Participants were about 6 times more likely to take too few pills than to take extra pills (18.8 vs. 3.3%). Adherence changed over time, initially worsening over the first 6 months of monitoring, which was followed by improvement beyond 6 months. Although clinicians were statistically better than chance at correctly labeling a participant’s adherence (odds ratio = 2.05, p = 0.015), their estimates often did not correlate with MEMS-cap data; clinicians judged participants to be “adherent” at 82.8% of visits that were categorized as moderately nonadherent using MEMS-cap data (≥20% nonadherence days). Similarly, at visits when participants were moderately nonadherent by MEMS, they self-reported perfect adherence 77.9% of the time.
Conclusions
These results suggest that patients may benefit from adherence counseling even when they claim to be taking their warfarin or the clinician feels they are doing so, particularly several months into their course of therapy.
KEY WORDS: patient adherence, warfarin, medication event monitoring system
INTRODUCTION
The anticoagulant warfarin is widely used for the prevention of thromboembolism, but it is often difficult to manage patients on the drug because of its narrow therapeutic index. Even among those treated at specialized anticoagulation clinics, it is estimated that patients are over- or underanticoagulated for as much as half of the time they are on the drug, putting them at significant risk for life-threatening bleeding complications or thromboembolism.1,2
Poor patient adherence to the prescribed drug regimen is often cited as an explanation for out-of-range international normalized ratio (INR) measurements.3,4 Despite this, there is little rigorous evidence of the level of adherence to warfarin, particularly among a broad spectrum of patients and anticoagulation practices.
One reason for the lack of data on adherence to warfarin is the difficulty in measuring adherence.5 To our knowledge, no studies to date have quantified adherence to warfarin over extended periods of time using medication event monitoring systems (MEMS) caps in large, broadly represented populations, nor compared this with patient report and clinician assessment.
Our goal in this study was to characterize patient adherence to warfarin using MEMS caps and to correlate these findings with both clinician assessment and patient self-report of adherence.
METHODS
Study Design, Setting, and Study Population
We conducted a prospective cohort study of subjects recruited from 3 anticoagulation clinics in Pennsylvania: the Hospital of the University of Pennsylvania (HUP) in Philadelphia, the Philadelphia Veterans Affairs Medical Center (PVAMC), and the Hershey Medical Clinic (HMC) in Hershey, PA. Details of the study design have previously been published.6 Briefly, subjects who were ≥21 years old and within 2 months of initiating warfarin therapy with a target INR of 2.0 to 3.0 were eligible for the study. All subjects were provided MEMS caps (Aardex, Zug, Switzerland) to monitor adherence. A minimum of 7 days of MEMS-cap use was required for inclusion in the cohort given the instability of adherence estimates with shorter durations of use.
Data Collection
Study participants were given MEMS caps for their warfarin pill bottles, which recorded the exact time and date the bottle was opened. Information on participant demographics was obtained by trained study interviewers using standardized interviews. Information on self-reported assessments of adherence also was collected by standardized study interview at all follow-up visits, in which participants were asked how many pills they skipped and how many extra pills they took since their last visit. Clinicians’ estimate of participant pill-taking was performed in accordance with standard of care at each site and was not modified for the study. At PVAMC, clinicians performed pill counts and then recorded whether they believed the participant was adherent or not based on these counts. At HUP and HMC, clinicians recorded their assessment of adherence based on their own assessment and patient reports to clinicians without considering pill count. Clinicians and patients were blinded to the MEMS-cap data but not to the INR level at the time of their assessment or reporting of adherence (and thus, these measurements of adherence were made after INR measurement). Because MEMS caps record continuously, we were able to obtain adherence data prior to the INR.
A large proportion of participants were accustomed to using 7-day pill organizers rather than medication bottles, which precluded use of the MEMS cap directly. These participants were instructed to open and then shut an empty bottle with a MEMS cap as a “diary” each time they opened their 7-day pill organizers to take their warfarin. If therapeutically appropriate, participants were assigned alternate-day dosing (ADD). Participants with ADD using the cap as a “diary” were instructed to open the cap once each time they removed a dose from their 7-day pill reminders, regardless of dosage. Participants with ADD using the cap directly on the bottle used a single bottle with a MEMS cap.
Measures of Adherence
The time of day at which participants “normally” took their medicines was not taken into account on a per participant basis. A “day” was strictly defined as the 24-h period from 3:00 am to 2:59 am for all participants, to allow for those who took their warfarin late at night.
The percent of days that the incorrect warfarin dose was taken by MEMS cap, PMEMS,incorrect, was defined as the number of days the participant either did not open the bottle with the MEMS cap when they were supposed to take a pill or opened it more than once divided by the number of days in the monitored period. Days for which the clinician instructed the participant to take no pills were also considered incorrect if the participant opened the bottle on that day. The percent of days overadherent, PMEMS,over, was defined as the number of days the participant opened the bottle more often than prescribed divided by the number of days in the monitored period. The percent of days underadherent, PMEMS,under, was defined as the number of days with no bottle openings divided by the number of days when the participant was instructed to take a pill. Because a participant cannot be underadherent on days when they are told to take zero pills, these days were excluded from the calculations for PMEMS,under.
For participant-reported adherence, we calculated self-reported percent incorrect Pself,incorrect by taking the number of pills skipped and extra and dividing by the number of days since the last clinic visit. Pself,incorrect was then used to compare self-reported adherence against adherence measured by the cap. We used 2 a priori cutoffs for Pself,incorrect: (1) a cutoff of 20% based on the mean adherence by pill cap measured in our pilot work and (2) a cutoff of 0% to dichotomize self-reported adherence to a yes/no question for the sake of comparison to the clinician assessment of adherence, which was collected in a yes/no fashion, and also to identify those who reported perfect adherence.
Data Analysis
Descriptive statistics for the adherence variables included means, medians, and standard deviations (SD). To measure how adherence changed over time, we examined the trend of adherence over each month of follow-up. In particular, PMEMS,incorrect was regressed on time (in months) since the first clinic visit. To capture the nonlinear time trend of the adherence outcome, we used polynomial terms of time (month, month2, month3...) in the model. We used a linear regression model with random subject effect with a first-order autoregressive correlation structure to accommodate the clustering of responses contributed by the same participant. Because of the lack of normality of PMEMS,incorrect, we also used a logit transformation to achieve approximate normality. Likelihood ratio tests were used to select the degree of polynomials because these models were nested. For the purposes of these trend analyses, data were truncated at 12 months because few participants were followed longer.
Clinician assessment of participant adherence at each visit was compared with MEMS-cap data using a generalized estimating equation (GEE) logistic regression model. Similar analyses were performed for participants’ self-reported adherence.
We have previously reported the results of the relationship between adherence as measured by MEMS caps and INR.6 However, we did not assess whether these associations were independent of either clinician assessment or patient reports of adherence. Therefore, we performed multivariable GEE regression analyses where the dependent variable was low INR and the independent variables included terms for MEMS-cap PMEMS,under and either clinician assessment or patient reports of adherence. There were too few visits with overadherence and too few high INRs to perform analyses for overanticoagulation.
All statistics were performed with SAS (version 9.1, SAS Institute, Cary, NC, USA) and SPSS (version 11.0.1, LEAD Technologies, Chicago, IL, USA). The Institutional Review Boards at all participating hospitals approved the study, and all participants provided informed consent.
RESULTS
Participants
The MEMS cap was offered to 188 participants and 161 (86%) agreed to use it. Sixteen of the 161 participants were excluded because they used the MEMS cap for <7 days. A total of 26,753 days (891.8 months) were monitored for the remaining 145 participants over 1,921 visits, with a mean follow-up of 184.5 days (SD 181.9 days; median 126 days). The mean interval between visits was 16.3 days (median 14 days; range 1–106 days). Baseline characteristics of the participants at the 3 anticoagulation clinics are shown in Table 1.
Table 1.
Patient Demographics at the 3 Clinic Sites
| Total | HUP | VAMC | HMC | |
|---|---|---|---|---|
| Participants, n (%) | 145 (100.0%) | 62 (42.8%) | 73 (50.3%) | 10 (6.9%) |
| Gender, n (%) | ||||
| Male | 107 (73.8%) | 30 (48.4%) | 70 (95.9%) | 7 (70.0%) |
| Female | 38 (26.2%) | 32 (51.6%) | 3 (4.1%) | 3 (30.0%) |
| Race, n (%) | ||||
| African American | 76 (52.4%) | 36 (58.1%) | 40 (54.8%) | 0 (0.0%) |
| White | 65 (44.8%) | 25 (40.3%) | 30 (41.1%) | 10 (100.0%) |
| Other | 4 (2.8%) | 1 (1.6%) | 3 (4.1%) | 0 (0.0%) |
| Age (years), n (%) | ||||
| Mean age (±SD) | 57.9 (±14.6) | 52.5 (±16.9) | 60.2 (±11.6) | 68.4 (±14.6) |
| <35 | 11 (7.6%) | 10 (16.1%) | 1 (1.4%) | 0 (0.0%) |
| 35–44 | 17 (11.7%) | 11 (17.7%) | 6 (8.2%) | 0 (0.0%) |
| 45–54 | 26 (17.9%) | 13 (21.0%) | 11 (15.1%) | 2 (20.0%) |
| 55–64 | 43 (29.7%) | 11 (17.7%) | 31 (42.5%) | 1 (10.0%) |
| 65–74 | 28 (19.3%) | 10 (16.1%) | 16 (21.9%) | 2 (20.0%) |
| >74 | 20 (13.8%) | 7 (11.3%) | 8 (11.0%) | 5 (50.0%) |
| Income, $ | ||||
| Mean income (±SD) | 20,405 (±12,075) | 23,678 (±15,591) | 17,715 (±7,082) | 18,214 (±10,074) |
| Education, n (%) | ||||
| Elementary (1–8 years) | 3 (2.1%) | 0 (0%) | 2 (2.7%) | 1 (11.1%) |
| High School (9–12 years) | 65 (45.1%) | 19 (30.7%) | 45 (61.6%) | 1 (11.1%) |
| College/Trade (13–16 years) | 59 (41.0%) | 31 (50.0%) | 24 (32.9%) | 4 (44.4%) |
| More than college (>17 years) | 17 (11.8%) | 12 (19.4%) | 2 (2.7%) | 3 (33.3%) |
| Employment status, n (%) | ||||
| Working | 41 (28.7%) | 23 (37.1%) | 16 (22.5%) | 2 (20.0%) |
| Unemployed | 18 (12.6%) | 8 (12.9%) | 10 (14.1%) | 0 (0%) |
| Retired | 57 (39.9%) | 19 (30.7%) | 31 (43.7%) | 7 (70.0%) |
| Disabled | 27 (18.9%) | 12 (19.4%) | 14 (19.7%) | 1 (10.0%) |
| Use of MEMS cap, n (%) | ||||
| On the bottle | 59 (40.7%) | 13 (21.0%) | 45 (61.6%) | 1 (10.0%) |
| As a diary | 86 (59.3%) | 49 (79.0%) | 28 (38.4%) | 9 (90.0%) |
| Mean duration of cap use (days) (±SD) | 184.5 (±181.9) | 168.8 (±177.5) | 211.1 (±190.8) | 87.8 (±82.1) |
n = number
Income per household member. Income data on 24 participants are missing, education data on 1 participant are missing, and employment data on 2 participants are missing
Adherence Behavior as Measured by MEMS Caps
On average, participants had an incorrect MEMS-cap opening on 21.8% of days (SD 21.1%; median 14.9%). Participants were 6 times more likely to miss pills (PMEMS,under mean 18.8%; SD 20.5%; median 11.6%) than to take extra pills (PMEMS,over mean 3.3%; SD 4.5%; median 1.9%). The distribution of PMEMS,incorrect is presented in Figure 1. Overall, 38.6% of patients had >20% incorrect pill bottle openings. There was no difference in PMEMS,incorrect or PMEMS,under for those using the MEMS cap on the bottle vs. as a diary (mean PMEMS,incorrect 21.1 vs. 22.2% for bottle vs. diary users; p = 0.542; mean PMEMS,under 17.1 vs. 20.0% for bottle vs. diary users; p = 0.243). Participants using the MEMS cap on the bottle were more likely to have extra bottle openings than participants using the cap as a diary (4.6 vs. 2.4%, respectively; p = 0.036).
Figure 1.
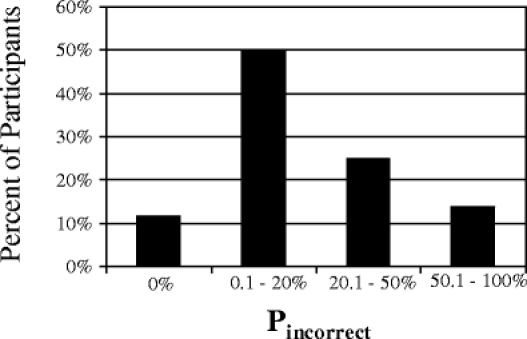
Distribution of the percent of days nonadherent (PMEMS,incorrect). There are 17 participants in the 0% group, 39 in the 0.1–10.0% group, 33 in the 10.1–20.0% group, 16 in the 20.1–30.0% group, 13 in the 30.1–40.0% group, 7 in the 40.1–50.0% group, and 20 in the >50% group.
Results for PMEMS,incorrect over time are presented in Figure 2. PMEMS,incorrect increased from 17.0 to 26.6% from months 1 to 6 and then decrease by month 12 to 17.1% (p < 0.001 for the quadratic term in a model with both month and month2). To determine whether this trend was because of more nonadherent subjects dropping out, sensitivity analyses were performed on the subset of participants who used the cap continuously from month 1 through months 9 or 12 (34 and 20 participants for 9 and 12 months, respectively). For both the 9- and 12-month subsets, adherence behaviors over time were similar to the group as a whole, with adherence worsening until month 6 and then improving between months 6 and 12 (data not shown).
Figure 2.
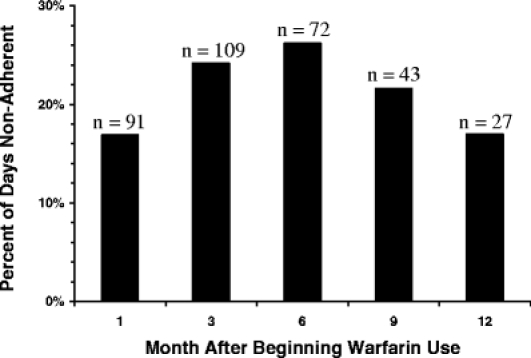
Percent of days nonadherent (PMEMS,incorrect) vs. month after beginning warfarin use. The sample size for each month is indicated by n above each bar.
Clinician Assessment of Adherence
Clinician assessment of participant adherence was compared against MEMS measured adherence. Results presented in Table 2 are on a per-visit basis and include multiple visits per participant. In general, clinicians were more likely to label participants adherent than not (717 of 812 visits, 88.3%). The odds of the clinician reporting that the participant was nonadherent were 2 times greater for visits at which the participant was nonadherent per the MEMS cap (using ≥20% incorrect openings as “nonadherent”) than for visits at which the participant was adherent per the MEMS cap [GEE-calculated odds ratio (OR) 2.05; 95% confidence interval (CI) 1.15–3.64; p = 0.015]. However, despite doing better than chance, clinicians often reported MEMS-cap-recorded, nonadherent participants as adherent. For example, for even the most noncompliant visits with PMEMS,incorrect ≥50%, clinicians thought participants were adherent over 80% of the time (93 of 111 visits; 83.8%).
Table 2.
Clinician Assessment of Adherence as Compared with MEMS-cap-measured Adherence
| Participant “nonadherent” per clinician | Participant “adherent” per clinician | |
|---|---|---|
| Participant nonadherence per MEMS cap using 2 different cutoff points | ||
| PMEMS,incorrect ≥ 20% (n = 303) | 17.2% (n = 52) | 82.8% (n = 251) |
| PMEMS,incorrect ≥ 50% (n = 111) | 16.2% (n = 18) | 83.8% (n = 93) |
| Participant adherence per MEMS cap using 2 different cutoff points | ||
| PMEMS,incorrect < 20% (n = 509) | 8.4% (n = 43) | 91.6% (n = 466) |
| PMEMS,incorrect < 50% (n = 701) | 11.0% (n = 77) | 89.0% (n = 624) |
n = number
Results presented are based on visits and do not adjust for clustering based on participant. The total number of visits at which clinicians reported on adherence was 812.
Subgroup analyses were performed comparing PVAMC (where pill counts were performed) to HUP/HMC to determine how pill counts might augment clinician assessment. Among the 163 PVAMC visits at which participants were nonadherent by the PMEMS,incorrect ≥20% cutoff, clinicians mislabeled participants as adherent less often [125 visits (76.7%)] than the 140 visits at HUP/HMC with PMEMS,incorrect ≥20% [126 visits labeled adherent (90.0%), p = 0.002].
Participant Assessment of Adherence
Similar to clinicians’ assessments, participants were more likely to say they were nonadherent when they were nonadherent by MEMS-cap data (Table 3; for PMEMS,incorrect ≥20% GEE-calculated OR 10.1; 95% CI 1.1–90.6; p = 0.038). However, participant reports of adherence still overestimated their adherence by MEMS caps. For example, participants who missed at least 50% of doses per the MEMS-cap-reported perfect adherence (no missed pills) at 78.5% of visits.
Table 3.
Participant Assessment of Adherence as Compared with MEMS-cap-measured Adherence
| Participant nonadherent per self-report Pself,incorrect > 0% | Participant adherent per self-report Pself,incorrect = 0% | |
|---|---|---|
| Participant nonadherence per MEMS cap using 3 different cutoff points | ||
| PMEMS,incorrect > 0% (n = 607) | 18.3% (n = 111) | 81.7% (n = 496) |
| PMEMS,incorrect ≥ 20% (n = 298) | 22.1% (n = 66) | 77.9% (n = 232) |
| PMEMS,incorrect ≥ 50% (n = 107) | 21.5% (n = 23) | 78.5% (n = 84) |
| Participant adherence per MEMS cap using 3 different cutoff points | ||
| PMEMS,incorrect = 0% (n = 197) | 2.0% (n = 4) | 98.0% (n = 193) |
| PMEMS,incorrect < 20% (n = 506) | 9.7% (n = 49) | 90.3% (n = 457) |
| PMEMS,incorrect < 50% (n = 697) | 13.2% (n = 92) | 86.8% (n = 605) |
n = number
Results presented are based on visits and do not adjust for clustering based on participant. The total number of visits at which participants reported on adherence was 804.
Relationship Between Adherence and INR
The relationships between PMEMS,under and low INRs (<2.0) were similar in analyses that adjusted for either clinician assessment or patient self-reported adherence compared with analyses that did not adjust for these factors.6 When adjusting for clinician assessment of adherence, the ORs (95% CIs) for 0.1–10, 10.1–20, 20.1–30, 30.1–50, and >50% underadherence by MEMS were 1.56 (0.89–2.75), 1.48 (0.79–2.78), 2.33 (1.25–4.35), 2.53 (1.48–4.33), and 2.58 (1.34–4.95), respectively (p value for trend <0.01). When adjusting for patient self-reported adherence, the ORs for 0.1–10, 10.1–20, 20.1–30, 30.1–50, and >50% underadherence by MEMS were 1.48 (0.86–2.55), 1.41 (0.76–2.62), 2.11 (1.16–3.85), 2.57 (1.45–4.54), and 2.24 (1.15–4.39), respectively (p value for trend <0.01).
DISCUSSION
Our findings demonstrate that nonadherence to warfarin occurs to a substantial degree in patients despite being closely monitored and counseled in specialized anticoagulation clinics. Participants appeared 6 times more likely to miss pills than to take extra pills, which would put them at greater risk of underanticoagulation and thromboembolism. In addition, adherence was found to decrease with time after initiation of warfarin use but rebound between 6 and 12 months. Adherence as measured by MEMS caps identified more nonadherence than either clinician assessment or patient self-reports and was associated with anticoagulation control, independent of these other methods of assessing adherence.
Few other studies have reported on adherence to anticoagulant therapy. A study using pill counts reported approximately 90% adherence with warfarin.7 Our study found a lower rate of adherence using MEMS caps, as would be expected given the known overestimation of adherence resulting from pill counts.8,9 Two small, short-term studies have made use of MEMS caps to characterize adherence to an anticoagulation regimen. One single-center study of phenprocoumon found better adherence than in our study (7.2% incorrect cap openings).8 However, the small size (n = 30) of this cohort, who were selected from among 6,500 treated at the clinic, and the short follow-up period (≤3 months) make comparisons with our multicenter, longer-term study difficult. Another study found 80% correct bottle openings among warfarin patients, similar to our findings.9 Unlike our study, this prior study included only patients selected for a randomized trial, was small (n = 40), and had a median duration of follow-up of only 83 days (vs. our median duration of 126 days). None of these prior studies described how adherence changed over time.
The reasons for the change in adherence over time are unclear but did not appear to be because of a dropout effect. Perhaps patients were more diligent when initiating therapy; later began to miss more pills; and then, as a result of loss of anticoagulation and perhaps increased clinician instructions, again became more adherent. These findings suggest that patients may benefit from interventions to improve adherence, particularly in the several months after they initiate warfarin, but certainly throughout their course of therapy because adherence is still poor even in later months. Also, although clinicians may focus on adherence barriers at the beginning of treatment, barriers may crop up during treatment. Therefore, clinicians must recognize that adherence needs to be readdressed throughout follow-up, even in patients who may be adherent early in their therapy.
Although clinicians were statistically better than chance at correctly labeling a participant as adherent or not compared to the MEMS cap, their estimate was often different from that assessed by MEMS caps. Although MEMS caps are not the perfect measure of adherence, studies of other medications have suggested that clinicians do tend to overestimate adherence.10–12 This finding highlights the need for clinicians to encourage appropriate pill-taking behavior, even if the patient appears to be doing so. Whereas clinicians were less likely to incorrectly label a patient adherent when they had information on pill counts, they still concluded that more than 75% of nonadherent participants were adherent. This underscores the inaccuracy of pill counts.13–15
Our data also suggest that self-report, at least as typically reported in clinical practice, may underestimate nonadherence in warfarin users. This finding is consistent with previous studies that have shown that patient self-reports can be inaccurate measures.13–15
In terms of the limitations of our study, analyses of participant adherence relied on the use of electronic pill caps, which may not reflect actual pill taking. Many participants did not use the MEMS caps directly on a pill bottle, although their MEMS-cap adherence was similar to those who did use the cap on their pill bottles. In addition, we could not determine whether patients prescribed different doses on different days actually took the correct dose, which would likely have underestimated nonadherence. In addition, participants knew they were being monitored, which may have improved their adherence. In general, however, electronic pill caps are considered the most accurate method of assessing pill-taking behavior.5,16 Furthermore, even accounting for clinician assessment and patient reports of adherence, MEMS-cap-measured adherence was still associated with anticoagulation control.
Our findings also may not be fully generalizable to warfarin users outside of the anticoagulation clinic population.17,18 Nonetheless, anticoagulation clinics are becoming the standard-of-care for patients on warfarin,18,19 and the inclusion of anticoagulation clinics minimizes confounding by variability in practice patterns. Moreover, our clinic sites provided geographic and socioeconomic diversity, thus enhancing generalizability.
Clinicians were not blinded to the INR, which might have influenced their assessment of adherence. Whether this led to an advantage in estimating adherence (i.e., if the INR was low in the setting of poor adherence) or a disadvantage (assuming good adherence because the INR was in range, which can occur if warfarin dose has been titrated to a patient’s consistent level of nonadherence), our findings underscore the difficulty of judging patient adherence even with monitoring of INR.
In conclusion, patients at specialized anticoagulation clinics incorrectly take their warfarin medication on approximately 20% of days of intended therapy, a clinically meaningful level of nonadherence.6 Adherence also declines significantly in the first several months of therapy. Finally, clinicians’ subjective impressions of patient adherence – even when based on the patient’s INR levels or pill counts – and patients’ self-reports do not correlate well with electronically measured adherence. To reduce rates of nonadherence, clinicians treating patients with warfarin should continue to emphasize strict adherence, even among patients whom they believe are adherent and throughout the course of therapy.
Acknowledgements
This study was supported by grants from the NIH (R01-HL66176) and Agency for Healthcare Research and Quality (P01-HS11530). Dr. Kimmel is supported by P20RR020741. The authors would like to thank Anita Hill, BS; Kiasha Huling, BA; Linda Bowman, BS, RN; Mitchell Laskin, RPh; Mabel Chin, PharmD; and Francis Herrmann, BS, RPh, for their dedicated field work; Joseph Gascho, MD, for serving as the site investigator at the Hershey Medical Center; and Sandy Barile for editorial assistance.
Conflict of Interest Dr. Kimmel has received research funding from GlaxoSmithKline and Pfizer, and has served as a consultant to Bayer, GlaxoSmithKline, Bristol-Meyers Squibb, and Pfizer, all unrelated to warfarin. Dr. Gross has received research funding from Bristol-Myers Squibb, unrelated to warfarin. Dr. Samaha has received grant support from Merck, Sharpe and Dome; Abbott; Kos Pharmaceuticals; and Aegerion, all unrelated to warfarin.
References
- 1.Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158:1641–7. [DOI] [PubMed]
- 2.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423–8. [DOI] [PubMed]
- 3.Kutner M, Nixon G, Silverstone F. Physicians’ attitudes toward oral anticoagulants and antiplatelet agents for stroke prevention in elderly patients with atrial fibrillation. Arch Intern Med. 1991;151:1950–3. [DOI] [PubMed]
- 4.Bush D, Tayback M. Anticoagulation for nonvalvular atrial fibrillation: effects of type of practice on physicians’ self-reported behavior. Am J Med. 1998;104:148–51. [DOI] [PubMed]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. [DOI] [PubMed]
- 6.Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med. 2007;167:229–35. [DOI] [PubMed]
- 7.Howard AF, Frewin DB, Leonello PP, Taylor WB. Compliance with anticoagulant drug therapy: a study on patients with prosthetic heart valves. Med J Aust. 1981;2:274–6. [PubMed]
- 8.Van der Meer FJ, Briet E, Vandenbroucke JP, et al. The role of compliance as a cause of instability in oral anticoagulant therapy. Br J Haematol. 1997;98:893–900. [DOI] [PubMed]
- 9.Laporte S, Quenet S, Buchmuller-Cordier A, et al. Compliance and stability of INR of two oral anticoagulants with different half-lives: a randomised trial. Thromb Haemost. 2003;89:458–67. [PubMed]
- 10.Butler JA, Peveler RC, Roderick P, et al. Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation. 2004;77:786–9. [DOI] [PubMed]
- 11.Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133:129–33. [DOI] [PubMed]
- 12.Gross R, Bilker WB, Friedman HM, Coyne JC, Strom BL. Provider inaccuracy in assessing adherence and outcomes with newly initiated antiretroviral therapy. AIDS. 2002;16(13):1835–7. [DOI] [PubMed]
- 13.Pullar T, Kumar S, Tindall H, Feely M. Time to stop counting the tablets? Clin Pharmacol Ther. 1989;46:163–8. [DOI] [PubMed]
- 14.Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–7. [DOI] [PubMed]
- 15.Urquhart J. Role of patient compliance in clinical pharmacokinetics. A review of recent research. Clin Pharmacokinet. 1994;27:202–15. [DOI] [PubMed]
- 16.Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32:345–56. [DOI] [PubMed]
- 17.Wilson SJ, Wells PS, Kovacs MJ, et al. Comparing the quality of oral anticoagulant management by anticoagulation clinics and by family physicians: a randomized controlled trial. CMAJ. 2003;169:293–8. [PMC free article] [PubMed]
- 18.Gallus AS. Towards the safer use of warfarin: an overview. J Qual Clin Pract. 1999;19:55–9. [DOI] [PubMed]
- 19.Ansell JE, Buttaro ML, Thomas OV, Knowlton CH. Consensus guidelines for coordinated outpatient oral anticoagulation therapy management. Anticoagulation Guidelines Task Force. Ann Pharmacother. 1997;31:604–15. [DOI] [PubMed]


